Abstract
Water loss either by desiccation or freezing causes multiple forms of cellular damage. The encysted embryos (cysts) of the crustacean Artemia franciscana have several molecular mechanisms to enable anhydrobiosis—life without water—during diapause. To better understand how cysts survive reduced hydration, group 1 late embryogenesis abundant (LEA) proteins, hydrophilic unstructured proteins that accumulate in the stress-tolerant cysts of A. franciscana, were knocked down using RNA interference (RNAi). Embryos lacking group 1 LEA proteins showed significantly lower survival than control embryos after desiccation and freezing, or freezing alone, demonstrating a role for group 1 LEA proteins in A. franciscana tolerance of low water conditions. In contrast, regardless of group 1 LEA protein presence, cysts responded similarly to hydrogen peroxide (H2O2) exposure, indicating little to no function for these proteins in diapause termination. This is the first in vivo study of group 1 LEA proteins in an animal and it contributes to the fundamental understanding of these proteins. Knowing how LEA proteins protect A. franciscana cysts from desiccation and freezing may have applied significance in aquaculture, where Artemia is an important feed source, and in the cryopreservation of cells for therapeutic applications.
Keywords: Late embryogenesis abundant (LEA) proteins, Intrinsically disordered proteins (IDPs), Desiccation tolerance, Freeze tolerance, Brine shrimp, RNA interference (RNAi)
Introduction
Survival in extreme or unpredictable environments is facilitated by tolerance of biotic and abiotic stresses. Anhydrobiotic organisms withstand loss of almost all cell water, surviving dry conditions for prolonged periods, even at subzero temperatures (Browne et al. 2002; Caprioli et al. 2004; Clegg 1986; Lapinski and Tunnacliffe 2003; Tyson et al. 2012). Encysted embryos (cysts) of the brine shrimp, Artemia franciscana, withstand cellular water contents lower than 2 %, and survive temperatures below −20 °C (Crowe et al. 1981; Hengherr et al. 2011). Cysts are produced oviparously in Artemia females where development stalls at gastrulation and embryos are coated with a rigid, chitinous shell impermeable to non-volatile compounds (de Chaffoy et al. 1978; Dai et al. 2011; Liang and MacRae 1999; Ma et al. 2013). Once released from females, the metabolic activity of cysts declines dramatically and they enter diapause, a reversible dormancy characterized by extreme stress tolerance (Clegg et al. 1996; Qiu and MacRae 2010).
Termination of cyst diapause requires cues that are species- and population-specific (Drinkwater and Crowe 1987; Van Stappen et al. 1998). Artemia cysts from the Great Salt Lake (GSL), UT terminate diapause efficiently in the laboratory when desiccated and subsequently incubated at −20 °C for up to 3 months, both substantial stresses (King and MacRae 2012). Alternatively, hydrogen peroxide (H2O2) terminates diapause in Artemia cysts from the GSL and other geographical locations (Robbins et al. 2010; Van Stappen et al. 1998; Veeramani and Baskaralingam 2011). The molecular pathways responding to termination cues have not been identified, but once diapause is broken A. franciscana embryos in favourable environments resume development, emerging from cracked cyst shells as membrane-enclosed prenauplii termed E1 and E2 (Go et al. 1990; Rafiee et al. 1986; Trotman et al. 1980). Prenauplii hatch upon membrane rupture to yield swimming nauplii which molt numerous times to become adults.
Accumulation of small compounds such as trehalose, in addition to molecular chaperones and late embryogenesis abundant (LEA) proteins, contribute to desiccation and freeze tolerance. A. franciscana embryos accrue these protective molecules as part of the developmentally programmed entrance into diapause, thereby stabilizing macromolecules and cell integrity under conditions of stress (Qiu and MacRae 2010). Trehalose functions as a water replacement molecule and colligative agents such as glycerol likely enhance osmotic stress tolerance and reduce damage from ice formation by lowering the freezing point of water (Clegg 1986; Storey and Storey 2013). Molecular chaperones, like the small heat shock protein (sHsp) p26, contribute substantially to cyst stress tolerance by preventing irreversible denaturation of other proteins (King and MacRae 2012). At least six group 1 LEA proteins or their mRNAs are upregulated in A. franciscana cysts (Table 1), along with several group 3 LEA proteins and their transcripts (Boswell et al. 2013; Hand et al. 2007; Menze et al. 2009; Sharon et al. 2009; Warner et al. 2010, 2012). Group 1 LEA proteins, containing the repeated 20 amino acid motif GGQTRREQLGEEGYSQMGRK, have not been detected in any animal other than Artemia (Hand et al. 2011; Tunnacliffe and Wise 2007).
Table 1.
Group 1 LEA proteins in A. franciscana
| LEA proteina | Repeatsb | NCBIc | Size, massd | Expression/functione | Ref.f |
|---|---|---|---|---|---|
| AfrLEA1-1mg, Mit | 8 | ACX81198, GQ406334 | 217 aa, 21 kDa |
[1] | |
| AfrLEA1-1g
(LEA-1a), Cyt |
8 | ABR67402, EF656614 | 182 aa, 21 kDa |
↑ in diapause (mRNA and protein) ↓ protein aggregation |
[1,2] |
| AfrLEA1-2 (LEA-1b), Cyt/mit | 7 | 150 aa, 19 kDa |
↑ in diapause (protein) | [1,3] | |
| AfrLEA1-3 (LEA-1c), Cyt | 6 | ADE45145, GU568033 | 142 aa, 15.5 kDa |
↑ in diapause (protein) | [1] |
| AfrLEA1-4 (LEA-1d) | 5 | ADE45146, GU568034 | 122 aa, 13 kDa |
↑ in diapause (protein) | [1] |
| AfrLEA1-5 | 4 | ABX89317, ES500255 | 102 aa, 10 kDa |
↑ in diapause (mRNA) | [1,3] |
| AfrLEA1-6 (LEA-1 g) |
2 | ADE45147, GU568035 | 62 aa, 5 kDa |
[1] |
aNomenclature based on Wu et al. 2011. The nomenclature used by authors who originally characterized the proteins is in parentheses. Cell location, cyt cytosol; mit mitochondria
bRepeats of the conserved 20 amino acid motif characteristic of group 1 LEA proteins
cNCBI (National Centre for Biotechnology Information) accession numbers
d aa amino acid
e ↑ increased abundance, ↓, decreased abundance
fReferences: [1] Warner et al. 2010, [2] Sharon et al. 2009, [3] Wu et al. 2011
gBoth sequences are highly similar and differ almost exclusively in the presence (ACX81198)/absence (ABR67402) of a mitochondrial localization sequence
LEA proteins are highly hydrophilic, intrinsically unstructured proteins associated with desiccation tolerance in anhydrobiotic animals, plant seeds and several microorganisms (Bahrndorff et al. 2009; Battista et al. 2001; Browne et al. 2002; Cornette et al. 2010; Erkut et al. 2013; Förster et al. 2009; Hand et al. 2007, 2011; Tunnacliffe et al. 2005). LEA proteins reduce desiccation- and freezing-induced protein aggregation in vitro (Chakrabortee et al. 2007; Furuki et al. 2012; Goyal et al. 2005; Reyes et al. 2005), protect protein function (Grelet et al. 2005) and stabilize membranes (Li et al. 2012; Tolleter et al. 2010). LEA proteins improve the desiccation tolerance of transgenic mammalian and Drosophila melanogaster cells (Chakrabortee et al. 2007; Marunde et al. 2013) and organisms such as maize (Amara et al. 2013) and yeast (Duan and Cai 2012). Several theories have been proposed to explain how LEA proteins protect cells from water loss, based on their high proportion of polar and charged amino acids, intrinsically unstructured nature, and the acquisition of secondary structure when dried (Hundertmark et al. 2012; Tunnacliffe and Wise 2007). LEA proteins may prevent protein aggregation by acting as molecular shields (Chakrabortee et al. 2012; Furuki et al. 2012; Hatanaka et al. 2013), protect membranes (Hincha and Thalhammer 2012; Li et al. 2012; Tolleter et al. 2010), contribute to vitrification, the formation of intracellular glasses (Buitink and LePrince 2004; Hengherr et al. 2011), act as “hydration buffers” that slow water loss (Manfre et al. 2006; Tompa et al. 2010), and reduce oxidative stress by serving as ion sinks (Mowla et al. 2006), although the latter is unlikely to contribute significantly to stress tolerance (Hand et al. 2011).
While numerous studies of LEA proteins have been performed in vitro, to our knowledge there are no previous examinations of animal group 1 LEA proteins in vivo. In this study, the role of group 1 LEA proteins in Artemia cysts was examined through loss-of-function experiments using RNA interference (RNAi). Cysts lacking group 1 LEA proteins had reduced viability following freezing and/or desiccation, suggesting these proteins protected against both stressors. Under the conditions tested, group 1 LEA proteins did not alter diapause termination by H2O2. The analysis of Artemia group 1 LEA protein in vivo, as described herein, contributes to the understanding of similar proteins in many organisms.
Materials and methods
Culture of A. franciscana
A. franciscana cysts from the GSL, UT (INVE Aquaculture, Inc., Ogden, UT, USA) were rehydrated with aeration at room temperature in filtered (22 μm) and autoclaved sea water from Halifax Harbour, Nova Scotia, Canada, hereafter called sea water. Hatched nauplii were harvested 24 to 48 h after hydration was initiated, and grown to adults in aerated sea water at room temperature. Cultures were fed daily with Isochrysis sp. (clone synonym T-Iso) from The Provasoli-Guillard National Center for Culture of Marine Phytoplankton, West Boothbay Harbor, ME, USA. Adult A. franciscana were maintained in sea water in six-well culture plates at room temperature (24 °C) during experiments unless otherwise specified.
Knockdown of group 1 LEA proteins in A. franciscana cysts
AfrLEA1-1 (Table 1, Fig. 1) was amplified from full length AfrLEA1-1 cDNA cloned in the pCR 2.1 vector (Invitrogen, Burlington ON, Canada) using 0.2 mM primers containing the T7 promoter (Table 2) and Platinum Taq DNA Polymerase (Invitrogen), and the following reaction conditions: 5 min at 94 °C, 30 cycles of 30 s at 94 °C, 30 s at 60 °C, and 1 min at 72 °C, with a final 10 min at 72 °C. GFP was amplified from the commercial vector pEGFP-N1 (Clontech, Mountain View CA, USA) using the above reaction conditions with Platinum Taq DNA Polymerase (Invitrogen) and 0.2 mM primers containing the T7 promoter (Table 2) (Zhao et al. 2012; King and MacRae 2012). PCR products were sequenced at The Centre for Applied Genomics DNA Sequencing Facility, Toronto Sick Kids Hospital, Toronto, ON, Canada. Five micrograms of each PCR product was used as template for the generation of AfrLEA1 and GFP dsRNA with the MEGAscript® RNAi kit (Ambion Applied Biosystems, Austin, TX, USA) according to manufacturer’s instructions. AfrLEA1-1 and GFP PCR and dsRNA products were resolved in 1.5 % agarose gels in 0.5× TBE (45 mM Tris, 45 mM boric acid, 1 mM EDTA, pH 8.5) at 90 V, stained with SYBR Safe® (Invitrogen), and examined with a DNR Bio-Imaging Systems MF-ChemiBIS 3.2 gel documentation system (Montreal Biotech, Montreal, QC, Canada). Absorbance was measured at 260 nm to determine dsRNA concentrations. Three volumes of AfrLEA1 and GFP dsRNA were separately added to 1 volume of 0.5 % phenol red in Dulbecco’s phosphate buffered saline (DPBS) for injection of females (King and MacRae 2012).
Fig. 1.
AfrLEA1-1 sequence. Nucleotide and amino acid sequences for the group 1 LEA protein, AfrLEA1-1, with eight repeats, are shown (accession #s EF656614, ABR67402) (Warner et al. 2010). The AfrLEA1 dsRNA is complementary to the shaded region of AfrLEA1-1. Start and stop codons are underlined. The first and eighth repeated motifs are boxed
Table 2.
Primers used for the production of dsRNA and for RT-qPCR
| Primer sequence (5′ to 3′) | ||
|---|---|---|
| Forward | Reverse | |
| dsRNA productiona | ||
| AfrLEA1 | TAATACGACTCACTATAGGGAAAGCTAAGCCGCCAAG | TAATACGACTCACTATAGGGAGTCTGCTCTTGACATTTGCT |
| GFP b | TAATACGACTCACTATAGGGAGACACATGAAGCAGCACGACTT | TAATACGACTCACTATAGGGAGAAGTTCACCTTGATGCCGTTTC |
| RT-qPCR | ||
| AfrLEA1 | GAAGTTCAAGCGTTCTCC | TAGCTTGACCACCTTTCC |
| α-tubulin c | CGACCATAAAAGCGCAGTCA | CTACCCAGCACCACAGGTCTCT |
Mature females carrying diapause-destined unfertilized eggs, identified by the presence of a shell gland (Liang and MacRae 1999), were injected in the egg sac with 60 to 80 ng of either AfrLEA1 or GFP dsRNA (King and MacRae 2012). Injections were performed as described (King and MacRae 2012) under an Olympus SZ61 stereomicroscope (Olympus Canada, Inc., Markham, ON, Canada) with a borosilicate micropipette pulled with a custom programmed P-97 Flaming/Brown Micropipette Puller (Sutter Instrument Co., Novato, CA, USA) and broken at 45° using a clean razor blade (Copf et al. 2004). Females were returned to sea water and observed for 2 h to ensure retention of phenol red and thus dsRNA (King and MacRae 2012). Each female was mated with a male 24 h post-injection.
Quantitation of AfrLEA1 mRNA by qPCR
A. franciscana cysts were harvested 10 days post-release from females injected with either AfrLEA1 or GFP dsRNA. RNA was extracted from 25 to 30 cysts by homogenization with a micropestle (Fisher Scientific, Ottawa, ON, Canada) in a 1.5 ml microtube with 100 μl TRIzol® (Invitrogen). cDNA was generated using 0.1 μg of RNA as template with SuperScript® III First-Strand Synthesis Kit for RT-PCR (Invitrogen) and oligo dT20 primers. All RNA preparations were also incubated without reverse transcriptase to ensure the absence of genomic DNA. qPCR was conducted with a QuantiTect® SYBR® Green PCR Kit (Qiagen, Mississauga, ON, Canada) in a Rotor-Gene RG-3000 system (Corbett Research, Sydney, NSW, Australia) using 0.5 μl cDNA as template (King et al. 2013). Primers for α-tubulin and AfrLEA1 (Table 2) were used at 1 mM. Four to six independent samples with two nested replicates were used in each experiment. AfrLEA1 cDNA copy numbers were determined from a standard curve of Ct values (R2 > 0.99), and normalized against α-tubulin (King et al. 2013). Primer fidelity was assessed by melting curve analysis.
Detection of group 1 LEA proteins by immunoprobing of western blots
Fifty to 75 cysts released from females injected with either AfrLEA1 or GFP dsRNA were harvested 10 days post-release from females and homogenized on ice with a micropestle (Fisher Scientific) in 1.5 ml microtubes containing 30 μl treatment buffer for SDS polyacrylamide gel electrophoresis (62.5 mM Tris, 2 % (w/v) SDS, 10 % (v/v) glycerol, 5 % (v/v) β-mercaptoethanol, 0.05 % (w/v) bromophenol blue, pH 6.8). Protein samples were placed in a boiling water bath for 5 min prior to centrifugation at 4 °C for 10 min at 10,000×g. The supernatants and PiNK Plus Prestained Protein Ladder (FroggaBio Inc., Toronto, ON, Canada) were resolved in 12.5 % SDS polyacrylamide gels at 30 mA for 60 min, and then transferred to Protran nitrocellulose membranes (GE Healthcare, Piscataway, NJ, USA). Immunoprobing of blots was as in King and MacRae (2012), except the primary antibody was rabbit anti-group 1 LEA protein antibody (Warner et al. 2010) diluted 1:5,000 in TBS. Secondary HRP-conjugated goat anti-rabbit IgG antibody (Sigma-Aldrich, Oakville, ON, Canada) was localized with ECL Plus Western Blotting Detection Reagents (GE Healthcare, Baie d’Urfé, QC, Canada) and a DNR Bio-Imaging Systems MF-ChemiBIS 3.2 gel documentation system.
Female survival and cyst development following knockdown of group 1 LEA proteins
Survival following successive brood releases was documented for females injected with dsRNAs. Cyst quantity, the time from fertilization defined by egg sac fusion to initial release of cysts, and the time required for complete brood discharge was determined for each of the first three broods released. Females were incubated in sea water at either room temperature (24 °C) or 18 °C. The latter was employed to assess if subtle differences in growth of females and cyst development were evident at lower temperatures.
Stress tolerance of A. franciscana cysts lacking group 1 LEA proteins
Cysts were collected 10 days after release from females injected with dsRNA, and excess sea water was removed with a Pasteur pipette. The cysts were either blotted dry with Kimwipes and incubated in 1.5 ml microtubes in Styrofoam containers at −20 °C for 12 weeks, or they were dried for 4 weeks at room temperature in a desiccator containing Indicating Drierite (Sigma-Aldrich) before freezing at −20 °C for 8 weeks. Cysts were then incubated in sea water at room temperature to quantify hatching, used as a measure of viability. Cysts were monitored for 5 days after the appearance of the last nauplius to ensure no further hatching occurred. Experiments were done in triplicate with 38 to 113 cysts in each replicate.
Cysts from females injected with either GFP or LEA dsRNA were collected by vacuum filtration on 45 μm Millipore filter discs (Billerica, MA, USA) after incubation at room temperature in sea water 13 to 14 weeks, immersed in 3 % H2O2 for 20 min, filtered and washed either two or five times in sea water at room temperature. Washed cysts were transferred to sea water and hatching was recorded until 5 days after the last nauplius appeared. The experiments were done in triplicate with 24 to 61 cysts in each replicate.
Statistical analyses
A one-tailed student’s t test (α = 0.05) was used to assess whether AfrLEA1 mRNA was more abundant in cysts from females injected with GFP versus AfrLEA1 dsRNA. One-way ANOVA was used to assess differences in brood size. The viability of cysts from females injected with either AfrLEA1 or GFP dsRNA after desiccation, freezing, and H2O2 exposure was compared using χ2 independence of factors tests.
Results
AfrLEA1-1 and GFP dsRNA
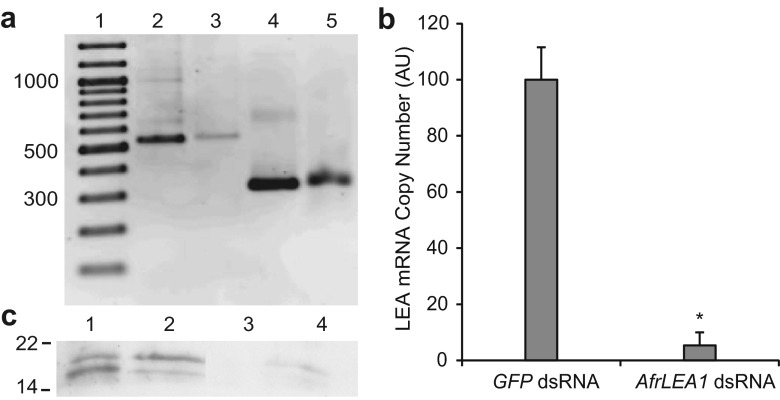
The AfrLEA1 dsRNA is complementary to 463 bp (75 %) of full length AfrLEA1-1 mRNA and it is comprised predominantly of a repeated 20 amino acid motif (Fig. 1). dsRNAs generated from AfrLEA1 and GFP cDNA were respectively about 500 and 300 bp, the expected sizes (Fig. 2a). GFP dsRNA is not complementary to AfrLEA1 mRNAs, nor to any other mRNA known to occur in Artemia (Zhao et al. 2012).
Fig. 2.
Knock down of group 1 LEA proteins in cysts by RNAi. a AfrLEA1 and GFP cDNA were amplified and dsRNAs were synthesized using PCR products. Reaction products were resolved in 1.5 % agarose and visualized with SYBR®Safe. Lane 1 GeneRuler 100 bp DNA Ladder (Thermo Scientific), sizes indicated on left in base pairs; 2 AfrLEA1 PCR product; 3 AfrLEA1 dsRNA; 4 GFP PCR product; 5 GFP dsRNA. b RT-qPCR was performed in duplicate with RNA from 25 to 30 cysts to determine AfrLEA1 and α-tubulin transcript copy number. AfrLEA1 copy numbers were normalized against α-tubulin and averaged for 4 and 6 cyst samples obtained from females injected with either GFP or AfrLEA1 dsRNA. AfrLEA1 copy number in cysts from females injected with GFP dsRNA was set at 100. Error bars represent standard deviation. The asterisk indicates that the mean copy number of group 1 LEA protein transcripts is statistically different from the group 1 LEA protein transcript copy number in cysts from females injected with GFP dsRNA (p < 0.05). c Protein extracts of 50 to 75 cysts from females injected with GFP dsRNA (1, 2) and AfrLEA1 dsRNA (3, 4) were resolved in SDS polyacrylamide gels, transferred to nitrocellulose, and probed with rabbit anti-group 1 LEA protein antibody followed by HRP-conjugated goat anti-rabbit IgG antibody and chemiluminescence. Molecular mass in kDa is on the left. Lanes 1 and 3, extracts of first brood cysts; 2 and 4, extracts of second brood cysts
Injection of Artemia females with AfrLEA1 dsRNA reduced group 1 LEA proteins in cysts
Artemia females injected with AfrLEA1 dsRNA released cysts with decreased AfrLEA1 mRNA when compared to cysts from females receiving GFP dsRNA, p = 0.0004 (Fig. 2b). As shown by probing of Western blots, group 1 LEA proteins of 21 and 19 kDa were detected in extracts of cysts from females injected with GFP dsRNA but not in extracts from the same number of cysts produced by females given AfrLEA1 dsRNA (Fig. 2c). Group 1 LEA proteins with molecular masses lower than 19 kDa were not observed in any samples.
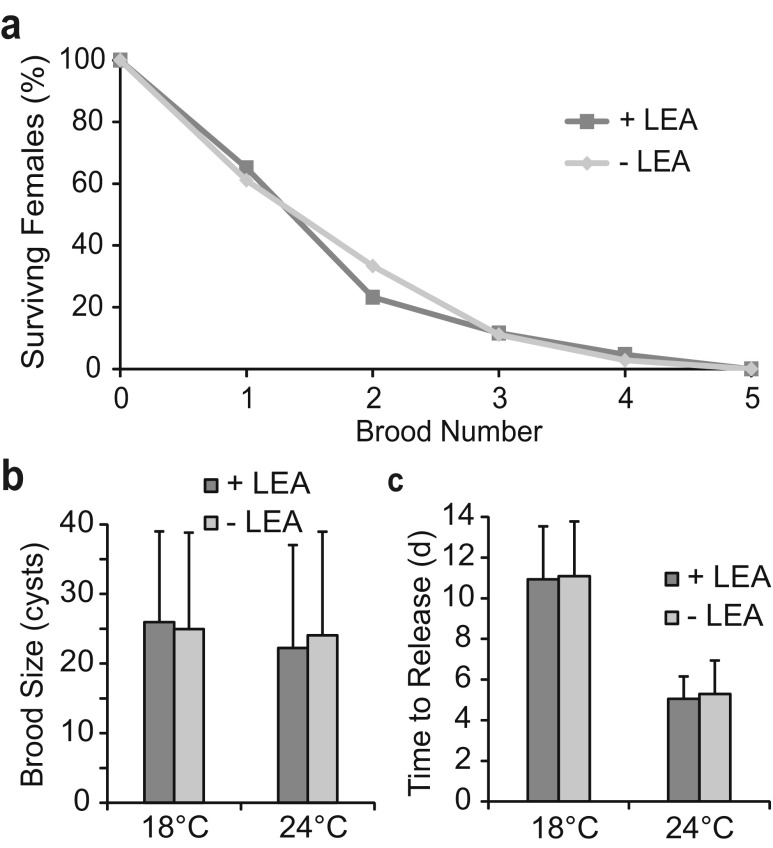
Group 1 LEA protein knockdown affects neither survival of A. franciscana females nor cyst development
A. franciscana females injected with either AfrLEA1 of GFP dsRNA survived equally well after successive brood releases (Fig. 3a) and had an average brood size of approximately 24 cysts, whether incubated at room temperature or 18 °C, p = 0.57 (Fig. 3b), with brood sizes ranging from 3 to 60 cysts. Diapause-destined embryos in females injected with either AfrLEA1 or GFP dsRNA developed at similar rates and were released from females 5 days following fertilization at 24 °C, p = 0.32, and after 11 days upon incubation at 18 °C, p = 0.79 (Fig. 3c). Broods were discharged from females in less than a day regardless of the dsRNA administered (data not shown).
Fig. 3.
Survival of Artemia females and cyst development were unaffected by dsRNAs. a Representative survival of 43 females injected with GFP dsRNA (+LEA) and 36 females injected with AfrLEA1 dsRNA (−LEA) at 18 °C. b Average brood sizes produced by females injected with either GFP dsRNA (+LEA) or AfrLEA1 dsRNA (−LEA) were calculated from first, second, and third broods at 24 °C (+LEA, 53 broods; −LEA, 54 broods) and 18 °C (+LEA, 39 broods; −LEA, 32 broods). c The average time from fertilization to cyst release from females injected with either GFP dsRNA (+LEA) or AfrLEA1 dsRNA (−LEA) was determined from first, second, and third brood releases at 18 °C (+LEA, 43 broods; −LEA, 34 broods) and 24 °C (+LEA, 70 broods; −LEA, 76 broods). d day. Error bars represent standard deviation. No statistical difference was observed between +LEA and −LEA (p > 0.05) in b or c
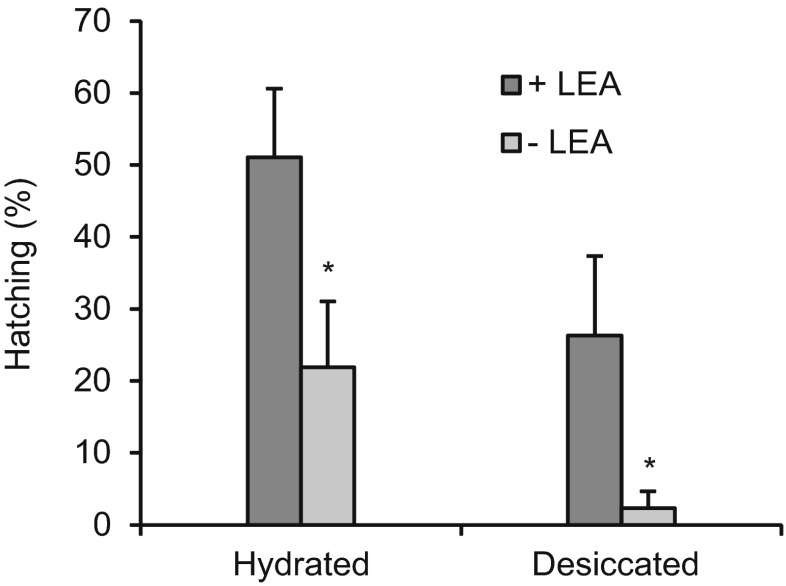
Group 1 LEA proteins were required for maximum resistance of cysts to freezing and desiccation
As indicated by hatching, cysts containing, as opposed to those lacking, group 1 LEA proteins had approximately two-fold higher viability after they were blotted with Kimwipes and incubated at −20 °C for 12 weeks, p = 0.02 (Fig 4, Hydrated). When cysts were desiccated for 4 weeks followed by 8 weeks freezing, those containing group 1 LEA proteins exhibited 11-fold higher viability than when the proteins were absent, p = 0.03 (Fig. 4, Desiccated). Regardless of group 1 LEA proteins, the viability of cysts blotted dry prior to freezing was higher than for cysts that were desiccated and frozen, p < 0.001 (Fig. 4). Specifically, cysts with LEA proteins were approximately two-fold more viable when blotted and frozen rather than desiccated and frozen, whereas this difference was nine-fold when group 1 LEA proteins were absent (Fig. 4).
Fig. 4.
Tolerance to desiccation and freezing decreased in cysts lacking group 1 LEA proteins. Hatching (%) of cysts from females injected with either GFP dsRNA (+LEA) or AfrLEA1 dsRNA (−LEA) either after blotting with Kimwipes and 12 weeks incubation at −20 °C (Hydrated), or 4 weeks desiccation at room temperature and 8 weeks incubation at −20 °C (Desiccated). Cysts were incubated in sea water at room temperature to determine viability. Experiments were done in triplicate for batches of first brood cysts; Hydrated, +LEA: n = 65, 60, 62; −LEA: n = 38, 53, 60; Desiccated, +LEA: n = 56, 113, 92; −LEA: n = 65, 87, 82. Error bars represent standard deviation. The asterisks indicate that the mean value of −LEA treatments is statistically different from the respective + LEA treatments (p < 0.05)
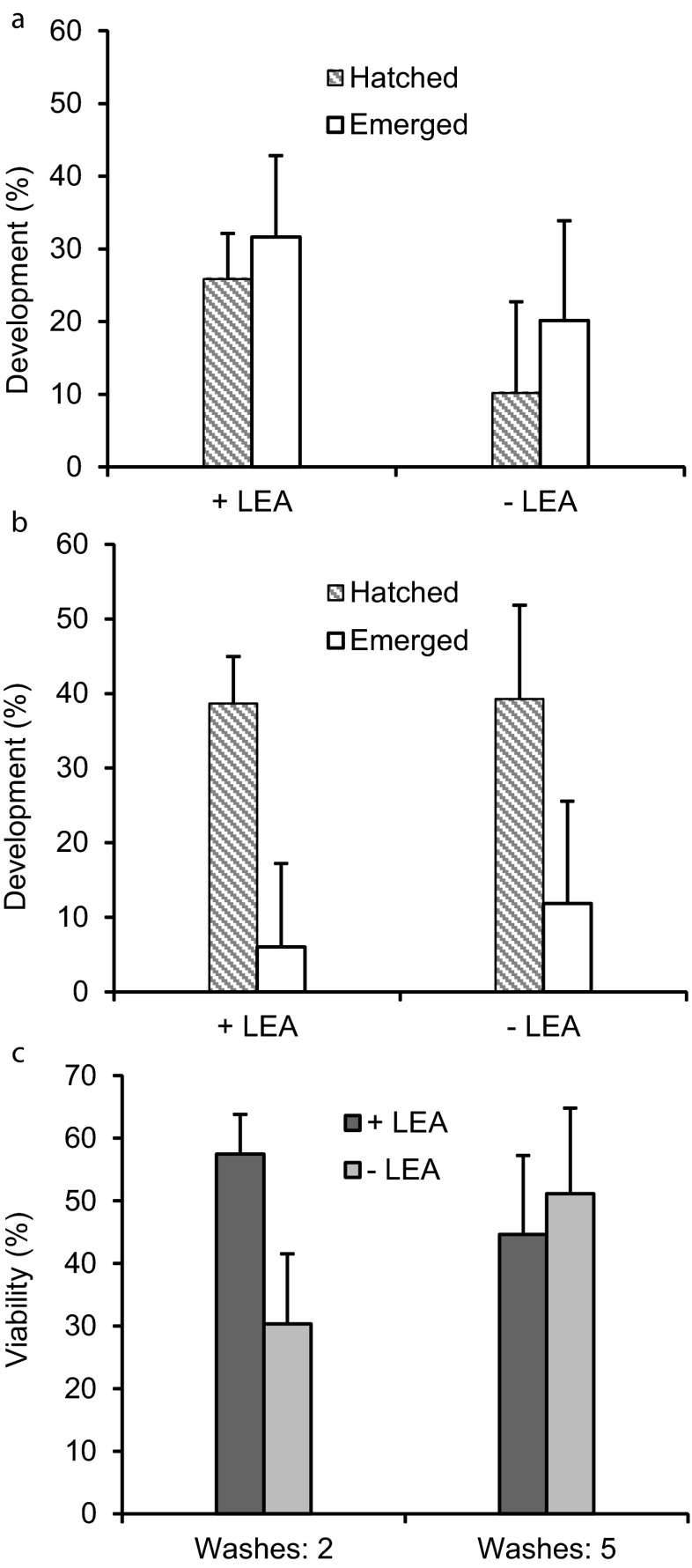
Group 1 LEA proteins did not affect the response of developing cysts to H2O2
When diapause was terminated by H2O2 exposure after 13 to 14 weeks incubation in sea water at room temperature some cysts hatched into swimming nauplii, whereas for other cysts the emergence of nauplii halted shortly after the shell cracked (E1) or with the membrane-enclosed nauplius attached to the shell (E2). In extreme cases of abnormal exit from the cyst shell the membrane-enclosed nauplii emerged backwards, with the tail rather than the head protruding from the cyst shell. All three forms of abnormal emergence were documented in cysts either containing or lacking group 1 LEA proteins.
Approximately 10 to 25 % of cysts released from injected females and incubated in sea water for 13 to 14 weeks produced swimming nauplii after exposure to H2O2, two washes with sea water, and incubation at room temperature in sea water for 7 days, regardless of the presence of group 1 LEA proteins, p = 0.63 (Fig. 5a). As well, the number of nauplii stalled at emergence (E1, E2, backwards) after two washes and incubation at room temperature for 7 days was not significantly different for cysts with or without LEA proteins, p = 0.51 (Fig. 5a). When washed five times after H2O2 treatment approximately 40 % of cysts with or without LEA proteins generated swimming nauplii p = 0.39 (Fig. 5b), while 6 to 12 % produced emerged nauplii, p = 0.42 (Fig. 5b). The viability of cysts washed two times and five times after H2O2 treatment was between 30 and 58 % (Fig. 5c). These values, determined by combining emerged and hatched nauplii were not significantly different for cysts containing or lacking group 1 LEA proteins.
Fig. 5.
Development of cysts after H2O2 exposure occurred independently of group 1 LEA proteins. Cysts either containing (+LEA) or lacking (−LEA) group 1 LEA proteins were incubated in sea water at room temperature for 13 to 14 weeks, exposed to H2O2, washed with sea water, and then incubated in sea water for 5 days after the last nauplius emerged. Hatched and emerged nauplii were counted. a Cysts were washed twice after H2O2 treatment. The experiment was done in triplicate for first brood cysts. +LEA: n = 40, 40, 30; 2, −LEA: n = 40, 70, 40. b Cysts were washed five times after H2O2 treatment. The experiment was done in duplicate for first brood cysts. +LEA: n = 61, 25; −LEA: n = 24, 26. c The viability (hatched + emerged nauplii) of +LEA and −LEA cysts washed either two or five times post-H2O2 treatment was determined from the samples in a and b. Error bars represent standard deviation. No statistical difference was observed between +LEA and −LEA (p > 0.05) in a, b, or c
Discussion
A. franciscana group 1 LEA proteins were characterized in vivo by RNAi. The AfrLEA1 qPCR primers amplified regions of AfrLEA1-1, -3, and -4 cDNA, indicating that the dramatic transcript reduction caused by RNAi involved the knockdown of multiple group 1 LEA protein mRNAs in cysts, likely due to their conserved repetitive sequences. In line with this observation, the two most abundant group 1 LEA proteins in cysts, AfrLEA1-1 and -2, were eliminated by injection of females with AfrLEA1 dsRNA. The absence of smaller group 1 LEA proteins on Western blots as observed previously (Warner et al. 2010), was probably due to the low amount of cyst extract applied to gels. Congruent with the lack of group 1 LEA proteins in adults (Warner et al. 2010), injection of females with AfrLEA1 dsRNA did not affect survival or reproductive capacity, the latter indicated by brood size. This contrasts with results obtained for ArHsp22, a stress-inducible sHsp in adult Artemia (Qiu and MacRae 2008). Injection of ArHsp22 dsRNA is fatal to Artemia adults (King et al. 2013). Group 1 LEA protein knock down also had no apparent effect on the timing of embryo development, even when experiments were performed at 18 °C to extend the observations. This result contrasts those obtained for two diapause-specific Artemia molecular chaperones where loss of the sHsp p26 raises the residence time of cysts in the brood sac and removal of artemin increases the duration of brood release (King and MacRae 2012; King et al. 2014).
Hatching was used as an indicator of viability in this work even though it is not possible to know if a non-hatched cyst is dead. However, from a biological perspective, a cyst that does not hatch is considered dead because it cannot grow and reproduce. The viability of cysts lacking group 1 LEA proteins was reduced significantly upon desiccation and/or freezing, supporting in vitro work that group 1 LEA proteins protect against water stress (Goyal et al. 2005; Hand et al. 2011). Artemia cysts have a high proportion of bound water (Crowe et al. 1981) perhaps due in part to the LEA proteins (e.g., Manfre et al. 2006). Bulk cytoplasmic water in fully hydrated Artemia cysts freezes at about −2 °C (Crowe et al. 1981), but “bound water”, associated with the surfaces of macromolecules and organelles tends to remain liquid, even at much colder temperatures (Ramløv and Hvidt 1992). Frozen hydrated cysts probably experienced ice formation, but retained liquid water with a high concentration of solutes (Crowe et al. 1981), a cold-induced desiccation. Damage due to cold- or drying-induced desiccation is reduced because LEA proteins act as molecular shields preventing aggregation of denatured proteins (Chakrabortee et al. 2012; Hatanaka et al. 2013) and they protect organelles such as mitochondria from freezing (Menze et al. 2009). Mitochondrially targeted AfrLEA1-1 and AfrLEA1-2 (Warner et al. 2010) may preserve membrane and protein integrity in this organelle, allowing cysts to quickly resume respiration upon termination of diapause.
When bulk cytoplasmic water is removed by desiccation the remaining intracellular or bound water does not freeze (Crowe et al. 1981; Ramløv and Hvidt 1992). Cysts desiccated prior to incubation at −20 °C are therefore expected to experience minimal freezing stress, and the dominant challenge to viability is desiccation. Some LEA proteins partially fold under low water conditions (Goyal et al. 2003; Hundertmark et al. 2012; Soulages et al. 2002), enhancing sugar glass formation and strength (Buitink and LePrince 2004; Hincha and Thalhammer 2012; Wolkers et al. 2001) and protecting membranes, although the latter has yet to be demonstrated for group 1 LEA proteins (Shimizu et al. 2010; Tolleter et al. 2010). High cyst mortality upon desiccation when group 1 LEA proteins were absent suggests a protective role for these proteins through intracellular glass formation (Hengherr et al. 2011) as is the case for chironomid larvae of Polypedilum vanderplanki (Sakurai et al. 2008).
Desiccation and freezing are significant threats to Artemia cysts, as shown by their death in the absence of either p26 (King and MacRae 2012) or group 1 LEA proteins when these stresses are experienced. There is an interplay between desiccation and freezing, with hydrated cysts generally less tolerant of freezing than desiccated cysts (Clegg and Trotman 2002; Crowe et al. 1981). However, in this study, a higher number of hydrated as opposed to desiccated cysts hatched after freezing. The rate of freezing may explain this result as slow freezing of hydrated Artemia cysts at −0.5 °C/min gives high hatching (Yoshida et al. 2011). In the experiments herein, hydrated cysts dried only with Kimwipes were placed in Styrofoam containers to reduce the rate of temperature decline upon transfer to −20 °C. In addition, desiccated cysts were frozen for 8 weeks while hydrated cysts were incubated for 12 weeks at −20 °C. High viability of cysts after long uninterrupted freezing occurred in laboratory experiments (Toxopeus and MacRae, unpublished). Based on temperatures experienced in the GSL (National Oceanic and Atmospheric Administration 2011), the ability to maintain diapause for long freezing periods is likely adaptive for overwintering cysts.
In the present study, there was no significant difference in diapause termination or viability upon exposure of cysts with or without group 1 LEA proteins to H2O2, a chemical previously shown to terminate diapause (Robbins et al. 2010; Veeramani and Baskaralingam 2011). However, the exit of nauplii from cysts was hindered by H2O2 during post-diapause development, resulting in membrane-enclosed nauplii stalled at emergence or which emerged backwards. It is probable that residual H2O2 entered embryos when cyst shells cracked and interfered with hatching. In support of this proposal, H2O2 at 0.003 % (1,060 μM) inhibits the emergence and hatching of nauplii (Robbins et al. 2010). Artemia cysts exhibit abnormal hatching when incubated in medium with either mercury, zinc, cadmium, or potassium cyanide (Bagshaw et al. 1986; Go et al. 1990; Pandey and MacRae 1991; Rafiee et al. 1986; Trotman et al. 1980).
Conclusions
Group 1 LEA proteins, as part of a suite of molecular defenses, have a profound effect on cyst response to desiccation and freezing, thereby contributing substantially to the stress tolerance of cysts. These proteins have not previously been associated with freezing stress in animals (Hand et al. 2011). Because the absence of group 1 LEA proteins is most detrimental when cysts are desiccated, these proteins likely contribute to intracellular glass formation. The ability of group 1 LEA proteins to protect Artemia embryos from desiccation and freezing coincides with the types of environmental changes experienced by Artemia and suggests therapeutic applications in the cryopreservation of human cells (Loi et al 2013).
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN/7661-2011) to THM, and an NSERC Julie Payette Scholarship and Killam Pre-doctoral Scholarship awarded to JT.
References
- Amara I, Capellades M, Ludevid MD, Pagès M, Goday A. Enhanced water stress tolerance of transgenic maize plants over-expressing LEA Rab28 gene. J Plant Physiol. 2013;170:864–873. doi: 10.1016/j.jplph.2013.01.004. [DOI] [PubMed] [Google Scholar]
- National Oceanic and Atmospheric Administration (2011). NOAA’s 1981-2010 Climate Normals. http://www1.ncdc.noaa.gov/pub/data/normals/1981-2010/products/station/USW00024127.normals.txt. Accessed 1 August 2013
- Bagshaw JC, Rafiee P, Matthews CO, MacRae TH. Cadmium and zinc reversibly arrest development of Artemia larvae. Bull Environ Contam Toxicol. 1986;37:289–296. doi: 10.1007/BF01607763. [DOI] [PubMed] [Google Scholar]
- Bahrndorff S, Tunnacliffe A, Wise MJ, McGee B, Holmstrup M, Loeshcke V. Bioinformatics and protein expression analyses implicates LEA proteins in the drought responses of Collembola. J Insect Physiol. 2009;55:210–217. doi: 10.1016/j.jinsphys.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Battista JR, Park M-J, McLemore AE. Inactivation of two homologues of proteins presumed to be involved in the desiccation tolerance of plants sensitizes Deinococcus radiodurans R1 to desiccation. Cryobiology. 2001;43:133–139. doi: 10.1006/cryo.2001.2357. [DOI] [PubMed] [Google Scholar]
- Boswell LC, Moore DS, Hand SC. Quantification of cellular protein expression and molecular features of group 3 LEA proteins from embryos of Artemia franciscana. Cell Stress Chaperones. 2013 doi: 10.1007/s12192-013-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne J, Tunnacliffe A, Burnell A. Anhydrobiosis: plant desiccation gene found in a nematode. Nature. 2002;416:38. doi: 10.1038/416038a. [DOI] [PubMed] [Google Scholar]
- Buitink J, Leprince O. Glass formation in plant anhydrobiotes: survival in the dry state. Cryobiology. 2004;48:215–228. doi: 10.1016/j.cryobiol.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Caprioli M, Katholm AK, Melone G, Ramløv H, Ricci C, Santo N. Trehalose in desiccated rotifers: a comparison between a bdelloid and a monogonont species. Comp Biochem Physiol A. 2004;139:527–532. doi: 10.1016/j.cbpb.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Chakrabortee S, Boschetti C, Walton LJ, Sarkar S, Rubinsztein DC, Tunnacliffe A. Hydrophilic protein associated with desiccation tolerance exhibits board stabilization function. Proc Natl Acad Sci USA. 2007;104:19073–18078. doi: 10.1073/pnas.0706964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabortee S, Tripathi R, Watson M, Kaminski Schierle GS, Kurniawan DP, Kaminski CF, Wise MJ, Tunnacliffe A. Intrinsically disordered proteins as molecular shields. Mol Biosys. 2012;8:210–219. doi: 10.1039/c1mb05263b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg JS. The physical properties and metabolic status of Artemia cysts at low water contents: the ‘water replacement hypothesis’. In: Leopold AC, editor. Membranes, metabolism and dry organisms. Ithaca: Comstock; 1986. pp. 169–187. [Google Scholar]
- Clegg JS, Trotman CNA. Physiology and biochemistry of Artemia ecology. In: Abatzopolous TJ, Beardmore JA, Clegg JS, Sorgeloos P, editors. Artemia: basic and applied biology. Dordrecht: Kluwer; 2002. pp. 129–170. [Google Scholar]
- Clegg JS, Drinkwater LE, Sorgeloos P. The metabolic status of diapause embryos of Artemia franciscana (SFB) Physiol Zool. 1996;69:49–66. [Google Scholar]
- Copf T, Schröder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci. 2004;101:17711–17715. doi: 10.1073/pnas.0407327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornette R, Kanamori Y, Watanabe M, Nakahara Y, Gusev O, Mistumasu K, Kadono-Okuda K, Shimomura M, Mita K, Kikawada T, Okuda T. Identification of anhydrobiosis-related genes from an expressed sequence tag database in the cryptobiotic midge Polypedilum vanderplanki (Diptera; Chironomidae) J Biol Chem. 2010;285:35889–35899. doi: 10.1074/jbc.M110.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, O’Dell SJ. Ice formation during freezing of Artemia cysts of variable water contents. Mol Physiol. 1981;1:145–152. [Google Scholar]
- Dai L, Chen D-F, Liu Y-L, Zhao Y, Yang F, Yang J-S, Yang W-J. Extracellular matrix peptides of Artemia cyst shell participate in protecting encysted embryos from extreme environments. PLoS ONE. 2011;6(6):e20187. doi: 10.1371/journal.pone.0020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaffoy D, de Maeyer-Criel G, Kondo M. On the permeability and formation of the embryonic cuticle during development in vivo and in vitro of Artemia salina embryos. Differentiation. 1978;12:99–109. doi: 10.1111/j.1432-0436.1979.tb00995.x. [DOI] [Google Scholar]
- Drinkwater LE, Crowe JH. Regulation of embryonic diapause in Artemia: environmental and physiological signals. J Exp Zool. 1987;241:297–307. doi: 10.1002/jez.1402410304. [DOI] [Google Scholar]
- Duan J, Cai W. OsLEA3-2, an abiotic stress gene of rice plays a key role in salt and drought tolerance. PLoS ONE. 2012;7(9):e45117. doi: 10.1371/journal.pone.0045117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkut C, Vasilj A, Boland S, Habermann B, Shevchenko A, Kurzchalia TV. Molecular strategies of the Caenorhabditis elegans dauer larva to survive extreme desiccation. PLoS ONE. 2013;8(12):e82473. doi: 10.1371/journal.pone.0082473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster F, Liang C, Shkumatov A, Beisser D, Engelmann JC, Schnölzer M, Frohme M, Müller T, Schill RO, Dandekar T. Tardigrade workbench: comparing stress-related proteins, sequence-similar and functional protein clusters as well as RNA elements in tardigrades. BMC Genomics. 2009;10:469. doi: 10.1186/1471-2164-10-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuki T, Shimizu T, Chakrabortee S, Yamakawa K, Hatanaka R, Takahashi T, Kikawada T, Okuda T, Mihara H, Tunnacliffe A, Sakurai M. Effects of Group 3 LEA model peptides on desiccation-induced protein aggregation. Biochim Biophys Acta. 2012;1824:891–897. doi: 10.1016/j.bbapap.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Go EC, Pandey AS, MacRae TH. Effect of inorganic mercury on the emergence and hatching of the brine shrimp Artemia franciscana. Mar Biol. 1990;107:93–102. doi: 10.1007/BF01313246. [DOI] [Google Scholar]
- Goyal K, Tisi L, Basran A, Browne J, Burnell A, Zurdo J, Tunnacliffe A. Transition from natively unfolded to folded state induced by desiccation in an anhydrobiotic nematode protein. J Biol Chem. 2003;278:12977–12984. doi: 10.1074/jbc.M212007200. [DOI] [PubMed] [Google Scholar]
- Goyal K, Walton LJ, Tunnacliffe A. LEA proteins prevent protein aggregation due to water stress. Biochem J. 2005;388:151–157. doi: 10.1042/BJ20041931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelet J, Benamar A, Teyssier E, Avelange-Macherel M-H, Grunwald D, Macherel D. Identification in pea seed mitochondria of a late embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol. 2005;137:157–167. doi: 10.1104/pp.104.052480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand SC, Jones D, Menze MA, Witt TL. Life without water: expression of plant LEA genes by an anhydrobiotic arthropod. J Exp Zool. 2007;307A:62–66. doi: 10.1002/jez.a.343. [DOI] [PubMed] [Google Scholar]
- Hand SC, Menze MA, Toner M, Boswell L, Moore D. LEA proteins during water stress: not just for plants anymore. Annu Rev Physiol. 2011;73:115–134. doi: 10.1146/annurev-physiol-012110-142203. [DOI] [PubMed] [Google Scholar]
- Hatanaka R, Hagiwara-Komoda Y, Furuki T, Kanamori Y, Fujita M, Cornette R, Sakurai M, Okuda T, Kikawada T. An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles. Insect Biochem Mol Biol. 2013;43:1055–1067. doi: 10.1016/j.ibmb.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Hengherr S, Schill RO, Clegg JS. Mechanisms associated with cellular desiccation tolerance in the animal extremophile Artemia. Physiol Biochem Zool. 2011;84:249–257. doi: 10.1086/659314. [DOI] [PubMed] [Google Scholar]
- Hincha DK, Thalhammer A. LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochem Soc Tran. 2012;40:1000–1003. doi: 10.1042/BST20120109. [DOI] [PubMed] [Google Scholar]
- Hundertmark M, Popova AV, Rausch S, Seckler R, Hincha DK. Influence of drying on the secondary structure of intrinsically disordered and globular proteins. Biochem Biophys Res Commun. 2012;417:122–128. doi: 10.1016/j.bbrc.2011.11.067. [DOI] [PubMed] [Google Scholar]
- King AM, MacRae TH. The small heat shock protein p26 aids development of encysting Artemia embryos, prevents spontaneous diapause termination and aids in stress. PLoS ONE. 2012;7(8):e43723. doi: 10.1371/journal.pone.0043723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, Toxopeus J, MacRae TH. Functional differentiation of small heat shock proteins in diapause-destined Artemia embryos. FEBS J. 2013;280:4761–4772. doi: 10.1111/febs.12442. [DOI] [PubMed] [Google Scholar]
- King AM, Toxopeus J, MacRae TH. Artemin, a diapause-specific chaperone, contributes to stress tolerance of Artemia cysts and influences their release from females. J Exp Biol. 2014 doi: 10.1242/jeb.100081. [DOI] [PubMed] [Google Scholar]
- Lapinski J, Tunncliffe A. Anhydrobiosis without trehalose in bdelloid rotifers. FEBS Lett. 2003;553:387–390. doi: 10.1016/S0014-5793(03)01062-7. [DOI] [PubMed] [Google Scholar]
- Li S, Chakraborty N, Borcar A, Menze MA, Toner M, Hand S. Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. Proc Natl Acad Sci USA. 2012;100:20859–20864. doi: 10.1073/pnas.1214893109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of small heat shock/α-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- Loi P, Iuso D, Czernik M, Zacchini F, Ptak G. Towards storage of cells and gametes in dry form. Trends Biotech. 2013;31:688–695. doi: 10.1016/j.tibtech.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Ma W-M, Li H-W, Dai Z-M, Yang J-S, Yang F, Yang W-J. Chitin-binding proteins of Artemia diapause cysts participate in formation of the embryonic cuticle layer of cyst shells. Biochem J. 2013;449:285–294. doi: 10.1042/BJ20121259. [DOI] [PubMed] [Google Scholar]
- Manfre AJ, Lanni LM, Marcotte WR. The Arabidopsis group 1 LATE EMBRYOGENESIS ABUNDANT protein ATEM6 is required for normal seed development. Plant Physiol. 2006;140:140–149. doi: 10.1104/pp.105.072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunde MR, Samarajeewa DA, Anderson J, Li S, Hand SC, Menze MA. Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J Insect Physiol. 2013;59:377–386. doi: 10.1016/j.jinsphys.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Menze MA, Boswell L, Toner M, Hand SC. Occurrence of mitochondria-targeted late embryogenesis abundant (LEA) gene in animals increases organelle resistance to water stress. J Biol Chem. 2009;284:10714–10719. doi: 10.1074/jbc.C900001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SB, Cuypers A, Driscoll SP, Kiddle G, Thomson J, Foyer CH, Theodoulou FL. Yeast complementation reveals a role for an Arabidopsis thaliana late embryogenesis abundant (LEA)-like protein in oxidative stress tolerance. Plant J. 2006;48:743–756. doi: 10.1111/j.1365-313X.2006.02911.x. [DOI] [PubMed] [Google Scholar]
- Pandey AS, MacRae TH. Toxicity of organic mercury compounds to the developing brine shrimp, Artemia. Ecotoxic Environ Saf. 1991;21:68–79. doi: 10.1016/0147-6513(91)90009-E. [DOI] [PubMed] [Google Scholar]
- Qiu Z, MacRae TH. ArHsp22, a developmentally regulated small heat shock protein produced in diapause-destined Artemia embryos, is stress inducible in adults. FEBS J. 2008;275:3556–3566. doi: 10.1111/j.1742-4658.2008.06501.x. [DOI] [PubMed] [Google Scholar]
- Qiu Z, MacRae TH. A molecular overview of diapause in embryos of the crustacean, Artemia franciscana. In: Clark M, Cerda J, Lubzens E, editors. Dormancy and resistance in harsh environments. Berlin: Springer; 2010. pp. 165–187. [Google Scholar]
- Rafiee P, Matthews CO, Bagshaw JC, MacRae TH. Reversible arrest of Artemia development by cadmium. Can J Zool. 1986;64:1633–1641. doi: 10.1139/z86-246. [DOI] [PubMed] [Google Scholar]
- Ramløv H, Hvidt A. Artemia cysts at subzero temperatures studied by differential scanning calorimetry. Cryobiology. 1992;29:131–137. doi: 10.1016/0011-2240(92)90013-R. [DOI] [Google Scholar]
- Reyes JL, Rodrigo M-J, Colmenero-Flores JM, Gil J-V, Garay-Arroyo A, Campos F, Salamini F, Bartels D, Covarrubias AA. Hydrophilins from distant organisms can protect enzymatic activities from water limitation effects in vitro. Plant Cell Environ. 2005;28:709–718. doi: 10.1111/j.1365-3040.2005.01317.x. [DOI] [Google Scholar]
- Robbins HM, Van Stappen G, Sorgeloos P, Sung YY, MacRae TH, Bossier P. Diapause termination and development of encysted Artemia embryos: roles for nitric oxide and hydrogen peroxide. J Exp Biol. 2010;213:1464–1470. doi: 10.1242/jeb.041772. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Furuki T, Akao K, Tanaka D, Nakahara Y, Kikawada T, Watanabe M, Okuda T. Vitrification is essential for anhydrobiosis in an African chironomid, Polypedilum vanderplanki. Proc Natl Acad Sci USA. 2008;105:5093–5098. doi: 10.1073/pnas.0706197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon MA, Kozarova A, Clegg JS, Vacratsis PO, Warner AH. Characterization of a group 1 late embryogenesis abundant protein in encysted embryos of the brine shrimp Artemia franciscana. Biochem Cell Biol. 2009;87:415–430. doi: 10.1139/O09-001. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Kanamori Y, Furuki T, Kikawada T, Okuda T, Takahashi T, Mihara H, Sakurai M. Desiccation-induced structuralization and glass formation of group 3 late embryogenesis abundant protein model peptides. Biochemistry. 2010;49:1093–1104. doi: 10.1021/bi901745f. [DOI] [PubMed] [Google Scholar]
- Soulages JL, Kim K, Walters C, Cushman JC. Temperature-induced extended helix/random coil transitions in a group 1 late embryogenesis-abundant protein from soybean. Plant Physiol. 2002;128:822–832. doi: 10.1104/pp.010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey KB, Storey JM. Molecular biology of freezing tolerance. Comp Physiol. 2013;3:1283–1308. doi: 10.1002/cphy.c130007. [DOI] [PubMed] [Google Scholar]
- Tolleter D, Hincha DK, Macherel D. A mitochondrial late embryogenesis abundant protein stabilizes model membranes in the dry state. Biochim Biophys Acta. 2010;1798:1926–1933. doi: 10.1016/j.bbamem.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Tompa K, Bokor M, Tompa P. Hydration of intrinsically disordered proteins from wide-line NMR. In: Uversky V, Longhi S, editors. Instrumental analysis of intrinsically disordered proteins: assessing structure and conformation. Hoboken: Wiley; 2010. pp. 345–368. [Google Scholar]
- Trotman CNA, Mansfield BC, Tate WP. Inhibition of emergence, hatching, and protein biosynthesis in embryonic Artemia salina. Dev Biol. 1980;80:167–174. doi: 10.1016/0012-1606(80)90506-0. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A, Wise MJ. The continuing conundrum of the LEA proteins. Naturwissenschaften. 2007;94:791–812. doi: 10.1007/s00114-007-0254-y. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A, Lapinski J, McGee B. A putative LEA protein, but no trehalose, is present in anhydrobiotic bdelloid rotifers. Hydrobiologia. 2005;546:315–321. doi: 10.1007/s10750-005-4239-6. [DOI] [Google Scholar]
- Tyson T, O’Mahony Zamora G, Wong S, Skelton M, Daly B, Jones JT, Mulvihill ED, Elsworth B, Phillips M, Blaxter M, Burnel AM. A molecular analysis in the anhydrobiotic nematode Panagrolaimus superbus using expressed sequence tags. BMC Res Notes. 2012;5:68–91. doi: 10.1186/1756-0500-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stappen G, Lavens P, Sorgeloos P. Effects of hydrogen peroxide treatment in Artemia cysts of different geographical origin. Arch Hydrobiol Spec Issues Advanc Limnol. 1998;52:281–296. [Google Scholar]
- Veeramani S, Baskaralingam V. Shell-bound iron dependant nitric oxide synthesis in encysted Artemia parthenogenetica embryos during hydrogen peroxide exposure. Biometals. 2011;24:1035–1044. doi: 10.1007/s10534-011-9462-1. [DOI] [PubMed] [Google Scholar]
- Warner AH, Miroshnychenko O, Kozarova A, Vacratsis PO, MacRae TH, Kim J, Clegg JS. Evidence for multiple group 1 late embryogenesis abundant proteins in encysted embryos of Artemia and their organelles. J Biochem. 2010;148:581–592. doi: 10.1093/jb/mvq091. [DOI] [PubMed] [Google Scholar]
- Warner AH, Chakrabortee S, Tunnacliffe A, Clegg JS. Complexity of the heat-soluble LEA proteome in Artemia species. Comp Biochem Physiol D. 2012;7:260–267. doi: 10.1016/j.cbd.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, McCready S, Brandt WF, Lindsey GG, Hoekstra FA. Isolation and characterization of a D-7 LEA protein from pollen that stabilizes glasses in vitro. Biochim Biophys Acta. 2001;1544:196–206. doi: 10.1016/S0167-4838(00)00220-X. [DOI] [PubMed] [Google Scholar]
- Wu G, Zhang H, Sun J, Liu F, Ge X, Chen W-H, Yu J, Wang W (2011) Diverse LEA (late embryogenesis abundant) and LEA-like genes and their responses to hypersaline stress in post-diapause embryonic development of Artemia franciscana. Comp Biochem Physiol B 160:32--39 [DOI] [PubMed]
- Yoshida T, Arii Y, Hino K, Sawatani I, Tanaka M, Takahashi R, Bando T, Mukai K, Fukuo K. High hatching rates after cryopreservation of hydrated cysts of the brine shrimp A. franciscana. Cryoletters. 2011;32:206–215. [PubMed] [Google Scholar]
- Zhao Y, Ding X, Xiang Y, Dai Z-M, Yang J-S, Yang W-J. Involvement of cyclin K posttranscriptional regulation in the formation of Artemia diapause cysts. PLoS ONE. 2012;7(2):e32129. doi: 10.1371/journal.pone.0032129. [DOI] [PMC free article] [PubMed] [Google Scholar]