Abstract
The 70-kDa family of heat-shock proteins (Hsp70) plays an important role in the host immunity, which is widely expressed in eukaryotic cells as a major chaperone protein. In the present study, the full-length complementary DNA (cDNA) of a second cognate cytosolic Hsp70 family member (MnHsc70-2) was cloned and characterized from Macrobrachium nipponense, which is an economically and nutritionally important crustacean. The cDNA was 2,717 bp, containing an open reading frame (ORF) of 1,950 bp, which encodes a protein of 649 amino acids with a theoretical molecular weight of 71.1 kDa and an isoelectric point of 5.27. Sequence alignment showed that the MnHsc70-2 shared 75–97 % identity with other heat-shock proteins. Compared to the previously identified cognate Hsp70 (MnHsc70-1) in M. nipponense, MnHsc70-2 showed quite different expression profiles under unstressed conditions in all tested tissues, including the hemocytes, heart, hepatopancreas, gill, intestine, nerve, and muscle. The phylogenetic analysis demonstrated that MnHsc70-2 showed the closest relationship with MnHsc70-1. Heat-inducibility assays showed that two isolated messenger RNAs (mRNAs) displayed different expression profiles in both the hepatopancreas and gill tissues. MnHsc70-1 mRNA expression level decreased at first and then increased to the normal level, whereas MnHsc70-2 mRNA level increased at first and then decreased. The expressions of two MnHsc70s showed substantial obvious heat-inducible regulation in both the hepatopancreas and gill. Under bacterial challenge by Aeromonas hydrophila, both MnHsc70-1 and MnHsc70-2 mRNA level was up-regulated moderately. The results suggested that two cognate Hsc70s may play essential functions in mediating responses to heat-shock and bacterial challenge.
Keywords: Macrobrachium nipponense, Heat-shock protein, Heat-shock treatment, Aeromonas hydrophila, Immune response
Introduction
Heat-shock proteins (Hsps), first described in Drosophila busckii in 1962 by Ritossa (Ritossa 1962), have been shown to be the most phylogenetically conserved proteins present in all prokaryotes and eukaryotes (Tsan and Gao 2004). Hsps perform essential biological functions under both normal and stress conditions (Kiang and Tsokos 1998; Sørensen et al. 2003). The primary function of Hsps appears to serve as molecular chaperones in which they recognize and bind to nascent polypeptide chains and partially folded intermediates of proteins, preventing their aggregation and misfolding, or as chaperonins that directly mediate protein folding (Hartl and Hayer-Hartl 2002). In addition to serving as molecular chaperones, Hsps have been implicated in autoimmune diseases (Pockley 2003), antigen presentation, and tumor immunity (Yue et al. 2011). Moreover, several studies have indicated that Hsps, acting as ligands for the toll-like receptors (TLR), are critical to the induction of both innate and adaptive immune responses (Asea et al. 2002; Vabulas et al. 2002).
Hsps have been classified into three major families according to their molecular weights: Hsp90 (85–90 kDa), Hsp70 (68–73 kDa), and low-molecular-weight Hsps (16–47 kDa) (Ming et al. 2010). As one of the most abundant and widely investigated families in higher eukaryotes, the Hsp70 family is made up of cytosolic Hsp70 including the inducible Hsp70 and the cognate Hsc70, glucose-regulated protein 78 (Grp78) located in the endoplasmic reticulum, and mitochondrion Hsp75 (Lindquist and Craig 1988). The inducible Hsp70s express at extremely low levels under normal conditions and increase significantly in response to stresses (Hartl 1996). However, the cognate Hsc70s are constitutively expressed under normal conditions and change relatively little on exposure to stressors (Kregel 2002; Park et al. 2001).
In recent years, many studies have focused on the Hsps of aquaculture animals due to their importance in coping with stress-induced denaturation of client proteins, as well as their essential roles including folding, assembly, degradation of other proteins, and the regulation of gene expression (Piano et al. 2005; Terasawa et al. 2005). Up to now, several Hsc70s have been cloned from many kinds of crustaceans, including the pacific white shrimp Litopenaeus vannamei (Wu et al. 2008), tiger shrimp Penaeus monodon (Lo et al. 2004), Chinese shrimp Fenneropenaeus chinensis (Jiao et al. 2004), and giant freshwater prawn Macrobrachium rosenbergii (Liu et al. 2004). However, there are only a little data on Hsc70 of the oriental river prawn, Macrobrachium nipponense.
It has been reported that some lower vertebrates and mammals appear to contain more than one isoform for both Hsp70 and Hsc70 (Ali et al. 1996b, 2003; Giebel et al. 1988; Walsh et al. 1994, 1997), but there is no report from a crustacean. In the present study, for the first time, we identified and characterized the second cytosolic member of the Hsp70 family (MnHsc70-2) from M. nipponense. The deduced amino acid sequences were compared with other known Hsp70s, and the distribution of MnHsc70-1 and MnHsc70-2 in various tissues was also investigated. By means of analysis of the expression changes in response to heat-shock and Aeromonas hydrophila challenge, we hope to learn if two cognate Hsc70s play different potential immune-related functions in M. nipponense.
Materials and methods
Animal and RNA extraction
The oriental river prawns, M. nipponense (2–3 g in weight, 4–5 cm in length) purchased from a market in Nanjing, China, were cultivated in 100-L aquaculture tanks at 15 °C with freshwater and an aeration system. The experimental prawns were fed with minced prawn once daily. The prawns were acclimated for 10 days before processing. The hemocytes and hepatopancreas from randomly selected specimens were checked using A. hydrophila diagnostic PCR (Pollard et al. 1990) and were all found to be PCR negative. Prawn health status was assessed daily during acclimation by monitoring both general activity and food intake.
RNA from different tissues was extracted using TRIzol Reagent (Invitrogen, USA) and then treated with RNase-free DNase I (Invitrogen, USA) following the manufacturer’s protocol. RNA quality was assessed by electrophoresis on 1.2 % agarose gel, and the total RNA concentration was determined by measuring the absorbance at 260 nm on a spectrophotometer.
cDNA synthesis and gene cloning
The complementary DNA (cDNA) was synthesized using about 5 μg total RNA using the Takara PrimerScript™ First Strand cDNA Synthesis kit (TaKaRa, China) according to the manufacturer’s instructions. Degenerate primers, MnHsc70-2F1 and MnHsc70-2R1, designed based on the highly conserved nucleotide region of other known Hsp70s (Luan et al. 2010) were used in the reverse-transcriptase polymerase chain reaction (RT-PCR). Amplification primers for MnHsc70-1 and MnHsc70-2 were shown in Tables 1 and 2.
Table 1.
Primers used in the RACE
| Name | Sequence (5′–3′) |
|---|---|
| MnHsc70-2F1 | CAGAACGACATGAAA(/G)CAT(/C)TG |
| MnHsc70-2R1 | GTGGGT(/C)TCGTTGATGATG(/A)C |
| MnHsc70-2R2 | TGACGACAGCATCCTT |
| MnHsc70-2R3 | CTTAATAAGCACCATTGAGGAG |
| MnHsc70-2R4 | GGTTTTGGTCTCTCCTTTGTAA |
| MnHsc70-2F2 | TGGGTGGTACTGTAAAGGATGCTGTCGT |
| MnHsc70-2F3 | GACTCCCAGCGTCAGGCCACCAAAGAT |
| AAP | GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG |
| AUAP | GGCCACGCGTCGACTAGTAC |
| Oligo-d(T)18 ACP | CTGTGAATGCTGCGACTACGA(T)18 |
| 3′-RACE primer | CTGTGAATGCTGCGACTACGA |
Table 2.
Primers used in the qRT-PCR
| Name | Sequence (5′–3′) | Annealing temperature (°C) | Size of product (bp) |
|---|---|---|---|
| MnHsc70-1QF | GTTATGACAGCACTTATTAA | 56 | 232 |
| MnHsc70-1QR | AGATACCATTGGCATCAATATC | ||
| MnHsc70-2QF | ATATTGATGCTAATGGTATTC | 57 | 207 |
| MnHsc70-2QR | TTGAAACAGTAAGACTCCAGGT | ||
| β-actin F | AATGTGTGACGACGAAGTAG | 56 | 176 |
| β-actin R | GCCTCATCACCGACATAA |
Rapid amplification of cDNA ends (RACE) of MnHsc70-2
About 5 μg of total RNA was reverse-transcribed with MnHsc70-2R2 primer to generate 5′-RACE template. MnHsc70-2R2 primer was designed based on the sequence amplified by the degenerate primers. For the 5′-RACE, part of the MnHsc70-2 gene was obtained using a 5′-RACE System (Invitrogen, USA) according to the manufacturer’s instructions. The primer set consisted of MnHsc70-2R3 with abridged anchor primer (AAP) for the first-run PCR and MnHsc70-2R4 with the abridged universal amplification primer (AUAP) for the second-run PCR. For the 3′-RACE, the RT-PCR was performed using the oligo-d(T)18 ACP primer with MnHsc70-2F2, and the nested PCR was performed using MnHsc70-2F3 with 3′-RACE primer. The amplified products were cloned into pMD19-T vector and sequenced by Springen (Nanjing) Biotechnology Company.
Sequence analysis
The cDNA sequences of MnHsc70-1 and MnHsc70-2 were analyzed using the basic local alignment search tool (BLAST) algorithm (http://www.ncbi.nlm.nih.gov/blast), and the deduced amino acid sequence was analyzed with the ExPASy tools (http://us.expasy.org/). The ClustalW multiple alignment program (http://www.ebi.ac.uk/clustalw/) was used for the multiple sequence alignment. A phylogenetic tree was constructed based on the amino sequences alignment by the neighbor-joining (NJ) algorithm embedded in the MEGA 5 program (Tamura et al. 2011). The reliability of the branching was tested by bootstrap resampling (1,000 pseudoreplicates).
Tissue expression of MnHsc70-1 and MnHsc70-2
Tissue distribution of MnHsc70-1 and MnHsc70-2 messenger RNAs (mRNA) in the hemocytes, heart, hepatopancreas, gill, intestine, nerve, and muscle was demonstrated by quantitative real-time PCR analysis. Total RNA was extracted as described above; 5 μg of total RNA was used to synthesize the first strand cDNA. For quantification of MnHsc70-1 and MnHsc70-2 expression, two pairs of gene-specific primers (MnHsc70-1QF, MnHsc70-1QR and MnHsc70-2QF, MnHsc70-2QR) were used, respectively. The primers β-actin F and β-actin R (Zhao et al. 2011) were used to amplify β-actin as an internal control (Table 2). The different gene expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen 2001). Statistical analysis was performed using SPSS software (Ver11.0). Data are presented as the mean ± standard error (n = 3). Statistical significance was determined by two-way ANOVA and post hoc Duncan multiple range tests.
Heat-shock treatment of M. nipponense
In order to study the effect of temperature on MnHsc70-1 and MnHsc70-2 gene expression, individuals were exposed to 25 °C for 12 h and then returned to the control temperature for a period of 48 h. Time course was performed at 0, 2, 6, 12, 24, or 48 h during the recovery period at 15 °C. The hepatopancreas and gill of five prawns at each sampling time were collected, respectively, frozen immediately in TRIzol Reagent, and stored at −80 °C until RNA extraction. Gene expression of MnHsc70-1 and MnHsc70-2 was determined by quantitative real-time RT-PCR.
Bacterial challenge
For bacterial challenge, the M. nipponense were cultivated at 25 °C for 10 days before processing. In the experimental group, the prawns were injected individually with 50 μl of bacterial suspension (104 cells/ml) which had been dissolved in 0.85 % NaCl solution. The bacterium A. hydrophila was purchased from China General Microbiological Culture Collection Center and was grown at 28 °C in a TSB medium. At the same time, the prawns injected with 50 μl saline (0.85 % NaCl) (pH = 7.0) were used as the control group. M. nipponense were sampled at 0, 1, 12, 24, 36, and 48 h post-injection. For each treatment and each exposure time, the hepatopancreas of five prawns were sampled, and total RNA was extracted as described above. Quantitative real-time RT-PCR was employed to determine expression profile of MnHsc70-1 and MnHsc70-2. In order to confirm the infection of the prawns, the hepatopancreas and hemolymph were chosen for bacterium culture. At the same time, PCR assay was also used to confirm the infection.
Results
Characterizations of MnHsc70-1 and MnHsc70-2 cDNA
MnHsc70-1 cDNA and amino acid sequences were found in GenBank accession number ABG45886. The full-length MnHsc70-1 cDNA was 2,553-bp long, including a 1,950-bp coding sequence and a 98- and 505-bp flanking region at the 5′ and 3′ ends, respectively. Different from MnHsc70-2 cDNA, the consensus polyadenylation site (AATAAA) was not found upstream of the polyA tail in MnHsc70-1. The deduced amino acid sequence encodes a 649 aa protein with a calculated molecular mass of 71.06 kDa and a pI of 5.27 (Fig. 1).
Fig. 1.
Nucleotide sequence and its deduced amino acid sequence of MnHsc70-1. Three characteristic Hsp70 family signatures are shown in bold letters; the putative ATP-GTP binding site is underlined and in italics; the putative bipartite nuclear localization signal (KK and RRLRT) is shown in a box; the potential non-organelle eukaryotic consensus motif RARFEEL is indicated in italics; three tetrapeptides of GGXP at the carboxyl terminal region are in gray; the conserved EEVD motif at the C-terminal is underlined
A fragment of 242 bp was generated by MnHsc70-2F1 and MnHsc70-2R1. The full-length MnHsc70-2 cDNA (GenBank accession number KC460343) was 2,717 bp in length including 252 bp in the 5′-untranslated region (UTR), an open reading frame (ORF) of 1,950 bp encoding a protein of 649 amino acids, and 515 bp in the 3′-untranslated region. A canonical polyadenylation signal (AATAAA) and a polyA tail were located within the 3′-UTR. The predicted molecular weight is 71.1 kDa with an estimated pI of 5.27 (Fig. 2).
Fig. 2.
Nucleotide sequence and its deduced amino acid sequence of MnHsc70-2. Three characteristic Hsp70 family signatures are shown in bold letters; the putative ATP-GTP binding site is underlined and in italics; the putative bipartite nuclear localization signal (KK and RRLRT) is shown in a box; the potential non-organelle eukaryotic consensus motif RARFEEL is indicated in italics; three tetrapeptides of GGXP at the carboxyl terminal region are in gray; the conserved EEVD motif at the C-terminal is underlined; and the consensus polyA signal is indicated by a double-underline
Homology analysis of MnHsc70-1 and MnHsc70-2
The BLAST analysis showed that two MnHsc70s amino acid sequences shared closest homology with the deduced proteins of other known constitutive Hsc70s. The alignment analysis between the MnHsc70-1 and MnHsc70-2 amino acid sequences showed 99 % identity. The MnHsc70-2 amino acid exhibited 94–97 % sequence identity with constitutive Hsc70s from other crustacean species, including M. rosenbergii Hsc70 (97 %), F. chinensis Hsc70 (94 %), P. monodon Hsc70 (94 %), and L. vannamei Hsc70 (94 %). Meanwhile, MnHsc70-2 was found to have 82 % homology with Procambarus clarkii Hsp70, 80 % with F. chinensis Hsp70, and 75 % with M. rosenbergii Hsp70.
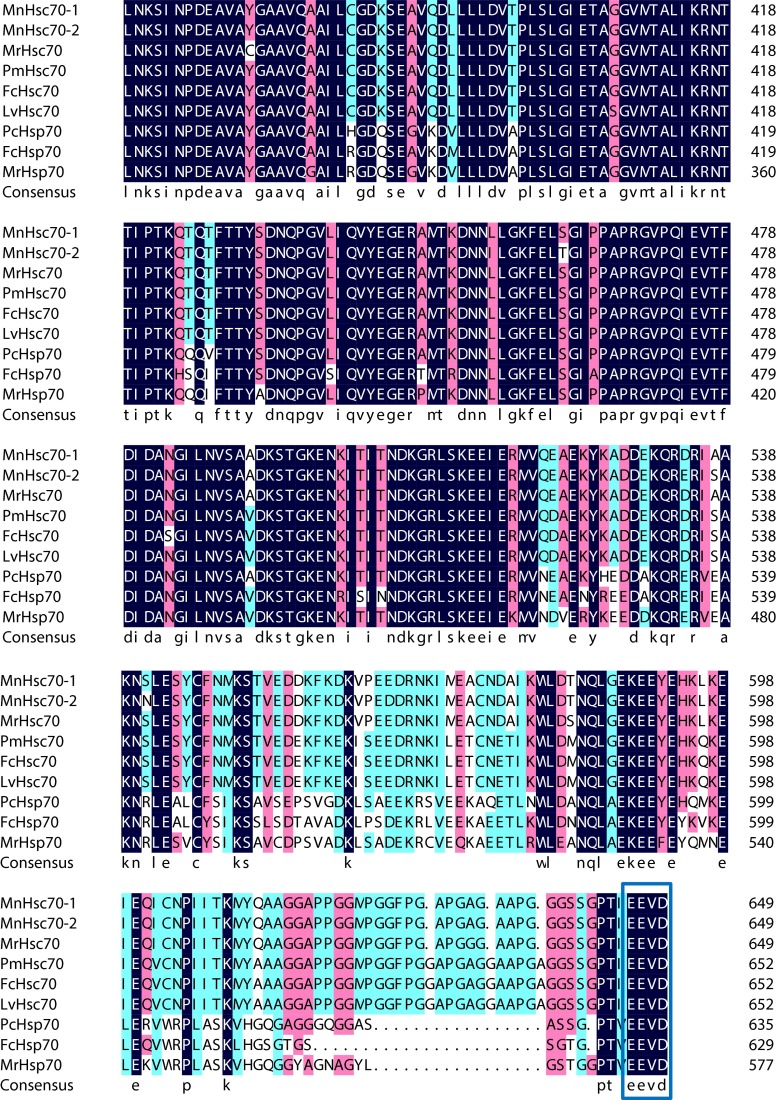
Deduced amino acid sequence alignment indicates that three highly conserved signatures including IDLGTTYS, IFDLGGGTFDVSIL, and IVLVGGSTRIPKIQK (Zdobnov and Apweiler 2001) were all identified in most organisms (Fig. 3). In addition, the potential non-organelle eukaryotic consensus motif RARFEEL and cytoplasmic characteristic motif EEVD were also identified.
Fig. 3.
Alignment result of the deduced amino acid sequences: MnHsc70-1, ABG45886; MnHsc70-2, KC460343; MrHsc70, AY466445; PmHsc70, AAQ05768; FcHsc70, AY748350; LvHsc70, ABP01681; PcHsp70, ABC01063; FcHsp70, FJ167398; and MrHsp70, AY466497. Three signatures are shown in red boxes. The potential non-organelle eukaryotic consensus motif RARFEEL and cytoplasmic characteristic motif EEVD are shown in the green and blue box, respectively
Phylogenetic analysis
A phylogenetic tree was constructed by analyzing the amino acid sequences of two MnHsc70s and HSPs from a variety of species (Fig. 4). The phylogenetic analysis, which was consistent with the alignment results, showed that MnHsc70-1 clustered with MnHsc70-2 on a unique branch. This branch showed the closest relationship with M. rosenbergii Hsc70 and other crustacean Hsc70s. In addition, Hsp70 and Hsc70s tended to cluster into two separate subclasses except for one especial sequence, which might show how Hsp70 family proteins evolved in the eukaryotic cells.
Fig. 4.
Phylogenetic tree showing the relationship of MnHsc70-1 and MnHsc70-2 amino acid sequences to other Hsp70 family members from a variety of species. Sequences used in the tree were followed by their GenBank accession number. The number at each node indicated the percentage of bootstrapping after 1,000 replications
Tissue distribution of MnHsc70-1 and MnHsc70-2
In order to compare the distribution of MnHsc70-1 and MnHsc70-2 mRNA in different tissues, total RNA was extracted from the hemocytes, heart, hepatopancreas, gill, intestine, nerve, and muscle. The relative expression level of gene was calculated by 2−ΔΔCT method, with the expression level of MnHsc70-1 mRNA in the hemocytes used as a calibrator and set at 1.0. The relative expression levels of other MnHsc70s mRNA at different tissues were indicated as n-fold differences relative to the calibrator. As determined by quantitative real-time PCR, the mRNA transcripts of MnHsc70-1 and MnHsc70-2 were widely detected in all examined tissues (Fig. 5). A predominant MnHsc70-1 transcript was found in the intestine, with slightly less in the hepatopancreas, and even less in other tissues. Different from MnHsc70-1, MnHsc70-2 transcript was most abundant in the hepatopancreas, with a moderate expression in the gill, intestine, and muscle, and a lower expression in the hemocytes, heart, and nerve. Significant differences between the two MnHsc70s were obvious, and a higher expression level of MnHsc70-2 was found in all tissues. Two-way analysis of variance showed that no significant difference was found among tissues within MnHsc70-1. For comparison between MnHsc70-1 and MnHsc70-2 within the same tissue, significant differences were found in all tested tissues except for the hemocytes.
Fig. 5.
Expression profiles of MnHsc70-1 and MnHsc70-2 mRNA in different tissues. Quantitative real-time RT-PCR was carried out with RNA samples from the hemocytes, heart, hepatopancreas, gill, intestine, nerve, and muscle of the M. nipponense. Different lowercase letters above the bars indicate significant differences (P < 0.05) at different tissues of the same group; asterisks above the bars show significant differences (P < 0.05) between two groups of the same tissue
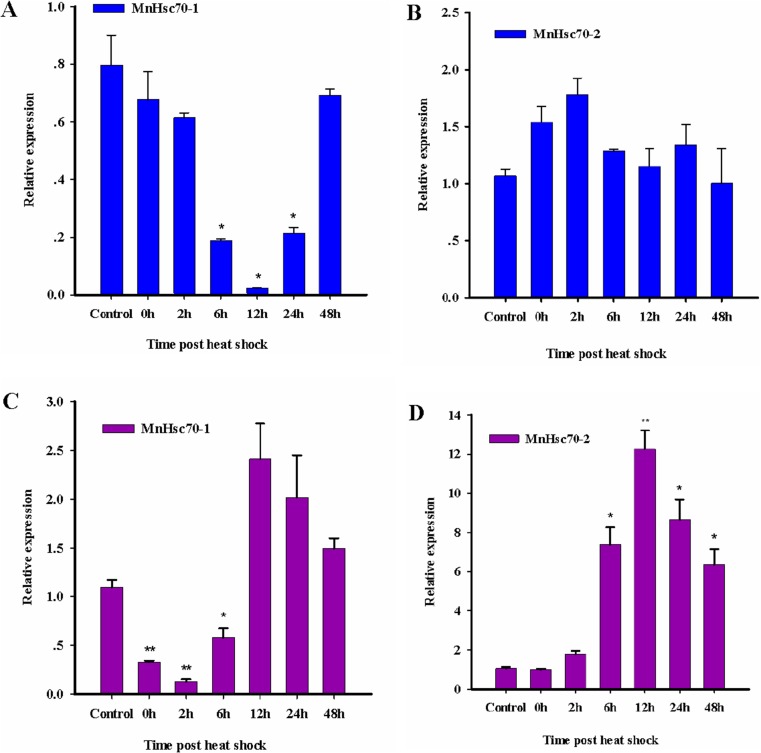
Expression analysis of MnHsc70-1 and MnHsc70-2 mRNA under heat shock
The expression profiles of MnHsc70-1 and MnHsc70-2 in the hepatopancreas and gill after heat-shock treatment are shown in Fig. 6. It was clear that the expression profile of MnHsc70-1 was quite different from that of MnHsc70-2 in both the hepatopancreas and gill tissues. In the hepatopancreas, MnHsc70-1 mRNA level decreased at first and then increased to the normal level, whereas MnHsc70-2 mRNA expression level increased at first and then decreased. MnHsc70-1 mRNA level slightly decreased at 0 h to 84.93 % of the control level. At 12 h, the mRNA expression level of MnHsc70-1 significantly decreased to 2.83 % of the control level. After that, MnHsc70-1 mRNA level increased gradually and reached the normal level at 48 h (Fig. 6a). MnHsc70-2 mRNA level increased moderately after heat shock and reached the highest level at 2 h which was 1.67-fold higher compared with that in the control group (Fig. 6b).
Fig. 6.
Real-time RT-PCR analyses of MnHsc70-1 and MnHsc70-2 expressions in M. nipponense under heat-shock treatment (n = 3). Graphs a and b represent expression profiles of MnHsc70-1 and MnHsc70-2 in the hepatopancreas, respectively. Graphs c and d represent expression profiles of MnHsc70-1 and MnHsc70-2 in the gill, respectively. Significant differences are indicated, *P < 0.05 and **P < 0.01. Expression of MnHsc70-1 and MnHsc70-2 mRNA is relative to β-actin
In the gill, MnHsc70-1 and MnHsc70-2 mRNA showed substantial obvious heat-inducible regulation. For MnHsc70-1 mRNA, the lowest level at 2 h was 11.69 % of the control group, and thereafter, it gradually increased. The MnHsc70-1 mRNA significantly increased at 12 h which was 2.20-fold compared with that in the control level (Fig. 6c). At 12 h, the mRNA expression level of MnHsc70-2 significantly increased to 11.67-fold and reached a peak (Fig. 6d).
Expression analysis of MnHsc70-1 and MnHsc70-2 mRNA after A. hydrophila challenge
The bacterium culture and PCR assay showed that the prawns had been successfully infected by A. hydrophila. After A. hydrophila challenge, the M. nipponense tail turned red, and subsequently, the swim foot and entire abdomen were affected. Fifty percent mortality was shown during this experiment. The temporal expression pattern of MnHsc70-1 and MnHsc70-2 in the hepatopancreas after being challenged by A. hydrophila was shown in Fig. 7. The results showed that after infection of A. hydrophila, two MnHsc70s mRNA levels increased slightly at first and then decreased. MnHsc70-1 mRNA levels moderately increased and reached a peak at 1 h, when the mRNA levels increased to 2.26-fold higher than that in the control. The expression of MnHsc70-2 was slightly up-regulated at 1 h and reached the highest level at 12 h post-injection, which was 2.72-fold higher than that in the control groups. Then, the expression level dropped as time progressed; the mRNA levels of MnHsc70-1 and MnHsc70-2 decreased to 21.04 and 75.9 % of the control at 24 h, respectively. Another up-regulation was detected at 36 h, and the expression returned to the normal level at 48 h. Although the MnHsc70-1 and MnHsc70-2 expression in the control group fluctuated slightly at different time points, no significant differences were found among them.
Fig. 7.
Relative expressions of MnHsc70-1 and MnHsc70-2 in the hepatopancreas at different time points under A. hydrophila challenge analyzed by quantitative RT-PCR. Graphs a and b represented expression profiles of MnHsc70-1 and MnHsc70-2, respectively. Asterisks indicate there was a significant difference between control prawns and bacteria-challenged prawns. Expression of MnHsc70-1 and MnHsc70-2 mRNA is relative to β-actin
Discussion
In this article, the gene encoding MnHsc70-2 was identified from M. nipponense, and the characteristics of two MnHsc70s were analyzed. Amino acid sequence analysis showed that both of the isolated cDNAs conserved the main typical structural features of eukaryotic cytoplasmic HSP70 family members. In addition, MnHsc70-1 and MnHsc70-2 sequences included two additional specific motifs indicative of the HSP70 cytosolic localization: a nonorganellar stress protein motif (RARFEEL) and the extreme C-terminal domain EEVD, which is involved in binding with different co-chaperones (Demand et al. 1998; Johnson et al. 1998). It has been suggested that the functional differences between inducible and constitutive HSP70 members include the α-helical subdomain, the tetrapeptide GGXP repeats, and the EEVD motif (Demand et al. 1998; Fuertes et al. 2004). The tetrapeptide motif GGXP repeats are thought to be involved in co-chaperone-binding activities of Hsc70 (Demand et al. 1998); and likewise, in our results, three tetrapeptides of GGXP at the carboxyl terminal were found in both MnHsc70-1 and MnHsc70-2.
There are a number of examples where more than one Hsc70 have been described from the same organism (Ali et al. 1996a, b, 2003; Graser et al. 1996; Santacruz et al. 1997). However, to our knowledge, there have been no reports of two or more Hsc70s in a crustacean organism, and this is the first time that two closely related Hsc70s have been identified in the oriental river prawn, M. nipponense. Furthermore, comparison between our peptide sequences and those of known Hsc70s in other crustaceans showed stronger homology than that of the heat-inducible form. A phylogenetic tree was constructed using the amino acid sequences of the MnHsc70s along with Hsc70 and Hsp70 sequences from a number of other species. As shown in Fig. 4, the MnHsc70s clusters closely with other crustacean Hsc70s on a unique branch of the tree, and they in turn form a larger node with Hsc70 sequences from Xenopus, human, Rattus, fish, and Crassostrea. Finally, taken together, Hsp70s and Hsc70s were clustered into two separate subclasses except for one especial sequence, which might show how Hsp70 family proteins evolved in the subcellular locations of eukaryotic cells.
Whether a heat-shock gene is heat-inducible (Hsp gene) or a cognate gene (Hsc gene) depends on the regulation of this gene in normal somatic cells: a heat-inducible gene is efficiently expressed only after heat induction, whereas a cognate gene has a high basal level of expression and is not or is weakly heat-inducible (David 1993). In our study, the expression of the MnHsc70 genes under unstressed conditions was performed by a quantitative real-time PCR method. Results revealed that two MnHsc70s mRNA transcripts were present in all tested tissues. The different expression profiles between MnHsc70-1 and MnHsc70-2 were obvious, and the level of MnHsc70 expression in different tissues of M. nipponense was also varied. Differential expression of Hsc70 also has been reported in the tissues of rats, Xenopus, carp, and shrimp (Ali et al. 1996a; Lo et al. 2004; O’Malley et al. 1985). The expression pattern of the two MnHsc70s led us to speculate that under normal conditions, the expression of MnHsc70-2 was dominant, while the expression of MnHsc70-1 was just supplementary.
In the present study, MnHsc70-1 exhibited a dramatic down-regulation in the hepatopancreas and gill after heat shock. By contrast, the expression of MnHsc70-2 was slightly up-regulated in the hepatopancreas, whereas the MnHsc70-2 of the gill was relatively sensitive to the stressor producing an 11.67-fold induction. The depression of Hsc70 has been demonstrated in Pacific oyster Crassostrea gigas (Boutet et al. 2003) and Chinese shrimp F. chinensis by cadmium exposure (Luan et al. 2010). It was demonstrated that the depression of Hsp70 by cadmium exposure might be regulated through a cAMP-responsive regulatory pathway (Vilaboa et al. 1995) which implied that the down-regulation of MnHsc70-1 may also be regulated by cAMP. In previous studies, Hsc70 expression in most species was not increased by heat-shock treatment (Kiang and Tsokos 1998). However, an increasing number of studies have demonstrated that heat-shock treatment does increase mRNA levels several fold (<20-fold) in a variety of cell types, including hemocytes of P. monodon (Lo et al. 2004), Pacific oyster C. gigas hemocytes (Gourdon et al. 2000), human peripheral blood monocytes (Jacquier-Sarlin et al. 1995), human lymphocytes (Hansen et al. 1991), and human HeLa cells (O’Malley et al. 1985). Several cognate Hsc70s increase several fold in stressed animals, indicating that they are moderately inducible (Tavaria et al. 1996).
In recent years, due to infection by various pathogenic microorganisms especially A. hydrophila (Shen et al. 2000), mass mortality of M. nipponense occurred, which caused heavy economic losses. It is necessary to better understand the mechanism of stress tolerance or pathogen resistance of M. nipponense. Previous studies showed that the expression levels of Hsp70 were significantly induced by pathogen stimulation (Cui et al. 2010; Rungrassamee et al. 2010). Likewise, Hsp70 showed specific immune responses against infectious agents; both MnHsc70-1 and MnHsc70-2 exhibited a moderate up-regulation when challenged with A. hydrophila. Similar expression profiles were found in previous studies: the expression of FcHsc70 was less sensitive to copper treatment (Luan et al. 2010), and no significant changes of Hsc70 were detected in the primary-cultured hepatocytes in rainbow trout under heavy metal exposure (Boone and Vijayan 2002).
In conclusion, two MnHsc70 members from M. nipponense have been identified and characterized for the first time. The expression levels of MnHsc70s under normal conditions are consistent with their cognate nature: the basal expression levels are substantial. The different expression profiles between MnHsc70-1 and MnHsc70-2 support the hypothesis that two Hsc70 genes have distinct biological tasks. The results presented here provide useful insights for investigating stress-related response in M. nipponense.
Acknowledgments
We appreciate Professor O. Roger Anderson (Columbia University) for editing the manuscript. This work was supported by the National Natural Sciences Foundation of China (NSFC no. 31170120; 31101926; 31200139; 31272686). A project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Agriculture Science and Technology Innovation Fund (JASTIF) CX (12)3066, Project for aquaculture in Jiangsu Province (no. PJ2011-65; Y2013-45; D2013-5-3; D2013-5-4), and NSFC for Talents Training in Basic Science (J1103507). Incubation Program for New Type Academic Ph.D Student of Nanjing Normal University.
Abbreviations
- Hsp70
Heat-shock protein 70
- Hsc70
Cognate cytosolic Hsp70
- HSPs
Heat-shock proteins
- TLR
Toll-like receptors
- Grp78
Glucose-regulated protein 78
- M. nipponense
Macrobrachium nipponense
- A. hydrophila
Aeromonas hydrophila
- RT-PCR
Reverse-transcriptase polymerase chain reaction
- RACE
Rapid amplification of cDNA ends
- AAP
Abridged anchor primer
- AUAP
Abridged universal amplification primer
- NJ
Neighbor-joining
- F. chinensis
Fenneropenaeus chinensis
- P. monodon
Penaeus monodon
- C. gigas
Crassostrea gigas
Footnotes
Highlights
1. This is first report of the second Hsc70 cDNA from crustacean organism (MnHsc70-2).
2. MnHsc70-2 was cloned, and the characteristics of two MnHsc70s were analyzed.
3. Heat-shock treatment was done to study expression profiles of two MnHsc70s.
4. Expression changes of two MnHsc70s were studied when challenged with A. hydrophila.
5. The results above implied that two Hsc70 genes have distinct biological tasks.
Contributor Information
Qingguo Meng, Phone: +86-25-85891955, Email: mlzzcld@gmail.com.
Wen Wang, Phone: +86-25-85891955, Email: njnuwang@263.net, Email: wenwang@njnu.edu.cn.
References
- Ali A, Salter-Cid L, Flajnik MF, Heikkila JJ. Isolation and characterization of a cDNA encoding a Xenopus 70-kDa heat shock cognate protein, Hsc70. I. Comp Biochem Physiol B. 1996;113:681–687. doi: 10.1016/0305-0491(95)02081-0. [DOI] [PubMed] [Google Scholar]
- Ali A, Salter-Cid L, Flajnik MJ, Heikkila JJ. Molecular cloning of a cDNA encoding a Xenopus laevis 70-kDa heat shock cognate protein, hsc70. II. Biochim Biophys Acta Gene Struct Expr. 1996;1309:174–178. doi: 10.1016/S0167-4781(96)00156-X. [DOI] [PubMed] [Google Scholar]
- Ali K, Dorgai L, Abraham M, Hermesz E. Tissue-and stressor-specific differential expression of two hsc70 genes in carp. Biochem Biophys Res Commun. 2003;307:503–509. doi: 10.1016/S0006-291X(03)01206-3. [DOI] [PubMed] [Google Scholar]
- Asea A, et al. Novel signal transduction pathway utilized by extracellular HSP70. Role of toll-like receptor (TLR) 2 and TLR4. Sci Signal. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Boone AN, Vijayan MM. Constitutive heat shock protein 70 (HSC70) expression in rainbow trout hepatocytes: effect of heat shock and heavy metal exposure. Comp Biochem Physiol C. 2002;132:223–233. doi: 10.1016/s1532-0456(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Boutet I, Tanguy A, Rousseau S, Auffret M, Moraga D. Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones. 2003;8:76–85. doi: 10.1379/1466-1268(2003)8<76:MIAEOH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Liu Y, Luan W, Li Q, Wu D, Wang S. Molecular cloning and characterization of a heat shock protein 70 gene in swimming crab (Portunus trituberculatus) Fish Shellfish Immunol. 2010;28:56–64. doi: 10.1016/j.fsi.2009.09.018. [DOI] [PubMed] [Google Scholar]
- David BM. Structure and mechanism of 70-kDa heat-shock-related proteins. Adv Protein Chem. 1993;44:67–98. doi: 10.1016/S0065-3233(08)60564-1. [DOI] [PubMed] [Google Scholar]
- Demand J, Lüders J, Höhfeld J. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol Cell Biol. 1998;18:2023–2028. doi: 10.1128/mcb.18.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes MA, Pérez JM, Soto M, Menéndez M, Alonso C. Thermodynamic stability of the C-terminal domain of the human inducible heat shock protein 70. BBA Proteins Proteome. 2004;1699:45–56. doi: 10.1016/j.bbapap.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Giebel LB, Dworniczak BP, Bautz E. Developmental regulation of a constitutively expressed mouse mRNA encoding a 72-kDa heat shock-like protein. Dev Biol. 1988;125:200–207. doi: 10.1016/0012-1606(88)90073-5. [DOI] [PubMed] [Google Scholar]
- Gourdon I, Gricourt L, Kellner K, Roch P, Escoubas J-M. Characterization of a cDNA encoding a 72 kDa heat shock cognate protein (Hsc72) from the Pacific oyster, Crassostrea gigas. Mitochondrial DNA. 2000;11:265–270. doi: 10.3109/10425170009033241. [DOI] [PubMed] [Google Scholar]
- Graser RT, Malnar-Dragojevic D, Vincek V. Cloning and characterization of a 70 kd heat shock cognate (hsc70) gene from the zebrafish (Danio rerio) Genetica. 1996;98:273–276. doi: 10.1007/BF00057591. [DOI] [PubMed] [Google Scholar]
- Hansen LK, Houchins J, O’Leary JJ. Differential regulation of HSC70, HSP70, HSP90α, and HSP90β mRNA expression by mitogen activation and heat shock in human lymphocytes. Exp Cell Res. 1991;192:587–596. doi: 10.1016/0014-4827(91)90080-E. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nat Biotechnol. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Jacquier-Sarlin MR, Jornot L, Polla BS. Differential expression and regulation of hsp70 and hsp90 by phorbol esters and heat shock. J Biol Chem. 1995;270:14094–14099. doi: 10.1074/jbc.270.23.14094. [DOI] [PubMed] [Google Scholar]
- Jiao C, Wang Z, Li F, Zhang C, Xiang J. Cloning, sequencing and expression analysis of cDNA encoding a constitutive heat shock protein 70 (HSC70) in Fenneropenaeus chinensis. Chin Sci Bull. 2004;49:2385–2393. [Google Scholar]
- Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates Hsp70/Hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–3686. doi: 10.1074/jbc.273.6.3679. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/S0163-7258(98)00028-X. [DOI] [PubMed] [Google Scholar]
- Kregel KC. Invited review: heat shock proteins: modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol. 2002;92:2177–2186. doi: 10.1152/japplphysiol.01267.2001. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig E. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang W-J, Zhu X-J, Karouna-Renier NK, Rao RK. Molecular cloning and expression of two HSP70 genes in the prawn, Macrobrachium rosenbergii. Cell Stress Chaperones. 2004;9:313–323. doi: 10.1379/CSC-40R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[delta][delta] CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lo W-Y, Liu K-F, Liao I-C, Song Y-L. Cloning and molecular characterization of heat shock cognate 70 from tiger shrimp (Penaeus monodon) Cell Stress Chaperones. 2004;9:332–343. doi: 10.1379/CSC-47R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan W, Li FH, Zhang JQ, Wen R, Li YT, Xiang JH. Identification of a novel inducible cytosolic Hsp70 gene in Chinese shrimp Fenneropenaeus chinensis and comparison of its expression with the cognate Hsc70 under different stresses. Cell Stress Chaperones. 2010;15:83–93. doi: 10.1007/s12192-009-0124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming JH, et al. Molecular cloning and expression of two HSP70 genes in the Wuchang bream (Megalobrama amblycephala Yih) Fish Shellfish Immunol. 2010;28:407–418. doi: 10.1016/j.fsi.2009.11.018. [DOI] [PubMed] [Google Scholar]
- O’Malley K, Mauron A, Barchas J, Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985;5:3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, et al. Genomic cloning of the Hsc71 gene in the hermaphroditic teleost Rivulus marmoratus and analysis of its expression in skeletal muscle: identification of a novel muscle-preferred regulatory element. Nucleic Acids Res. 2001;29:3041–3050. doi: 10.1093/nar/29.14.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano A, Franzellitti S, Tinti F, Fabbri E. Sequencing and expression pattern of inducible heat shock gene products in the European flat oyster, Ostrea edulis. Gene. 2005;361:119–126. doi: 10.1016/j.gene.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Pollard D, Johnson W, Lior H, Tyler S, Rozee K. Detection of the aerolysin gene in Aeromonas hydrophila by the polymerase chain reaction. J Clin Microbiol. 1990;28:2477–2481. doi: 10.1128/jcm.28.11.2477-2481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Cell Mol Life Sci. 1962;18:571–573. doi: 10.1007/BF02172188. [DOI] [Google Scholar]
- Rungrassamee W, Leelatanawit R, Jiravanichpaisal P, Klinbunga S, Karoonuthaisiri N. Expression and distribution of three heat shock protein genes under heat shock stress and under exposure to Vibrio harveyi in Penaeus monodon. Dev Comp Immunol. 2010;34:1082–1089. doi: 10.1016/j.dci.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Santacruz H, Vriz S, Angelier N. Molecular characterization of a heat shock cognate cDNA of zebrafish, hsc70, and developmental expression of the corresponding transcripts. Dev Genet. 1997;21:223–233. doi: 10.1002/(SICI)1520-6408(1997)21:3<223::AID-DVG5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Shen J, et al. Studies on the pathogens of bacterial diseases of Macrobrachium nipponense. J Zhejiang Ocean Univ. 2000;3:222–224. [Google Scholar]
- Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6:1025–1037. doi: 10.1046/j.1461-0248.2003.00528.x. [DOI] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker’s guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:AHSGTT>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa K, Minami M, Minami Y. Constantly updated knowledge of Hsp90. J Biochem. 2005;137:443–447. doi: 10.1093/jb/mvi056. [DOI] [PubMed] [Google Scholar]
- Tsan M-F, Gao B. Heat shock protein and innate immunity. Cell Mol Immunol. 2004;1:274–279. [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the toll/interleukin-1 receptor signal pathway. Sci Signal. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Vilaboa NE, Calle C, Pérez C, de Blas E, García-Bermejo L, Aller P. cAMP increasing agents prevent the stimulation of heat-shock protein 70 (HSP70) gene expression by cadmium chloride in human myeloid cell lines. J Cell Sci. 1995;108:2877–2883. doi: 10.1242/jcs.108.8.2877. [DOI] [PubMed] [Google Scholar]
- Walsh D, Li K, Zeng F, Zhe L, Edwards M (1994) Heat shock genes and cell cycle regulation during early mammalian development. Environ Med 38:1–6
- Walsh D, Li Z, Wu Y, Nagata K. Heat shock and the role of the HSPs during neural plate induction in early mammalian CNS and brain development. Cell Mol Life Sci. 1997;53:198–211. doi: 10.1007/PL00000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Sun Y, Lei L, Xie S. Molecular identification and expression of heat shock cognate 70 (HSC 70) in the pacific white shrimp Litopenaeus vannamei. Mol Biol. 2008;42:234–242. doi: 10.1134/S002689330802009X. [DOI] [PubMed] [Google Scholar]
- Yue X, Liu B, Sun L, Tang B. Cloning and characterization of a hsp70 gene from Asiatic hard clam Meretrix meretrix which is involved in the immune response against bacterial infection. Fish Shellfish Immunol. 2011;30:791–799. doi: 10.1016/j.fsi.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Zdobnov EM, Apweiler R. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics. 2001;17:847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen L, Qin J, Wu P, Zhang F, Li E, Tang B. MnHSP90 cDNA characterization and its expression during the ovary development in oriental river prawn, Macrobrachium nipponense. Mol Biol Rep. 2011;38:1399–1406. doi: 10.1007/s11033-010-0243-7. [DOI] [PubMed] [Google Scholar]