Abstract
The conversion of muscle into meat is a complex process of major concern for meat scientists due to its influence on the final meat quality. The aim of this study was to investigate the occurrence of autophagic processes in the conversion of muscle into meat. Our findings demonstrated, for the first time, the occurrence of autophagic processes in the muscle tissue at early postmortem period (2 h to 24 h) in both beef breeds studied (Asturiana de los Valles and Asturiana de la Montaña) showing significant time-scale differences between breeds, which could indicate a role of this process in meat maturation. These breeds have different physiological features: while Asturiana de los Valles is a meat-specialized breed showing high growth rate, an elevated proportion of white fibers in the muscle and low intramuscular fat level, Asturiana de la Montaña is a small- to medium-sized rustic breed adapted to less-favored areas, showing more red fibers in the muscle and a high intramuscular fat content.
Keywords: Asturiana de la Montaña, Asturiana de los Valles, autophagy, beef, cathepsins, meat aging
Introduction
In the case of fresh meat, tenderness is considered the factor that most influences the consumer’s purchase choice. However, meat tenderness is a highly variable attribute and is dependent on many different intrinsic and extrinsic factors during the life of the animal. Thus, little is still known about the early changes occurring in the muscle tissue after the animal’s death.
In the postmortem muscle the anoxia situation caused by the sudden end of blood flow will drastically reduce energy production in the cells, most of which was provided by ATP generated in the mitochondria in an oxygen-dependent process. Under this situation, the mitochondrial electron transport chain is inhibited, which results in redox changes and increases of reactive oxygen species (ROS) that produce oxidative stress. 1
In response to this oxidative challenge, cells may trigger different signaling pathways, such as physiological turnover of organelles via autophagy or it may result in the complete destruction of the cell via one or more of the programmed cell death (PCD) pathways that could be activated if the viability of the cell cannot be maintained.
To date, only apoptosis (previously referred to as type I PCD) has been considered, by the meat scientist, as the most likely process of cell death in the postmortem muscle. Ouali et al. 2 , 3 hypothesize that the hypoxic/ischemic conditions that are induced through the process of the animal’s death and exsanguination could activate caspases and apoptosis in the skeletal muscle, similarly to what is observed during neuronal or cardiac ischemia. They propose that apoptosis and its effects on cell structure and proteins could provide partial answers to still unexplained variability of meat tenderness as well as explain some early changes occurring in the muscle tissue. Since then, many studies have pointed out the importance of considering apoptosis to understand beef aging and tenderization. 4 - 9
Autophagy is considered a specialized form of lysosomal proteolysis, where part of the cytoplasm or entire organelles are sequestered into double-membrane vesicles called autophagosomes, which fuse with lysosomes, resulting in the degradation of their contents by lysosomal cathepsins. 10 Autophagic cell death (previously referred to as type II PCD), is a mechanism equivalent to apoptosis in that the end result is cell death, in addition to the traditional, well-established view that autophagy is an important protective mechanism for cells under stress such as starvation via provision of nutrients and removal of protein aggregates and damaged mitochondria. 11 , 12
Autophagic processes have been described under starvation, 13 cancer, 14 and aging, 15 but the possible relationship between autophagy and meat tenderization has not been suggested thus far. Autophagy is one of the cellular defense mechanisms activated in response to excessive ROS production. Indeed, ROS act as signaling molecules in the early events of autophagy induction. 16 As to skeletal muscle, ROS have been implicated in the induction of autophagy in muscle atrophy, disuse, and aging. 17 , 18 Moreover, previous research from our group has demonstrated that the oxidative stress caused by ROS with regard to lipids and proteins of beef muscles at early postmortem stages is directly related to meat tenderization; 19 , 20 therefore a more in-depth study on this subject could provide some explanations for the still unexplained aspects of meat quality variability.
In this article, several autophagic markers such as BECN1/Beclin 1, MAP1LC3/LC3, CTSD/cathepsin D and CTSB/cathepsin B and their ratio 21 - 23 were studied in 2 beef breeds of different animal production purposes: Asturiana de los Valles (AV) and Asturiana de la Montaña (AM). AV is a breed with a high growth rate, 24 an elevated proportion of white fibers in the longissimus dorsi (LD) muscle 25 and a low intramuscular fat (IMF) level. 26 Conversely, AM is a small- to medium-sized rustic breed, 24 adapted to less favored mountain areas, with a high proportion of red fibers and high IMF content. 25 , 26
The aim of the present study was to evaluate the possible presence of autophagy during the first 24 h of muscle tenderization and to evaluate if this process could be different depending on the animal breed, thus influencing the final meat quality.
Results
Total antioxidant activity
There were significant differences along the postmortem period (P < 0.001) and between the breeds at 2 h (P < 0.001), 12 h (P < 0.01) and 24 h (P < 0.001) postmortem, with meat from the AM breed showing higher values of total antioxidant activity (TAA) (Table 1).
Table 1. Effect of breed (in rows) and aging time (in columns) on meat TAA (mg trolox mg−1 protein).
| Breed | |||
|---|---|---|---|
| Aging time | AV | AM | Sign. |
| 2 h | 1.332 b | 1.944 b | *** |
| 12 h | 1.317 b | 1.511 a | ** |
| 24 h | 0.080 a | 1.303 a | *** |
| Sign. | *** | *** | |
Within a breed, means in the same column followed by different letters are significantly different at P < 0.05; **P < 0.01; ***P < 0.001.
Cathepsin activities
The cathepsin activities studied (CTSB and CTSD) revealed a similar pattern during the early postmortem period in both breeds, showing an increase in activity from 2 to 12 h postmortem and decreasing thereafter (Table 2) with higher activity in the AM meat at the very short postmortem time compared with the AV breed.
Table 2. Effect of breed (in rows) and aging time (in columns) on the activity of CTSB, CTSD, and CTSD/CTSB ratio.
| Breed | ||||
|---|---|---|---|---|
| Variable | Aging time | AV | AM | Sign. |
| CTSB | 2 h | 2.868 | 2.765 a | NS |
| (mU mg−1 protein) | 12 h | 3.465 | 3.920 b | * |
| 24 h | 3.220 | 3.758 b | ** | |
| Sign. | NS | *** | ||
| CTSD | 2 h | 12.620 a | 17.010 b | *** |
| (U mg−1 protein) | 12 h | 14.152 b | 17.000 b | *** |
| 24 h | 12.937 a | 15.118 a | ** | |
| Sign. | ** | * | ||
| Ratio CTSD/CTSB | 2 h | 4.425 | 6.225 b | * |
| 12 h | 4.100 | 4.325 ab | NS | |
| 24 h | 4.025 | 4.025 a | NS | |
| Sign. | NS | ** | ||
Within a breed, means in the same column followed by different letters are significantly different at P < 0.05; NS: not significant, *P < 0.05, **P < 0.01; ***P < 0.001.
In relation to the CTSD/CTSB activity ratio (Table 2), the results showed significant differences between the breeds (P < 0.05) at 2 h postmortem, the ratio being higher for the meat samples from the AM breed. This ratio gradually decreased in both breeds, being significant (P < 0.01) for the AM breed, until it reached similar values to the AV specimens at 24 h.
Autophagy markers: BECN1 and LC3
BECN1 was detected in all of the samples studied by western blot (Fig. 1), with similar levels of expression in both breeds (Fig. 1) and a similar postmortem pattern. BECN1 expression increased with aging from 2 h to 12 h, with this increase being more intense and significant (P < 0.05) in meat from the AM breed (Table 3). However, a significant decrease of BECN1 expression was observed later, at 24 h postmortem, in both breeds (P < 0.05 for AV and P < 0.01 for AM) (Table 3).
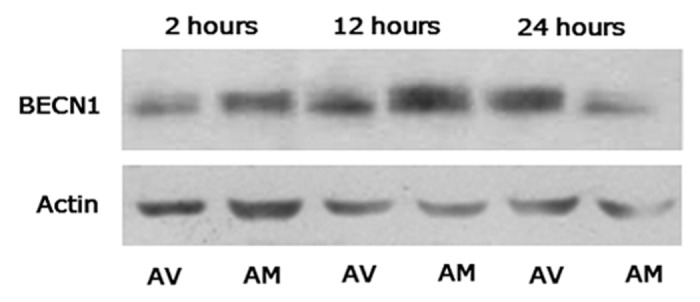
Figure 1. Immunoblot analysis of BECN1 and actin expression in meat from AV and AM breeds along the postmortem period in the study (2 h to 24 h).
Table 3. Effect of breed (in rows) and aging time (in columns) on BECN1 expression (Optical Density, in arbitrary units).
| Breed | |||
|---|---|---|---|
| Aging time | AV | AM | Sign. |
| 2 h | 1.133 b | 1.005 b | NS |
| 12 h | 1.376 b | 1.609 c | NS |
| 24 h | 0.541 a | 0.485 a | NS |
| Sign. | * | ** | |
Within a breed, means in the same column followed by different letters are significantly different at P < 0.05; *P < 0.05, **P < 0.01.
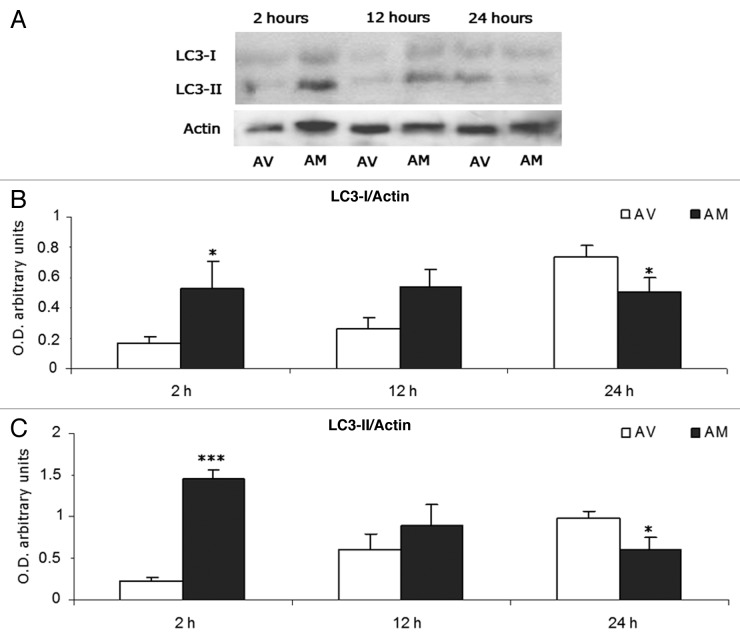
In contrast, the immunoanalysis for the LC3 autophagic marker (Fig. 2A) revealed 2 bands corresponding to the cleaved, cytosolic form LC3-I (18 kDa) and the subsequently lipidated form LC3-II (16 kDa). Semiquantitative densitometry showed significant differences between both breeds (Fig. 2B and C). Thus, meat from the AM breed presented significantly higher values of LC3-I (Fig. 2B, P < 0.05) and LC3-II (Fig. 2C, P < 0.001) at 2 h postmortem. These differences between the breeds decreased gradually, following a similar trend that was described for the CTSD/CTSB ratio. Moreover, at 24 h the AV breed showed significantly higher values for both LC3-I and LC3-II (P < 0.05).
Figure 2. (A) Immunoblot analysis of LC3-I, LC3-II and actin expression in meat from AV and AM breeds along the postmortem period in the study (2 h to 24 h). (B) Semi-quantitative optical density (arbitrary units) of LC3-I expression normalized to actin. (C) Semi-quantitative optical density (arbitrary units) of LC3-II expression normalized to actin. Significant differences between breeds at a given aging time are expressed as *P < 0.05 and ***P < 0.001.
Discussion
Meat aging is one of the most important processes for obtaining a satisfactory degree of meat tenderness, which is decisive for the consumer’s acceptability and subsequent intention to repurchase. Tenderization is largely a consequence of enzymatic degradation of key myofibrillar and cytoskeletal proteins that, under in vivo conditions, maintain the structural integrity of myofibrils. However, this is a complex process influenced by many factors and several of them still remain unknown.
After the hypothesis of Ouali et al. 2 that the hypoxic/ischemic conditions induced through the process of the animal’s death and exsanguination could activate caspases and apoptosis in the skeletal muscle, many studies have pointed out the importance of considering apoptosis to understand beef aging and tenderization.
In this sense, Laville et al. 5 have observed a significant increase of proteolytic products of proteins that constitute the mitochondrial membrane (from the inner and outer membranes) in the soluble fraction of tender meat, in the examination of proteome changes during meat aging of tender and tough meat from Charolais young bulls. Detection of these products occurred very early during the postmortem conditioning period and thus could not have been derived from meat aging. These data indicate an increase in mitochondrial membrane disruption in the tender group, which is known to occur early in the intrinsic apoptotic pathway and is subsequently involved in caspase activation. In agreement with this, some recent evidence has shown the development of apoptosis, by rapid phosphatidylserine externalization, cell shrinkage, and a progressive degradation of actin (a protein proposed to be a good marker of apoptosis) during the 72 h following exsanguination in rat muscle. 27 Also, Cao et al. 7 describe hallmarks of apoptosis such as chromatin condensation and margination, typical apoptotic DNA fragmentation and apoptotic bodies formation, in different skeletal muscles of bulls between day 1 and 4 after slaughter, concluding on the one hand that in postmortem muscle, apoptosis takes place.
Moreover, several studies have focused on determining whether caspases are active in the muscle tissue during meat conditioning and whether they cleave proteins found within myofibril structures. Caspase activity changes across the postmortem conditioning period of pork, chicken, and beef, with the highest activities always detected in the early postmortem phase. 8 , 28 - 30 Changes in CASP3/caspase 3 and CASP7, as well as CASP9 activity during the early phase of conditioning of porcine LD muscle have been identified to have a negative relationship with shear force; thus, the more caspase activation, the greater the change and the lower the shear force value, and therefore the higher the level of meat tenderness. 8 However, other authors 31 when examining changes in CASP3 activity and protein expression in beef muscle samples during postmortem aging, only detected the inactive isoform of CASP3, not the active one, and no association between caspase isoforms and shear force values was found. Thus, these contradictory results only further highlight our need for continued research to increase our understanding of the process of conversion of muscle into meat.
On the other hand, due to the oxidative conditions derived from exsanguination during the animal’s death, cells may trigger physiological turnover of organelles via autophagy 16 however, this process has not been considered by meat scientist to date. This article shows the occurrence of autophagy in muscle cells from the LD of yearling bulls during early postmortem meat aging (2 h to 24 h).
Previous research from our group had demonstrated the oxidative damage caused by ROS through measurements of protein damage and lipid peroxidation in the muscular tissue of the same breeds (AV and AM) during the postmortem stage of meat aging and also the subsequent antioxidant response. 19 The data obtained in the present study showed significant differences in the capability of the muscle cells of different beef breeds (the meat-specialized AV and the rustic AM) to counteract oxidative stress at the pre-rigor phase. Thus, meat from the AM animals had a higher antioxidant capability (as measured by the TAA) than the AV meat, which could indicate that oxidative stress had less harmful effects in the meat from AM than AV. This protection against oxidative stress that the rustic breed showed could be the reason for the slow tenderization process that this breed presents in comparison to AV 32 because its resistance against cellular death due to hypoxia can be longer.
The occurrence of autophagy has been studied by our group in different controversial conditions in organs that suffer high levels of oxidative stress (similar conditions to slaughter), such as the flank organ 23 and harderian gland in Syrian hamsters, 33 and under different cell stress conditions, such as hypoxia. 34 Based upon our experience, autophagy seemed an obvious candidate response to be triggered in muscular tissue after an animal’s death.
It is worth noting that in the present work, due to the experimental system limitations, standard autophagy flux assays could not be performed, therefore direct autophagy monitoring was not possible, however a set of selected biomarkers of autophagy were examined in muscle cells in response to slaughter and, consequently, under the sudden absence of oxygen, and during conditions of oxidative stress and nutrient starvation.
Our results showed, for the first time, not only the occurrence of autophagic processes but also clear differences in the pathways followed during the maturation phase of meat coming from 2 beef breeds and corroborate our hypothesis, showing faster and more intense indication of autophagy in the AM breed than in the AV breed. Additionally, we observed clear differences in cathepsin activities and, thereby, in the activity ratio between both breeds.
Cathepsins contribute to meat quality later in the maturation process, when lysosomes are disrupted and pH conditions are favorable for acidic enzymes. 32 , 35 , 36 However, lysosomal permeabilization and cathepsin release is often an early event in cell response to oxidative stress, not only in the autophagic processes but also in the apoptotic cascade 37 directly, as cathepsins cleave and activate caspases or indirectly by triggering mitochondrial dysfunction and subsequent release of mitochondrial proteins. 37 - 39 The magnitude of oxidative stress determines the degree of lysosomal destabilization and consequently whether reparative autophagy, apoptosis, or necrosis will follow.
The ratio of the CTSD/CTSB activity is an interesting marker of lysosomal viability. Compared with the same tissue under control conditions or to a different tissue in the same organism, alterations in the CTSD/CTSB activity ratio reflect peculiarities in the autophagy-lysosomal pathway. 21 , 40 Therefore, the appearance of higher levels of this ratio may be one indication of the initiation of autophagy (lysosome)-dependent cell death. In our study, the CTSD/CTSB ratio was higher in AM than in AV muscle tissue at the very early postmortem time (2 h) (Table 2); thus, the AM breed appears to trigger autophagy more rapidly, as has been previously suggested, and, accordingly, cellular degradation and, eventually, meat maturation is postponed. This is in accordance with previous reports showing a retarded tenderization rate of meat from rustic breeds, such as AM. 41
In the present study, autophagy occurrence in the muscle tissue at early postmortem aging (within first 24 h) was demonstrated by the presence of specific autophagic markers, such as BECN1 and LC3. BECN1, the first gene identified that is involved in autophagy in mammalian cells, is a BCL2-interacting protein, localized to the trans-Golgi network. It is a part of a complex with the class III PtdIns 3-kinase protein that plays an important role in promoting autophagy. Our results showed similar expression levels of BECN1 in meat from AM and AV breeds (Table 3). Interestingly, ROS-induced autophagy is partially dependent upon the BECN1 complex. 42 LC3 is an autophagosomal ortholog of yeast Atg8. LC3 is recruited onto phagophore membranes during autophagic cell death and is essential for amino-acid starvation-induced autophagy. An LC3-modification, requiring cleavage and lipidation (LC3-I to LC3-II) is essential for the formation of autophagosomes 43 and, therefore, LC3-II, is considered an autophagosomal marker.
Our results support the occurrence of a macroautophagic process in the pre-rigor phase of meat, as the LC3-II band was found in the muscle extracts of both breeds, although with significant differences: AM showed significantly higher LC3-II and LC3-I expression at the very early postmortem time (2 h, in accordance with the CTSD/CTSB ratio), with this trend being maintained at 12 h. These results suggest an earlier autophagic activation in the muscle of AM breed. Based upon our results, AM tissue showed stronger evidence of the autophagic process as an adaptive response to maintain cell survival under stress conditions. It is clear that this strategy may have important effects on the meat maturation process because autophagy delays the disintegration of the Z-disk, the loss of the transversal alignment of the sarcomeres and the longitudinal splitting of myofibers, with important consequences in muscular destruction and meat tenderization.
This delay in meat tenderization in AM is in accordance with several previous reports that rustic beef breeds show a slower tenderization pattern than those of meat-specialized breeds. 32 , 41 , 44
Herein, we have described for the first time variations in the autophagic-lysosomal pathway of postmortem muscle cells, which are directly related to the oxidative stress status of cells and are probably involved in the first steps of meat tenderization. Our findings indicate that autophagy seems to be involved in the pre-rigor phase of meat aging in certain beef breeds with significant differences in autophagy development in both breeds, which could be related to specific genotypes. This discovery may have important implications to improve the understanding of the processes involved in the conversion of muscle into meat and in the search for biomarkers of meat quality.
Materials and Methods
Animals and sampling procedure
Sixteen yearling bulls of 2 local breeds from Northern Spain: AV, as a meat-specialized breed and AM as a rustic breed, were studied. Eight animals from each breed were used.
The death of the animals occurred in a commercial abattoir following approved European Union procedures. After death and dressing (2 h postmortem), hot carcasses were transferred to a cold room at 4 °C and were kept there for 24 h postmortem. From each animal, LD muscle samples of 20 g were taken at 2, 12, and 24 h postmortem, snap frozen in liquid nitrogen and stored at −80 °C until used for different analyses. It has been assumed that muscle samples taken at 2 h postmortem represent the basal levels for oxidative and autophagic markers, as this was the shortest time at which carcass tissues could be extracted due to the standard commercial abattoir procedures and that measurements of pH and temperature of the 2 h postmortem muscle were similar to the ones found in the living tissue. 45
Tissue extraction
Muscle tissue (1 g per animal) at each aging time (2, 12, and 24 h postmortem) was thawed and homogenized according to the procedure described by Caballero and coworkers. 32 The protein content of the supernatant fraction was measured by the Bradford method 46 at 595 nm using a spectrophotometer (Uvikon 930, Kontron Instruments).
Total antioxidant activity
The TAA was determined using the ABTS/H202/HRP method for food samples, 47 as modified for animal samples. 48 The results are expressed in equivalents of mg Trolox mg−1 protein.
Cathepsin activities
Cysteine proteinase CTSB (EC 3.4.22.1) was assayed fluorimetrically (CytofluorTM 2350, Millipore) according to the method of Barret, 49 with minor modifications. 50 The aspartate proteinase, CTSD (EC 3.4.23.5), was assayed spectrophotometrically (Uvikon 930, Kontron Instruments) at 280 nm according to the procedure described by Takahashi and Tang, 51 with minor modifications. 32 The CTSD/CTSB activity ratio was also calculated for each biological type and aging time.
Immunoblotting
Immunoblottings were developed as is described in Coto-Montes et al. 23 using primary antibodies against BECN1 (sc-10086), actin (sc-1615) (Santa Cruz Biotechnology) and LC3 (Calbiochem, MBL, PD014), each previously diluted 1:1,000 in blocking buffer. The results were normalized to actin. The levels of BECN1, LC3-I and LC3-II, and actin were quantitatively analyzed using Quantity One v. 5.5.1. (Bio-Rad Laboratories, Inc.).
Statistical analysis
All of the variables, the CTSB and CTSD activities, the CTSD/CTSB activity ratio and the immunoblotting results, were analyzed with ANOVA using the General Lineal model procedure of SPSS v. 15.0 (2006) also considering breed, aging time, and their interaction as the main effects. Once an interaction between the breed and aging was established, the effect of the breed and aging time (with the animal as random factor) were tested. When significant, differences with aging were analyzed by means of the Tukey post-hoc test (the Games-Howell test when variances were not homogeneous).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the staff of Animal Production Systems of SERIDA for skilled management of animals and carcasses and Dr JM López (University of Oviedo, Spain) for his valuable suggestions. We are members of the INPROTEOLYS network. This work was supported by: FISS-06-RD06/0013/0011; RTA2007-00087-C02; BFU2010-20919 and FEDER funds. MG-M acknowledges a predoctoral fellowship from the Ministerio de Ciencia e Innovación. IV-N has a Marie Curie postdoctoral contract from the EU. DDG-C is a FICYT predoctoral fellow from the Principado de Asturias. Financial support from the University of Oviedo is also acknowledged.
Glossary
Abbreviations:
- AM
Asturiana de la Montaña
- AV
Asturiana de los Valles
- BECN1
Beclin1
- CTSD
cathepsin D
- CTSB
cathepsin B
- CASP3
caspase 3
- CASP7
caspase 7
- CASP9
caspase 9
- IMF
intramuscular fat
- MAP1LC3 (LC3)
microtubule-associated protein 1 light chain 3
- LD
longissimus dorsi
- PCD
programmed cell death
- ROS
reactive oxygen species
- TAA
total antioxidant activity
References
- 1. Freeman BA, Crapo JD. . Biology of disease: free radicals and tissue injury. Lab Invest 1982; 47:412 - 26; PMID: 6290784 [PubMed] [Google Scholar]
- 2. Ouali A, Herrera-Mendez CH, Coulis G, Becila S, Boudjellal A, Aubry L, Sentandreu MA. . Revisiting the conversion of muscle into meat and the underlying mechanisms. Meat Sci 2006; 74:44 - 58; http://dx.doi.org/ 10.1016/j.meatsci.2006.05.010; PMID: 22062715 [DOI] [PubMed] [Google Scholar]
- 3. Herrera-Mendez CH, Becila S, Boudjellal A, Ouali A. . Meat ageing: Reconsideration of the current concept. Trends Food Sci Technol 2006; 17:394 - 405; http://dx.doi.org/ 10.1016/j.tifs.2006.01.011 [DOI] [Google Scholar]
- 4. Bernard C, Cassar-Malek I, Le Cunff M, Dubroeucq H, Renand G, Hocquette JF. . New indicators of beef sensory quality revealed by expression of specific genes. J Agric Food Chem 2007; 55:5229 - 37; http://dx.doi.org/ 10.1021/jf063372l; PMID: 17547415 [DOI] [PubMed] [Google Scholar]
- 5. Laville E, Sayd T, Morzel M, Blinet S, Chambon C, Lepetit J, Renand G, Hocquette JF. . Proteome changes during meat aging in tough and tender beef suggest the importance of apoptosis and protein solubility for beef aging and tenderization. J Agric Food Chem 2009; 57:10755 - 64; http://dx.doi.org/ 10.1021/jf901949r; PMID: 19860418 [DOI] [PubMed] [Google Scholar]
- 6. Zapata I, Zerby HN, Wick M. . Functional proteomic analysis predicts beef tenderness and the tenderness differential. J Agric Food Chem 2009; 57:4956 - 63; http://dx.doi.org/ 10.1021/jf900041j; PMID: 19449808 [DOI] [PubMed] [Google Scholar]
- 7. Cao J, Sun W, Zhou G, Xu X, Peng Z, Hu Z. . Morphological and biochemical assessment of apoptosis in different skeletal muscles of bulls during conditioning. J Anim Sci 2010; 88:3439 - 44; http://dx.doi.org/ 10.2527/jas.2009-2412; PMID: 20525926 [DOI] [PubMed] [Google Scholar]
- 8. Kemp CM, Parr T, Bardsley RG, Buttery PJ. . Comparison of the relative expression of caspase isoforms in different porcine skeletal muscles. Meat Sci 2006; 73:426 - 31; http://dx.doi.org/ 10.1016/j.meatsci.2005.12.009; PMID: 22062480 [DOI] [PubMed] [Google Scholar]
- 9. Kemp CM, Parr T. . Advances in apoptotic mediated proteolysis in meat tenderisation. Meat Sci 2012; 92:252 - 9; http://dx.doi.org/ 10.1016/j.meatsci.2012.03.013; PMID: 22546815 [DOI] [PubMed] [Google Scholar]
- 10. Bursch W. . The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 2001; 8:569 - 81; http://dx.doi.org/ 10.1038/sj.cdd.4400852; PMID: 11536007 [DOI] [PubMed] [Google Scholar]
- 11. Shen HM, Codogno P. . Autophagy is a survival force via suppression of necrotic cell death. Exp Cell Res 2012; 318:1304 - 8; http://dx.doi.org/ 10.1016/j.yexcr.2012.02.006; PMID: 22366289 [DOI] [PubMed] [Google Scholar]
- 12. Erman A, Resnik N, Romih R. . Autophagic activity in the mouse urinary bladder urothelium as a response to starvation. Protoplasma 2013; 250:151 - 60; http://dx.doi.org/ 10.1007/s00709-012-0387-5; PMID: 22407469 [DOI] [PubMed] [Google Scholar]
- 13. Mizushima N. . Autophagy in protein and organelle turnover. Cold Spring Harb Symp Quant Biol 2011; 76:397 - 402; http://dx.doi.org/ 10.1101/sqb.2011.76.011023; PMID: 21813637 [DOI] [PubMed] [Google Scholar]
- 14. Grandér D, Panaretakis T. . Autophagy: cancer therapy’s friend or foe?. Future Med Chem 2010; 2:285 - 97; http://dx.doi.org/ 10.4155/fmc.09.155; PMID: 21426194 [DOI] [PubMed] [Google Scholar]
- 15. Caballero B, Coto-Montes A. . An insight into the role of autophagy in cell responses in the aging and neurodegenerative brain. Histol Histopathol 2012; 27:263 - 75; PMID: 22237704 [DOI] [PubMed] [Google Scholar]
- 16. Scherz-Shouval R, Elazar Z. . ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 2007; 17:422 - 7; http://dx.doi.org/ 10.1016/j.tcb.2007.07.009; PMID: 17804237 [DOI] [PubMed] [Google Scholar]
- 17. Mammucari C, Rizzuto R. . Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev 2010; 131:536 - 43; http://dx.doi.org/ 10.1016/j.mad.2010.07.003; PMID: 20655326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, et al. . Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab 2008; 8:425 - 36; http://dx.doi.org/ 10.1016/j.cmet.2008.09.002; PMID: 19046573 [DOI] [PubMed] [Google Scholar]
- 19. Coto-Montes A, Caballero B, Sierra V, Vega-Naredo I, Tomás-Zapico C, Hardeland R, et al. . Actividad de los principales enzimas antioxidantes durante el periodo de oreo de culones de la raza asturiana de los valles. ITEA 2004; 100A:43 - 55 [Google Scholar]
- 20. Caballero B, Sierra V, Vega-Naredo I, Tomás-Zapico C, Rodríguez-Colunga MJ, Tolivia D, et al. . Enzimas antioxidantes en la maduración de carne de vacuno procedente de dos cabañas autóctonas asturianas. ITEA 2006; 102:288 - 303 [Google Scholar]
- 21. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. . Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008; 4:151 - 75; PMID: 18188003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vega-Naredo I, Coto-Montes A. . Physiological autophagy in the Syrian hamster Harderian gland. Methods Enzymol 2009; 452:457 - 76; http://dx.doi.org/ 10.1016/S0076-6879(08)03627-6; PMID: 19200898 [DOI] [PubMed] [Google Scholar]
- 23. Coto-Montes A, Tomás-Zapico C, Martínez-Fraga J, Vega-Naredo I, Sierra V, Caballero B, Huidobro-Fernández C, Soria-Valles C, Tolivia D, Rodríguez-Colunga MJ. . Sexual autophagic differences in the androgen-dependent flank organ of Syrian hamsters. J Androl 2009; 30:113 - 21; http://dx.doi.org/ 10.2164/jandrol.108.005355; PMID: 18930906 [DOI] [PubMed] [Google Scholar]
- 24. Albertí P, Sañudo C, Olleta JL, Ripoll G, Ertbjerg P, Christensen M, et al. . Live weight, body size and carcass characteristics of young bulls of fifteen European breeds. Livest Sci 2008; 114:19 - 30; http://dx.doi.org/ 10.1016/j.livsci.2007.04.010 [DOI] [Google Scholar]
- 25. Gil M, Serra X, Gispert M, Angels Oliver M, Sañudo C, Panea B, Olleta JL, Campo M, Oliván M, Osoro K, et al. . The effect of breed-production systems on the myosin heavy chain 1, the biochemical characteristics and the colour variables of Longissimus thoracis from seven Spanish beef cattle breeds. Meat Sci 2001; 58:181 - 8; http://dx.doi.org/ 10.1016/S0309-1740(00)00150-9; PMID: 22062114 [DOI] [PubMed] [Google Scholar]
- 26. Aldai N, Murray BE, Oliván M, Martínez A, Troy DJ, Osoro K, Nájera AI. . The influence of breed and mh-genotype on carcass conformation, meat physico-chemical characteristics, and the fatty acid profile of muscle from yearling bulls. Meat Sci 2006; 72:486 - 95; http://dx.doi.org/ 10.1016/j.meatsci.2005.08.016; PMID: 22061732 [DOI] [PubMed] [Google Scholar]
- 27. Becila S, Herrera-Mendez CH, Coulis G, Labas R, Astruc T, Picard B, et al. . Postmortem muscle cells die through apoptosis. Eur Food Res Technol 2010; 213:485 - 93; http://dx.doi.org/ 10.1007/s00217-010-1296-5 [DOI] [Google Scholar]
- 28. Kemp CM, King DA, Shackelford SD, Wheeler TL, Koohmaraie M. . The caspase proteolytic system in callipyge and normal lambs in longissimus, semimembranosus, and infraspinatus muscles during postmortem storage. J Anim Sci 2009; 87:2943 - 51; http://dx.doi.org/ 10.2527/jas.2009-1790; PMID: 19420232 [DOI] [PubMed] [Google Scholar]
- 29. Pulford DJ, Dobbie P, Fraga Vazquez S, Fraser-Smith E, Frost DA, Morris CA. . Variation in bull beef quality due to ultimate muscle pH is correlated to endopeptidase and small heat shock protein levels. Meat Sci 2009; 83:1 - 9; http://dx.doi.org/ 10.1016/j.meatsci.2008.11.008; PMID: 20416615 [DOI] [PubMed] [Google Scholar]
- 30. Chen L, Feng XC, Lu F, Xu XL, Zhou GH, Li QY, Guo XY. . Effects of camptothecin, etoposide and Ca2+ on caspase-3 activity and myofibrillar disruption of chicken during postmortem ageing. Meat Sci 2011; 87:165 - 74; http://dx.doi.org/ 10.1016/j.meatsci.2010.10.002; PMID: 21055882 [DOI] [PubMed] [Google Scholar]
- 31. Underwood KR, Means WJ, Du M. . Caspase 3 is not likely involved in the postmortem tenderization of beef muscle. J Anim Sci 2008; 86:960 - 6; http://dx.doi.org/ 10.2527/jas.2007-0549; PMID: 18156354 [DOI] [PubMed] [Google Scholar]
- 32. Caballero B, Sierra V, Oliván M, Vega-Naredo I, Tomás-Zapico C, Álvarez-García O, et al. . Activity of cathepsins during beef aging related to mutations in the myostatin gene. J Sci Food Agric 2007; 87:192 - 9; http://dx.doi.org/ 10.1002/jsfa.2683 [DOI] [Google Scholar]
- 33. Vega-Naredo I, Caballero B, Sierra V, Huidobro-Fernández C, de Gonzalo-Calvo D, García-Macia M, Tolivia D, Rodríguez-Colunga MJ, Coto-Montes A. . Sexual dimorphism of autophagy in Syrian hamster Harderian gland culminates in a holocrine secretion in female glands. Autophagy 2009; 5:1004 - 17; http://dx.doi.org/ 10.4161/auto.5.7.9610; PMID: 19736526 [DOI] [PubMed] [Google Scholar]
- 34. Soria-Valles C, Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernández C, Gonzalo-Calvo D, et al. . Antioxidant response to variations of oxygen in harderian gland of different species of Spalax. Can J Zool 2010; 88:803 - 7; http://dx.doi.org/ 10.1139/Z10-049 [DOI] [Google Scholar]
- 35. Nagaraj NS, Anilakumar KR, Santhanam K. . Changes in the calpain-calpastatin and cathepsin (B, B+L, H and D) during post-mortem storage of goat muscles. J Food Biochem 2002; 26:75 - 89; http://dx.doi.org/ 10.1111/j.1745-4514.2002.tb00051.x [DOI] [Google Scholar]
- 36. Thomas AR, Gondoza H, Hoffman LC, Oosthuizen V, Naudé RJ. . The roles of the proteasome, and cathepsins B, L, H and D, in ostrich meat tenderisation. Meat Sci 2004; 67:113 - 20; http://dx.doi.org/ 10.1016/j.meatsci.2003.10.001; PMID: 22061124 [DOI] [PubMed] [Google Scholar]
- 37. Bröker LE, Kruyt FAE, Giaccone G. . Cell death independent of caspases: a review. Clin Cancer Res 2005; 11:3155 - 62; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-2223; PMID: 15867207 [DOI] [PubMed] [Google Scholar]
- 38. Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jäättelä M, Kroemer G. . Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med 2003; 197:1323 - 34; http://dx.doi.org/ 10.1084/jem.20021952; PMID: 12756268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Conus S, Simon HU. . Cathepsins: key modulators of cell death and inflammatory responses. Biochem Pharmacol 2008; 76:1374 - 82; http://dx.doi.org/ 10.1016/j.bcp.2008.07.041; PMID: 18762176 [DOI] [PubMed] [Google Scholar]
- 40. Tomás-Zapico C, Caballero B, Sierra V, Vega-Naredo I, Alvarez-García O, Tolivia D, Rodríguez-Colunga MJ, Coto-Montes A. . Survival mechanisms in a physiological oxidative stress model. FASEB J 2005; 19:2066 - 8; PMID: 16186173 [DOI] [PubMed] [Google Scholar]
- 41. Sierra V, Guerrero L, Fernández-Suárez V, Martínez A, Castro P, Osoro K, Rodríguez-Colunga MJ, Coto-Montes A, Oliván M. . Eating quality of beef from biotypes included in the PGI “Ternera Asturiana” showing distinct physicochemical characteristics and tenderization pattern. Meat Sci 2010; 86:343 - 51; http://dx.doi.org/ 10.1016/j.meatsci.2010.05.007; PMID: 20665984 [DOI] [PubMed] [Google Scholar]
- 42. Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. . Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 2008; 90:313 - 23; http://dx.doi.org/ 10.1016/j.biochi.2007.08.014; PMID: 17928127 [DOI] [PubMed] [Google Scholar]
- 43. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. . LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720 - 8; http://dx.doi.org/ 10.1093/emboj/19.21.5720; PMID: 11060023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sañudo C, Macie ES, Olleta JL, Villarroel M, Panea B, Albertí P. . The effects of slaughter weight, breed type and ageing time on beef meat quality using two different texture devices. Meat Sci 2004; 66:925 - 32; http://dx.doi.org/ 10.1016/j.meatsci.2003.08.005; PMID: 22061026 [DOI] [PubMed] [Google Scholar]
- 45.Swatland J. On-line monitoring of meat quality. In: Kerry J, Kerry J, Ledward D. eds. Meat processing. Improving quality. Cambridge, England: Woodhead Publishing Limited, 2007:193-216. [Google Scholar]
- 46. Bradford MM. . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 54; http://dx.doi.org/ 10.1016/0003-2697(76)90527-3; PMID: 942051 [DOI] [PubMed] [Google Scholar]
- 47. Arnao MB, Cano A, Acosta M. . The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 2001; 73:239 - 44; http://dx.doi.org/ 10.1016/S0308-8146(00)00324-1 [DOI] [Google Scholar]
- 48. de Gonzalo-Calvo D, Neitzert K, Fernández M, Vega-Naredo I, Caballero B, García-Macía M, Suárez FM, Rodríguez-Colunga MJ, Solano JJ, Coto-Montes A. . Differential inflammatory responses in aging and disease: TNF-alpha and IL-6 as possible biomarkers. Free Radic Biol Med 2010; 49:733 - 7; http://dx.doi.org/ 10.1016/j.freeradbiomed.2010.05.019; PMID: 20639132 [DOI] [PubMed] [Google Scholar]
- 49. Barrett AJ. . Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J 1980; 187:909 - 12; PMID: 6897924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schreurs FJ, van der Heide D, Leenstra FR, de Wit W. . Endogenous proteolytic enzymes in chicken muscles. Differences among strains with different growth rates and protein efficiencies. Poult Sci 1995; 74:523 - 37; http://dx.doi.org/ 10.3382/ps.0740523; PMID: 7761338 [DOI] [PubMed] [Google Scholar]
- 51. Takahashi T, Tang J. . Cathepsin D from porcine and bovine spleen. Methods Enzymol 1981; 80:565 - 81; http://dx.doi.org/ 10.1016/S0076-6879(81)80045-6; PMID: 7341918 [DOI] [PubMed] [Google Scholar]