Abstract
The crucial issue for defining successful natural killer (NK)-based anticancer therapy is the ability of tumor cells to activate resistance mechanisms leading to escape from NK-mediated killing. It is now well established that such mechanisms are likely evolved under hypoxia in the tumor microenvironment. Here, we show that hypoxia-induced autophagy impairs breast cancer cell susceptibility to NK-mediated lysis and that this impairment is reverted by targeting autophagy. We provide evidence that activation of autophagy in hypoxic cells is involved in selective degradation of the pro-apoptotic NK-derived serine protease GZMB/granzyme B, thereby blocking NK-mediated target cell apoptosis. Our in vivo data validate the concept that targeting autophagy in cancer cells promotes tumor regression by facilitating their elimination by NK cells. This study provides a cutting-edge advance in our understanding of how hypoxia-induced autophagy impairs NK-mediated lysis and might pave the way for formulating more effective NK-based antitumor therapy by combining autophagy inhibitors.
Keywords: hypoxia, autophagy, granzyme B, breast cancer, natural killer
Tumor hypoxia is associated with tumor growth, malignant progression, and resistance to therapy, and becomes a central issue in cancer treatment. Hypoxic cells activate signaling pathways that regulate proliferation, angiogenesis, and death. Cancer cells have adapted these pathways, allowing tumors to survive and grow under hypoxia. Recently, hypoxia in the tumor microenvironment has been reported to suppress the antitumor immune response and to enhance tumor escape from immune surveillance. In line with this concept, we showed that hypoxic breast cancer cells are less susceptible to NK-mediated lysis than normoxic cells. More interestingly, we demonstrated that the resistance of hypoxic cancer cells to NK-mediated killing is strikingly dependent on autophagy activation, as genetic inhibition of autophagy is sufficient to suppress this resistance and restore NK-mediated killing of hypoxic cells. Furthermore, we showed that hypoxia is not a prerequisite event for autophagy-dependent induction of tumor escape from NK. Indeed, we observed that, similar to hypoxia-induced autophagy, starvation-induced autophagy is also able to impair tumor susceptibility to NK-mediated killing. Our results highlight autophagy as a key determinant in tumor cell evasion from NK-mediated killing.
It is well established that a dynamic and precisely coordinated balance between activating and inhibitory receptors governs NK cell activation programs. In our model, no significant differences are observed in the expression of activating and inhibitory receptors on the surface of NK cells, and in the expression of their ligands (except HLA class I molecules) at the surface of normoxic and hypoxic target cells. While the causal mechanism underlying the increase in HLA class I in hypoxic cells remains elusive, we demonstrated, using an HLA class I blocking antibody, that the resistance of hypoxic tumor cells occurs independently of upregulated-HLA class I molecules. Furthermore, we could not observe any defect in the ability of NK cells to secrete cytotoxic granules toward hypoxic or normoxic cells. Together, our results provide additional clues regarding the critical role of autophagy as an intrinsic mechanism that makes hypoxic tumor cells less sensitive to NK cell attack.
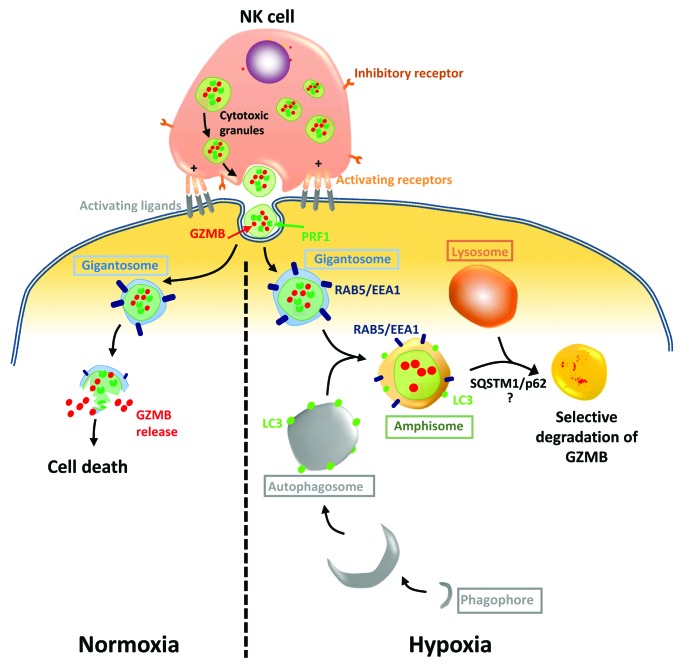
As cancer cells have evolved multiple mechanisms of resistance in order to outmaneuver an effective immune response and escape from immune cell killing, we next focused on autophagy as an intrinsic resistance mechanism operating in hypoxic cells. NK cells recognize and kill their targets by several mechanisms including the release of cytotoxic granules containing PRF1/perforin and GZMB. It has been recently proposed that PRF1 and GZMB enter target cells by endocytosis and traffic to enlarged endosomes called “gigantosomes.” Subsequently, PRF1 forms pores in the gigantosome membrane, allowing for the gradual release of GZMB and the initiation of apoptotic cell death. The fusion between early endosomes and autophagic vacuoles to form amphisomes seems to be a prerequisite in some cases for the formation of autolysosomes. In keeping with this, we attempted to analyze GZMB content in hypoxic tumor cells. We hypothesized that during intracellular trafficking, GZMB could be exposed to a high risk of being targeted to amphisomes and thereby degraded by autophagy in the lysosomal compartment. Several lines of data reported in this study support such a mechanism: i) the level of NK-derived GZMB detected in hypoxic cells is significantly lower than that in normoxic cells; ii) inhibition of autophagy or lysosomal hydrolases restores the level of GZMB and subsequently restores NK-mediated lysis of hypoxic cells; and iii) NK-derived GZMB is detected in LC3- and RAB5-positive cellular compartments, suggesting its presence within amphisomes in hypoxic cells. Based on these findings, we proposed a mechanism by which GZMB may be degraded by autophagy during its intracellular trafficking leading to cancer cell escape form NK cell attack (Fig. 1).
Figure 1. Selective degradation of NK-derived GZMB by autophagy in hypoxic tumor cells. Following the recognition of their targets NK cells secrete cytotoxic granules containing PRF1, GZMB, and other hydrolytic enzymes to the target cells. These granules enter target cells and traffic to enlarged endosomes marked by RAB5 or EEA1, which are called “gigantosomes.” In normoxic cells, PRF1 forms pores in the gigantosome membrane, allowing the release of GZMB and the initiation of cell death. Under hypoxia, excessive autophagy in target cells leads to the fusion of autophagosomes with gigantosomes containing PRF1 and GZMB and the formation of structures marked with LC3 and RAB5-EEA1 called amphisomes. Although PRF1 is detected in the same subcellular compartment as GZMB, the fusion of amphisomes with lysosomes leads to specific degradation of GZMB in a SQSTM1/p62-dependent manner. The degradation of GZMB by autophagy leads to hypoxic tumor cell escape from NK-mediated killing.
Autophagy has long been considered as a bulk degradation cell process. However, several studies have reported that autophagy can be a selective degradation process under stress conditions. The molecular basis of selective autophagy involves several cargo protein receptors such as SQSTM1/p62, NBR1, OPTN and CALCOCO2/NDP52, which are able to interact with ubiquitinated proteins and target them into phagophores in order to be degraded in lysosomes. Based on this, an important issue arises from our results: Is GZMB selectively degraded by autophagy or it is just an “innocent victim” which is subject to a bulk nonspecific degradation in hypoxic tumor cells under excessive autophagy? While much remains to be learned mechanistically, several lines of data reported in this study support the selective degradation of GZMB by autophagy: i) The level of GZMB in hypoxic cells is restored by targeting SQSTM1; and ii) even if PRF1 is detected in the same subcellular compartment as GZMB in hypoxic cells, we do not observe any difference in its expression level compared with normoxic cells.
In light of our in vitro observations, we investigated whether targeting autophagy enhances in vivo an NK-mediated antitumor immune response. We used BALB/c and C57BL/6 mice transplanted with syngeneic murine 4T1 breast adenocarcinoma and B16-F10 melanoma tumor cells, respectively. We first demonstrated that NK cells control in vivo B16-F10 and 4T1 tumor development, as the depletion of host NK cells significantly increases tumor growth. There is a significant reduction of tumor volume in autophagy-defective B16-F10 and 4T1 cells presumably as a consequence of potentiation of tumor cell killing by NK cells. Therefore, it would be interesting to investigate whether this improvement is related to the increase in the infiltration/recruitment of different immune subsets in the tumor bed or to the decrease of immune suppressive cells such as T regulatory cells, myeloid-derived suppressor cells or tumor-associated macrophages. Whether targeting autophagy affects the ability of cancer cells to induce an immunosuppressive microenvironment, including the secretion of various immunosuppressive cytokines, also remains an issue of great interest.
Overall, the present study underlines the inhibition of autophagy as a cutting-edge approach to overcome the suppressive effect of the hypoxic tumor microenvironment on the antitumor immune response. This study highlights the importance of integrating autophagy inhibitors as a potential therapeutic approach to improve NK-based cancer immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grants from Public Research Center for Health (REC-LHCE-2009-0201) and “Foundation Cancer” Luxembourg (FC/2012/02) to BJ and “Association de Recherche sur le Cancer” to SC. JB is a recipient of an AFR grant (2009-1201) from the “Fonds National de la Recherche,” Luxembourg.