Abstract
Jones and Klin recently found that the well-known decreased fixations to eyes in children with autism spectrum disorder are not present throughout infancy; instead a decline in eye fixations between 2 and 6 months predicts diagnosis. This decline is the earliest behavioral pattern linked to autism to date.
How do humans become skilled social communicators? The road to expertise in social communication may begin simply, with visual preferences present during early infancy that prioritize important channels of social information, like faces and eyes [1,2,3,4].
If early socially relevant perceptual preferences play a role in typical social communicative development, these preferences may be atypical in clinical populations impaired in this domain such as individuals with Autism Spectrum Disorder (ASD). Children and adults with ASD show decreased fixation on the eye region of faces relative to their typically developing peers [5]. This deficit in socially typical gaze, observable in some studies by 6 months of age [6], is perhaps the most highly replicated phenomenon in the scientific literature on autism. The developmental origins and trajectory of atypical social gaze in autism, however, are unknown. A congenital-deficit hypothesis [e.g., 1,5,7] predicts that this characteristic decreased fixation on faces and eyes should be present from birth.
Recent research efforts have focused on atypical attention to social stimuli in early infancy as possible behavioral markers of ASD. Although ASD is not yet reliably diagnosed until toddlerhood, the higher prevalence of behaviors characteristic of ASD such as atypical social interest and engagement in high-risk later-born siblings (approximately 19% of whom are diagnosed with ASD [8]) has allowed researchers to examine possible markers in the first year of life in these high-risk siblings (e.g., [4]).
Jones and Klin [1] are the first to use the “babysibs” approach to measure social gaze densely from early infancy (2 months) to age of diagnosis (3 years). In an impressively designed and executed longitudinal study, Jones and Klin [1] report important new data about social visual preferences in early infancy that predict clinical outcomes of ASD at age 3. They show that a decline in fixation time to social stimuli – specifically a region of interest comprising the upper face including the eyes – between 2 and 6 months predicts clinical outcome with considerable accuracy (80–99%).
In contrast to the prevailing congenital-deficit hypothesis [5,7], Jones and Klin found that the well-known reduction of fixation time to eyes seen in children with ASD – a reduction of approximately 50% relative to typically developing (TD) peers at 2 years of age [1] – was not congenital. Instead, 2-month-old infants who were later diagnosed with ASD fixated the eye region of faces much more similarly to their TD peers than did older infants.
Jones and Klin’s data shift us away from a simple deficit model of autism to one characterized by active processes of developmental change. A key next question is how to characterize these fundamentally developmental processes.
We suggest two possible scenarios of how autism manifests during infancy, inspired by Jones and Klin’s paper. The key distinction between these accounts is whether infants begin life with a typical or an atypical pattern of social attention. We hope that laying out these alternatives will encourage further research on the developmental origins of perceptual preferences for social stimuli.
One possibility, highlighted by Jones and Klin in their paper, is that children who later develop autism begin life with visual preferences for faces in the ‘normative range’, but show rapid decline in eye fixations between 2 and 6 months. If these infants begin life with visual attention to faces ‘initially intact’, we have grounds to hope to keep these infants on the path to typical development. Such a ‘decline’ model of autism is an important and novel hypothesis that deserves replication and further study.
A second, more speculative possibility is that there may be a congenital difference in gaze behavior in infants who are later diagnosed with ASD, but in a direction opposite to that predicted by a deficit theory. The reported data (Figure 1A versus B) suggest that the group developing ASD may begin life with greater than normal levels of looking to the eye region, broadly consistent with a ‘social-imbalance’ hypothesis. Face-related visual attention may have an optimal, adaptive level such that both hypo- and hyperattentive behaviors can derail typical development. Homeostasis is ubiquitous throughout biological systems with disrupted balance resulting in pathologies throughout the body (e.g., [9]) and the central nervous system (e.g., [10]). Conceiving of ASD as the triggering of allostatic processes (correction and, potentially, overcorrection) in response to homeostatic imbalance would suggest a different initial neural foundation [10] and starting point for treatment than would be predicted by a decline model. Additionally, a decline model is silent about whether very early behaviors can predict level of severity or affectedness on the autism spectrum. By contrast, in a social-imbalance account greater deviation from normative levels may predict greater levels of decline in eye looking and consequently greater severity of autism affectedness.
Figure 1.
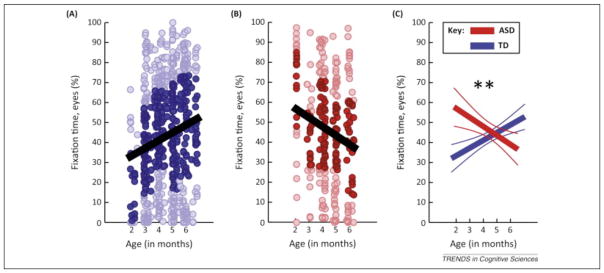
Developmental patterns of fixation to the eye region of faces. (A) Raw data for eye fixations across age in a typically developing (TD) group (blue). (B) Raw data for eye fixations across age in a group that develops autism spectrum disorder (ASD) (red). (C) Hierarchical linear model (HLM) summary of data with mean trend lines and 95% confidence intervals, with significant interaction between diagnosis and age (P=0.002). From Extended Data Figure 2 in [1].
Improved treatment possibilities for autism depend on a better understanding of early neural foundations of social perception and, with these new data, Jones and Klin move us toward a better understanding. Although we now have reason to doubt a congenital-deficit model of autism, it will require additional study of even earlier stages of infancy to establish whether infants begin life with a typical or an atypical pattern of social attention and what mechanisms of developmental change can explain the declines seen across the first several months of life. Further research is necessary to establish whether this pattern also holds for females and for the broader population who develop ASD. In addition, extremely young infants pose significant technical challenges to the acquisition of high-quality eye-tracking data. Care is needed in interpreting and generalizing measurements from this population, especially where hypotheses predict fixation of specific features (eyes) that comprise a small portion of the region of interest specified. We hope that Jones and Klin’s important and surprising data will inspire researchers to enrich the pool of measures made in the first months of life and to consider new viewpoints on the development of autism.
Acknowledgments
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD072018 awarded to A.V.
References
- 1.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senju A, Johnson MH. Atypical eye contact in autism: Models, mechanisms and development. Neuroscience & Biobehavioral Reviews. 2009;33(8):1204–1214. doi: 10.1016/j.neubiorev.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Gliga T, Csibra G. Seeing the face through the eyes: a developmental perspective on face expertise. Progress in Brain Research. 2007;164:323–339. doi: 10.1016/S0079-6123(07)64018-7. [DOI] [PubMed] [Google Scholar]
- 4.Elsabbagh M, Johnson MH. Getting answers from babies about autism. Trends in Cognitive Sciences. 2010;14(2):81–87. doi: 10.1016/j.tics.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Pickles A, Baron-Cohen S, Bolton P, Johnson MH BASIS Team 7. Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Current Biology. 2012;22(4):338–42. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klin A, Jones W, Schultz R, Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2003;358(1430):345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jameson SC. Maintaining the norm: T-cell homeostasis. Nature Reviews Immunology. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 10.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annual Review of Neuroscience. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]


