Abstract
This study provides a histological comparison of the mature regenerated and original tail of the lizard Anolis carolinensis. These data will provide a framework for future studies of this emerging model organism whose genome was recently published. This study demonstrated that the cartilage skeleton of the regenerated tail enclosed a spinal cord with an ependymal core, but there was no evidence that dorsal root ganglia or peripheral nerves are regenerated. The cartilage tube contained foramina that allowed the vasculature to cross, but was otherwise a rigid structure. The original tail has muscle groups arranged in quadrants in a regular pattern that attach to the vertebral column. The regenerated tail has irregular muscle bundles of variable number that form unusual attachments to each other and to the cartilage tube. Furthermore, the data show that there was increased connective tissue within the muscle bundles. Implications for functionality of the regenerated tail and for future biomechanical studies are discussed.
Introduction
Lizards have evolved the remarkable abilities to both autotomize, or self-amputate, their tails when threatened (Vitt 1981; Reichman 1984; Bellairs & Bryant, 1985; Goss 1987; Arnold 1988; Maginnis, 2006) and then to regenerate a functional tail (reviewed in Alibardi, 2010). Members of the Polychrotidae, particularly the green anole, Anolis carolinensis, have been particular favorites for tail regeneration studies (Jamison, 1964; Maderson & Licht, 1968; Cox, 1968, Simpson, 1968; Zika, 1969; Licht & Howe, 1969; Simpson, 1970; Egar et al., 1970; Maderson & Salthe, 1971; Turner & Simpson, 1973; Chlebowski et al., 1973; Bellairs & Bryant, 1985; Simpson & Duffy, 1994; Alibardi, 1995a; Alibardi, 1995b; 2010; Alibardi & Lovicu, 2009). Anolis carolinensis is found throughout the southeastern United States and this made it a readily available lizard model for a wide range of studies, including research focusing on developmental and reproductive biology, behavioral ecology, and neurobiology. Further adding to the value of A. carolinensis as an emerging lizard model, is the recent publication of the whole genome (Alföldi et al., 2011), the first non-avian reptile sequenced, together with an increasing number of deep transcriptomes that are newly available (Kusumi et al., 2011; Eckalbar et al., 2012). Collectively, these make A. carolinensis a powerful model to integrate anatomical, histological, and molecular analyses to investigate the tail regeneration process (Losos, 2009).
The process of lizard tail regeneration has been divided into four stages: wound healing, lasting up to 10 days post-autotomy (dpa); formation of a cone of mesenchymal and ependymal cells, ranging from 10–15 dpa; tail growth, ranging from 15–25 dpa; and finally, maturation of the tail, studied primarily from 25–60 dpa but with a currently unidentified end-point (reviewed in Alibardi, 2010). Tail regeneration produces a tail with a hyaline cartilage tube that encloses a spinal cord with an ependymal cell core. Groups of myoblasts form around the cartilage tube and give rise to myofibers. Some groups have described these myofibers as organized in segmental myomeres that mature to form a complete layer of muscles deep to the regenerating dermis (Simpson 1970; Mufti and Iqbal 1975).
Despite decades of studies in the A. carolinensis model system, a comparative histological atlas has not been generated for the original and regenerated tail. An atlas of the original tail would provide a foundation for further investigation into the functional anatomical and biomechanical processes of tail autotomy, while complementary in-depth histological analysis of the regenerated tail would highlight the key features that are divergent from the original structure. To address these deficiencies, this study provides a detailed analysis of the tissues in regenerated versus original tails in photomicrographic form.
Materials and Methods
Animals and collection of original and regenerated tails
Male and female adult A. carolinensis lizards were purchased from Charles D. Sullivan, Inc. (Nashville, TN) or Marcus Cantos Reptiles (Fort Myers, FL). The average snout-vent length of lizards received was 55.15+/- 6.97 mm. Anoles were housed at 70% humidity in a Percival incubator (Boone, IA) and14 hours at 28°C daylight and 10 hours at 19°C night. Anoles were fed crickets once every two days, with weekly Rep-cal calcium and Vitamin D supplementation (Repcal Research Labs, Los Gatos, CA). Water was provided by daily mist spraying of artificial plant surfaces. All animals were maintained according to Institutional Animal Care and Use Committee guidelines at Arizona State University. Autotomy was induced in A. carolinensis using a standard procedure. A point 5 cm from the base of the base of the tail was identified, and the tail was firmly held just distal of this location, while the lizard was allowed to otherwise move freely on a flat surface. Slight pressure was applied until the tail was released by autotomy.
Fixation and paraffin embedding
Original and regenerated tails were fixed overnight in 4% paraformaldehyde with constant shaking at 4C. For original tail samples, bone was decalcified using Calciclear (National Diagnostics, Atlanta, GA) with shaking at 4°C for 4 days. The Calciclear was changed every 24 hours. Original and regenerated tails were then dehydrated through graded alcohols and cleared in xylenes. Following this process they were embedded in paraffin wax. The entire tail for each animal was sectioned into 5 micron sections using a microtome and placed on HistoBond slides (Fisher Scientific, Fair Lawn, NJ).
Hematoxylin and Eosin (H&E) staining
Sections were dewaxed in xylenes and rehydrated through graded alcohols to dH2O. Slides were incubated in Harris hematoxylin, and washed. The slides were then incubated in acid alcohol (0.4% HCl in 70% EtOH) and ammonia water (1% in dH2O) followed by a wash. The slides were then washed sequentially in 25% and 50% ethanol and counterstained with eosin. Sections were then dehydrated through graded alcohols and xylenes and mounted in Permount (Fisher Scientific). Hematoxylin stains nuclei and nucleoli blue and eosin stains proteins in the cytoplasm and extracellular matrix pink/red. Adipocytes, myelin, and other highly hydrophic cells will remain clear.
Gomorri's trichrome stain
Sections were dewaxed in xylenes and rehydrated through graded alcohols. Sections were post-fixed in Bouin's solution that was preheated to 58°C. The slides were then washed in running tap water until the tissue was no longer yellow, followed by a wash in dH2O. Slides were stained in modified Weigert's iron hematoxylin (Solution A: hematoxylin crystals, C.I. 75290 in 90% EtOH; Solution B: 62% aqueous FeCl3, dH2O, and HCl) and washed in dH2O. This was followed by incubation in acid alcohol solution (0.5% HCl in 70% EtOH). The slides were then counterstained with trichrome (chromotrope 2R, C.I. 16570, fast green FCF, acetic acid, and phosphotungstic acid). Sections were differentiated in acetic acid water solution (0.5% glacial acetic acid), and rinsed in dH2O. Sections were then dehydrated in graded alcohols and xylenes and mounted in Permount (Fisher Scientific). This preparation will stain connective tissues and collagen green-blue. Muscle, keratin and cytoplasm are red and nuclei will be blue/black.
Verhoeff-van Gieson staining for elastic fibers
Sections were dewaxed in xylenes and rehydrated through graded alcohols. Sections were stained in Verhoeff's solution (5% alcoholic hematoxylin, 10% FeCl3, and Weigert's iodine solution) and then rinsed in tap water. Slides were differentiated in 2% FeCl3; differentiation was stopped by rinsing the slides multiple times in tap water. The slides were then placed in 5% Na2S2O3 followed by a wash in running tap water for 5 minutes. Sections were counterstained with van Gieson's solution (1% acid aqueous acid fuchsin in saturated picric acid). Sections were dehydrated through graded alcohol solutions and xylenes and mounted using Permount (Fisher Scientific). The van Gieson's preparation stains connective and collagen-dense tissues pink to red, nuclei black, and remaining tissues, such as ependymal cells and muscle, brown.
Results
Since the goal of this study was to carry out a comparative histological analysis of the tissues in the autotomous region of the original and regenerated tail, we sought to determine the time point at which the growth rate had reached a minimum and the regeneration process was complete. Previous reports have typically characterized regeneration and growth rate to 45 dpa (Maderson & Licht, 1968) but there was no plateau in the average length. To determine the appropriate time point after autotomy to carry out our analysis of the fully regenerated tail, we serially measured the lengths of regenerating A. carolinensis tails post-autotomy. The regenerated length should be comparable across animals given the relatively constant position of the induced autotomy breakpoint (see Materials and Methods). Since, regeneration can be influenced by environment (Bustard et al., 1968; Bellairs and Bryant 1985; Alibardi 2010), constant humidity and day and night temperatures were maintained throughout the analysis (see Materials and Methods). From 30 to 60 dpa there was a significant increase in average length of the regenerated tails, indicating active growth and regeneration. However, the average lengths of the regenerated tails from 60 dpa to 120 dpa demonstrated no significant difference (Fig. 1). Based on these data, we concluded that tail regeneration was complete by 60 dpa so regenerated tails utilized in our studies were 60-90 dpa.
Figure 1. Average tail length at extended days post-autotomy.
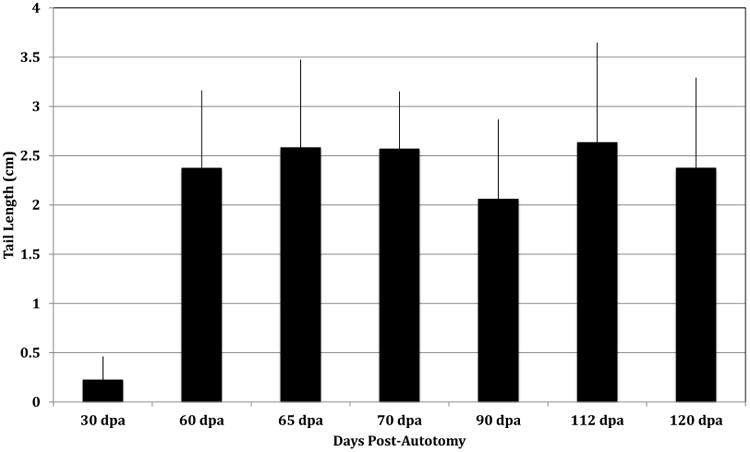
Regenerated tails were serially measured to determine the time at which maximum length reaches a plateau. Average tail lengths were graphed +/- s.d. to estimate the rate of growth. The average lengths demonstrated a general trend of increasing length between 60 and 120 dpa, but no significant differences were identified between the average length from 60-120 dpa. A total of 72 samples were analyzed by one-way ANOVA and confirmed with the post-hoc Tukey test: 30 dpa, n=8; 60 dpa, n=21; 65 dpa, n=5; 70 dpa, n=3; 95 dpa, n=11; 112 dpa, n=14; 120 dpa, n=10.
Structure of the vertebrae and spinal cord of the original tail
The structure of the caudal vertebrae simplifies as one moves distally down the tail (Fig. 2A-C). In the autotomous region of the tail, the more proximal caudal vertebrae are characterized by a prominent neural spine and robust zygapophyseal processes (Fig. 2A). These processes serve as attachment sites for muscles and the intramuscular septa. The spinal cord is enclosed by the neural arch. These vertebrae also have a relatively large centrum, located ventral to the neural arch (Figs. 2A, B). The more distal caudal vertebrae are characterized by a reduced centrum and a reduced or absent neural spine (Fig. 2C, D). This simplification of the vertebrae and loss of elaborate processes along the tail has been noted in almost all lizards (Etheridge 1967; Bellairs and Bryant, 1985). Along the length of the tail, the paired lymphatic trunks, caudal artery, and caudal vein maintain a position ventral to the centra (Figs. 2A-D). Perivertebral adipose tissue is also present in all sections, deep to the muscle and adjacent to the vertebrae (Fig. 2C). It has been suggested that nonautotomous lizards lack adipose tissue in the caudal regions of their tails and that the fat layer may be important for autotomy (Shepard and Bellairs, 1972).
Figure 2. Histology of the original tail in A. carolinensis.

Representative transverse sections of the original tail at the levels shown in the lizard diagram, stained with hematoxylin and eosin at 40× magnification.
A. Transverse section demonstrating the elaborate zygapophyseal processes and neural spine of a more proximal vertebra. Perivertebral adipose tissue is located deep to the muscle bundles. Muscle bundles are organized around the circumference of the tail.
B. Transverse section demonstrating the centrum and neural arch; this section is immediately adjacent to that seen in A. The bony centrum with marrow cavity is visible immediately ventral to the neural arch. In this section, the zygapophyses are no longer detectable but there is no change in the prominence of the neural spine.
C. Transverse section demonstrating a more distal vertebra. There is a reduced neural spine and centrum attached to the neural arch. The caudal vasculature is present on the ventral side. Tendons arise from the muscle bundles and attach to the neural arch. There is less perivertebral adipose tissue, compared to more proximal segments.
D. Transverse section demonstrating a simple neural arch that encloses the spinal cord and ventrally positioned caudal vasculature. The lines roughly indicate the quadrants that contain four muscle bundles, formed by the horizontal septa and dorsal and ventral intramuscular septa.
Structure of the cartilage endoskeleton and regenerated spinal cord of the regenerated tail
In the regenerated tail, a hyaline cartilage tube surrounds the regenerated spinal cord with ependymal cells lining a narrow lumen known as the central canal (Fig. 3A-D). The cartilage tube was characterized by a number of randomly spaced foramina (Fig. 3A-E). Foramina were found in every regenerated tail examined, increasing in number in more distal sections. The cartilage tube and foramina were encased by a collagen rich perichondrium (Fig. 3C), indicating that the foramina are not artifacts, but form as a part of the regenerative process. The foramina served as passageways for blood vessels (Fig. 3D, E). However, neither nerve roots nor the ependymal cells pass through them (Fig. 3A-E).
Figure 3. Organization of the cartilage tube and ependymal core of the regenerated tail.
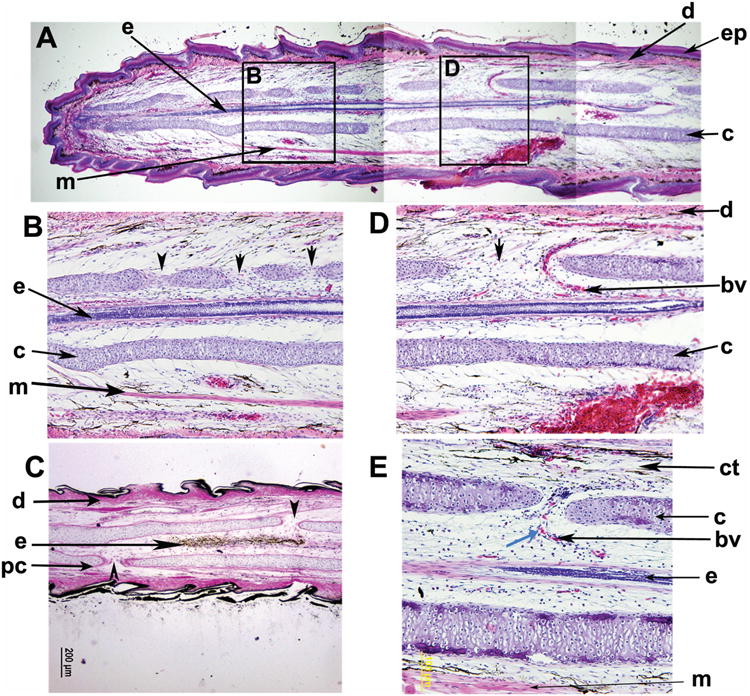
A. A composite photograph comprised of sagittal section images from a 65 dpa tail. The tissues comprising the regenerated tail were organized in layers from medial to lateral: ependymal cells (e), regenerated cartilage skeleton (c), muscle fibers (m), dermis (d), and epidermis (ep). H&E stain, 40× magnification.
B. Detail of box B from panel A. The ependymal cells (e) formed a core at the center of the cartilage skeleton (c). Interestingly, the cartilage had a series of openings or foramina (black arrowheads) but they were not bilaterally symmetrical, nor evenly spaced. H&E stain, 100× magnification
C. Visualization of 90 dpa regenerated tail by van Gieson's stain demonstrated that the perichondrium (pc) is collagen rich and surrounds the cartilage tube, including the edges of the foramina (black arrowheads). The collagen rich dermis (d) deep to the regenerating epidermis and scales was evident. The van Gieson's preparation stains connective and collagen-dense tissues pink to red, nuclei black, and remaining tissues, such as the ependymal cells (e), brown. 40× magnification, 90 dpa
D. Detail of box D from panel A. The ependymal cells are typically a single axial structure at the center of the cartilage tube (c). Vasculature (bv) passes through the cartilage tube foramen, d=dermis, H&E stain, 100× magnification
E. Detail of a blood vessel (bv) entering the cartilage tube (c) through a foramen (black arrowhead). Nucleated red blood cells (blue arrowhead) were visible within the vessel, which branches after entering through the foramen. ct=connective tissue, H&E stain, 200× magnification.
Within the cartilage tube, the ependymal cells line the central canal (Fig. 4A, B). The meninges surround the spinal cord and consisted of a collagen rich inner layer, adjacent to the ependymal cells, and an outer, loose connective tissue layer (Fig. 4B, D, F). This has been observed previously and the inner meningeal layer was shown to be an outgrowth of the original meninges (Simpson 1968; Egar et al., 1970). Interestingly, supernumerary spinal cords with ependymal cores were present in several regenerating tails (Fig. 4E-H). In some specimens, the accessory structures were encased in the cartilage tube and did not appear to communicate with the exterior (Fig. 4E, F). In other tails, the twin spinal cords and ependymal cells were closely juxtaposed within the central lumen of the cartilage (Fig. 4G, H).
Figure 4. Organization of the spinal cord and ependymal cell core in the regenerated tail.

A. Transverse section of a 90 dpa regenerated tail, indicating the ependymal cells that line the central canal of the regenerated spinal cord within the cartilage tube. H&E, 100× magnification
B. Sagittal section of a 65 pda regenerated tail. Two layers of meninges surround the regenerated spinal cord within the cartilage tube. 100× magnification, H&E stain.
C, D Transverse sections of a 90 dpa regenerated tail stained with Gomorri's trichrome, demonstrating increased connective tissue (green-blue stain) that was intercalated within the regenerating muscle (purple-red). Collagen rich meninges surround the regenerated spinal cord. 40× magnification (C) and 200× magnification (D), size bars are as indicated.
E, F. Transverse sections of a 90 dpa regenerated tail. Supernumerary regenerated spinal cords with ependymal cores were observed in regenerated tails. In these cases of duplications, each regenerated spinal cord and ependymal core are surrounded by meninges and were localized within a distinct canal within the cartilage tube. In F, red arrowheads indicate tendons extending from the cartilage toward the muscle. 40× magnification (E) and 200× magnification (F), Trichrome stain,
G, H. Transverse section of a 65 dpa regenerated tail. Axial duplication of ependymal cores within the regenerating spinal cord were observed within the cartilage tube. Trichrome stain, 40× magnification (G), 200× magnification (H).
Structure of the muscles of the original tail
Sixteen muscle bundles are present throughout the original tail (Fig. 2A-C). These segmental muscles are arranged into quadrants by connective tissue septa, as demonstrated in Figure 2D. Each quadrant contained four muscle bundles and robust tendons connected the muscle bundles to the neural arch (Fig. 2C). The architecture of the original tail musculature was further examined using van Gieson's elastic stain to differentiate muscle from tendon and connective tissue (Figs. 5A, B). Each muscle bundle was organized around a central myoseptum and was surrounded by a collagen rich epimysium (Fig. 5B). As noted above, perivertebral adipose tissue was present deep to the muscle, adjacent to the vertebral column (Fig. 5A).
Figure 5. Organization of connective tissue and muscle groups in the original tail.
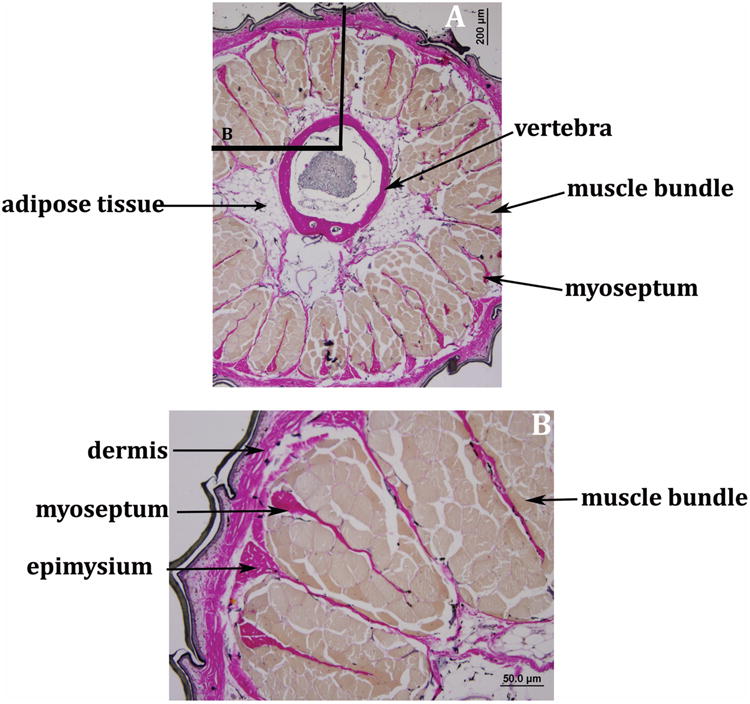
A. Transverse section of the original tail with van Gieson's stain showing symmetric, evenly spaced muscle bundles (brown stain) deep to the dermis (pink stain) and superficial to the vertebra enclosing the spinal cord as well as the caudal vasculature. Perivertebral adipose tissue is noted, 40× magnification.
B. Detail of box in panel A. Muscle bundles (brown stain) were organized with a central myseptum (pink stain) within each muscle group. The epimysium (pink stain) is a collagen rich layer around each muscle group. van Gieson's stain 100× magnification, size bars are as indicated.
Structure of the muscles of the regenerated tail
Previous reports suggest a segmental arrangement to the regenerated muscle (reviewed in Bellairs and Bryant, 1985). We found that in the regenerated tail, the muscle bundles were non-uniform in size and lack the organization seen in the original tail (Fig. 6A-C). Muscle bundles are irregularly spaced and quadrants are no longer discernable (Fig. 6A). The lack of central myosepta was also evident; in contrast, there was an increase in the amount of connective tissue within each muscle bundle (Fig. 6B, C). Peripheral nerves were detected in proximity to muscle bundles, but were not derived from the regenerated spinal cord (Fig. 6C). In order to further examine the connective tissue within the muscle bundles, a series of transverse sections were prepared using Gomorri's trichrome stain in the more distal regenerated tail. In this preparation, collagen-rich tissue (e.g., connective tissue) stains blue green, and muscle stains red. This preparation further highlighted the lack of organization and the loss of the central myosepta and the abundance of connective tissue in the muscle of the regenerated tail (Fig. 7A-D). Additionally, in more distal sections of the regenerated tail, connective tissue within the muscle bundles increases; in some tails, it replaces all of the skeletal muscle in certain muscle bundles (Fig. 7C-D). This connective tissue intercalated within the muscle bundles may represent supernumerary myosepta.
Figure 6. Organization of connective tissue and muscle groups in the regenerated tail.

A. Transverse section of a 65 dpa regenerated tail, showing multiple disorganized myosepta within each muscle bundle, in contrast to the single myoseptum present in the original tail (Figure 5). The muscle bundles were unevenly spaced and the quadrant organization was lost. Arrowheads show tendons that extended from the muscle bundles to the cartilage. There was loose connective tissue between the muscle and the cartilage, but no adipose tissue. 40× magnification, van Gieson's stain.
B. Transverse section of a 90 dpa regenerated tail at a more distal level, showing increased amounts of connective tissue or myosepta intercalated within the muscle groups. No adipose tissue was observed, in contrast to the original tail. 40× magnification, van Gieson's stain.
C. Detail of box in Panel A. Peripheral nerves were detected in close proximity to muscle bundles. However, no nerves were found to originate from the regenerated spinal cord. Arrowheads indicate tendons extending from the muscle bundles to the cartilage. 200× magnification, van Gieson's stain.
D. Detail of box in Panel B. Compared to connective tissue in the original tail, the regenerated tail displays supernumerary myosepta in the muscle bundles. The dermis is also thickened in the regenerated tail. 100× magnification, van Gieson's stain.
Figure 7. Organization of the regenerated tail muscle along the proximal to distal axis.
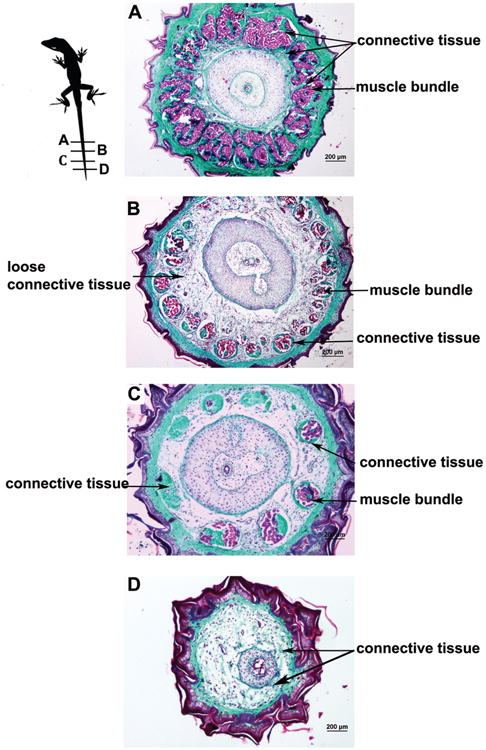
Transverse sections from a 65 dpa regenerated tail stained with Gomorri's trichrome are shown with size bars.
A. At the most proximal level, muscle groups (pink stain) were deeply intertwined with connective tissue (green stain) due to an increased number of myosepta. No adipose tissue was observed. 40× magnification
B. At more distal levels, the proportional cross-sectional area of skeletal muscle groups within the regenerated tail decreased. There was an increased amount of less dense connective tissue between the muscle groups and the cartilage that did not stain green with Gomorri's trichrome. Note the irregularity of the size and spacing of the muscle bundles compared to the original tail (Figure 1). 40× magnification
C. Further distally, the cross sectional area of muscle bundles remains low but the amount of less dense connective tissue was decreased. The radially organized muscle bundles were small and disorganized, and in some bundles, connective tissue replaced muscle entirely. Note that there were fewer radial muscle groups at this level than more proximally in A or B. 40× magnification.
D. At the most distal levels, muscle bundles are almost entirely replaced by connective tissue. Loose connective tissue and anastamosing collagen fibers comprise the space between the dermis (green stain) and the cartilage tube. 40× magnification
Critical differences in the structure of the muscle in the original versus the regenerated tail are also apparent in sagittal sections. In the original tail, the muscle was organized into a series of nested cones, forming myomeres with associated myosepta (Fig. 8A, B), as has been described by others (Cox, 1968). In the regenerated tail, the muscle groups are elongated and irregularly arranged (Fig. 8C). Myosepta were evident, but they did not form nested muscle bundles such as those in the original tail (Fig. 8C, D). In the original tail, the muscles attached directly to the vertebrae via tendons or indirectly via the connective tissue septa (Fig. 8A). In the regenerated tail, the muscles demonstrated a variety of attachments, including tendinous connections to the cartilage tube and tendinous connections to one another (Fig. 8D). As noted above, connective tissue replaces skeletal muscle in some tails; however, in a minority of tails the muscle bundles extend to the tip of the regenerated tail (Fig. 8E).
Figure 8. Organization and attachment of musculature in the original versus regenerated tails.

A. Sagittal section of the original tail demonstrating the organization of muscles as cones that intercalate to form a myomere. All muscles in the original tail attach to the vertebrae either indirectly (via the intermuscular septa) or via tendons. van Gieson's stain, 40× magnification
B. Sagittal section of the original tail demonstrating the organization of the muscle. Myosepta delineate the myomeres, and the pinnate muscle fibers radiate from the central myosepta of each muscle. van Gieson's stain, 40× magnification.
C. Sagittal section of a 90 dpa regenerated tail demonstrating the lack of nested intercalated myomeres. The muscle fibers are organized into long groups that extend to the tip of the regenerated tail. The tendon of one muscle group attaches head on to the tendon of the next. The muscle fibers are still arranged in a pinnate fashion. van Gieson's stain, 40× magnification.
D. Sagittal section of a 65 dpa regenerated tail demonstrating the tendinous connections. In this section, there is an example of a tendon attaching to another tendon and a tendon attaching to the cartilage tube. van Gieson's stain, 100× magnification.
E. Sagittal section of a 65 dpa regenerated tail. Muscle bundles extend toward the tip of the regenerating tail. At the distal end of the tail, the muscles do not extend toward the cartilage but attach to the dermis. H&E stain, 40× magnification.
Discussion
The regenerated spinal cord is contained within the cartilage tube but can display ependymal tube axial duplications
The process of tail regeneration has been described in a number of lizard species, with several studies focusing on the process in A. carolinensis (Jamison, 1964; Maderson & Licht, 1968; Cox, 1968, Simpson, 1968; Zika, 1969; Licht & Howe, 1969; Simpson, 1970; Egar et al., 1970; Maderson & Salthe, 1971; Turner & Simpson, 1973; Chlebowski et al., 1973; Bellairs & Bryant, 1985; Simpson & Duffy, 1994; Alibardi, 1995a; Alibardi, 1995b; Alibardi & Lovicu, 2009; Alibardi, 2010). However, very little data exists regarding the fully regenerated tail, as most accounts focus on earlier stages of regeneration. With the sequencing of the A. carolinensis genome (Alföldi et al., 2011) and release of multiple RNA-Seq based transcriptomes (Eckalbar et al., 2012), there is a unique opportunity to integrate histological and anatomical findings with molecular studies in this lizard model species. Previous studies in A. carolinensis and Scincella lateralis have demonstrated that the ependyma plays a crucial role in inducing the regeneration of the cartilage (Kamrin & Singer, 1955; Simpson, 1970; Cox, 1969). The ependymal cells, which are the population of cells lining the central canal, grow directly from the spinal cord and no dedifferentiation has been reported for nervous tissues in A. carolinensis, Sphaerodactylus goniorhynchus, S. argus and Lygosoma laterale tail regeneration (Hughes & New, 1959; Cox, 1969; Simpson, 1968). The A. carolinensis regenerated spinal cord and ependymal core have been reported to have two meningeal layers associated with it–an inner layer that is continuous with the original spinal cord and an outer layer that looks like loose mesenchyme. Others have reported the inner meningeal layer is continuous with the original meningeal layer of the spinal cord and grows out from the stump (Simpson, 1968; Egar et al., 1970); our findings are consistent with these data. We detected branching or duplication of the spinal cord and associated ependymal core in many regenerated tails, but consistent with previous reports, no ependymal cells or nerve axons were detected exiting the cartilage tube (Fig. 4). The functional implication of the duplicated ependymal cells, if any, is not clear. We observed peripheral nerve axons in the regenerated tail, but no new dorsal root ganglia, suggesting that these neuronal cell bodies are localized proximal to the autotomy break point. This would be consistent with previous reports that studied neural regeneration in S. goniorhynchus, S. argus, Lacerta muralis and A. carolinensis indicating that all functional peripheral axons are derived from above the breakpoint and that there was a lack of regenerated dorsal root ganglia (Hughes & New, 1959; Pannese, 1962; Simpson 1970; Egar et al., 1970; Bellairs & Bryant, 1985).
The regenerated hyaline cartilage tube is not segmented but is perforated by foramina
In the regenerated lizard tail, a tube of hyaline cartilage replaces the articulated vertebrae for endoskeletal support (reviewed in Alibardi, 2010). This cartilage forms around the ependymal cell core that grows out from the severed spinal cord post-autotomy. Comprehensive sagittal and transverse section analysis (Figs. 3 & 4) identified multiple cartilage tube foramina, especially in the more distal portion of the regenerated A. carolinensis tails; however, these foramina were not observed in any repeating pattern and were not symmetrically organized with a contralateral aperture (Fig. 3). Examination of the foramina demonstrated that blood vessels were the only detectable tissue that crossed out of the ependymal tube (Fig. 3). Thus, the cartilage tube does not have any discernible segmental construction and provides endoskeletal rigidity as a single tubular structure. These histological observations will aid in the design of future biomechanical studies testing the function of the regenerated tails.
Organization of the regenerated axial muscle groups
Previous studies have described the organization of muscle in the original lizard tail as consisting of two dorsal quadrants, with epaxial muscle groups, and two ventral quadrants with the hypaxial muscle groups. Each quadrant contains four muscle bundles (Fig. 2) (reviewed in Alibardi 2010). Based on this study, it is clear that the organization of the regenerated muscle fibers is distinctly different than that of the original tail. In contrast to the original tail, the regenerated muscle bundles are not arranged into quadrants and the number of bundles varies as one moves distally along the regenerated tail (Figs. 6 & 7). Furthermore, individual regenerated muscle bundles lack a single central myoseptum (Fig. 5); instead, there was an increased amount of connective tissue embedded within each muscle bundle. These supernumerary myosepta are irregularly organized and variable in number within each muscle group (Figs. 6 & 7). The amount of connective tissue increases distally along the regenerated tail and in some cases completely replaces the muscle tissue in the bundle (Fig. 7). The connective tissue may act as a scaffold for the development of the regenerated muscle, although our studies do not identify any significant increase in tail length after 60 dpa (Fig. 1).
Axial skeletal muscle and tendons are derived from different embryological tissues, i.e., the dermamyotome vs. the syndetome (reviewed in Eckalbar et al., 2012). In the original tail, all muscles attached via tendons to the vertebrae of the axial skeleton. Previous reports indicated that there was no attachment of muscle to the cartilage skeleton of regenerated tails in Lacertids (Bellairs and Bryant, 1985). In the regenerated tail, we were surprised to find that there were muscle attachments to the cartilage skeleton, and there were also muscle groups that attached only to each other (Fig. 8). This muscle-to-muscle tendon attachment is distinctly different than that which is found in the original tail. The muscle bundles are long, and lack the nested cone arrangement of adjacent muscle bundles in the original tail (reviewed in Bryant & Bellairs, 1985). Previous studies in several species of lizard, including S. goniorhynchus, S. argus, Lampropholis delicata, and A. carolinenesis, reported that regenerated muscle develops as myomeres that interdigitate in a segmental fashion (Hughes and New, 1959, Bellairs and Bryant, 1985; Alibardi, 1995); however, our observations do not support any segmental organization of the regenerated tail musculature after 60 dpa.
Implications for the function of the regenerated versus original tail
Externally, the regenerated tail appears amazingly similar to the original tail, apart from differences in skin color and scale pattern. However, while the tail as a whole is regenerated, the internal structure of the regenerated tail is novel and quite distinct from that of the original tail. As described above, there are substantial differences in the structure of the regenerated compared to the original tail including: an endoskeleton consisting of a rigid hyaline cartilage tube vs. articulated vertebrae; regenerated muscle bundles with a high connective tissue content that are loosely organized radially around the cartilage tube vs. highly organized segmental muscle groups organized into quadrants; a limited regenerated spinal cord with an ependymal core supporting axonal growth vs. a larger and more complex spinal cord with segmental sensory, motor, and autonomic nerve roots; and limited peripheral nerve axons in the regenerated tail vs. segmentally organized dorsal root ganglia. Given these vast differences in internal structure, detailed comparisons of original versus regenerated tail function would prove insightful. It will be particularly interesting, considering the unusual attachments of muscles and the rigid cartilage skeleton, to understand how the regenerated tail moves. The data from this study can provide the basis to design biomechanical studies and neuromuscular assays to uncover the functional capabilities and limitations of the regenerated tail in A. carolinensis, a valuable lizard model.
Acknowledgments
This work was supported by Contract 1113 from the Arizona Biomedical Research Commission (KK) and grant R21 RR031305 from the National Center for Research Resources and the Office of Research Infrastructure Programs of the National Institutes of Health (KK). The authors wish to thank Walter Eckalbar, Natalia Emmert, Jesse King, Inbar Maayan, Glenn Markov, Terry Ritzman, and Bianca Zietal for technical support.
Grant Sponsor: Arizona Biomedical Research Commission
Grant Number: 1113 (Kusumi)
Grant Sponsor: National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health
Grant Number: R21 RR031305 (Kusumi)
Literature Cited
- Alfodi J, Di Palma F, Grabherr M, Williams C, Kong L, Mauceli E, Russell P, Lowe CB, Glor RE, Jaffe JD, Ray DA, Boissinot S, Shedlock AM, Botka C, Castoe TA, Colbourne JK, Fujita MK, Moreno RG, ten Hallers BF, Haussler D, Heger A, Heiman D, Janes DE, Johnson J, de Jong PJ, Koriabine MX, Lara M, Novick PA, Organ CL, Peach SE, Poe S, Pollock DE, de Queiroz K, Sanger T, Searle S, Smith JD, Smith Z, Swofford R, Turner-Maier J, Wade J, Young S, Zadissa A, Edwards SV, Glenn TG, Schneider CJ, Losos JB, Lander ES, Breen M, Ponting CM, Lindblad-Toh K. The genome of the green anole lizard and a comparative analysis with birds and mammals. Nature. 2011;477:587–591. doi: 10.1038/nature10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. Development of the axial cartilaginous skeleton in the regenerating tail of lizards. Bull Assoc Anat (Nancy) 1995;79(244):3–9. [PubMed] [Google Scholar]
- Alibardi L. Muscle differentiation and morphogenesis in the regenerating tail of lizards. J Anat. 1995;186(Pt 1):143–151. [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. A model system with implications for tissue regeneration in mammals. Berlin Heidelberg: Springer-Verleg; 2010. Morphological and Cellular aspects of tail and limb regeneration in Lizards. [PubMed] [Google Scholar]
- Alibardi L, Toni M. Wound Keratins in the Regenerating Epidermis of Lizard Suggest that the Wound Reaction is Similar in the Tail and Limb. J Exp Zool. 2005;303a:845–860. doi: 10.1002/jez.a.213. [DOI] [PubMed] [Google Scholar]
- Araujo TH, Pavla deFaria F, Katchburian E, Haapalainen EF. Ultrastructural changes in skeletal muscle of the tail of the lizard Hemidactylus mabouia immediately following autotomy. Acta Zoologica. 2010;91:440–446. [Google Scholar]
- Arnold EN. Caudal autotomy as a defense. In: Gans C, Huey RB, editors. Biology of the Reptilia, Ecology B. New York: Alan R. Liss, Inc; 1988. pp. 235–273. [Google Scholar]
- Bellairs A d'A, Bryant SV. Autotomy and regeneration in reptiles. In: Gans C, Huey RB, editors. Biology of the Reptilia, Development B. New York: John Wiley and Sons; 1985. pp. 303–410. [Google Scholar]
- Bustard HR. Temperature Dependent Tail Autotomy Mechanism in Gekkonid Lizards. Herpetologica. 1968;24:127–130. [Google Scholar]
- Chlebowski JS, Przbylski RJ, Cox PG. Ultrastructural studies of lizard (Anolis carolinensis) myogenesis in vitro. Developmental Biology. 1973;33:80–99. doi: 10.1016/0012-1606(73)90166-8. [DOI] [PubMed] [Google Scholar]
- Cox PG. Some Aspects of Tail Regeneration in the Lizard, Anolis carolinensis I. A Description based on Histology and Autoradiography. J Exp Zool. 1968;171:127–150. [Google Scholar]
- Cox PG. Some aspects of tail regeneration in the lizard, Anolis carolinensis. II. The role of the peripheral nerves. J Exp Zool. 1969:151–160. [Google Scholar]
- Eckalbar WL, Lasku E, Infante CR, Elsey RM, Markov GJ, Allen AN, Corneveaux JJ, Losos JB, Denardo DF, Huentelman MJ, Wilson-Rawls J, Rawls A, Kusumi K. Somitogenesis in the anole lizard and alligator reveals evolutionary convergence and divergence in the amniote segmentation clock. Dev Biol. 2012;363:308–319. doi: 10.1016/j.ydbio.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Egar M, Simpson SB, Singer M. The Growth and Differentiation of the Regenerating Spinal Cord of the Lizard, Anolis carolinensis. J Morph. 1970;131:131–152. doi: 10.1002/jmor.1051310202. [DOI] [PubMed] [Google Scholar]
- Goss RJ. Why mammals don't regenerate—or do they? News Physiol Sci. 1987;2:112–115. [Google Scholar]
- Hughes A, New D. Tail regeneration in the geckonid lizard, Sphaerodactylus. J Embryol Exp Morph. 1959;7:281–302. [PubMed] [Google Scholar]
- Jamison J. Regeneration subsequent to intervertebral amputation in lizards. Herpetologica. 1964;20:145–149. [Google Scholar]
- Kamrin RP, Singer M. The influence of the spinal cord in regeneration of the tail of the lizard, Anolis carolinensis. J Exp Zool. 1955;12:611–627. [Google Scholar]
- Kusumi K, Kulathinal RJ, Abzhanov A, Boissinot S, Crawford NG, Faircloth BC, Glenn TC, Losos JB, Menke DB, Poe S, Sanger TJ, Schneider C, Stapley J, Wade J, Wilson-Rawls J. Developing a community-based genetic nomenclature for anole lizards. BMC Genomics. 2011;12:554. doi: 10.1186/1471-2164-12-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht P, Howe N. Hormonal dependence of tail regeneration in the lizard Anolis carolinensis. J Exp Zool. 1969;171:75–83. [Google Scholar]
- Losos JB. Lizards in an evolutionary tree: Ecology and adaptive radiation of anoles. University of California Press; Berkeley, CA: 2009. [Google Scholar]
- Maderson PFA, Licht P. Factors influencing rates of tail regeneration in the lizard Anolis carolinensis. Cell Mol Life Sci. 1968;24:1083–1086. doi: 10.1007/BF02138764. [DOI] [PubMed] [Google Scholar]
- Maderson PF, Salthe SN. Further observations on tail regeneration in Anolis carolinensis (Iguanidae, Lacertilia) J Exp Zool. 1971;177:185–189. doi: 10.1002/jez.1401770206. [DOI] [PubMed] [Google Scholar]
- Maginnis TL. The costs of autotomy and regeneration in animals: a review and framework for future research. Behav Ecol. 2006;17:857–872. [Google Scholar]
- McLean KE, Vickaryous MK. A novel amniote model of epimorphic regeneration: the leopard gecko, Eublepharis macularius. BMC Dev Biol. 2011;11:50. doi: 10.1186/1471-213X-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti SA, Iqbal J. Tail regeneration after amputation in Hemidactylus flaviairidis. Pakistan J ZooI. 1975;7:15–28. [Google Scholar]
- Pannese E. Detection of neurofilaments in the perikaryon of hypertrophic nerve cells. J Cell Biol. 1962;13:457–467. doi: 10.1083/jcb.13.3.457. In Lacerta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman OJ. Evolution of regeneration capabilities. Am Nat. 1984;123:752–763. [Google Scholar]
- Sanger TJ, Hime PM, Johnson MA, Diani J, Losos JB. Laboratory protocols for husbandry and embryo collection of Anolis lizards. Herpetol Rev. 2008;39:58–63. [Google Scholar]
- Shah RV, Chakko TV. Histological observations on the normal and regenerating tail of the house lizard, Hemidactylus flavaiairidis. J Anim Morph Physiol. 1968;15:26–39. [Google Scholar]
- Sheppard L, Bellairs A d'A. The mechanism of autotomy in Lacerta. Br J Herpet. 1972;4:276–286. [Google Scholar]
- Simpson SB. Analysis of tail regeneration in the lizard Lygosomala terale. I Initiation of regeneration and cartilage differentiation: the role of the ependyma. In: Thornton CS, Bromley SC, editors. J Morph. Vol. 114. Stroudsburg: Dowden, Hutchinson and Ross; 1964. pp. 425–436. Reprinted in: Vertebrate Regeneration. 1973. [DOI] [PubMed] [Google Scholar]
- Simpson SB. Regeneration of the lizard tail. In: Kiortsis V, Trampusch HAL, editors. Regeneration in Animals and Related Problems. Amsterdam: North-Holland; 1965. pp. 431–443. [Google Scholar]
- Simpson SR., Jr Morphology of the Regenerated Spinal Cord in the Lizard, Anolis carolinensis. J Comp Neur. 1968;134:193–210. doi: 10.1002/cne.901340207. [DOI] [PubMed] [Google Scholar]
- Simpson SB., Jr Studies on Regeneration of the Lizard's Tail. Am Zool. 1970;10:157–165. doi: 10.1093/icb/10.2.157. [DOI] [PubMed] [Google Scholar]
- Simpson S, Jr, Duffy M. The lizard spinal cord: a model system for the study of spinal cord injury and repair. Progress in brain research. 1994;103:229–241. doi: 10.1016/s0079-6123(08)61139-5. [DOI] [PubMed] [Google Scholar]
- Turner J, Singer M. Some Morphological And Ultrastructural Changes In Ependyma Of Amputation Stump During Early Regeneration Of Tail In Lizard, Anolis-carolinensis. J Morphol. 1973;140:257–269. doi: 10.1002/jmor.1051400302. [DOI] [PubMed] [Google Scholar]
- Vitt LI. Tail autotomy and regeneration in the tropical skink, Mabuya heathi. J Herpetol. 1981;15:454–457. [Google Scholar]
- Werner YL. Regeneration of the caudal axial skeleton in a gekkonid lizard (Hemidactylus) with particular reference to the “latent” period. Acta Zool. 1967;48:103–125. [Google Scholar]
- Zika J. A histological study of the regenerative response in a lizard, Anolis carolinensis. Journal of Experimental Zoology. 1969;172:1–9. doi: 10.1002/jez.1401720102. [DOI] [PubMed] [Google Scholar]


