Abstract
The development of HER2-targeting agents has dramatically altered the natural history of HER2-positive breast cancer and is often cited as a prime example of the effectiveness of molecularly targeted therapy. Emerging data suggests that the remarkable clinical efficacy of these agents may be related to their ability to target the breast cancer stem cell (BCSC) population. A new study suggests that the regulation of BCSC by HER2 may extend to breast cancers that do not display HER2 gene amplification. In these tumors, HER2 is selectively expressed in the CSC population and this expression is regulated by the tumor microenvironment. In mouse models, trastuzumab blocked growth of these “HER2-negative” tumors when administered in the adjuvant setting but had no effect on established tumors. These studies provide a potential biological explanation for retrospective analysis of clinical trials which surprisingly suggest that the clinical benefits of adjuvant trastuzumab may extend to women currently classified as “HER2-negative. In addition to having significant implications for breast cancer therapy, these studies suggest the need to reevaluate the role of HER2 in regulating CSCs in other tumor types. Furthermore, these studies suggest that effective adjuvant therapies may need to target the CSC population.
Breast Cancer: A poster child for molecularly targeted therapeutics
The therapy of breast cancer provides one of the best examples of the clinical benefits of molecularly targeted therapeutics. Development of these therapies has resulted from realization that the spectrum of breast cancer encompasses distinct molecular subtypes, which have characteristic gene expression profiles, natural histories, and which respond to different therapies. These molecular classifications also reflect major drivers of these cancer subtypes including the steroid hormone receptors, ER and PR, and the growth factor receptor HER2. The development of reliable hormone receptor and HER2 assays have led to the classification of breast cancers as ER, PR+, HER2+ or triple-negative (TN). The use of hormonal therapy for ER+ disease and HER2 targeted agents for HER2+ disease represents one of the greatest advances in clinical oncology and illustrates the tremendous potential of molecularly targeted therapeutics. In fact, the use of hormonal agents and HER2 targeting agents in the adjuvant setting accounts for a major portion of the significant decrease in breast cancer mortality over the past 20 years (1, 2).
The plot thickens
Despite the success of hormonal and HER2 targeting agents, many women with breast cancer, who receive these agents in the adjuvant setting still relapse. Furthermore, almost all women with advanced breast cancer develop resistance to these targeted therapies, as well as to chemotherapy and as a result, metastatic breast cancer remains incurable. The selection of therapy based on molecular subtype of breast cancer assumes that cell populations within an individual tumor are homogeneous and, thus, will uniformly respond to these treatments. This assumption has been challenged by the cancer stem cell hypothesis which posits that many cancers, including breast cancer, are hierarchically organized and driven by a population of cells that displays stem cell properties. Furthermore, there is accumulating evidence that these “cancer stem cells (CSCs)” mediate tumor metastasis and by virtue of their relative resistance to chemotherapy and radiation therapy contribute to treatment relapse (3, 4). Breast CSCs can be identified by virtue of their expression of marker proteins such as CD44+/CD24− or aldehyde dehydrogenase (5, 6). CSCs which constitute a small subset of cells in human breast cancers give rise to other CSCs through the process of self-renewal, as well as generate the non-stem cell populations forming the tumor bulk. Although CSCs and bulk tumor cells may share genetic signatures, they display distinct gene expression patterns by virtue of epigenetic regulation. As a result, CSCs and bulk cell populations within an individual tumor may be driven by distinct pathways. This suggests that effective therapy may require selective targeting of these distinct cell populations. This level of molecular heterogeneity is superimposed on genetic clonal heterogeneity generated by genetic instability.
The HER family of growth factors plays an important role in breast development and mammary carcinogenesis. Although HER2 itself has no known ligand, it forms heterodimers with ligand activated EGFR, HER3 and HER4. Previous studies utilizing the mouse models of conditional HER2 knockout in the mammary gland have demonstrated that HER2 is required for normal mammary ductal morphogenesis (7).
The HER2 gene is amplified in approximately 20% of human breast cancers, a molecular subtype associated with an aggressive clinical course with early development of metastasies. The development of HER2 targeting agents such as trastuzumab has led to a dramatic alteration in the natural history of this disease (8). Preclinical studies, as well as clinical trials, have shown that in women with advanced breast cancer, the clinical benefit of HER2 targeted therapies are limited to women whose breast cancers display HER2 gene amplification. This observation has led to the routine testing of breast cancer samples for HER2 overexpression by immunochemistry and fluorescence in situ hybridization (FISH) (9). Based on studies in advanced breast cancer demonstrating that the potentiation of tumor regression by HER2 targeting agents was limited to HER2+ breast cancers, entry into adjuvant trials utilizing these agents was limited to this patient population. Adjuvant trials demonstrated that addition of the HER2 blocking agent trastuzumab to cytotoxic chemotherapy resulted in a remarkable 50% reduction in disease recurrence compared to patients receiving chemotherapy alone (10-12). However, the conventional wisdom that only HER2+ patients benefit from adjuvant trastuzumab was challenged by a study published by Paik, et al. in the New England Journal of Medicine in 2008. In this retrospective study, clinical samples accrued to NSABP B31, a pivotal trastuzumab adjuvant trial were reanalyzed for HER2 gene amplification in a central laboratory. This reanalysis identified 174 cases which although originally reported as HER2+ actually lacked HER2 gene amplification (13). Surprisingly, analysis of outcome data revealed that these “HER-2-negative” patients benefited as much from adjuvant trastuzumab as did patients whose tumors displayed HER2 gene amplification. These findings were confirmed in similar analysis of an independent trastuzumab adjuvant study (14). Although provocative, these studies are limited by their retrospective nature and a randomized prospective phase III trial, NSABP B47 is currently in progress to determine whether the clinical benefits of adjuvant trastuzumab extend to women whose tumors do not display HER2 amplification. Although the molecular mechanisms which might account for the clinical efficacy of adjuvant trastuzumab in women with “HER2-negative “ breast cancer are not known, recent preclinical findings by our group suggest that this clinical observation may be explained by the cancer stem cell model.
HER2 and breast cancer stem cells
Several lines of evidence indicate that HER2 is an important regulator of the CSC population in HER2+ breast cancers (13). We previously demonstrated that HER2 overexpression increases, and HER2 blockade decreases the CSC population in breast cancer cell lines and mouse xenografts (15). Furthermore, in human breast cancers there is a correlation between HER2 amplification and CSC frequency as assessed by expression of the breast CSC marker ALDH-1(5). In contrast to cytoxic chemotherapy, neoadjuvant HER2 blockade reduces the CSC population resulting in significantly increased complete pathologic response rate compared to chemotherapy alone (16). In addition, in contrast to chemotherapy, administration of the EGFR/HER2 blocker, Lapatinib in the neoadjuvant setting reduced the breast CSC population (4). In a new study published in Cancer Research, we demonstrate that HER2 also plays an important role in regulating the CSC population in luminal breast cancers that do not display HER2 amplification and therefore are currently classified as HER2-negative (16). In these tumors, HER2 is selectively expressed in and drives the CSC population. Since CSCs constitute only a small fraction of the total tumor population, analysis of whole tumors masks this measurement of HER2 expression. A recent report that HER2 expressing cells in luminal “HER2-negative” breast cancers are radiation resistant provides further evidence for such a model (17). Our studies also raise questions regarding current clinical practices for HER2 assessment. Tumors are routinely evaluated by IHC on a scale from 0-3+ with the latter being considered HER2+. Those that are 2+ are further examined by FISH and if amplified are also considered positive. FISH analyses of HER2 gene amplification is considered to be the “gold standard” for classification of breast cancers as “HER2-positive” a designation that has dictated treatment selection.
Our studies suggest that contrary to this dichotomous model, HER2 expression in breast tumors follows a distribution related to molecular sub-type with luminal tumors expressing an intermediate level of HER2 compared to claudin-low/basal (low) and HER2-amplified (high) (16). These data are consistent with previous reports demonstrating a bimodal association of clinical outcomes with HER2 expression levels (18, 19). Those studies indicated that, when HER2 expression was quantitated with enzyme-linked immunosorbent or immunofluorescent in situ assays, very low and very high HER2 protein levels in breast cancer patients had a poorer outcome than those with intermediate expression. Consistent with our findings, the HER2 low group was associated with TN the HER2 high group with HER2-positive and the intermediate HER2 group with luminal ER+ breast cancer. Our pre-clinical studies predict that women with luminal breast cancer in which HER2 and the CSC marker ALDH1 are co-expressed in the same cells will benefit most from adjuvant trastuzumab. This hypothesis can be directly tested in prospective clinical trials such as NSABP B47.
Regulation of HER2 expression by the tumor microenvironment
If HER2 expression in CSCs does not require HER2 gene amplification, what regulates HER2 expression in these cells? We demonstrate that the bone microenvironment at sites of bone metastasis is able to induce expression of HER2 in a process regulated by RANK ligand which is produced by bone osteoblasts. It has previously been demonstrated that normal breast stem cells are regulated by RANK ligand which is produced in response to progesterone elevation during pregnancy (20, 21). In TN basal breast cancers, it has been shown that RANK ligand produced by bone osteoblasts is capable of stimulating self-renewal of CSCs through activation of NF-κB (7). Our studies expand upon this concept by demonstrating that RANK ligand is also able to regulate CSCs in luminal breast cancers through induction of HER2 expression (22). The RANK ligand inhibitory antibody denosumab is currently approved for treatment of bone metastasis in breast and other cancers. Ongoing randomized clinical trials are in progress to determine whether administration of denosumab in the adjuvant setting reduces recurrence in women with early stage breast cancer. The ability of RANK ligand to regulate CSC populations in bone micrometastasis suggests a potential mechanism that might produce clinical benefit in this setting. By comparing HER2 protein expression in patient biopsies from primary luminal breast tumors and bone metastasies, we demonstrated a significant increase in HER2 protein expression in the bone metastasies compared to matched primary tumors. Furthermore, as indicated by FISH analysis, this increase in HER2 expression was not due to selection of clones of HER2 amplified cells (16). These observations are consistent with previous studies that reported a significant discordance in HER2 protein expression, between primary tumors, circulating tumor cells and metastatic lesions (9, 23). A number of these studies utilized FISH to assess HER2 gene copy number and thus may have missed regulation of HER2 protein expression by the tumor microenvironment. However, a recent report demonstrated that 89% of women with “HER2-negative” breast cancer had circulating tumor cells (CTCs) that expressed HER2 protein. Furthermore, trastuzumab decreased recurrence in these patients, an effect associated with reduction in HER2 expressing CTC(s) (24).
Interaction of HER2 and other signaling pathways in the regulation of breast CSCs
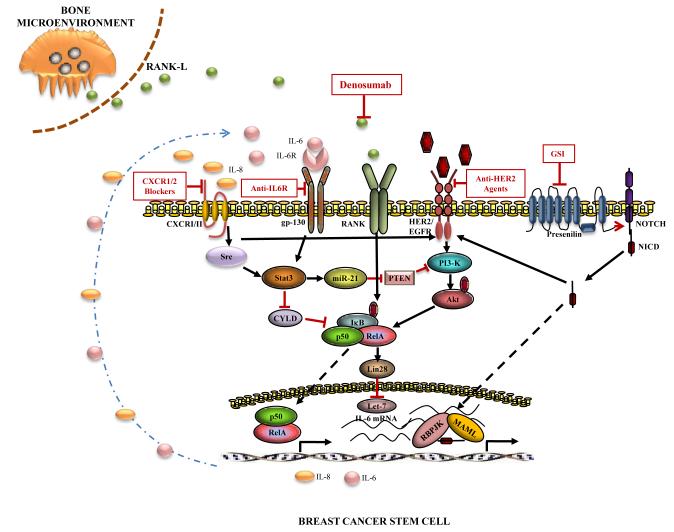
We have previously reported that HER2 regulates breast CSC through a cell intrinsic process involving signaling through the PI3 kinase, Akt and Wnt pathways (25). In contrast to the adjuvant setting, the majority of patients with advanced HER2-positive breast cancer develop resistance to trastuzumab. Many of these trastuzumab resistant patients respond to newer HER2 targeting drugs such as Pertuzumab or the trastuzumab drug conjugate T-DM1. Furthermore immunotherapeutic approaches including T-cell therapy targeting HER2 have been developed (26). In addition, loss of expression of the tumor suppressor gene PTEN is frequently associated with resistance to HER2 targeting agents. In preclinical models, we demonstrated that PTEN knockdown in HER2-positive breast cancer cells generates trastuzumab resistant CSCs through activation of an inflammatory loop mediated by NF-κB and IL-6 (27). It has been proposed that the trastuzumab resistance in HER2+ER− (basal) compared to HER2+ER+ (luminal) breast tumors may relate to CSC regulatory pathways (28). In addition, recent studies have demonstrated that HER2 activating mutations may contribute to trastuzumab resistance (29). HER2 may also interact with other CSC regulatory pathways. For example, it has been recently shown that in HER2-positive breast cancers HER2 interacts with CXCR1, a receptor for the cytokine IL-8 (30), which has been shown to be selectively expressed in breast CSCs (31). The Notch pathway, another regulator of breast CSCs, regulates HER2 expression (32, 33). Together, these studies suggest that multiple cell extrinsic and cell intrinsic pathways, including HER2, interact to regulate breast CSCs. These pathways provide potential targets for therapeutic intervention as illustrated in Figure1. A number of early stage clinical trials targeting these CSC regulatory pathways are in progress.
Figure 1.
Pathways regulating BCSCs. Molecular pathways interacting with HER2 regulate self-renewal of BCSC. Therapeutic agents inhibiting these pathways are shown.
HER2 and CSCs in other tumor types
The demonstration that HER2 regulates breast CSCs in the absence of gene amplification suggests the possibility that similar signaling pathways may exist in other tumor types. Indeed, it has been recently shown in hormone refractory prostate cancer that these CSCs are regulated by a pathway involving α6β4 integrin which amplifies signaling through the HER2 and c-Met pathways (29). HER2 has also been reported to be expressed in a number of other solid malignancies including those of the bladder and ovary (34). In these tumors, expression of HER2 protein generally occurs in the absence of gene amplification. It remains to be determined whether HER2 regulates CSCs and thus whether HER2 blockade will be a useful clinical strategy in these tumor types. It will be important to consider the CSC model when designing clinical trials to access the efficacy of HER2 blockade in these tumors. Trials utilizing tumor regression as a measure of efficacy may miss important effects of HER2 blockade on CSC populations. Lessons learned from HER2 targeted therapies in breast cancer may prove useful in designing clinical trials for these other cancers.
Implications for development of adjuvant therapies
Although HER2 targeted therapies have demonstrated clinical benefit in breast cancer when utilized in both advanced and adjuvant settings, the use of adjuvant trastuzumab has had the greatest impact on breast cancer mortality reducing tumor recurrence by approximately 50%. Since the natural history of HER2-positive breast cancer includes a propensity for early metastasis, the likelihood of cure for patients who remain disease-free after five years is great. This raises the intriguing possibility that effective targeting of CSCs in the adjuvant setting may have similar dramatic effects in other cancer types. If the lessons learned from development of HER2-targeted therapies in breast cancer apply to other tumor types, we may need to alter the current paradigm for development of adjuvant cancer therapies. This paradigm selects agents based on their ability to cause regression of advanced tumors. These agents are then administered in the adjuvant setting after removal of the primary tumor. The cancer stem cell model calls this strategy into question since regression of advanced tumors largely reflects effects on bulk tumor populations while growth of tumors from microscopic foci at metastatic sites may be mediated by CSCs. This model posits that only CSCs possess sufficient self-renewal capacity to form clinically significant macrometastasis from these micrometastatic foci. Since CSC and bulk tumor populations may be driven by different pathways, it will be important to utilize agents that target CSC regulatory pathways in the adjuvant setting. If lessons learned from HER2-targeted therapies apply to other tumor types then administration of effective CSC targeting therapies in the adjuvant setting should reduce recurrence improving patient outcome.
Acknowledgments
Conflict of interest: MSW has financial holdings and is a scientific advisor for OncoMed Pharmaceuticals, is a scientific advisor for Verastem, Paganini and MedImmune and receives research support from Dompe Pharmaceuticals and MedImmune.
HK receives research funding from MedImmune.
References
- 1.Harris CA, Ward RL, Dobbins TA, Drew AK, Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol. 2011;22:1308–17. doi: 10.1093/annonc/mdq593. [DOI] [PubMed] [Google Scholar]
- 2.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–84. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–3. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–9. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 5.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, et al. RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res. 2012;72:2879–88. doi: 10.1158/0008-5472.CAN-12-0044. [DOI] [PubMed] [Google Scholar]
- 8.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 9.Lipton A, Kostler WJ, Leitzel K, Ali SM, Sperinde J, Weidler J, et al. Quantitative HER2 protein levels predict outcome in fluorescence in situ hybridization-positive patients with metastatic breast cancer treated with trastuzumab. Cancer. 2010;116:5168–78. doi: 10.1002/cncr.25430. [DOI] [PubMed] [Google Scholar]
- 10.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 13.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 14.Perez EA, Reinholz MM, Hillman DW, Tenner KS, Schroeder MJ, Davidson NE, et al. HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol. 2010;28:4307–15. doi: 10.1200/JCO.2009.26.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–30. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 17.Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D, et al. HER2-associated radiation resistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dressler LG, Berry DA, Broadwater G, Cowan D, Cox K, Griffin S, et al. Comparison of HER2 status by fluorescence in situ hybridization and immunohistochemistry to predict benefit from dose escalation of adjuvant doxorubicin-based therapy in node-positive breast cancer patients. J Clin Oncol. 2005;23:4287–97. doi: 10.1200/JCO.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Eppenberger-Castori S, Kueng W, Benz C, Caduff R, Varga Z, Bannwart F, et al. Prognostic and predictive significance of ErbB-2 breast tumor levels measured by enzyme immunoassay. J Clin Oncol. 2001;19:645–56. doi: 10.1200/JCO.2001.19.3.645. [DOI] [PubMed] [Google Scholar]
- 20.Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–7. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 21.Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 22.Cao N, Li S, Wang Z, Ahmed KM, Degnan ME, Fan M, et al. NF-kappaB-mediated HER2 overexpression in radiation-adaptive resistance. Radiat Res. 2009;171:9–21. doi: 10.1667/RR1472.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabi A, Di Benedetto A, Metro G, Perracchio L, Nistico C, Di Filippo F, et al. HER2 protein and gene variation between primary and metastatic breast cancer: significance and impact on patient care. Clin Cancer Res. 2011;17:2055–64. doi: 10.1158/1078-0432.CCR-10-1920. [DOI] [PubMed] [Google Scholar]
- 24.Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23:1744–50. doi: 10.1093/annonc/mds020. [DOI] [PubMed] [Google Scholar]
- 25.Korkaya H, Paulson A, Charafe-Jauffret E, Ginestier C, Brown M, Dutcher J, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rainusso N, Brawley VS, Ghazi A, Hicks MJ, Gottschalk S, Rosen JM, et al. Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther. 2012;19:212–7. doi: 10.1038/cgt.2011.83. [DOI] [PubMed] [Google Scholar]
- 27.Korkaya H, Kim GI, Davis A, Malik F, Henry NL, Ithimakin S, et al. Activation of an IL6 inflammatory loop mediates trastuzumab resistance in HER2+ breast cancer by expanding the cancer stem cell population. Mol Cell. 2012;47:570–84. doi: 10.1016/j.molcel.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Castillo B, Oliveras-Ferraros C, Vazquez-Martin A, Cufi S, Moreno JM, Corominas-Faja B, et al. Basal/HER2 breast carcinomas: Integrating molecular taxonomy with cancer stem cell dynamics to predict primary resistance to trastuzumab (Herceptin) Cell Cycle. 2013;12:225–45. doi: 10.4161/cc.23274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 Mutations in HER2 Gene Amplification Negative Breast Cancer. Cancer Discov. 2013;3:224–37. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G, et al. Targeting CXCR1/2 Significantly Reduces Breast Cancer Stem Cell Activity and Increases the Efficacy of Inhibiting HER2 via HER2-dependent and - independent Mechanisms. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–97. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magnifico A, Albano L, Campaner S, Delia D, Castiglioni F, Gasparini P, et al. Tumor-initiating cells of HER2-positive carcinoma cell lines express the highest oncoprotein levels and are sensitive to trastuzumab. Clin Cancer Res. 2009;15:2010–21. doi: 10.1158/1078-0432.CCR-08-1327. [DOI] [PubMed] [Google Scholar]
- 33.Korkaya H, Wicha MS. HER-2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res. 2009;15:1845–7. doi: 10.1158/1078-0432.CCR-08-3087. [DOI] [PubMed] [Google Scholar]
- 34.Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146:264–75. doi: 10.1016/j.jconrel.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]