Abstract
Arsenic is a well established human carcinogen and is associated with a variety of cancers including those of the skin. Paradoxically, arsenic has also been used, amid at low doses, in the treatment of leukemia for over a century. Here we demonstrate that low to moderate concentrations of arsenite (2–10 μM) that has little or no effect on normal melanocytes may induce apoptosis of human melanomas including highly metastatic ones despite their low surface Fas levels. The two prerequisites that dictate apoptotic response of melanomas upon arsenite treatment are low nuclear NF-κB activity and an endogenous expression of tumor necrosis factor α. Under these conditions, melanoma cells acquired sensitivity to tumor necrosis factor α-mediated killing. On the other hand, signaling pathways including those of phosphatidylinositol 3-kinase-AKT, MEK-ERK, and JNK play a protective role against arsenite-induced oxidative stress and apoptosis in melanoma cells. Suppression of these pathways dramatically accelerates arsenite-induced apoptosis. Taken together, these data could provide potential approaches to sensitize melanomas to the cytotoxic effects of arsenite through modulating the signaling pathways.
Arsenical compounds are environmental toxins with multiple effects in animal and human populations. Chronic low levels of exposure to arsenite (0.5–5 μM) may result in the induction of skin, lung, bladder, and kidney cancer (1). Environmental arsenic contamination is a serious problem in some regions of the world. For example, almost 50 million people are at risk in Bangladesh where both chronic and acute arsenic poisoning as well as increased cancer incidence have been reported previously (2). Higher doses of arsenite (>5 μM) produce highly damaging effects in different tissues because of its induction of apoptosis and necrosis. Such dose-dependent effects in a tissue-specific manner have enabled arsenic to be used for the treatment of certain types of cancer including acute promyelocytic leukemia and multiple myelomas through the induction of programmed cell death (3–5). There are two important biochemical aspects of arsenite-mediated effects in the cell. (i) Arsenite acts as a sulfhydryl reagent that binds to the free thiol (–SH) group of enzymes and inhibits their functions (6). (ii) Arsenite induces production of reactive oxygen species at high levels with subsequent development of oxidative stress, which affects multiple targets in the cell (2, 7, 8). The most prominent target of enzymatic inhibition by arsenite is IκB kinase β (IKKβ)1 (9–11). This results in the suppression of NF-κB transcription factor activation followed by the dramatic change in the anti-apoptotic functions of the cells (12–14). Simultaneously, as a consequence of oxidative stress, stress-induced kinase pathways (MKK6-MAPK p38, MKK4/7-JNK, and MEK-ERK1/2) up-regulate the AP-1 (Jun-Fos, Jun-ATF2) transcription factors and induce AP-1-dependent gene expression (15–18). The third family of the master transcription regulators that is also involved in regulation via oxidative stress is the STAT family (19, 20). Arsenic compounds may suppress STAT3 activation and dramatically change growth and survival of multiple myeloma cells (21).
Many therapeutic approaches including γ-irradiation and chemotherapeutic drug treatment have been proposed to reactivate apoptosis in cancer cells. Human malignant melanomas, an often deadly form of skin cancer due to the lack of effective treatment options, possess numerous genetic and epigenetic mechanisms that suppress apoptosis, which allows tumor survival after treatment (22–24). In this study, we elucidated pro-apoptotic activities induced by arsenite in human melanomas and demonstrated that arsenite-mediated NF-κB inhibition and simultaneous endogenous expression of death receptor ligand TNFα (25) sensitize melanoma cells to undergo TNFα-mediated apoptosis.
EXPERIMENTAL PROCEDURES
Materials
Sodium arsenite was obtained from Sigma. Pharmacological inhibitors LY294002, PD98059, SB203580, and U0126 were obtained from Calbiochem, and SP600125 was purchased from Biomol (Plymouth Meeting, PA). Caspase inhibitors Z-VAD-fmk, Ac-IETD-CHO (an inhibitor of caspase-8 and caspase-6), and Ac-LEHD-CHO (an inhibitor of caspase-9) were purchased from Calbiochem. Precast SDS-polyacrylamide gels were purchased from Bio-Rad (Hercules, CA).
Cell Lines
Human melanoma cell lines WM35, SBcl2, LU1205 (also known as 1205lu), WM9, WM793 (26–29), and OM431, mouse melanoma cell line SW1 (30), and normal human fibroblast TIG-3 were maintained in DMEM supplemented with 10% fetal bovine serum, L-glutamine, and antibiotics. FEMX, HHMSX, and LOX human melanoma lines (31) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics. Normal human melanocytes were maintained in TICVA medium.
Transfection and Luciferase Assay
The luciferase reporter gene containing three GAF DNA-binding sequence elements from the Ly6E gene was described previously (32). The NF-κB luciferase reporter containing two κB binding sites, Jun2-Luc reporter and vector thymidine kinase-Luc (33), were used for determination of NF-κB and AP-1 trans-activation. Additional reporter constructs used were as follows: −1.7 kb of FASpr-Luc (34); −453 kb of FASLpr-Luc (35); −615 TNFpr-Luc; and its mutated variants (36), −1.6 kb of TRAILpr-Luc (37). Transient transfection of different reporter constructs (0.5 μg) together with expression vectors (0.5 μg) and pCMV-β-galactosidase (0.25 μg) into 5 × 105 melanoma cells was performed using Lipofectamine (Invitrogen). Proteins were prepared for β-galactosidase and luciferase analysis 16 h after transfection. Luciferase activity was determined using the luciferase assay system (Promega, Madison, WI) and was normalized based on β-galactosidase levels. Expression vectors encoding IKKβS178E/S181E (activator of NF-κB pathway) (38) and super-repressor HAIκBαΔN (39) were used as indicated.
Treatment and Apoptosis Studies
Cells were exposed to arsenite (1–50 μM) in the medium for 6–48 h. Specific inhibitors of PI3K-AKT LY294002 (50 μM), of MEK-ERK PD98059 (50 μM) or U0126 (5 μM), of JNK SP600125 (10–20 μM), and of MAPK p38 SB203580 (5–10 μM) were used with or without 5–10 μM arsenite. Inhibitors were added to media 30 min before arsenite treatment. Antibodies against TNFα (BD Biosciences), FasL and TRAIL (Alexis, San Diego, CA), were added (1–5 μg/ml) 1 h before arsenite treatment. Apoptosis was assessed by quantifying the percentage of hypodiploid nuclei undergoing DNA fragmentation (40) or by quantifying the percentage of Annexin V-FITC-positive cells (BD Biosciences). Flow-cytometric analysis was performed on a FACSCalibur flow cytometer (BD Biosciences) using the CellQuest program.
Resistance to Treatment
Cells (400/well) were placed in triplicate on 6-well plates 12 h before treatment. For analysis of clonogenic survival of melanoma cells after treatment (16 h) with arsenite alone or in the combinations with the specific inhibitors of signaling pathways, colonies were stained with crystal violet solution 10 days after treatment. The percentage of colony-forming efficiency (in relation to values of untreated control cells) was calculated.
Western Blot Analysis
Cell lysates (50–100 μg of protein) were resolved on 10% SDS-PAGE and processed according to standard protocols. The antibodies used were polyclonal anti-phospho-c-Jun (Ser73), anti-c-Jun, anti-phospho-p44/42 MAPK (Thr202/Tyr204), anti-p44/42 MAPK, anti-phospho-AKT (Ser473), anti-AKT, anti-phospho-p38 MAPK (Thr180/Tyr182), anti-p38 MAPK, anti-poly(ADP-ribose) polymerase (Cell Signaling, Beverly, MA), and monoclonal anti-β-actin (Sigma) (optimal dilutions of Abs were 1:1000–1:10,000). The secondary Abs (anti-rabbit or anti-mouse) were conjugated to horseradish peroxidase (dilution 1:5000–1:10,000). Signals were detected using the ECL system (Amersham Biosciences).
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed for detection of NF-κB and AP-1 binding activity as described previously (41) using labeled double-strand oligonucleotides 5′-AGCTTGGG-GACTTTCCAGCCG-3′ and 5′-AGCTTGATGAGTCAGCCG-3′, respectively (binding sites are underlined).
RESULTS
Role of NF-κB in Arsenite-induced Apoptosis of Human Melanomas
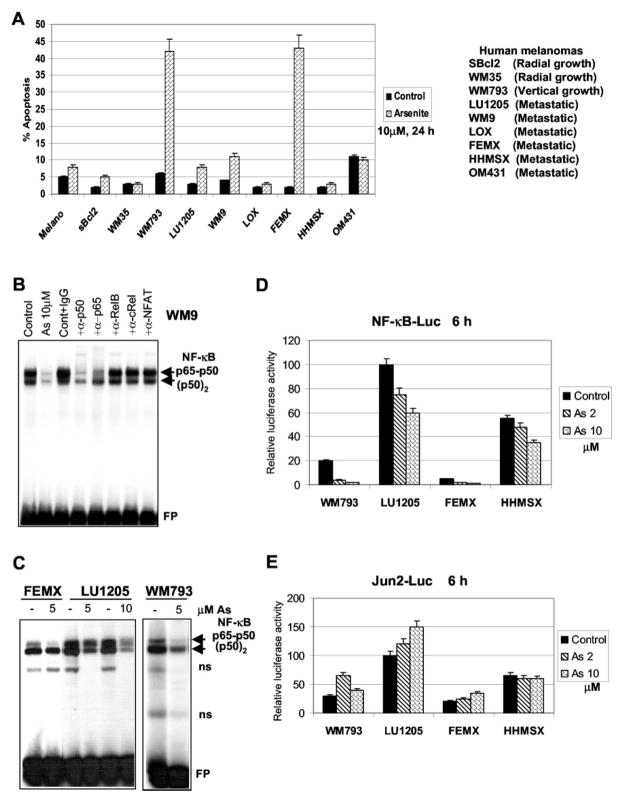
The first step of our study was to find out how arsenite treatment affects apoptosis of human melanomas. We used low to moderate doses of arsenite (2–10 μM) that were successfully applied for treatment of acute promyelocytic leukemia and multiple myelomas through induction of programmed cell death (3–5). It should be noted that high doses of arsenite (100–200 μM) may induce different signaling pathways (42). Cell cycle-apoptosis studies as well as routine morphological examinations revealed that melanocytes and early-phase (radial growth) melanomas WM35 and sBcl2 were resistant to arsenite (5–10 μM)-induced apoptosis (Fig. 1A). We used several additional lines of human melanomas at different phases of cancer development to further elucidate the effects of arsenite on induction of apoptosis. Melanoma cell lines responded differently to arsenite (10 μM) treatment. WM793 and FEMX cells developed apoptosis after 24 h of exposure, whereas other lines were relatively resistant to treatment (Fig. 1A).
Fig. 1. Differential response of human melanomas to arsenite treatment: a correlation with NF-κB activity.
A, apoptotic response of melanocytes (Melano) and indicated melanoma cell lines after 10 μM arsenite treatment. Cell cycle-apoptosis analysis was performed with PI staining and FACS analysis of cells 24 h after arsenite treatment. Results of four independent experiments are presented. B, NF-κB binding activity of nuclear fraction of WM9 melanoma cells. DNA-binding reactions were pretreated with 0.5 μl of indicated antisera to different NF-κB subunits. Basal and arsenite-affected levels (lane 2) (10 μM arsenite, 12 h) of nuclear NF-κB DNA binding activity were determined by EMSA. Two main NF-κB complexes, the upper band p65-p50 and the lower band p50-p50, are indicated. FP, free labeled oligonucleotide probe. C, basal and arsenite-affected levels of nuclear NF-κB DNA binding activity were determined by EMSA in indicated melanoma lines 6 h after treatment with 5 or 10 μM arsenite. Two main NF-κB complexes, the upper band p65-p50 and the lower band p50-p50, are indicated. D and E, melanoma cells were transiently transfected with NF-κB-Luc or Jun2-Luc (0.5 μg) reporter in the presence of β-galactosidase (0.25 μg). 16 h after transfection, cells were not treated or treated with 2 and 10 μM arsenite for 6 h. Normalized Luc activity is indicated. Error bars represent mean ± S.D. from four independent experiments.
A correlation was observed between the relatively low basal levels of NF-κB (p65-p50) DNA binding/transacting activities in WM793 and FEMX cells (Fig. 1, C and D) and the strong apoptotic response following arsenite treatment (Fig. 1A). Transcription factor NF-κB controls expression of many genes with survival and anti-apoptotic functions (43). Moderate to high basal levels of nuclear NF-κB DNA binding activity were found in several melanomas (Fig. 1, B and C). Pretreatment of DNA-binding reactions with specific antibodies to the NF-κB subunits allowed the identification of two major bands, the classical p65-p50 and p50-p50 NF-κB complexes (Fig. 1B). The other NF-κB combinations were present in the nuclear fractions at substantially lower levels. Furthermore, arsenite treatment for 6 h additionally caused a substantial decrease in the nuclear NF-κB DNA binding activity determined by EMSA for WM9, LU1205, FEMX, and WM793 cells (Fig. 1, B and C) and the corresponding decrease in transactivation of the NF-κB-dependent promoter activities (Fig. 1D). In contrast, all of the melanoma cell lines examined have moderate to high basal levels of the AP-1-dependent activity (Jun2-Luc), which could be additionally increased after arsenite treatment (Fig. 1E).
To determine whether NF-κB (p65-p50) activation restores cell resistance to arsenite treatment, we used immortalized normal human TIG-3 fibroblasts with high efficiency of transfection and WM793 melanoma cells. Both cell lines have low basal NF-κB (p65-p50) activity. Transfection of permanently active IKKβS178E/S181E (canonical activator of the NF-κB pathway) (38) in TIG-3 cells results in strong activation of NF-κB (p65-p50) (Fig. 2, A and C). In contrast, transfection and expression of super-repressor IκBαΔN (Fig. 2B) (39) caused suppression of basal NF-κB activity (Fig. 2C). TIG-3 cells transfected with NF-κB activator, IKKβS178E/S181E, possess increased resistance to arsenite treatment compared with the control cells (Fig. 2D), whereas NF-κB inhibitor, IκBαΔN, up-regulated arsenite-induced apoptosis in TIG-3 cells (Fig. 2D). Similarly, levels of arsenite-induced apoptosis were down-regulated in WM793 melanoma cells following transfection of permanently active IKKβS178E/S181E (Fig. 2, E, G, and F), whereas IκBαΔN up-regulated arsenite-induced apoptosis in these cells (Fig. 2, F–H). LU1205 metastatic melanoma with high basal levels of NF-κB activity was relatively resistant to arsenite-induced apoptosis at low or moderate concentrations of arsenite (Figs. 1A and 2F). Additionally, LU1205/IκBαΔN cells, which were stably transfected with super-repressor IκBαΔN (39) and demonstrated permanent down-regulation of nuclear NF-κB activity and sensitivity to TNF-mediated killing (44), were used. These cells possess a remarkably increased apoptotic response 24 h after arsenite treatment from 1 to 33% of apoptotic cells (data not shown) because of an acquired sensitivity to TNF-mediated killing. Hence, as a result of the suppression of basal NF-κB activity, both fibroblasts and melanoma cells can acquire increased sensitivity to arsenite-induced apoptosis, whereas reactivation of NF-κB may reduce levels of arsenite-induced apoptosis.
Fig. 2. Effect of modulation of nuclear NF-κB activities on arsenite-induced apoptosis.
A, nuclear NF-κB DNA binding activities of human TIG-3 cells transfected with the empty vector or IKKβ expression construct. FP, free oligonucleotide probe. B, Western blot analysis of HA-IκBαΔN levels after transfection and expression in TIG-3 cells. Anti-HA Ab was used. C, TIG-3 cells were transiently transfected with NF-κB-Luc reporter construct (0.5 μg) and indicated expression vector (0.5 μg) in the presence of 0.25 μg of pCMV-β-galactosidase. 18 h after transfection, cells were treated with 5 μM arsenite for 6 h. The normalized ratio of luciferase activity to β-galactosidase is shown. D, levels of apoptosis in transfected TIG-3 cells following arsenite treatment were detected with PI staining and FACS analysis. E, nuclear NF-κB DNA binding activities of WM793 melanoma cells transfected with the empty vector or IKKβ expression construct. F, Western blot analysis of HA-IκBαΔN levels after transfection and expression in WM793 cells. Anti-HA Ab was used. G, WM793 cells were transiently transfected with NF-κB-Luc reporter construct (0.5 μg) and indicated expression vector (0.5 μg) in the presence of 0.25 μg of pCMV-β-galactosidase. 18 h after transfection, cells were treated with 5 μM arsenite for 6 h. The normalized ratio of luciferase activity to β-galactosidase is shown. H, levels of apoptosis in transfected WM793 cells following arsenite treatment were detected with PI staining and FACS analysis. Error bars represent mean ± S.D. from four independent experiments.
Arsenite Induces TNFα-mediated Apoptosis in WM793 Early Melanoma Cells
Early melanoma WM793 cells demonstrated notable induction of Jun2-Luc, TNFα-, and TRAIL-promoter activities (but not FasL-promoter activity), which are parallel to the inhibition of NF-κB-dependent DNA binding and promoter activity (Fig. 3A) following arsenite (5 μM) treatment. Up-regulation of endogenous TNFα expression and suppression of NF-κB activity by arsenite created a canonical case for induction of TNFα-mediated apoptosis (12, 45). Indeed, these cells possess dose-dependent sensitivity to arsenite 24 and 48 h after treatment by developing apoptosis (Fig. 3B). Introduction of anti-TNFα-inhibitory Ab (5 μg/ml) but not anti-FasL or anti-TRAIL Abs into cell cultures partially suppressed arsenite-induced apoptosis in WM793 cells (Fig. 3C). Advanced apoptosis of WM793 cells (determined by counting cells with hypodiploid nuclear DNA levels) was also blocked by Z-VAD-fmk (Fig. 3C) that illustrated an involvement of caspases, typical features of this process. Both Ac-IETD-CHO (an inhibitor of caspase-8 and caspase-6) and Ac-LEHD-CHO (an inhibitor of caspase-9) partially suppressed arsenite-induced apoptosis, indicating that both death receptor/caspase-8-mediated apoptotic cascade and mitochondrial caspase-9-dependent pathway operate during arsenite-induced apoptosis in WM793 melanoma cells (Fig. 3C). The combination of two caspase inhibitors had the same negative effect on apoptosis as Ac-IETD-CHO alone, indicating that caspase-8 and caspase-9 function in parallel.
Fig. 3. Arsenite treatment induces TNFα-mediated apoptosis in WM793 human melanoma.
A, WM793 cells were transiently transfected with indicated Luc reporter constructs (0.5 μg) in the presence of 0.25 μg of pCMV-β-galactosidase. 18 h after transfection, cells were treated with 5 or 10 μM arsenite for 6 h. The normalized ratio of luciferase activity to β-galactosidase is shown. B, dose-dependent induction of apoptosis in WM793 cells by arsenite. Apoptosis analysis was performed with PI staining and FACS analysis of cells 24 and 48 h after treatment. C, apoptotic analysis of WM793 cells, which were treated with 5 μM arsenite in the presence of nonspecific IgG, inhibitory anti-TNFα, anti-FasL, or anti-TRAIL Abs (5 μg/ml). FACS analysis was performed with cells stained with PI. A percentage of apoptotic cells 24 h after treatment is indicated. Effects of caspase inhibitors on arsenite-induced apoptosis are as follows: arsenite (5 μM); arsenite (10 μM); Z-VAD-fmk (50 μM); Ac-IETD-CHO (50 μM); and Ac-LEHD-CHO (50 μM). As, arsenite. Error bars represent mean ± S.D. from four independent experiments.
Beside NF-κB pathway, two other cell signaling pathways, Ras-Raf-MEK-ERK and PI3K-AKT, play critical roles in the general regulation of cell survival. Furthermore, MKK3/6-MAPK p38-ATF2 and MKK4/7-JNK-c-Jun signaling pathways may perform either pro-apoptotic or anti-apoptotic functions depending of cell type, nature, and intensity of signaling (12, 46). A characteristic feature of arsenite treatment (5 μM) is up-regulation of ERK1/2 activities in WM793 cells, which was suppressed by MEK inhibitor PD98059 (50 μM) (Fig. 4A). By contrast, no arsenite-induced AKT activation was observed in WM793 cells. Co-treatment of WM793 cells with arsenite and an inhibitor of PI3K-AKT, LY294002 (50 μM), additionally decreased low basal phospho-AKT levels (Fig. 4A). Total MAPK p38 and phospho-p38 levels were up-regulated after arsenite treatment (Fig. 4A). The 5–10 μM SB203580 (p38-ATF2 inhibitor) did not affect p38 phosphorylation but suppressed ATF2 phosphorylation/activation and Jun2-Luc reporter activity (data not shown). Well known activation of MKK7-JNK by arsenite treatment (15, 18) was observed in WM793 cells monitoring phosphorylation of c-Jun, the main target of JNK. An inhibitor of JNK, SP600125, partially suppressed c-Jun phosphorylation (Fig. 4A).
Fig. 4. Inhibition of the main survival pathways up-regulates arsenite-induced apoptosis in WM793 melanoma cells.
A, Western blot analysis of phospho-ERK, phospho-AKT, phospho-p38, and phospho-c-Jun levels following arsenite (5 μM) treatment. B, effects of LY294002 (50 μM) (LY), PD98059 (50 μM) (PD), and SB203580 (10 μM) (SB) on arsenite (5 μM)-induced apoptosis of WM793 cells. Levels of apoptosis were determined by Annexin V-FITC + PI staining and FACS analysis 12 h after treatment. Z-VAD-fmk (50 μM) was used for suppression of apoptosis. C, effects of LY294002 (50 μM), PD98059 (50 μM), U0126 (5 μM), SB203580 (10 μM), and SP600125 (20 μM) (SP) on arsenite (5 μM)-induced apoptosis of WM793 cells. Levels of apoptosis were determined with PI staining and FACS analysis of cells 24 h after treatment. Results are based on four independent experiments. As, arsenite.
We evaluated the early apoptotic commitment of WM793 cells using Annexin V-FITC staining and FACS analysis (Fig. 4B). Notable levels of early apoptosis were detected after 6 h of 5 μM arsenite treatment. The percentage of late apoptotic cells (FITC+ PI+) increased after 12 h of treatment. In the presence of LY294002 or PD98059, development of apoptosis and secondary necrosis was also accelerated. Effects of SP600125 (not shown) and SB203580 on the early stages of arsenite-induced apoptosis were not well pronounced. By contrast, Z-VAD-fmk, a universal caspase inhibitor, effectively suppressed the progress of apoptosis (Fig. 4B). Finally, 24 h after treatment, we observed dramatic accelerating effects of LY294002 or PD98059 on arsenite-induced apoptosis in WM793 cells, whereas SP600125 (inhibitor of JNK) and SB203580 (inhibitor of MAPK p38) were less effective (Fig. 4C). U0126 (5 μM), an additional MEK1/2 inhibitor, was also very effective in the up-regulation of arsenite-induced apoptosis (Fig. 4C). These data convincingly demonstrated a protective role of the basal PI3K-AKT and inducible MEK-ERK activities against the development of arsenite-induced TNFα-mediated apoptosis of early melanoma WM793.
Arsenite Induces TNFα-mediated Apoptosis in FEMX Meta-static Melanoma Cells
The common features of metastatic FEMX cells and early melanoma WM793 cells are low basal NF-κB activity (see Fig. 1C) and endogenous expression of TNFα (44, 47). Arsenite (5 μM) treatment up-regulated AP-1 DNA binding activity, AP-1-dependent (Jun2-Luc) reporter activity, and c-Jun phosphorylation, which was suppressed by 10 μM SP600125 (JNK inhibitor) (Fig. 5, A, B, and D). Up-regulation of TNFpr-Luc activity in FEMX cells after arsenite treatment could be suppressed by 5–10 μM SB203580 (Fig. 5D) via inhibition of MAPK p38-ATF2, a critical regulator of TNFα expression (48). The effect of 10–20 μM SP600125 on the TNFα promoter activity was less pronounced (Fig. 5A). Up-regulation of TNFα expression took place in the context of substantial down-regulation of NF-κB activities by arsenite (see Fig. 1, C and D) that accelerated arsenite-induced apoptosis of FEMX cells. Furthermore, arsenite treatment (3 h) substantially up-regulated ERK activity, slightly increased AKT activity, and had modest positive effects on phospho-p38 levels in FEMX cells (Fig. 5C). Despite arsenite-induced activation of survival pathways, metastatic FEMX cells developed a rapid apoptotic response to arsenite, which was detected with Annexin V-FITC plus PI staining (Fig. 5E) or with cell cycle-apoptosis analysis (Fig. 6A).
Fig. 5. Anti-apoptotic role of PI3K-AKT and MEK-ERK pathways in FEMX melanoma cells.
A, arsenite treatment up-regulates nuclear AP-1 DNA binding activity, which was determined by EMSA, in LU1205 and FEMX cells. FP, free labeled oligonucleotide probe. Cells were non-treated or treated 3 h with 10 μM arsenite in the presence of SP600125 (20 μM) (SP) or PD98059 (50 μM) (PD). Cont, control. B, Western blot analysis of phospho-c-Jun and control c-Jun levels following treatment of LU1205 and FEMX cells with 10 μM arsenite alone or together with SP600125 for 3 h. C, Western blot analysis of phospho-AKT, phospho-ERK, and phospho-p38 levels following arsenite (10 μM) treatment alone or in the presence of indicated inhibitors. D, Jun2-Luc and TNFpr-Luc activity in transiently transfected FEMX cells following treatment with 10 μM arsenite alone or in the presence of PD98002 (50 μM), SB203580 (10 μM) (SB), or SP600125 (10 μM). E, effects of LY294002 (LY) and PD98059 (50 μM) on arsenite (10 μM)-induced apoptosis of FEMX cells. A caspase inhibitor Z-VAD-fmk (50 μM) was used as indicated. Apoptosis analysis was performed by Annexin V-FITC + PI staining and FACS analysis. Results of a typical experiment are presented. As, arsenite.
Fig. 6. Arsenite treatment induces TNFα-mediated apoptosis in FEMX human metastatic melanoma.
A, effects of LY294002 (50 μM) (LY), PD98059 (50 μM) (PD), U0126 (5 μM), and SP600125 (20 μM) (SP) on 10 μM arsenite-induced apoptosis in FEMX cells. FACS analysis was performed with PI-stained melanoma cells. A percentage of apoptotic cells 24 h after treatment is indicated. Cont, control. B, apoptosis analysis of FEMX cells treated with 5 μM arsenite, arsenite together with PD98059 (50 μM), and arsenite together with U0126 (5 μM) in the presence of nonspecific IgG or inhibitory anti-TNFα monoclonal Ab (5 μg/ml). FACS analysis was performed with PI-stained melanoma cells. A percentage of apoptotic cells 24 h after treatment is indicated. C, Western blot analysis of the poly(ADP-ribose) polymerase (PARP) cleavage during arsenite-induced apoptosis 12 or 16 h after treatment. Cells were treated with 10 μM arsenite or arsenite together with PD98059 (50 μM). D, effects of caspase inhibitors Z-VAD-FMK (50 μM), Ac-IETD-CHO (50 μM), and Ac-LEHD (50 μM) on arsenite-induced apoptosis. FACS analysis was performed with PI-stained melanoma cells. A percentage of apoptotic cells 24 h after treatment is indicated. Error bars represent mean ± S.D. from four independent experiment. As, arsenite.
However, the number of apoptotic and secondary necrotic FEMX cells was substantially increased by the combined treatment of arsenite with LY294002 or with PD98059/U0126, which indicates some protective role of PI3K-AKT and MEK-ERK in arsenite-induced cell death (Figs. 5E and 6A). Combined treatment of FEMX cells with arsenite and SP600125 also caused a moderate acceleration of apoptosis, whereas SB203850 slightly suppressed apoptosis (Fig. 6A), probably via suppression of TNFα expression (Fig. 5D). A difference between FEMX and WM793 cells was linked with distinct effects of suppression of MAPK p38 on arsenite-induced apoptosis of these cells: modest positive effects for WM793 cells (see Fig. 4C) and slight negative effects for FEMX cells (Fig. 6A). Inhibitory anti-TNFα monoclonal Ab partially suppressed arsenite-induced as well as (arsenite + PD98059 or arsenite + U0126)-induced apoptosis of FEMX cells (Fig. 6B), demonstrating a role of TNFα-mediated signaling in arsenite-induced apoptosis of human metastatic melanoma cells. Arsenite, either alone or in the combination with PD98059 (Fig. 6C) or with LY294002 (not shown), induced cleavage of poly(ADP-ribose) polymerase, a characteristic feature of caspase-3-mediated apoptosis. A universal inhibitor of caspases Z-VAD-fmk suppressed arsenite-induced apoptosis in FEMX cells. Both Ac-IETD-CHO (an inhibitor of caspase-8) and Ac-LEHD-CHO (an inhibitor of caspase-9) partially suppressed apoptosis in FEMX cells, indicating the role of caspase-8 and caspase-9 in the mediation of arsenite-induced apoptosis (Fig. 6D) as was observed in WM793 early melanoma cells (Fig. 3C). Interestingly, the combination of both caspase inhibitors was more effective for suppression of arsenite-induced apoptosis of FEMX cells than each individual inhibitor. The results obtained demonstrated that arsenite may be a very effective pro-apoptotic agent for some aggressive melanomas, especially when additional inhibition of survival pathways is performed.
Modulation of Survival Pathways in LU1205 Cells
Meta-static melanoma LU1205, which is resistant against arsenite-induced apoptosis, was previously generated from the arsenite-sensitive vertical growth phase melanoma, WM793 (29). There are several important differences between these melanoma lines including basal levels of NF-κB activity that are up-regulated in LU1205 cells (see Fig. 1, C and D) and are involved in the protection against arsenite-induced killing. LU1205 cells are also characterized by modest levels of basal ERK activity because of the presence of braf-activating mutation T1796A (47, 49). LU1205 cells have high basal levels of nuclear AP-1 (Fig. 7A). As expected, 10–20 μM SP600125 (JNK inhibitor) partially suppressed basal and arsenite-induced levels of AP-1 activity and c-Jun phosphorylation (Fig. 7, A and B). Treatment with 10–20 μM SP600125 alone induced dramatic changes in the cell cycle (G2/M arrest) of LU1205 cells (data not shown). When SP600125 was combined with arsenite, they started to increase apoptotic levels 18–48 h after treatment (Fig. 7D).
Fig. 7. The inhibitors of cell signaling pathways alter a susceptibility to arsenite-induced apoptosis in LU1205 melanoma cells.
A, arsenite treatment up-regulates nuclear AP-1 DNA binding activity, which was determined by EMSA, in LU1205 cells. FP, free labeled oligonucleotide probe. Cells were non-treated or treated for 3 h with 10 μM arsenite in the presence of SP600125 (20 μM) or PD98059 (50 μM). B, Western blot analysis of phospho-c-Jun and control c-Jun levels following treatment of LU1205 and FEMX cells with 10 μM arsenite alone or together with SP600125 for 3 h. C, Western blot analysis of phospho-p38, phospho-AKT, and phospho-ERK1/2 levels following arsenite treatment alone or in the presence of indicated inhibitors. D, effects of LY294002 (50 μM) (LY), PD98059 (50 μM) (PD), SP600125 (20 μM) (SP), and U0126 (5 μM) treatment on LU1205 cell cycle and apoptosis induced by 10 μM arsenite 18 and 48 h after treatment. Cell cycle-apoptosis analysis was performed with PI-stained melanoma cells by FACS. E, colony-forming efficiency of LU1205 cells, which had been treated with indicated inhibitors alone or in the presence of 10 μM arsenite for 16 h, was analyzed 10 days later. As, arsenite.
Arsenite treatment did not up-regulate MAPK p38 activity, which was already present at high basal levels in LU1205 cells (Fig. 7C), and SB203580 (p38-ATF2 inhibitor) did not affect arsenite-induced apoptosis (Fig. 7D). In contrast, arsenite notably activated AKT and ERK1/2 in LU1205 cells (Fig. 7C). However, LY2940029 (inhibitor of PI3K-AKT) or PD98059 (MEK inhibitor) separately were not effective for the rapid up-regulation of arsenite-induced apoptosis in LU1205 cells. The simultaneous suppression of two survival pathways, PI3K-AKT and MEK-ERK, by the combination of 50 μM LY294002 and 50 μM PD98059 induced TRAIL-dependent apoptosis in LU1205 cells 48 h after treatment (47). Arsenite co-treatment substantially accelerated LY294002+PD98059-induced apoptosis that was easily detectable by cell cycle-apoptosis analysis 18–48 h after treatment (Fig. 7D). U0126 (5 μM), an additional MEK1/2 inhibitor, was also very effective in blocking arsenite-induced ERK activation (Fig. 7C) and, in the combination with LY294002, in up-regulation of arsenite-induced apoptosis (Fig. 7D). We also assessed clonogenic survival of LU1205 cells following treatment with indicated inhibitors with or without arsenite. As expected, LY294002+PD98059 or LY294002+U0126 combined with arsenite substantially decreased cell survival (Fig. 7E). These data demonstrated that combinations of specific inhibitors of the cell signaling pathways with arsenite may dramatically increase susceptibility of resistant metastatic melanoma cells to apoptosis. The obvious difference between early phase WM793 and metastatic LU1205 cells in the induction of PI3K-AKT pathway appears to be connected with the activity of PTEN, an endogenous inhibitor of this pathway (50). PTEN expression is impaired in LU1205, allowing the complete induction of the PI3K-AKT pathway in these metastatic cells (47).
Suppression of Survival Pathways and Up-regulation of Arsenite-induced Apoptosis in Resistant Melanomas
We used the specific inhibitors of survival pathways to overcome a resistance or accelerate arsenite-induced apoptosis in several additional resistant melanomas. Arsenite treatment in combination with either 50 μM LY294002 or 50 μM PD98059 dramatically increased the apoptotic response of WM35 cells (Fig. 8A). Such treatment, however, had relatively mild effects on melanocytes (data not shown). MEK1/2 inhibitor, U0126 (5 μM), affected WM35 cells similar to PD98059 (data not shown). 10–20 μM SP600125 (inhibitor of JNK) was equally effective for a modest up-regulation of arsenite-induced apoptosis in both melanocytes and WM35 cells (data not shown). Together, these data indicate a protective role of PI3K-AKT and MEK-ERK and, to some extent, a protective of JNK pathways against arsenite-induced programmed death of WM35 melanoma cells. We used two metastatic melanomas (WM9 and HHMSX) that were highly resistant to arsenite (10 μM) treatment (Fig. 8A). These melanoma lines have moderate to high basal NF-κB activity (see Fig. 1B for WM9 cells) and active AKT (51). WM9 cells required suppression of at least one of the survival pathways (by LY294002 or by PD98059) to induce apoptosis following 10 μM arsenite treatment (Fig. 8A) as was previously observed for WM35 cells. HHMSX melanoma cells could be sensitized to 10 μM arsenite-induced apoptosis only after the simultaneous blocking of two survival pathways by LY294002 + PD98059 treatment (Fig. 8A) that was similar to LU1205 cells.
Fig. 8. Anti-apoptotic role of PI3K-AKT and MEK-ERK pathways in human and mouse melanomas.
A, inhibition of PI3K-AKT pathway by LY294002 (50 μM) (LY), MEK-ERK by PD98059 (50 μM) (PD), or simultaneous inhibition of both pathways by LY + PD sensitizes indicated melanoma cells to arsenite-induced apoptosis. Cell cycle-apoptosis analysis was performed 24 h after treatment. B, Western blot analysis of SW1 melanoma cell lines stably transfected with empty vector (pBabe-puro) or expression vector encoding AKTmyr. C, regulation of arsenite-induced apoptosis by active AKTmyr in transfected SW1 and FEMX cells. As, arsenite.
SW1 mouse metastatic melanoma, on the other hand, was sensitive to arsenite treatment. We previously established the control cell line, SW1-puro, stably transfected with an empty vector pBabe-puro and SW1-AKTmyr cells stably transfected with a vector encoding permanently active AKTmyr (51). The SW1-AKTmyr cells had increased levels of both total AKT and phospho-AKT (Fig. 8B). Apoptosis analysis revealed a dramatic decrease of arsenite-induced apoptosis in these cells (Fig. 8C) as well as an increased resistance of these cells to several types of apoptotic stimulation (data not shown). We also used previously established FEMX-AKTmyr cells for these studies (51). Despite high levels of AKT activity, these cells demonstrated only a partial suppression of arsenite-induced apoptosis (Fig. 8C), probably because of low levels of NF-κB activity. Taken together, the data obtained on the induction of apoptosis by arsenite treatment of melanomas demonstrate the importance of the low basal NF-κB activity and an additional suppression of PI3K-AKT and MEK-ERK survival pathways for the development of apoptosis in human melanomas.
DISCUSSION
Melanoma is the most aggressive form of skin cancer and is often resistant to most forms of treatment (23). Metastatic melanomas possess multiple genetic and epigenetic control mechanisms to block the apoptotic potential of chemotherapeutic drug treatment or γ-irradiation. An interesting example of such regulations is the silencing of the Fas death receptor expression in malignant melanomas (52–54). There is a profound necessity to develop alternative approaches in the treatment of this often fatal disease including effective induction/acceleration of programmed cell death despite suppression of Fas-mediating death signaling. In this regard, arsenic compounds have been successfully used as inducers of stress and apoptosis for treatment of several forms of leukemia and some solid tumors (4). It seems that IKK-NF-κB suppression is one of the main targets of arsenite treatment (9, 10, 14).
Metastatic melanomas produce many cytokines and growth factors (including TNFα, transforming growth factor-β, and TRAIL) to support their autonomous growth and to suppress the immune system (55). The unique features of low dose arsenite treatment, the down-regulation of NF-κB activity, and activation of Jun/ATF2-TNFα expression, thereby inducing TNFα-mediated apoptosis in some metastatic melanomas, have been demonstrated in this study. Stress-induced expression of TNFα was previously observed in mice treated with arsenite (56). TNFα serves as “non-professional” death ligand, which can induce death pathways in the specific conditions of very low NF-κB activity (57). Arsenite treatment of melanomas (as was observed in this study) can create such permissive conditions. Besides the NF-κB pathway, two other signaling pathways, Ras-Raf-MEK-ERK and PI3K-AKT, are critically involved in the regulation of cell survival. On the other hand, MKK6-MAPK p38-ATF2 and MKK7-JNK-c-Jun signaling pathways may play either a pro-apoptotic or an anti-apoptotic role depending of cell type, nature, and intensity of signaling (12, 46). In this study, we demonstrated that simultaneous treatment of resistant melanomas by arsenite and specific inhibitor(s) of survival signaling pathways resulted in the dramatic increase of the apoptotic response, providing a rationale for the future therapy.
Moderate and high dose arsenite treatments target mitochondria and also could be used for induction of cancer cell death (58, 59), although in this case damaging effects have been also detected in normal cells.2 Many melanomas are known to produce TRAIL (60) and do not respond to exogenously applied TRAIL, thereby abrogating the hope of the possibility of exploiting the apoptotic effects of TRAIL for this type of cancer. However, a parallel suppression of the expression of anti-apoptotic proteins like cFLIP by cycloheximide or by the specific RNA interference may dramatically improve this situation (61, 62). We previously observed the development of TRAIL-mediated apoptosis in LU1205 melanoma cells following suppression of both PI3K-AKT and MEK-ERKs by a mixture of LY294002 and PD98059 inhibitors (47). Further treatment of melanoma cells with arsenite, which suppresses NF-κB activity and NF-κB-dependent gene transcription including the FLIP gene (63, 64), accelerated the induction of apoptosis.
In contrast to its negative effects on NF-κB, arsenite treatment up-regulates MAPKs and AP-1 activity (18). One of the important consequences of an increase in AP-1/ATF2-dependent transcription is the induction of heme oxygenase-1 (HO-1). HO-1 is a universal hallmark of oxidative stress and a protein with strong anti-apoptotic potential that partially neutralizes pro-apoptotic functions of arsenite (65–69). Regulation of HO-1 expression is very complex and based on activities of several transcription factors in addition to the AP-1 proteins. In general, there are two sides of AP-1-dependent gene expression: (i) anti-apoptotic and positive regulation of cell proliferation (46) and (ii) pro-apoptotic regulation through the control of expression of proteins involved in the generation of death signaling like FasL, TNFα, or DR5 (36, 70, 71). A delicate balance that emerges after arsenite treatment is an increase in the anti-apoptotic, rather than pro-apoptotic AP-1-dependent gene expression that results in a significant increase of arsenite-induced apoptosis of c-jun−/− fibroblasts.2 Furthermore, a dramatic inhibition of HO-1 expression in melanomas by LY294002 connects the PI3K-AKT survival pathway with HO-1 induction.2 The PI3K-AKT signaling pathway regulates many normal cell functions, such as proliferation, survival, and growth. Hyperactivation of this pathway has been observed in a wide range of tumors (72) including melanomas (27, 47). The role of the PI3K-AKT pathway in carcinogenesis has been intensively investigated with special attention to the silencing of PTEN, an internal inhibitor of PI3K signaling (73). It should be mentioned that both PI3K-AKT (74) and MEK-ERK (75) pathways may be linked with the activation of the NF-κB pathway, although the precise input of these collateral pathways in the regulation of NF-κB activation in melanomas should be re-evaluated. The new interesting connection is the negative control of PTEN expression by NF-κB that allows the full activation of AKT in cells with high NF-κB activity (76). Cancer progression appears to have at least two possible mechanisms of suppression of PTEN functions, mutations or silencing via the negative control by NF-κB.
The other critical targets of survival signaling pathways are proteins of the STAT family of transcription factors (20). Suppression of STAT3 activation by arsenite may change numerous functions in cells including inhibition of the IL-6 gene expression (4) that induces cell cycle arrest and subsequent cell death. IL-6 suppression induced by arsenite in melanomas was not the primary subject of this study; however, its successful application for multiple myeloma treatment (21) provides a strong impetus for a similar approach in the sensitization of some IL-6-dependent melanomas. As an alternative approach, which operates through an IL-6-independent mechanism, the combined treatment of resistant myelomas has been recently described (77, 78). The checkpoint abrogator UCN-01 was very effective in combination with MEK1/2 inhibitors (77) or with the inhibitors of the NF-κB pathway (78) for induction of apoptosis. This approach is similar to our strategy for up-regulation of melanoma apoptosis by the simultaneous treatment of cancer cells with arsenite (as an inhibitor of the NF-κB activity) and a specific inhibitor of the MEK-ERK or the PI3K-AKT pathway.
Acknowledgments
We thank Drs. M. Herlin, O. Fodstad, R. Halaban, L. Owen-Schaub, and Z. Ronai for the cell lines and Dr. V. Adler, Dr. A. Chan, Dr. S. Fuchs, M. Hall, and S. Baker for critical reading of the paper.
Footnotes
This work was supported by National Institutes of Health Grant ES 11804, Superfund Grant P42 ES 10349, and Environmental Center Grant P30 ES 09089.
The abbreviations used are: IKK, inhibitor nuclear factor κB kinase; AP-1, activator protein-1; ATF2, activating transcription factor 2; EMSA, electrophoretic mobility shift assay; ERK, extracellular signal-regulated kinase; HO-1, heme oxygenase-1; FITC, fluorescein isothiocyanate; JNK, Jun N-terminal kinase; FACS, fluorescence-activated cell sorter; NF-κB, nuclear factor κB; IκB, inhibitor of NF-κB; HA, hemagglutinin; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PI, propidium iodide; TNFα, tumor necrosis factor α; TRAIL, TNF-related apoptosis-inducing ligand; STAT, signal transducers and activators of transcription; Luc, luciferase; Ab, antibody; Z, benzyloxycarbonyl; fmk, fluoromethyl ketone; IL, interleukin; MEK, MAPK/ERK kinase; MKK, MAPK kinase; Ac-IETD-CHO, N-acetyl-Ile-Glu-Thr-Asp-CHO (aldehyde); Ac-LEHD-CHO, N-acetyl-Leu-Glu-His-Asp-CHO (aldehyde); pr, promoter; PTEN, phosphatase and tensin homolog deleted from chromosome 10.
V. N. Ivanov and T. K. Hei, unpublished observations.
References
- 1.Chen CJ, Chen CW, Wu MM, Kuo TL. Br J Cancer. 1992;66:888–892. doi: 10.1038/bjc.1992.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu SX, Athar M, Lippai I, Waldren C, Hei TK. Proc Natl Acad Sci U S A. 2001;98:1643–1648. doi: 10.1073/pnas.031482998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 4.Hayashi T, Hideshima T, Anderson KC. Br J Haematol. 2003;120:10–17. doi: 10.1046/j.1365-2141.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao WL, Chen SJ, Shen Y, Xu L, Cai X, Chen GQ, Shen ZX, Chen Z, Wang ZY. Leuk Lymphoma. 2001;42:1265–1273. doi: 10.3109/10428190109097751. [DOI] [PubMed] [Google Scholar]
- 6.Snow ET. Pharmacol Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- 7.Hei TK, Liu SX, Waldren C. Proc Natl Acad Sci U S A. 1998;95:8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Cai JF, Chiu JF. J Cell Biochem. 2002;87:29–38. doi: 10.1002/jcb.10269. [DOI] [PubMed] [Google Scholar]
- 9.Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. J Biol Chem. 2000;275:36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 10.Roussel RR, Barchowsky A. Arch Biochem Biophys. 2000;377:204–212. doi: 10.1006/abbi.2000.1770. [DOI] [PubMed] [Google Scholar]
- 11.Hershko DD, Robb BW, Hungness ES, Luo G, Hasselgren PO. J Cell Biochem. 2002;84:687–698. doi: 10.1002/jcb.10083. [DOI] [PubMed] [Google Scholar]
- 12.Karin M, Lin A. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 13.Amit S, Ben-Neriah Y. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 14.Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C, Bommert K, Dorken B. Blood. 2003;102:1028–1034. doi: 10.1182/blood-2002-04-1154. [DOI] [PubMed] [Google Scholar]
- 15.Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Martindale JL, Holbrook NJ, Liu Y. Mol Cell Biol. 1998;18:5178–5188. doi: 10.1128/mcb.18.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig S, Hoffmeyer A, Goebeler M, Kilian K, Hafner H, Neufeld B, Han J, Rapp UR. J Biol Chem. 1998;273:1917–1922. doi: 10.1074/jbc.273.4.1917. [DOI] [PubMed] [Google Scholar]
- 18.Bode AM, Dong Z. Crit Rev Oncol Hematol. 2002;42:5–24. doi: 10.1016/s1040-8428(01)00215-3. [DOI] [PubMed] [Google Scholar]
- 19.Levy DE, Darnell JE., Jr Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 20.Darnell JE., Jr Nat Rev Cancer. 2002;2:740–749. doi: 10.1038/nrc906. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi T, Hideshima T, Akiyama M, Richardson P, Schlossman RL, Chauhan D, Munshi NC, Waxman S, Anderson KC. Mol Cancer Ther. 2002;1:851–860. [PubMed] [Google Scholar]
- 22.Chin L. Nat Rev Cancer. 2003;3:559–570. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- 23.Soengas MS, Lowe SW. Oncogene. 2003;22:3138–3151. doi: 10.1038/sj.onc.1206454. [DOI] [PubMed] [Google Scholar]
- 24.Ivanov VN, Bhoumik A, Ronai Z. Oncogene. 2003;22:3152–3161. doi: 10.1038/sj.onc.1206456. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal BB. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 26.Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M. Melanoma Res. 1997;7(Suppl 2):S35–S42. [PubMed] [Google Scholar]
- 27.Li G, Kalabis J, Xu X, Meier F, Oka M, Bogenrieder T, Herlyn M. Oncogene. 2003;22:6891–6899. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Satyamoorthy K, Herlyn M. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- 29.Berking C, Takemoto R, Schaider H, Showe L, Satyamoorthy K, Robbins P, Herlyn M. Cancer Res. 2001;61:8306–8316. [PubMed] [Google Scholar]
- 30.Hill LL, Shreedhar VK, Kripke ML, Owen-Schaub LB. J Exp Med. 1999;189:1285–1294. doi: 10.1084/jem.189.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Myklebust AT, Helseth A, Breistol K, Hall WA, Fodstad O. J Neurooncol. 1994;21:215–224. doi: 10.1007/BF01063770. [DOI] [PubMed] [Google Scholar]
- 32.Wen Z, Zhong Z, Darnell JE., Jr Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 33.van Dam H, Huguier S, Kooistra K, Baguet J, Vial E, van der Eb AJ, Herrlich P, Angel P, Castellazzi M. Genes Dev. 1998;12:1227–1239. doi: 10.1101/gad.12.8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan H, Bartos DP, Owen-Schaub LB. Mol Cell Biol. 1999;19:2098–2108. doi: 10.1128/mcb.19.3.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtz-Heppelmann CJ, Algeciras A, Badley AD, Paya CV. J Biol Chem. 1998;273:4416–4423. doi: 10.1074/jbc.273.8.4416. [DOI] [PubMed] [Google Scholar]
- 36.Rhoades KL, Golub SH, Economou JS. J Biol Chem. 1992;267:22102–22107. [PubMed] [Google Scholar]
- 37.Baetu TM, Kwon H, Sharma S, Grandvaux N, Hiscott J. J Immunol. 2001;167:3164–3173. doi: 10.4049/jimmunol.167.6.3164. [DOI] [PubMed] [Google Scholar]
- 38.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 39.Brockman JA, Scherer DC, McKinsey TA, Hall SM, Qi X, Lee WY, Ballard DW. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 41.Ivanov V, Fleming TJ, Malek TR. J Immunol. 1994;153:2394–2406. [PubMed] [Google Scholar]
- 42.Huang C, Ma WY, Li J, Dong Z. Cancer Res. 1999;59:3053–3058. [PubMed] [Google Scholar]
- 43.Karin M, Cao Y, Greten FR, Li ZW. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 44.Ivanov VN, Fodstad O, Ronai Z. Oncogene. 2001;20:2243–2253. doi: 10.1038/sj.onc.1204314. [DOI] [PubMed] [Google Scholar]
- 45.Beg AA, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 46.Shaulian E, Karin M. Nat Cell Biol. 2002;4:E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 47.Krasilnikov M, Ivanov VN, Dong J, Ronai Z. Oncogene. 2003;22:4092–4101. doi: 10.1038/sj.onc.1206598. [DOI] [PubMed] [Google Scholar]
- 48.Tsai EY, Yie J, Thanos D, Goldfeld AE. Mol Cell Biol. 1996;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuveson DA, Weber BL, Herlyn M. Cancer Cell. 2003;4:95–98. doi: 10.1016/s1535-6108(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Goel V, Haluska FG. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 51.Ivanov VN, Krasilnikov M, Ronai Z. J Biol Chem. 2002;277:4932–4944. doi: 10.1074/jbc.M108233200. [DOI] [PubMed] [Google Scholar]
- 52.Bullani RR, Wehrli P, Viard-Leveugle I, Rimoldi D, Cerottini JC, Saurat JH, Tschopp J, French LE. Melanoma Res. 2002;12:263–270. doi: 10.1097/00008390-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Ivanov VN, Bhoumik A, Krasilnikov M, Raz R, Owen-Schaub LB, Levy D, Horvath CM, Ronai Z. Mol Cell. 2001;7:517–528. doi: 10.1016/s1097-2765(01)00199-x. [DOI] [PubMed] [Google Scholar]
- 54.Ivanov VN, Lopez Bergami P, Maulit G, Sato TA, Sassoon D, Ronai Z. Mol Cell Biol. 2003;23:3623–3635. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussein MR, Haemel AK, Wood GS. J Pathol. 2003;199:275–288. doi: 10.1002/path.1300. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Kadiiska MB, Liu Y, Lu T, Qu W, Waalkes MP. Toxicol Sci. 2001;61:314–320. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- 57.Varfolomeev EE, Ashkenazi A. Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 58.Costantini P, Jacotot E, Decaudin D, Kroemer G. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 59.Larochette N, Decaudin D, Jacotot E, Brenner C, Marzo I, Susin SA, Zamzami N, Xie Z, Reed J, Kroemer G. Exp Cell Res. 1999;249:413–421. doi: 10.1006/excr.1999.4519. [DOI] [PubMed] [Google Scholar]
- 60.Hersey P, Zhang XD. Nat Rev Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- 61.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 62.Siegmund D, Hadwiger P, Pfizenmaier K, Vornlocher HP, Wajant H. Mol Med. 2002;8:725–732. [PMC free article] [PubMed] [Google Scholar]
- 63.Kreuz S, Siegmund D, Scheurich P, Wajant H. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caltabiano MM, Koestler TP, Poste G, Greig RG. J Biol Chem. 1986;261:13381–13386. [PubMed] [Google Scholar]
- 66.Deramaudt BM, Remy P, Abraham NG. J Cell Biochem. 1999;72:311–321. [PubMed] [Google Scholar]
- 67.Lee PJ, Camhi SL, Chin BY, Alam J, Choi AM. Am J Physiol. 2000;279:L175–L182. doi: 10.1152/ajplung.2000.279.1.L175. [DOI] [PubMed] [Google Scholar]
- 68.Kietzmann T, Samoylenko A, Immenschuh S. J Biol Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 69.Yih LH, Peck K, Lee TC. Carcinogenesis. 2002;23:867–876. doi: 10.1093/carcin/23.5.867. [DOI] [PubMed] [Google Scholar]
- 70.Kolbus A, Herr I, Schreiber M, Debatin KM, Wagner EF, Angel P. Mol Cell Biol. 2000;20:575–582. doi: 10.1128/mcb.20.2.575-582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guan B, Yue P, Lotan R, Sun SY. Oncogene. 2002;21:3121–3129. doi: 10.1038/sj.onc.1205430. [DOI] [PubMed] [Google Scholar]
- 72.Vivanco I, Sawyers CL. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 73.Luo J, Manning BD, Cantley LC. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 74.Romashkova JA, Makarov SS. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 75.Dhawan P, Richmond A. J Biol Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim S, Domon-Dell C, Kang J, Chung DH, Freund JN, Evers BM. J Biol Chem. 2004;279:4285–4291. doi: 10.1074/jbc.M308383200. [DOI] [PubMed] [Google Scholar]
- 77.Dai Y, Landowski TH, Rosen ST, Dent P, Grant S. Blood. 2002;100:3333–3343. doi: 10.1182/blood-2002-03-0940. [DOI] [PubMed] [Google Scholar]
- 78.Dai Y, Pei XY, Rahmani M, Conrad DH, Dent P, Grant S. Blood. 2004;103:2761–2770. doi: 10.1182/blood-2003-09-3037. [DOI] [PubMed] [Google Scholar]