Abstract
Lipopolysaccharide (LPS), also known as endotoxin due to its severe pathophysiological effects in infected subjects, is an essential component of the outer membrane (OM) of most Gram-negative bacteria. LPS is synthesized in the bacterial inner membrane, a process that is now well understood. In contrast, the mechanism of its transport to the outer leaflet of the OM has remained enigmatic. We demonstrate here that the OM protein, known as increased membrane permeability (Imp) or organic solvent tolerance protein, is involved in this process. An Imp-deficient mutant of Neisseria meningitidis was viable and produced severely reduced amounts of LPS. The limited amount of LPS that was still produced was not accessible to LPS-modifying enzymes expressed in the OM or added to the extracellular medium. We conclude therefore that Imp mediates the transport of LPS to the cell surface. The role of Imp in LPS biogenesis and its high conservation among Gram-negative bacteria make it an excellent target for the development of novel antibacterial compounds.
Gram-negative bacteria are enclosed by a cell envelope consisting of an inner membrane (IM) and an outer membrane (OM), separated by the periplasm. The IM is a phospho-lipid bilayer, whereas the OM is an asymmetrical bilayer, containing phospholipids in the inner leaflet and lipopolysaccharide (LPS) in the outer leaflet. LPS consists of a hydrophobic membrane anchor, lipid A, substituted with a nonrepeating oligosaccharide, the core region. In many bacteria, the core region is extended with the O antigen, a repeating oligosaccharide. The lipid A-core region and the O antigen are synthesized as separate units at the cytoplasmic leaflet of the IM. Almost all of the enzymes involved in their biosynthesis have been identified in Escherichia coli (1, 2). The transport of the lipid A-core moiety to the periplasmic side of the IM is mediated by the MsbA protein, an ATP-binding cassette family transporter (3), whereas flipping of O antigen units over the IM can be facilitated by several distinct mechanisms (1). At the periplasmic side of the IM, the O antigen is ligated to the lipid A-core region. The next step, transport of the fully assembled LPS through the periplasm and across the OM, remains an entirely elusive aspect of LPS biogenesis (1, 2). Recently, Omp85, an essential OMP, was suggested to be involved in this process (4). However, we found a severe OMP assembly defect in a Neisserial Omp85 mutant (5). This phenotype, together with the affinity of Omp85 for OMPs (5) and the presence of omp85 homologs in Gram-negative bacteria lacking LPS, are much more consistent with a role of this protein in OMP assembly, with only an indirect role in LPS transport. Braun and Silhavy (6) identified another essential OMP in E. coli, depletion of which resulted in the formation of aberrant membranes. Missense mutations in the gene encoding this 87-kDa OMP, called increased membrane permeability (Imp) or organic solvent tolerance protein, were already known to affect OM permeability (7, 8). Hence, we considered the possibility that this protein might be the missing link in LPS biogenesis.
LPS is an essential OM component in E. coli, but not in Neisseria meningitidis, which can exist without LPS as demonstrated by the viability of lpxA mutants, in which the gene involved in the first step in LPS biosynthesis is disrupted (9, 10). These lpxA mutants are completely devoid of LPS. Therefore, N. meningitidis is a very suitable organism to identify components of LPS biogenesis. Imp homologs were identified in the sequenced genomes of N. meningitidis strains MC58 (11) and Z2491 (12), and designated NMB0280 and NMA2207, respectively. In the present study, we investigated LPS synthesis and localization in a Neisserial imp mutant.
Experimental Procedures
Bacterial Strains and Growth Conditions. N. meningitidis H44/76, a serotype B strain, came from our laboratory collection. The H44/76 lpxA mutant (9) and the H44/76-derived strain HA3003, expressing lpxA from the tac promoter (13), were generously provided by L. Steeghs and P. van der Ley (Netherlands Vaccine Institute, Bilthoven, The Netherlands). N. meningitidis was grown on GC agar (Oxoid, Basingstoke, U.K.) plates containing Vitox (Oxoid) and antibiotics when appropriate (kanamycin, 100 μg/ml; chloramphenicol, 5 μg/ml) in candle jars at 37°C. Cultures were grown in tryptic soy broth in plastic flasks at 37°C with shaking. To obtain fully sialylated LPS, 80 μM cytidine 5′ monophospho-N-acetyl neuraminic acid (CMP-NANA; Sigma) was added for 2 h to the medium of bacteria growing in the mid-log phase. To induce expression of pagL, pEN11-pagL-containing strains were grown for 16 h on GC plates containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). E. coli strains DH5α and TOP10F′ (Invitrogen) were used for routine cloning. E. coli was propagated on LB plates. Antibiotics were added in the following concentrations: kanamycin, 50 μg/ml; chloramphenicol, 25 μg/ml; and erythromycin, 200 μg/ml.
Construction of Plasmids and Mutants. Details of constructions are provided in full in Supporting Materials and Methods and Table 1, which are published as supporting information on the PNAS web site. In brief, we used the sequence of NMB0279 and NMB0280 from N. meningitidis strain MC58 (http://www.tigr.org) to design primers to clone and subsequently inactivate the imp gene in strain H44/76. For complementation experiments, the imp gene from H44/76 was amplified by PCR and cloned into a Neisseria-replicative plasmid, behind a tandem lac promoter-operator (tac-lacUV5) sequence (5). The imp mutant was transformed with the resulting plasmid, designated pEN11-Imp, by coincubation of bacteria with plasmid for 6 h on a plate (5). Transformants were selected on plates containing chloramphenicol and the presence of pEN11-Imp was verified by PCR. Plasmid pEN11-pagL was obtained by subcloning the pagL gene of Bordetella bronchiseptica from plasmid pPagL(Bb) (J.G., L. Steeghs, J. ten Hove, A. de Jong, P. vander Ley, and J.T., unpublished work) into a similar Neisserial replicative plasmid. This plasmid was used to transform H44/76 L8 immunotype. Correct transformants were identified by PCR and the imp gene was subsequently inactivated.
Gel Electrophoresis and Immunoblotting. SDS/PAGE under denaturing or seminative conditions and immunoblotting were performed as described (5). For denaturing conditions, samples were boiled in SDS/PAGE sample buffer containing 2% SDS and 2.5% 2-mercaptoethanol before electrophoresis, whereas for seminative conditions, the sample buffer contained only 0.1% SDS and no 2-mercaptoethanol, and the samples were not heated before electrophoresis. For LPS evaluation, samples were boiled in denaturing SDS/PAGE sample buffer and subsequently incubated with 0.5 mg/ml proteinase K at 55°C for 1 h. After boiling for 10 min, samples were analyzed on N-tris(hydroxymethyl)methylglycine (Tricine)-SDS/PAGE (14), followed by silver staining (15).
Neuraminidase Treatment. Bacteria from 1 ml of a mid-log phase culture were pelleted and washed with buffer A (20 mM Na2HPO4/NaH2PO4/150 mM NaCl/5 mM MgCl2/5 mM CaCl2, pH 6.0). Bacteria were resuspended in 0.5 ml of buffer A and 0.2 units/ml neuraminidase (type V, Clostridium perfringens, Sigma N-2876) was added for 60 min at 37°C. Next, bacteria were pelleted and processed for Tricine-SDS/PAGE. Cell envelopes were similarly treated with 0.2 units/ml neuraminidase in buffer A.
Isolation of Cellular Fractions. Cell envelopes were prepared as described (5). IMs and OMs were separated by isopycnic sucrose-gradient centrifugation according to Masson and Holbein (16) or, alternatively, according to the procedure of Shell et al. (17). Cell-free culture supernatant was obtained by centrifugation, first for 15 min at 6,000 × g and subsequently for 2 h at 100,000 × g. Proteins and LPS were precipitated from the supernatants with 7% trichloroacetic acid. The precipitates were collected by centrifugation at 20,000 × g for 30 min and washed with acetone.
LPS Isolation and Quantification. LPS was isolated from H44/76 L3 immunotype by the hot-phenol extraction method described by Westphal and Jann (18). The LPS content of cell envelopes was determined by 3-deoxy-d-manno-octulosonic acid measurement as described (19). Data shown represent the means ± SD of two measurements.
PagL Assay. Cell envelopes were prepared from bacteria grown for 16 h on plates. Similar amounts of cell envelopes, as inferred from similar total protein content, were incubated in 50 mM Hepes, pH 8.0/0.1% Triton X-100/0.5 M NaCl/0.75 nmol L3-LPS in a volume of 10 μl at 37°C for 18 h (20). Assays were terminated by boiling in SDS/PAGE sample buffer. Protein content of cell envelopes was measured by using the Pierce BCA protein assay after boiling the samples in 0.1% SDS.
Fluorescence Microscopy. Bacteria were stained with the live/dead kit (Molecular Probes) according to the manufacturers protocol and were observed by using a Zeiss Axioskop 2 microscope. Data shown represent the means ± SD of three independent experiments.
Antibodies. The imp gene of H44/76 without its signal sequence encoding part was expressed in E. coli strain BL21(DE3) (Novagen). Details of the construction are provided in full in Supporting Materials and Methods. The Imp protein, accumulating in inclusion bodies, was isolated (21) and used for immunization of rabbits at Eurogentec. Mouse monoclonal anti-FbpA and anti-PorA (MN23G2.38) antibodies were gifts from B. Kuipers (Netherlands Vaccine Institute).
Identification of Imp Homologs. The sequence of the N. meningitidis MC58 imp gene NMB0280 (www.tigr.org) was used as a query to search microbial genomes for Imp homologs by using blast.
Results
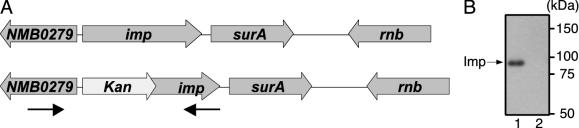
Imp Is Not Essential in N. meningitidis. A Neisserial imp mutant was constructed by allelic replacement of the imp gene in strain H44/76 with a copy containing a deletion-insertion mutation (Fig. 1A). Kanamycin-resistant transformants were tested by PCR for the absence of an intact copy of the imp gene and the presence of the imp::kan allele. Correct transformants were readily obtained, demonstrating that in N. meningitidis, in contrast with E. coli (6), imp is not an essential gene. The absence of the Imp protein in the mutants was confirmed by immunoblotting (Fig. 1B).
Fig. 1.
Construction of an imp mutant strain. (A) Genomic organization of the imp locus in the wild-type and imp mutant. NMB0279 is annotated as a conserved hypothetical protein in the MC58 database (www.tigr.org). The survival protein A (surA) gene encodes a periplasmic chaperone involved in OMP biogenesis. rnb, ribonuclease II. Arrows delineate the DNA fragment used for transformation to obtain the mutant. (B) Immunoblot of cell envelopes of wild-type (lane 1) and imp mutant (lane 2) separated by SDS/PAGE and probed with anti-Imp antibodies. Molecular size markers are indicated in kDa.
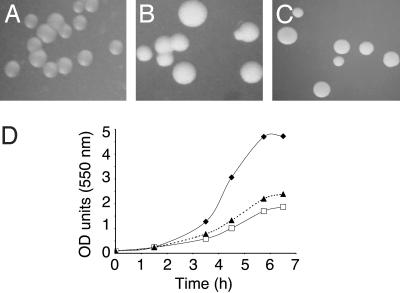
Phenotype of a Neisserial imp Mutant. A striking feature of the imp mutant was its intense colony opacity compared to wild-type colonies (Fig. 2 A and B), a property also apparent for the LPS-deficient lpxA mutant (Fig. 2C). Furthermore, similar to the LPS-deficient strain (13), the imp mutant bacteria grew slower than wild-type bacteria (Fig. 2D). Exponentially growing bacteria were stained with a combination of DNA-binding dyes, SYTO green which enters all cells, and propidium iodide, a red stain, which only enters cells with compromised membranes. The percentage of bacteria stained red was 8 ± 5% for the imp mutant and 0.1 ± 0.02% for the wild-type bacteria, indicating that an increased proportion of the mutant bacteria possessed a compromised membrane, although the vast majority appeared still intact. Thus, the slower growth rate could be due to faster dying of the imp mutant bacteria.
Fig. 2.
Characteristics of an N. meningitidis imp mutant. (A–C) Colony morphology of wild-type (A), imp mutant (B), and lpxA mutant (C) bacteria. Colonies were observed with a binocular microscope by using the shiny side of a flexible mirror. (D) Growth curve of wild-type (♦), imp mutant (□), and lpxA mutant (▴) bacteria in tryptic soy broth.
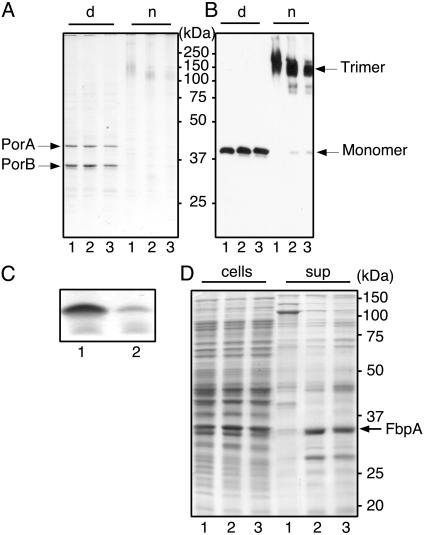
Analysis of the protein profiles of whole-cell lysates (Fig. 3D) or cell envelopes (Fig. 3A) in SDS/PAGE showed no marked differences between wild-type and imp mutant bacteria, at least not among the visible, major OMPs. The major OMPs of N. meningitidis are the trimeric porins PorA and PorB. These trimers are very stable and do not dissociate into monomers during seminative SDS/PAGE (5). When we analyzed cell envelopes of wild-type, imp, and lpxA mutant bacteria in seminative conditions, most of the PorA protein from all three strains was present in its trimeric form, as shown by immunoblotting (Fig. 3B). Interestingly, the PorA trimers from the lpxA mutant migrated slightly faster, suggesting that LPS remained associated with PorA trimers from the wild-type strain during the procedure. Only a small amount of monomeric PorA was detected in the imp mutant as well as in the lpxA mutant (Fig. 3B). Thus, PorA and PorB are present in normal levels and are assembled correctly. In contrast, Tricine-SDS/PAGE analysis showed that the cellular LPS content was dramatically decreased in the imp mutant (Fig. 3C), a finding corroborated by the 3-deoxy-d-manno-octulosonic acid content of cell envelopes: the imp mutant and wild-type cell envelopes contained 6.4 ± 0.5 and 95 ± 5.0 nmol of 3-deoxy-d-manno-octulosonic acid per mg of protein, respectively. The LPS of the imp mutant migrated at a similar position in the gel as wild-type LPS (Fig. 3C), suggesting that its structure was unaltered. Analysis of the extracellular growth medium by Tricine SDS/PAGE did not reveal enhanced release of LPS by the imp mutant (data not shown). However, its extracellular protein profile was very different from the wildtype, but similar to the profile of the lpxA mutant (Fig. 3D). The extracellular protein profiles of the imp and lpxA mutants were different from their cellular profiles (Fig. 3D). Furthermore, the major protein present in the medium of the mutants was an ≈35-kDa protein, which could be identified by immunoblotting as FbpA (data not shown), a periplasmic iron transporter (22). These results indicate periplasmic leakage, rather than total cell lysis, taking place in the imp and lpxA mutants, a phenomenon also reported for E. coli mutants expressing reduced amounts of LPS (23).
Fig. 3.
Protein and LPS profiles of wild-type (lanes 1), imp mutant (lanes 2), and lpxA mutant (lanes 3) bacteria. (A and B) Cell envelopes were analyzed by SDS/PAGE in denaturing (d) or seminative (n) conditions. Gels were stained with Coomassie blue (A) or were blotted and probed with anti-PorA antibody (B). (C) Equal amounts of proteinase K-treated cell lysates were subjected to Tricine-SDS/PAGE and stained with silver to visualize LPS. (D) Lanes labeled “cells” contain equal numbers of bacteria, based on OD. Lanes labeled “sup” contain equal volumes of culture supernatants precipitated with trichloroacetic acid. Samples were subjected to SDS/PAGE and were stained with Coomassie blue. Molecular size markers (in kDa) are indicated.
Complementation of the imp mutation by introduction of the imp gene on a plasmid under the control of an IPTG-regulatable promoter resulted in complete restoration of all wild-type phenotypic traits described above in the presence of IPTG (data not shown), demonstrating that the imp mutant phenotype is directly related to Imp deficiency and not a consequence of a polar effect of the mutation on the downstream-located surA gene. Thus, the imp mutant demonstrates a similar phenotype as the lpxA mutant, which is indicative of a role for Imp in LPS biogenesis. In contrast to the lpxA mutant, however, the imp mutant still produced a low amount of apparently full-length LPS. The presence of intact LPS molecules argues against a defect in LPS biosynthesis in the imp mutant. The reduction in the level of LPS may result, rather, from feedback inhibition on LPS synthesis due to stalled LPS transport.
Membrane Separations. To localize the LPS produced in the imp mutant, we performed sucrose-gradient density centrifugation to separate IMs and OMs. Despite many attempts using different protocols, we never obtained satisfactory membrane separations, even of wild-type cells. The IM marker, lactate dehydrogenase, and the OM marker, the porins, fractionated reasonably well to lower- and higher-density sucrose fractions, respectively. However, LPS did not specifically cofractionate with the porins and was found in almost every fraction of the gradient (data not shown). Difficulties with Neisserial membrane separations were appreciated (16). As with the LPS of the wild-type strain, the LPS of the imp mutant was found in almost every fraction of the gradient (data not shown), but because of the nonconclusive results with the wild-type membranes, conclusions could not be drawn from these results. Immunoblotting of the gradient fractions revealed that Imp cofractionated with the porins (data not shown), indicating an OM localization of Imp in N. meningitidis, which is consistent with its reported localization in E. coli (6). To assess LPS localization in the imp mutant, we designed alternative methods.
Surface Accessibility of LPS. Neisseriae do not synthesize O antigen. The terminal oligosaccharide portion of the core of Neisserial LPS is variable due to phase-variable expression of the glycosyltransferases involved. Consequently, many different so-called LPS immunotypes exist. The L8 immunotype contains three monosaccharide moieties in its α-chain. The L3 immunotype has a similar α-chain but extended with a lacto-N-neotetraose unit, which can be further extended with a sialic acid residue. Meningococci are capable of sialylating the lacto-N-neotetraose unit by using endogenously produced CMP-NANA as substrate or by using this nucleotide sugar when added to the growth medium (24). The sialic acid residue can be removed from LPS with neuraminidase (25). We used this feature to assess the cell surface location of LPS.
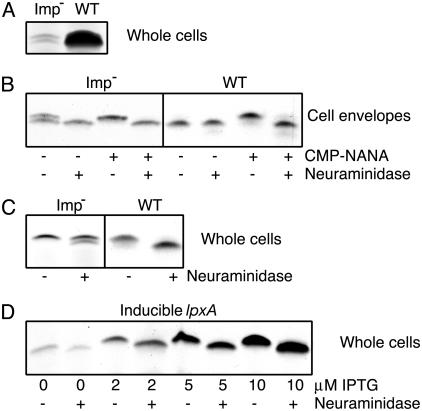
The results described so far were obtained with an L8 immunotype that cannot be sialylated (24). To exploit the neuraminidase assay, we constructed an imp mutant in an L3 background. The phenotype of this mutant, in terms of colony opacity, growth characteristics, release of periplasmic protein (data not shown), and low LPS content (Fig. 4A), was identical to that of the L8 imp mutant. The LPS of the L3 imp mutant appeared in silver-stained Tricine-SDS/PAGE gels as two bands (Fig. 4 A and B). After neuraminidase treatment of cell envelopes, all LPS migrated at the lower position (Fig. 4B), demonstrating that the higher band corresponds to sialylated LPS. After growth of the mutant in the presence of CMP-NANA, all LPS migrated at the higher position, and was converted to the lower migrating form upon neuraminidase treatment of cell envelopes (Fig. 4B). Thus, the L3 imp mutant produces LPS with a full-length α-chain, which can be sialylated and subsequently be desialylated with neuraminidase. Wild-type bacteria produced detectable sialylated LPS only when CMP-NANA was added to the growth medium (Fig. 4B). Apparently, the endogenous CMP-NANA levels are rate-limiting when regular high levels of LPS are produced. Endogenously produced CMP-NANA is used as a common pool both for capsule formation and LPS sialylation; therefore, the LPS of sialic acid capsule producing N. meningitidis strains is only partially sialylated (26).
Fig. 4.
Surface accessibility of LPS. Shown are silver-stained Tricine-SDS/PAGE gels containing samples treated with proteinase K before loading. (A) Equal amounts of proteinase K-treated cell lysates of the indicated strains were loaded on the same gel. (B) Cell envelopes of bacteria grown the presence or absence of CMP-NANA. Where indicated, the cell envelopes were treated with neuraminidase before electrophoresis. (C) Intact bacteria grown in the presence of CMP-NANA were treated with neuraminidase and subsequently processed for Tricine-SDS/PAGE. In B and C, five times as much material of the imp mutant samples, based on protein content or OD, was loaded compared with wild-type samples. Gels containing the imp mutant samples were developed longer to obtain optimal visibility of the LPS bands. (D) The inducible lpxA mutant was grown in the presence of the IPTG concentrations indicated plus CMP-NANA. Intact cells were treated with neuraminidase as indicated. Equal amounts of cell lysates, based on OD, were analyzed on the same gel.
To test whether LPS was exposed at the cell surface, we treated intact bacteria grown in the presence of CMP-NANA with neuraminidase. Only a minor part of LPS was desialylated in the intact imp mutant cells, indicating that most of the LPS was not accessible to neuraminidase at the cell surface (Fig. 4C). The small amount of LPS that was accessible possibly resulted from the leakiness of the mutant cells, as revealed by their enhanced protein release (Fig. 3D). In contrast, sialylated LPS present in wild-type cells was, as far as detectable in the gels, completely desialylated and thus fully exposed at the cell surface as expected (Fig. 4C). To address whether the difference in neuraminidase accessibility between wild-type and imp mutant bacteria was influenced in any way by the large difference in total LPS present, we performed similar assays in a strain where lpxA expression is regulatable with IPTG (13). This strain was grown in the presence of CMP-NANA and various concentrations of IPTG. Expression of LPS depended on the IPTG concentration used, although we detected some LPS even in the absence of IPTG (Fig. 4D); apparently, the IPTG-inducible promoter was not completely silent. Nevertheless, at all different LPS levels, cell surface localization of LPS was evident as inferred from its full accessibility to neuraminidase in intact cells (Fig. 4D). These data further validate the assay used and therefore strengthen our conclusion that LPS is mostly absent from the cell surface in the imp mutant. Thus, Imp functions in LPS transport to the outer leaflet of the OM.
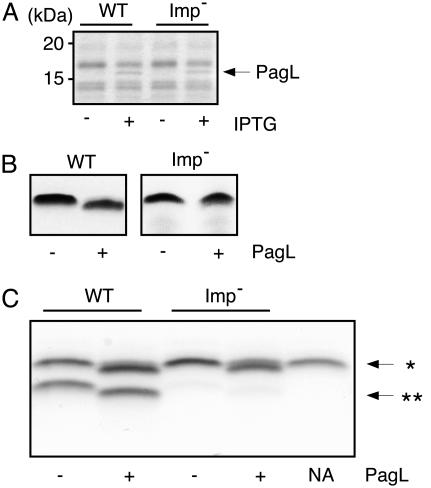
Modification of LPS in the OM. Certain bacterial species, such as Salmonella typhimurium or Pseudomonas aeruginosa, can covalently modify their LPS within the OM. The enzyme PagP adds a palmitate moiety to LPS, whereas PagL can remove an acyl chain (20, 27). These enzymes are OM proteins (OMPs). Therefore, modification of LPS by either of these enzymes can be regarded as a marker for OM localization of LPS (27). We searched the meningococcal genomes for PagL or PagP homologs without success. To enable us to use PagL activity as a marker for LPS localization, we introduced a plasmid containing a pagL homolog of B. bronchiseptica under control of a lac promoter into the H44/76 L8 immunotype strain. Growth of the resulting strain with IPTG resulted in the presence of a 17-kDa protein in the cell envelope, which is consistent with the calculated molecular weight of PagL (Fig. 5A). Furthermore, induction of PagL expression resulted in the faster migration of LPS in Tricine-SDS/PAGE, as expected after removal of an acyl chain from the LPS (Fig. 5B). Thus, B. bronchiseptica PagL expressed in N. meningitidis was functional. Next, the imp gene was deleted in the strain containing the pagL plasmid. Although PagL was normally expressed upon IPTG induction in the resulting strain (Fig. 5A), the electrophoretic mobility of its LPS was unaltered (Fig. 5B), indicating that the LPS produced in this strain was not accessible to PagL in the OM. Previously, it was reported that LPS depletion has no effect on assembly of OMPs in N. meningitidis (13), but to verify that the PagL produced in the imp mutant was functional, we determined PagL activity in membrane preparations. Cell envelopes containing or lacking PagL were incubated with purified LPS of the L3 immunotype and the modification of this LPS was assessed by Tricine-SDS/PAGE (Fig. 5C). L8-LPS present in the cell envelopes could be discriminated from the added L3-LPS by its faster migration in the gels. Cell envelopes of PagL producing wild-type and imp mutant strains showed equal capacities to modify the added LPS, demonstrating that also the imp mutant produced a functional PagL enzyme (Fig. 5C). Thus, in live imp mutant cells, the endogenously produced LPS is not accessible to PagL, and therefore not present at the cell surface, providing further evidence to the notion that Imp functions in the transport of LPS.
Fig. 5.
PagL-mediated LPS modification in wild-type and imp mutant strains expressing pagL of B. bronchiseptica. PagL expression was induced (+)ornot induced (-) by IPTG. (A) Cell envelopes were analyzed by SDS/PAGE and Coomassie staining. Only the relevant portion of the gel is shown. (B) Tricine-SDS/PAGE analysis of proteinase K-treated cell lysates. Five times as much proteinase K-treated cell lysate, based on OD, was loaded of the imp mutant samples compared with the wild-type samples. The gel containing the imp mutant samples was developed longer to obtain optimal visibility of the LPS bands. (C) Silver-stained Tricine-SDS/PAGE gels showing in vitro PagL activity. Purified L3-LPS (*) was incubated in a detergent-containing buffer for 18 h at 37°C with L8-LPS (**)-containing cell envelopes prepared from wild-type and imp mutant strains, containing pagL on a plasmid. Similar amounts of assay mixes were loaded in all lanes. NA, no cell envelopes were added.
Imp Homologs in Other Bacteria. Genes involved in the biogenesis of well conserved structures such as LPS are likely highly conserved. This concept appears to be true for the imp gene, because homologs could be found in most Gram-negative, but not in Gram-positive bacteria (6). The absence of an imp homolog in some Gram-negative bacteria appears to correlate with the absence of LPS, because we were unable to find imp homologs in bacteria that possess an OM, but lack LPS biosynthesis genes (1), such as Thermotoga maritima, Deinococcus radiodurans, and the spirochaetes Borrelia burgdorfferi and Treponema pallidum. This observation further reinforces the concept of Imp functioning as an LPS transporter.
Discussion
LPS is an essential component of the OM of most Gramnegative bacteria and a causative agent of severe septic shock in humans. Its biogenesis has been studied for a long time, resulting in the identification of many enzymes involved in its biosynthesis. However, the final step of LPS biogenesis, i.e., the transport of completed LPS molecules from the periplasmic leaflet of the IM to the bacterial cell surface, has remained elusive. We have now identified for the first time, to our knowledge, a protein required for this LPS transport pathway. A Neisserial imp mutant produced drastically reduced amounts of full-length LPS. Although we were unable to determine exactly the cellular location of the limited amount of LPS that accumulated in the imp mutant, we have clearly shown that this LPS is not present at the cell surface. The lack of modification by PagL and the poor accessibility to neuraminidase provide two independent lines of evidence for this notion. The desialylation of a small portion of the LPS observed when whole cells of the imp mutant were treated with neuraminidase was mostly likely due to the diminished membrane integrity in these bacteria, which may have been reinforced by the repeated centrifugation and respuspension steps required for this assay. Because Imp itself is an OMP, as shown by its presence in purified Neisserial (this study) and E. coli (6) OMs, Imp is likely the transporter that mediates the flip-flop of LPS over the OM, although an additional role in transport through the periplasm cannot be excluded at this stage.
Braun and Silhavy (6) reported that depletion of Imp in a conditional E. coli mutant resulted in the appearance of highdensity membranes found in sucrose gradient fractionations. This higher density might result from an increased protein to lipid ratio. Consistently, whereas OMP assembly appeared unaffected by Imp depletion, both in E. coli (6) and in N. meningitidis (this study), we have demonstrated now that Imp depletion results in decreased levels of LPS in the OM, thus changing the protein:lipid ratio. Also, the observations that missense mutations in the E. coli imp gene resulted in increased sensitivity to hydrophobic agents (7, 8) can now be understood: these mutants likely suffered from reduced levels of LPS, a property known to affect the integrity of the OM (23).
Previously, another essential OMP, Omp85, has been suggested to be involved in LPS transport (4). However, we have demonstrated a strong OMP assembly defect in an Omp85-depleted strain (5). Thus, any effect of Omp85 depletion on LPS biogenesis might be a consequence of the misassembly of Imp. Furthermore, the demonstration of an interaction of Omp85 with nonnative porin (5), the presence of an omp85 homolog in Gram-negative bacteria lacking LPS biosynthesis genes, the high conservation of Imp in Gram-negative bacteria, except in those that lack LPS-biosynthesis genes (this study), and the recent demonstrations that an Omp85 homolog is involved in OMP assembly in mitochondria as well (28–30), all argue for a direct role of Omp85 in OMP assembly and of Imp in LPS transport. With the identification of the functions of Omp85 and Imp, major progress in understanding the biogenesis of the bacterial OM can now be made.
We contribute the strongly decreased amounts of LPS present in the imp mutant to some type of feedback inhibition on LPS biosynthesis. A similar inhibition has been reported in capsule biogenesis mutants. Deletion of the OM transporter for E. coli group I capsule (Wza) inhibited capsule production although the biosynthetic enzymes were still present (31). In N. meningitidis, deletion of the capsule transporter CtrA in the OM also resulted in diminished capsule production (32). This feedback regulation was not due to altered transcription levels of capsular biosynthetic enzymes (33). Further insights into the mechanisms behind capsular and LPS feedback inhibition are lacking but are certainly an interesting topic for future studies.
The Imp protein is an attractive target for the development of novel antibacterial substances, in light of its high conservation, cell surface localization and essential role in most Gram negatives. Additionally, Neisserial imp mutant strains might be useful as vaccine strains. Neisserial vaccines consist of OM vesicles that are treated with detergents to remove the majority of LPS to prevent toxic reactions in vaccinees. This procedure unfortunately removes also potentially important vaccine components such as cell-surface-exposed lipoproteins. Vaccines prepared in this way contain ≈7% of the normal LPS levels (34), which is similar to the level of LPS left in the imp mutant. Thus, deletion of the imp gene in a vaccine strain relieves the need for detergent extraction and thereby the loss of potentially important vaccine components.
Supplementary Material
Acknowledgments
We thank R. van Boxtel for 3-deoxy-d-manno-octulosonic acid measurements. This work was supported by European Community Grant QLRT-1999-CT-00359 (to M.P.B.); the Netherlands Research Council for Chemical Sciences with financial aid from the Netherlands Technology Foundation; and the Research Council for Earth and Life Sciences (B.T.), with financial aid from the Netherlands Organization for Scientific Research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LPS, lipopolysaccharide; OM, outer membrane; IM, inner membrane; OMP, OM protein; CMP-NANA, cytidine 5′ monophospho-N-acetyl neuraminic acid; Imp, increased membrane permeability; IPTG, isopropyl-β-d-thiogalactopyranoside; Tricine, N-tris(hydroxymethyl)methylglycine.
References
- 1.Raetz, C. R. H. & Whitfield, C. (2002) Annu. Rev. Biochem. 71, 635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gronow, S. & Brade, H. (2001) J. Endotoxin Res. 7, 3-23. [PubMed] [Google Scholar]
- 3.Zhou, Z., White, K. A., Polissi, A., Georgopoulos, C. & Raetz, C. R. H. (1998) J. Biol. Chem. 273, 12466-12475. [DOI] [PubMed] [Google Scholar]
- 4.Genevrois, S., Steeghs, L., Roholl, P., Letesson, J.-J. & van der Ley, P. (2003) EMBO J. 22, 1780-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voulhoux, R., Bos, M. P., Geurtsen, J., Mols, M. & Tommassen, J. (2003) Science 299, 262-265. [DOI] [PubMed] [Google Scholar]
- 6.Braun, M. & Silhavy, T. J. (2002) Mol. Microbiol. 45, 1289-1302. [DOI] [PubMed] [Google Scholar]
- 7.Sampson, B. A., Misra, R. & Benson, S. A. (1989) Genetics 122, 491-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aono, R., Negishi, T. & Nakajima, H. (1994) Appl. Environ. Microbiol. 60, 4624-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steeghs, L., den Hartog, R., den Boer, A., Zomer, B., Roholl, P. & van der Ley, P. (1998) Nature 392, 449-450. [DOI] [PubMed] [Google Scholar]
- 10.Albiger, B., Johansson, L. & Jonsson, A. B. (2003) Infect. Immun. 71, 155-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tettelin, H., Saunders, N. J., Heidelberg, J., Jeffries, A. C., Nelson, K. E., Eisen, J. A., Ketchum, K. A., Hood, D. W., Peden, J. F., Dodson, R. J., et al. (2000) Science 287, 1809-1815. [DOI] [PubMed] [Google Scholar]
- 12.Parkhill, J., Achtman, M., James, K. D., Bentley, S. D., Churcher, C., Klee, S. R., Morelli, G., Basham, D., Brown, D., Chillingworth T., et al. (2000) Nature 404, 502-506. [DOI] [PubMed] [Google Scholar]
- 13.Steeghs, L., de Cock, H., Evers, E., Zomer, B., Tommassen, J. & van der Ley, P. (2001) EMBO J. 24, 6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesse, A. J., Campagnari, A. A., Bittner, W. E. & Apicella, M. A. (1990) J. Immunol. Methods 126, 109-117. [DOI] [PubMed] [Google Scholar]
- 15.Tsai, C. M. & Frasch, C. E. (1982) Anal. Biochem. 119, 115-119. [DOI] [PubMed] [Google Scholar]
- 16.Masson, L. & Holbein, B. E. (1983) J. Bacteriol. 154, 728-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shell, D. M., Chiles, L., Judd, R. C., Seal, S. & Rest, R. F. (2002) Infect. Immun. 70, 3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westphal, O. & Jann, J. K. (1965) Methods Carbohydr. Chem. 5, 83-91. [Google Scholar]
- 19.Van Alphen, L., Verkleij, A., Leunissen-Bijvelt, J. & Lugtenberg, B. (1978) J. Bacteriol. 134, 1089-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trent, M. S., Pabich, W., Raetz, C. R. H. & Miller, S. I. (2001) J. Biol. Chem. 276, 9083-9092. [DOI] [PubMed] [Google Scholar]
- 21.Dekker, N., Merck, K., Tommassen, J. & Verheij, H. M. (1995) Eur. J. Biochem. 232, 214-219. [DOI] [PubMed] [Google Scholar]
- 22.Ferreirós, C., Criado, M. T. & Gómez, J. A. (1999) Comp. Biochem. Physiol. 123, 1-7. [DOI] [PubMed] [Google Scholar]
- 23.Nurminen, M., Hirvas, L. & Vaara, M. (1997) Microbiology 143, 1533-1537. [DOI] [PubMed] [Google Scholar]
- 24.Kahler, C. & Stephens, D. C. (1998) Crit. Rev. Microbiol. 24, 281-334. [DOI] [PubMed] [Google Scholar]
- 25.Ram, S., Sharma, A. K., Simpson, S. D., Gulati, S., McQuillen, D. P., Pangburn, M. K. & Rice, P. A. (1998) J. Exp. Med. 187, 743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahler, C., Martin, L. E., Shih, G. C., Rahman, M. M., Carlson, R. W. & Stephens, D. S. (1998) Infect. Immun. 66, 5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raetz, C. R. H. (2001) J. Endotoxin Res. 7, 73-78. [PubMed] [Google Scholar]
- 28.Gentle, I., Gabriel, K., Beech, P., Waller, R. & Lithgow, T. (2004) J. Cell. Biol. 164, 19-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paschen, S. A., Waizenegger, T., Stan, T., Preuss, M., Cyrklaff, M., Hell, K., Rapaport, D. & Neupert, W. (2003) Nature 426, 862-866. [DOI] [PubMed] [Google Scholar]
- 30.Kozjak, V., Wiedemann, N., Milenkovic, D., Lohaus, C., Meyer, H. E., Guiard, B., Meisinger, C. & Pfanner, N. (2003) J. Biol. Chem. 278, 48520-48523. [DOI] [PubMed] [Google Scholar]
- 31.Nesper, J., Hill, C. M. D., Paimen, A., Harauz, G., Beis, K., Naismith, J. H. & Whitfield, C. (2003) J. Biol. Chem. 278, 49763-49772. [DOI] [PubMed] [Google Scholar]
- 32.Swartley, J. S., Ahn, J. H., Liu, L.-J., Kahler, C. M. & Stephens, D. S. (1996) J. Bacteriol. 178, 4052-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzeng, Y.-L., Swartley, J. S., Miller, Y. K., Nisbet, R. E., Liu, L.-J., Ahn, J. H. & Stephens, D. S. (2001) Infect. Immun. 69, 2502-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fredriksen, J. H., Rosenqvist, E., Wedege, E., Bryn, K., Bjune, G., Froholm, L. O., Lindbak, A. K., Mogster, B., Namork, E., Rye, U., et al. (1991) NIPH Ann. 14, 67-79. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.