Abstract
Objective
To investigate the serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1), and Paraoxonase-1 (PON-1) during fertility treatment of women with endometriosis (Endo), PCOS or unexplained infertility (Unexpl).
Methods
Thirty-six patients with Endo, PCOS or Unexpl undergoing controlled ovarian stimulation for IVF or IUI were consented and their serum, on day-3 (baseline) and at the end of FSH treatment (peak), was collected and investigated for levels of TNF-α, IL-6, MCP-1, and PON-1. Correlations, ANOVA and Student's t-test were used for statistical analysis.
Results
Peak serum levels of IL-6, MCP-1 and PON-1 were positively correlated to E2 peak levels. TNF-α levels were inversely correlated to estradiol levels and they were lower in patients who ultimately became pregnant when compared to non-pregnant (P < 0.05). Mean TNF-α levels were significantly higher in Unexpl group (P < 0.05). The mean levels of IL-6, and MCP-1 were significantly (p < 0.05) higher in women with PCOS compared with Endo and Unexpl. No differences were found between the three clinical groups in patient’s age, BMI, Day-3 FSH, PON-1 and pregnancy outcome.
Conclusion
Circulating cytokine levels were influenced by ovarian stimulation, as demonstrated by increased levels of IL-6, MCP-1 and PON-1, and decreased level of TNF-α at the end of controlled ovarian stimulation. While evidence of relationship between circulating cytokines with mild endometriosis was not found, PCOS was associated with elevated serum IL-6 and MCP-1 but lower TNF-α concentration. Unexplained infertility was associated with elevated TNF-α level. No relationship between serum PON-1 concentration and PCOS, mild endometriosis or unexplained infertility was noted.
Keywords: TNF-α, IL-6, MCP-1, PON-1, Endometriosis, PCOS, Unexplained infertility, Ovarian stimulation, IUI, IVF
Introduction
It is well established that many cytokines are critically important for normal female reproductive processes, such as follicular development, ovulation, fertilization, implantation and normal pregnancy (1–3). Also, there is increasing consensuses that cytokines may play a role in the pathophysiology of endometriosis, polycystic ovary syndrome (PCOS) and unexplained infertility (4–7). Endometriosis and PCOS are two of the most common gynecological disorders in women during their reproductive years. Both of these conditions, in addition to unexplained infertility, were the most common reason for women seeing reproductive endocrinologist for fertility treatment. Tumor Necrosis Factor-α has been shown to facilitate ovulation and fertilization process whereas IL-6 plays an important role in endometrial function and in implantation (2,3). Some research have suggested that chemotactic cytokines such as monocyte chemotactic protein-1 (MCP-1) may be involved in follicular development, ovulation, as well as in corpus luteum formation and demise (8). Deficiency of human serum PON-1 has been correlated with oxidative stress and reproductive diseases (9). Considering the complex known and unknown cellular and molecular mechanisms involved in pathophysiology of these clinical conditions, we aimed to compare the serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), MCP-1, and Paraoxonase-1 (PON-1) before and after controlled ovarian stimulation for IVF or IUI. Recently we performed a prospective study to analyze differences in serum levels of PON-1, Superoxide dismutases (SOD), Glutathione Peroxidase (GPx), IL-6, 8-Isoprostane, and fatty acids with regard to ovarian stimulations and its relation to pregnancy outcome in women undergoing IVF or IUI (10). In this study, we investigated the association of additional cytokines and an oxidative stress biomarker with endometriosis, PCOS, and unexplained infertility. A secondary objective was to assess whether gonadotropin-induced changes in circulating levels of tumor necrosis factor- α, interleukin-6, monocyte chemotactic protein-1 and paraoxonase-1 will affect the pregnancy outcome in women undergoing controlled ovarian stimulation for IUI.
Methods and methods
Patients
This study was approved by the Institutional review board (IRB) of the Medical Center of Central Georgia-Mercer University School of Medicine. Our study group consisted of 36 infertile women (mean age ± SD: 33.7 ± 4.7 years, range 26–44 years). The infertile patients were divided into three infertility etiology groups, unexplained infertility (19.4 %), PCOS (38.9 %), and endometriosis (41.7 %). All patients were in good health with normal thyroid, hepatic, and renal functions, and all had experienced spontaneous onset of puberty and normal sexual development. Inclusion criteria for selected patients included the presence of ovaries, regular menstrual cycle, nonsmoker, and good general health. Unexplained infertility was defined here as a diagnosis made in couples in whom standard investigations including semen analysis, tests of ovulation and tubal patency are normal. None of Unexpl patients in this study have history of PCOS or endometriosis. Patients with endometriosis-associated infertility were diagnosed by laparoscopy and classified according to the original American Society for Reproductive Medicine guidelines (11). Ninety three percent (14 out of 15) of the endometriosis patients had stage I or II (minimal/mild), only one had stage III moderate/severe endometriosis. Eighty-nine percent (32 out of 36) of the patients were treated with IUI and four had IVF-ICSI treatment. All patients underwent controlled ovarian hyperstimulation by recombinant human follicle-stimulating hormone (rFSH, Follistim) daily for 7–8 consecutive days and follicular development was monitored by serial transvaginal ultrasonography and serum E2 concentrations. Dosage of gonadotropin had been calculated individually for each patient considering patient's age, body mass index, ovarian pattern, menstrual cycles, basal FSH, Anti-Mullerian hormone (AMH), and E2, and response to previous controlled ovarian stimulations. An hCG injection was given to trigger the final stages of oocyte maturation and ultrasound-guided oocyte retrieval was performed 34–36 h later. Standard insemination of oocytes was performed using IVF or intracytoplasmic sperm injection (ICSI) procedures according to indications and sperm quality. Fertilization and cleavage was assessed daily and the embryos were classified according to their morphological appearance. Embryos were transferred at day-3 or 5. All IUI patients were given rFSH (Follistim) on cycle day 3 of cycle and monitored similar to IVF patients. The dose of gonadotropins was adjusted according to ovarian response. When the largest follicle(s) reached a mean diameter of 18 mm, ovulation was induced with 10,000 U of hCG. A single IUI was performed 36–38 h later with a smith insemination catheter. Clinical pregnancy was defined as a positive serum β-hCG (>25 mIU/mL) on day 14 after insemination and by transvaginal ultrasonography detection of fetal heart beat(s) by 7 weeks gestational age.
Samples collection and measurement of serum markers
Blood samples were collected and processed immediately by centrifugation at 3000× rpm for 10 min. The clear serum was used for hormone analysis and then frozen stored in liquid nitrogen at −196 °C. Frozen samples were shipped in dry ice to the Department of Clinical Laboratory and Nutritional Sciences, College of Health Sciences, University of Massachusetts Lowell for the measurement of the cytokines, chemokines and antioxidant enzyme. Serum concentrations of TNF-α, MCP-1 and IL-6 were determined using ELISA kits purchased from Bio-Legend, San Diego, CA. Plasma PON-1 arylesterase activity was measured using 1 mM ρNPA as substrate, as assessed from the formation of ρ-nitrophenol at 410 nm at 37 °C. Typically, aliquots of 10 μl of plasma were placed in microtiter plate wells in triplicate; the reaction was initiated by the addition of the substrate ρNPA, to yield a final concentration of 1 mM in PBS buffer containing calcium and magnesium. After mixing, the plate was read immediately to establish 0 time values, and the reactions were incubated at 37 °C. Readings were recorded at the end of 30 min. PON activity was calculated using the molar absorbitivity (12).
Statistical analysis
Statistical analysis was performed by using SPSS software (version 21.1; SPSS Inc., Chicago, IL). Patient demographic data were evaluated by descriptive statistics and variables were given as mean + SEM and compared using a Student’s t-test. The different etiologies of infertility were analyzed with paired samples t-test and ANOVA. Pearson’s correlation analysis was used to evaluate the relationship between peak E2 and variables in different etiologies of infertility. Statistical differences of P ≤ 0.05 were considered significant.
Results
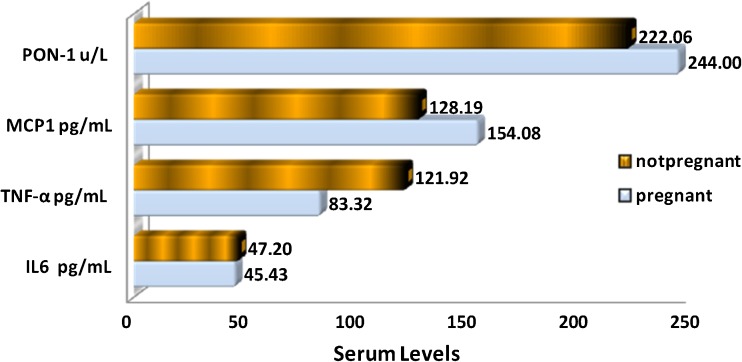
Effects of ovarian stimulation on TNF-α, IL-6, MCP-1 and PON-1 levels of all women, regardless of etiology, were shown in Table 1. The mean levels of serum factors of patients undergoing ovarian stimulation with rFSH collected at baseline and peak time, just prior to hCG injection, were compared and correlated to peak E2 values. There was significant increase (p < 0.05) in the levels of MCP-1, PON1 and IL-6 after ovarian stimulation in all patients. And this increase was positively correlated to E2 levels. In contrast, TNF-α levels were significantly (P < 0.05) lower after ovarian stimulation, and they were inversely correlated to E2 levels. The relationship between the mean level of serum factors and pregnancy outcome of IUI patients is shown in Fig. 1. We did not include IVF-ICSI patients data in our pregnancy outcome analysis because the number of patients in this category was small (n = 4). Pregnancy rate was negatively affected by the presence of high serum TNF-α level. There was no association between serum IL-6, MCP-1, and PON-1 levels and pregnancy rate.
Table 1.
The levels of serum E2, IL-6, MCP-1, PON1 and TNF-α at baseline prior to hormonal stimulation and on the day of hCG injection. IL-6 = Interleukin-6, PON = Paraoxonase, MCP-1 = Monocyte Chemotactic Protein-1, and TNF-α = Tumor Necrosis Factor-α
| Parameters | Before | After stimulation | p value |
|---|---|---|---|
| IL-6 pg/mL | 22.6 ± 3.0 | 42.3 ± 5.4 | P < 0.05 |
| MCP1 (pg/mL) | 120.3 ± 5.0 | 150.9 ± 8.8 | P < 0.05 |
| PON-1 u/L | 244.2 ± 12.9 | 287.6 ± 11.6 | P < 0.05 |
| TNF-α (pg/mL) | 120.8 ± 13.7 | 92.2 ± 7.6 | P < 0.05 |
| E2 (pg/ml) | 36.6 ± 2.8 | 855 ± 87.4 | P < 0.05 |
Fig. 1.
The relationship between mean serum levels of IL-6, MCP-1, PON1, TNF-α and pregnancy outcome of IUI patients. IL-6 = Interleukin-6, PON = Paraoxonase, MCP-1 = Monocyte Chemotactic Protein-1, and TNF-α = Tumor Necrosis Factor-α
Baseline demographic characteristics and hormone concentrations of the studied patients, listed according to the three etiology groups, are presented in Table 2. There were no differences between the three etiology groups in patient’s age, BMI, Day-3 FSH, baseline and peak E2, Progesterone or pregnancy outcome. Comparison between the three etiology groups on mean levels of TNF-α, IL-6, MCP-1 and PON at baseline and peak E2 levels were summarized in Table 3. Baseline IL-6 was higher whereas TNF-α was significantly (P < 0.05) lower in PCOS patients compared to unexplained. Women with PCOS exhibited a significantly (P < 0.05) high peak serum of and MCP-1 compared with the unexplained infertility or Endometriosis. The mean level of IL-6 and MCP-1 at peak E2 was significantly (p < 0.05) higher in women with PCOS compared with endometriosis and Unexplained. Peak TNF-α levels were significantly higher in unexplained than Endometriosis women (P < 0.05). No differences between the three etiology groups in baseline and peak serum levels of PON-1.
Table 2.
Comparison of patient characteristics in women with Endometriosis, PCOS or unexplained infertility (Mean ± SEM)
| Parameter | Endometriosis (Mean ± SEM) | PCOS (Mean ± SEM) | Unexplained (Mean ± SEM) | p value |
|---|---|---|---|---|
| Age (years) | 31.9 ± 1.1 | 32.7 ± 2.0 | 35.9 ± 1.1 | NS |
| BMI (kg/M2) | 26.2 ± 1.2 | 28.3 ± 2.2 | 27.7 ± 1.7 | NS |
| Day-3 FSH (mIU/mL) | 7.7 ± 0.4 | 4.9 ± 0.8 | 7.6 ± 0.4 | NS |
| Baseline E2 | 37.7 ± 3.1 | 34.5 ± 3.3 | 37.6 ± 6.0 | NS |
| E2 (pg/ml) @ hCG injection | 877 ± 136 | 720 ± 118 | 886 ± 158 | NS |
| P4 (ng/ml) @ hCG injection | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 | NS |
| Pregnant (%) | 46.7 | 42.9 | 42.9 | NS |
Table 3.
Comparison of baseline and peak serum IL-6, MCP-1, PON1 and TNF-α levels in women with endometriosis, PCOS or unexplained infertility (Mean± SEM). IL-6 = Interleukin-6, PON = Paraoxonase, MCP-1 = Monocyte Chemotactic Protein-1, and TNF-α = Tumor Necrosis Factor-α
| Parameter | Endometriosis (Mean ± SEM) | PCOS (Mean ± SEM) | Unexplained (Mean ± SEM) | p value | |
|---|---|---|---|---|---|
| Baseline: | IL-6 pg/mL | 23.9 ± 4.9 | 30.1 ± 10.7b | 18.3 ± 3.4a | P < 0.05 |
| MCP1 (pg/mL) | 123.3 ± 7.7 | 131.7 ± 14.9 | 112.6 ± 7.2 | NS | |
| PON-1 u/L | 199.2 ± 16.1 | 201.8 ± 26.0 | 196.4 ± 10.7 | NS | |
| TNF-α (pg/mL) | 113.7 ± 18.8 | 99.3 ± 20.5a | 163.7 ± 26.1b | P < 0.05 | |
| Peak: | IL-6 pg/mL | 39.0 ± 8.9a | 58.0 ± 11.7b | 39.4 ± 8.2a | P < 0.05 |
| MCP1 (pg/mL) | 133.3 ± 12.0a | 180.6 ± 21.9b | 146.5 ± 14.4a | P < 0.05 | |
| PON-1 u/L | 222.5 ± 15.4 | 257.3 ± 38.3 | 260.6 ± 22.2 | NS | |
| TNF-α (pg/mL) | 79.3 (±18.8)a | 99.3 ± 20.5b | 102.1 ± 14.1b | P < 0.05 | |
Discussion
The ovarian cycle resembles an inflammatory process and the expressions of pro-inflammatory cytokines have been reported to increase during certain phases of menstrual cycle (1–3, 13, 14, 15). Ovarian stimulation is known to induce rapid changes in endogenous E2 and P4 which are not seen during a normal menstrual cycle, but little is known about the possible effect these changes may have on serum cytokines. In the present study, we investigated the serum concentrations of three pro-inflammatory cytokines (IL-6, TNF-α, MCP-1) and an antioxidant enzyme (PON-1) at two points: at the time of the baseline ultrasound before the initiation of gonadotropin treatment (usually day 2 or 3 of menstrual cycle), and secondly at peak follicular growth when the decision was made to administer hCG to induce ovulation. We found that ovarian stimulation causes significant increased in serum levels of IL-6, MCP-1 and PON-1 and decreased of TNF-α in infertile women with mild endometriosis, PCOS, or unexplained infertility. These findings are comparable to other studies that have compared cytokine levels in stimulated and non-stimulated ovarian cycles (8,13,15, 18). In unstimulated menstruating women, MCP-1 and TNF-α levels were reported higher in the follicular phase and lower in the late ovulatory and luteal phase (8,13); however, no difference in IL-6 level between the follicular phase and the luteal phases were noted (16,17). Souter et al. (18) showed that during controlled ovarian simulation, TNF-α concentrations increase at the mid follicular phase; whereas, IL-6 concentrations increase at the luteal phase of the cycle. In addition, other investigators (15) found an inverse correlation between concentrations of TNF-α and that of E2 during stimulated cycles. These finding in conjunction with other studies, support the hypothesis that cytokines secretion is modulated by gonadotropin stimulations and elevated E2 (2,8,10,13,14,19). The reported fluctuation of serum cytokine levels in different research studies may be due to their complicated cell-mediated mechanisms of synthesis and the differences in hormonal regulation from one woman to another.
With regard to PON-1, we found that its serum level increased after gonadotropin stimulation in women with mild endometriosis, PCOS, or unexplained infertility and the increase was positively correlated with serum E2 concentration. To our knowledge, this enhanced effect has never been reported elsewhere in the literature. PON-1 is a known potent antioxidant serum enzyme that is normally synthesized in the liver and circulates in the blood associated with HDL (High Density Lipoprotein) (9,12). Its role in human reproduction and why exogenous gonadotropin increases its activity is unknown. The observed increase after gonadotropin stimulation suggests possible de novo production in ovarian granulosa cells. Browne et al. (20) have detected PON-1 arylesterase activity in human follicular fluid of IVF patients and reported a positive association between high PON-1- level with oocyte and embryo quality. That finding, in addition to our research, suggest potential utility in measuring circulating PON-1 as a biomarker in clinical diagnosis or therapeutic management of oxidative stress.
This study was also aimed at assessing whether measuring cytokines and an oxidative stress biomarker during ovarian stimulation might provide new tool for screening endometriosis, PCOS, or unexplained infertility. In this regard, we have to acknowledge the main limitations of this study; only milder forms of endometriosis were investigated. The study results, despite these limitations, suggest a potential role for circulating TNF-α, IL-6, MCP-1 and PON-1 in pathophysiology of endometriosis, PCOS, or unexplained infertility. Our data showed that PCOS group had higher IL-6 and lower TNF-α when compared with the endometriosis and unexplained groups, both before and after, exogenous gonadotropins. The same group had higher MCP-1 levels when compared with endometriosis and unexplained groups at peak E2. These data agree with the finding of several other studies that revealed association of PCOS with increased pro-inflammatory cytokines and chemokines such as IL-6, TNF-α and MCP-1 (15,21,22). In contrast, several other studies (7,23,24) including a recent meta-analysis, have showed no difference in the serum levels of IL-6 or TNF-α between women with PCOS and age and BMI matched controls (25). These studies suggest that obesity, and not PCOS status per se, was a major determinant of the circulating TNF-α, and IL-6 (26). Women with PCOS are a heterogeneous cohort, some of them are obese with insulin-resistant and others are not. In our study, there were no differences between the three etiology groups in patient’s age, BMI and weight; none of the participants were obese. Regardless of these conflicting data, the results of this pilot study may represent an initial step for further studies to clarify the possible role of IL-6, MCP-1 and TNF in the pathogenesis of PCOS.
Regarding endometriosis, we did not find statistical difference in circulating IL-6, TNF-α, or MCP-1, between our endometriosis patients (note, most of them were diagnosed with minimal/mild stage of the disease) and the unexplained group. That finding supports other studies that found modest or no association between minimal/mild endometriosis with altered serum levels of cytokines or oxidative stress markers (27–30). Unlike the finding in mild endometriosis, patients with chronic moderate/severe endometriosis have been associated with elevated levels of various serum biomarkers including IL-6, TNF-α, MCP-1 and PON-1 (6,19,27,29,31,32, 33). Because of that, different types of biomarkers may be needed for the diagnosis of different stages and types (superficial, deep, cyst) of endometriosis (34, 35).
Similar to the cytokines, we observed no differences between serum PON-1 levels in mild endometriosis and all participating groups. In the same way, Bragatto et al. (28) found no difference in the serum levels of PON-1 activity between women with endometriosis in minimal/mild and moderate/severe stages. In contrast, Verti et al. (29) reported that PON-1 activity was significantly higher in women with moderate/severe endometriosis compared to women with minimal/mild disease and controls, and in women with minimal/mild endometriosis compared with control groups. The authors conclude that PON-1 activity can be used as a diagnostic test to detect endometriosis. Although we didn’t have a control group, the baseline serum level of PON-1 in our study was 199.2 ± 16.1 U/l which is not different from the levels reported in Verit et al.(29) control (183.7 ± 22.3 U/l) (27). PON1 enzyme activity is essential for the physiological function of several key metabolic pathways; and several studies have confirmed the antioxidant and atheroprotector role of PON-1 and observed that reduced PON-1 activity in adults is associated with various diseases such as diabetes mellitus, renal failure, obesity, and cardiovascular disease (36–38). However, there is no clear evidence that women with endometriosis, PCOS or unexplained infertility have more atherosclerosis than the general population (9). These conflicting data suggest that much is still unknown regarding the causes and mechanism involved in endogenous production of PON-1 and other antioxidants.
Our results also show a relationship between elevated TNF-α concentration with unexplained infertility compared with other etiologies. This relationship is also found in other cytokine studies involving unexplained infertility patients (39–41). However, the unexplained diagnosis may inherently include bias since it could include patients who, in fact, have male factor, tubal infertility, premature ovarian ageing and immunological factors (39). Nevertheless, in this study, the unexplained group excluded any patient with endometriosis or PCOS, which are known to interfere with ovarian development and possibly cytokine production.
In conclusion, this study demonstrated that circulating TNF-α, IL-6, MCP-1 and PON-1 were influenced by exogenous hormone administration for ovarian stimulation. While evidence of relationship between circulating cytokines with mild endometriosis was not found, PCOS was associated with elevated serum IL-6 and MCP-1, but lower TNF-α concentration. Unexplained infertility was associated with elevated TNF-α level. No relationship between serum PON-1 concentration and PCOS, endometriosis or unexplained infertility was noted. Further studies including a large percentage advanced stages of endometriosis and a fertile control group are needed to validate these data.
Acknowledgments
The authors wish to thank all the staff at Central Georgia Fertility Institute, specially Cynthia Clower, RNC and Deanna Nelsen, RN, BSN, for their enthusiastic support. This work was partially supported by a faculty startup grant from the University of Massachusetts Lowell to M.G.
Footnotes
Capsule summary
The same group had higher MCP-1 levels when compared with endometriosis and unexplained groups at peak E2. Unexplained infertility was associated with elevated TNF-α level. PON-1 serum level increased after gonadotropin stimulation in all patient groups and the increased level was positively correlated with serum E2 concentration.
Contributor Information
Abdelmoneim Younis, Phone: +1-478-7577888, FAX: +1-478-7577887, Email: moneims@gmail.com.
Mahdi Garelnabi, Phone: +1-978-9344528, FAX: +1-978-9343025, Email: Mahdi_Garelnabi@uml.edu.
References
- 1.Adashi EY. The potential relevance of cytokines to ovarian physiology. J Steroid Biochem Mol Biol. 1992;43:439–444. doi: 10.1016/0960-0760(92)90082-T. [DOI] [PubMed] [Google Scholar]
- 2.Brannstrom M. Potential role of cytokines in ovarian physiology: the case for Interleukin-1. In The Ovary (Elsevier). 2004: 261–271.
- 3.Zolti M, Ben-Rafael T, Meirom R, Shemesh M, Bider D, Mashiach S, et al. Cytokine involvement in oocytes and early embryos. Fertil Steril. 1991;56:265–272. doi: 10.1016/s0015-0282(16)54483-5. [DOI] [PubMed] [Google Scholar]
- 4.Premkumar B, Agarwal A. Female Infertility and Assisted Reproduction: Impact of Oxidative Stress –Current Women’s Health Reviews, 2012, 8: 183–207.
- 5.Demir B, Guven S, Guven ES, Atamer Y, Gul T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am J Repro Immunol. 2009;62:261–7. doi: 10.1111/j.1600-0897.2009.00734.x. [DOI] [PubMed] [Google Scholar]
- 6.Vassiliadis S., k Relakis, Papageorgiou, & i. Athanassakis. Endometriosis and infertility: A multi-cytokine imbalance versus ovulation, fertilization and early embryo development. Clinical & Develop Immunology, 2005; 12(2): 125–129. [DOI] [PMC free article] [PubMed]
- 7.Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: their relationship to insulin resistance and body mass index. Dis Markers. 2009;26:163–70. doi: 10.1155/2009/465203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahm-Kähler P, Runesson E, Karin A, Brännström M. Monocyte chemotactic protein-1 in the follicle of the menstrual and IVF cycle. Mol Hum Reprod. 2006;12:1–6. doi: 10.1093/molehr/gah256. [DOI] [PubMed] [Google Scholar]
- 9.Marsillach J, Checa MA, Pedro-Botet J, Carreras R, Joven J, Camps J. Paraoxonase-1 in female infertility: a possible role against oxidative stress-induced inflammation. Fertil Steril. 2010;94:1132–4. doi: 10.1016/j.fertnstert.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 10.Younis A, Clower C, Nelsen D, Butler W, Carvalho A, Hok E, Garelnabi M. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J Assist Reprod Genet. 2012;29(10):1083–1089. doi: 10.1007/s10815-012-9831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94:1609–1615. doi: 10.1016/j.fertnstert.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Jaichander P, Selvarajan K, Garelnabi M, Parthasarathy S. Induction of paraoxonase 1 & apolipoprotein A-I gene expression by aspirin. J Lipid Res. 2008;49(10):2142–8. doi: 10.1194/jlr.M800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buscher U, Chen FC, Kentenich H, Schmiady H. Cytokines in the follicular fluid of stimulated and nonstimulated human ovaries; is ovulation a suppressed inflammatory reaction? Hum Reprod. 1999;14(1):162–166. doi: 10.1093/humrep/14.1.162. [DOI] [PubMed] [Google Scholar]
- 14.Brännström M, Friden B, Jasper M, Norman R. Variations in peripheral blood levels of immunoreactive tumor necrosis factor alpha (TNF-α) throughout the menstrual cycle and secretion of TNF-α from the human corpus luteum. Eur J Obs Gyn Repro Biol. 1999;83:213–217. doi: 10.1016/S0301-2115(99)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Amato G, Conte M, Gherardo M, Lalli E, Vitolo G, Tucker A, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101:1177–82. doi: 10.1016/S0029-7844(03)00233-3. [DOI] [PubMed] [Google Scholar]
- 16.Chiu KM, Arnaud AD, Ju J, Mayes D, Bacchetti P, Weitz S, et al. Correlation of estradiol, parathyroid hormone, interleukin-6, and soluble interleukin-6 receptor during the normal menstrual cycle. Bone. 2000;26:79–85. doi: 10.1016/S8756-3282(99)00243-4. [DOI] [PubMed] [Google Scholar]
- 17.Angstwurm MW, Gartner R, Ziegler-Heitbrock HW. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine. 1997;9:370–374. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- 18.Souter I, Huang A, Martinez-Maza O, Breen EC, De Cherney AH, Chaudhuri G, Nathan L. Serum levels of soluble vascular cell adhesion molecule-1, tumor necrosis factor-α, and interleukin-6 in in vitro fertilization cycles. Fertil Steril. 2009;91(5):2012–2019. doi: 10.1016/j.fertnstert.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Sarapik A., Velthut A., Haller-Kikkatalo K, Faure G, Marie-Christine, Carvalho M, Bittencourt, Fred´ericMassin, Raivo Uibo, and Salumets. Follicular Proinflammatory Cytokines and Chemokines as Markers of IVF Success. Clinical and Developmental Immunology. 2012; 606459, 10 pages [DOI] [PMC free article] [PubMed]
- 20.Browne RW, Shelly WB, Bloom MS, Ocquel AJ, Sandler JR, Huddleston HG. Distributions of high-density lipoprotein particle components in human follicular fluid and sera and their associations with embryo morphology parameters during IVF. Hum Reprod. 2008;23:1884–1894. doi: 10.1093/humrep/den183. [DOI] [PubMed] [Google Scholar]
- 21.Babayof R, Margalioth E, Huleihel M, Amash A, Zyllber-Haran E, Gal M, et al. Serum inhibin A, VEGF and TNF-a levels after triggering oocyte maturation with GnRH agonist compared with HCG in women with polycystic ovaries undergoing IVF treatment: a prospective randomized trial. Hum Reprod. 2006;21:1260–5. doi: 10.1093/humrep/dei475. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism. 2009;58:954–62. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol. 2009;71:652–658. doi: 10.1111/j.1365-2265.2009.03523.x. [DOI] [PubMed] [Google Scholar]
- 24.Duleba AJ, Dokras A. Is PCOS an inflammatory process? Fertil Steril. 2012;97(1):7–12. doi: 10.1016/j.fertnstert.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escobar-Morreale H, Luque-Ramírez M, González F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95:1048–1058. doi: 10.1016/j.fertnstert.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohlig M, Spranger J, Osterhoff M, et al. The polycystic ovary syndrome per se is not associated with increased chronic inflammation. Eur J Endocrinol. 2004;150:525–532. doi: 10.1530/eje.0.1500525. [DOI] [PubMed] [Google Scholar]
- 27.Pellicer A, Albert C, Mercader A, et al. The follicular and endocrine environment in women with endometriosis: local and systemic cytokine production. Fertil Steril. 1998;70:425–431. doi: 10.1016/S0015-0282(98)00204-0. [DOI] [PubMed] [Google Scholar]
- 28.Bragatto FB, Barbosa CP, Christofolini DM, Peluso C, dos Santos AA, Mafra FA, Cavalcanti V, Hix S, Bianco B. There is no relationship between Paraoxonase serum level activity in women with endometriosis and the stage of the disease: an observational study. Reprod Health. 2013;10:32–37. doi: 10.1186/1742-4755-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verit FF, Erel O, Celik N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum Reprod. 2008;23:100–104. doi: 10.1093/humrep/dem340. [DOI] [PubMed] [Google Scholar]
- 30.Matorras R, Rodriguez F, Pijoan JI, Etaxanojaurequi A, Neyso JZ, Elorriaga MA, et al. Women who are not exposed to spermatozoa and infertile women have similar rates of stage I endometriosis. Fertil Steril. 2001;76:923–8. doi: 10.1016/S0015-0282(01)02833-3. [DOI] [PubMed] [Google Scholar]
- 31.Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, et al. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–31. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 32.Melo A, Rosa-e-Silva C, Rosa-e-Silva A, Poli-Neto O, Ferriani R, Vieira C. Unfavorable lipid profile in women with endometriosis. Fertil Steril. 2010;10:2433–2436. doi: 10.1016/j.fertnstert.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 33.Mihalyi A, Gevaert O, Kyama CM, Simsa P, Pochet N, De Smet F, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. 2010;25:654–664. doi: 10.1093/humrep/dep425. [DOI] [PubMed] [Google Scholar]
- 34.Fassbender A, Vodolazkaia A, Saunders P, Lebovic D, Waelkens E, Bart De M, D'Hooghe T. Biomarkers of endometriosis. Fertil Steril. 2013;99:1135–1145. doi: 10.1016/j.fertnstert.2013.01.097. [DOI] [PubMed] [Google Scholar]
- 35.Tortorella C1, Piazzolla G1, Matteo M2, Pinto V2, Tinelli R2, Sabbà C1, Fanelli M1, Cicinelli E3. Interleukin-6, interleukin-1b, & tumor necrosis factor a in menstrual effluents as biomarkers of chronic endometritis. Fertil Steril. 2014; 101:242–247. [DOI] [PubMed]
- 36.Gur M, Aslan M, Yildiz A, Demirbag R. Paraoxonase and arylesterase activities in coronary artery disease. Eur J Clin Invest. 2006;10:779–787. doi: 10.1111/j.1365-2362.2006.01727.x. [DOI] [PubMed] [Google Scholar]
- 37.Dursun P, Demirtas E, Bayrak A, Yarali H. Decreased serum paraoxonase 1 (PON1) activity: an additional risk factor for atherosclerotic heart disease in patients with PCOS? Hum Reprod. 2006;21:104–108. doi: 10.1093/humrep/dei284. [DOI] [PubMed] [Google Scholar]
- 38.Baskol G, Aygen E, Erdem F, Caniklioğlu A, Narin F, Şahin Y, Kaya T. Assessment of paraoxonase 1, xanthine oxidase and glutathione peroxidase activities, nitric oxide and thiol levels in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2012;91:326–330. doi: 10.1111/j.1600-0412.2011.01337.x. [DOI] [PubMed] [Google Scholar]
- 39.Ray A, Shah A, Gudi A. Unexplained infertility: an update and review of practice. Reprod Bio Med Online. 2012;24(6):591–602. doi: 10.1016/j.rbmo.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Markert UR, Morales-Prieto DM, Fitzgerald JS. Understanding the link between the IL-6 cytokine family and pregnancy: implications for future therapeutics. Expert Rev Clin Immunol. 2011;7(5):603–609. doi: 10.1586/eci.11.60. [DOI] [PubMed] [Google Scholar]
- 41.Demir B, Guven S, Guven ES, Atamer Y, Gul T. Serum IL-6 level may have role in the pathophysiology of unexplained infertility. Am J Repr Immunol. 2009;62:261–7. doi: 10.1111/j.1600-0897.2009.00734.x. [DOI] [PubMed] [Google Scholar]