Abstract
Purpose
The present study by using different growth factors was aimed to develop the best practical culture condition for purification of goat undifferentiated SSCs and their colonization under in vitro and in vivo conditions.
Methods
The enzymatically isolated SSCs obtained from one month old goat testes were cultured in DMEM plus FCS supplemented with different sets of growth factors (GDNF, LIF, bFGF, and EGF) for 2 weeks. At the end of each week, the morphological characteristics of cells and colonies alongside with purification rate of undifferentiated type A spermatogonia were evaluated by immunocytochemical staining and flow cytometry.
Results
The number and size of colonies in treatment groups were significantly (P < 0.01) higher than corresponding values in control group. In immunocytochemical evaluation, the proportion of KIT and PGP9.5 positive cells were significantly (P < 0.001) higher in control and treatment groups, respectively.
Conclusions
The culture medium comprising all four growth factors, especially the one supplemented with the higher concentration of GDNF, was superior to the other groups with respect to the population of undifferentiated type A spermatogonia and its propagation in culture system. Additionally, goat SSCs could colonize within the mouse testis following xenotransplantation.
Keywords: PGP9.5, KIT, Goat, Spermatogonial stem cell, Growth factors, Mouse
Introduction
Despite the economical importance of the goat industry and its importance in production of transgenic animals, there are still many more issues that should be investigated. The duration of goat spermatogenesis is about 47.7 days comprising of 4.5 seminiferous epithelium cycle of 10.6 days. During this process the germinal cells produce many proliferating cells in a transit amplifying compartment [1]. The committed proliferating cells differentiate and eventually produce large numbers of terminally differentiated cells with fully specialized capabilities [1, 2]. Spermatogonial stem cells are unipotent adult stem cells responsible for the maintenance of the fertility throughout the entire life of the male [2]. These cells can proliferate rapidly when the testis is damaged by chemicals or radiation, whereas under physiological conditions, these cells divide slowly to produce both stem cells and progenitor cells [3, 4]. SSCs transplantation into the seminiferous tubules of an infertile male can establish donor-derived spermatogenesis and produce spermatozoa that transmit the donor haplotype to progeny [5]. Additionally, as long as, they are cultured in appropriate conditions, they can acquire pluripotency and differentiate into derivatives of the three embryonic germ layers including sperm [6–8].
Studying SSCs population and associated niche in vitro and also genetically manipulation and transplantation of these SSC further provide a model to better understand adult stem cell biology, decipher the mechanisms that control SSC functions and modify the germ line for transgenesis [8–13]. Since, SSCs are present in extremely low numbers, the ability to study their self-renewal and biological characteristics requires large enough populations of pure SSCs and an in vitro system that supports this process in enriched biologically active populations of cells [2, 14]. Interestingly, using a chemically defined medium, combination of growth factors and by way of continuous subculturing could study accurately and directly the growth factor-regulated molecular mechanisms of stem cell in vitro culture system [15, 20].
Among different SSCs growth factors, the most important factor involved in the regulation of SSC renewal and proliferation is glial cell line derived neurotrophic factor (GDNF). This small molecule is normally secreted under the influence of FSH, growth factors and cytokines [16, 19] by Sertoli cells [4, 21]. GDNF is introduced as a crucial self-renewal factor for normal spermatogenesis in vivo [16, 21, 22] and in vitro maintenance [15, 16, 18, 22–24]. Additionally, exposure to the other growth factors such as basic fibroblast growth factor (bFGF), leukemia inhibitory factor (LIF) and epidermal growth factor (EGF) allow for long-term spermatogonia maintenance and their expansion in vitro and consider as cell-extrinsic effectors of SSCs activity.
To obtain a pure or highly enriched stem cell population, we used percoll density gradient because the previous study demonstrated that the isolated SSCs in this method had more typical morphological characteristics and more intensity of immunocytochemical reactivity than different methods of goat type A spermatogonia purification [26]. And also to determine the percentage of purified type A, two specific markers was used. One such a marker is c-kit, the receptor for stem cell factor (SCF), which is expressed by some Aal, A1-A4, In, and B spermatogonia [27, 28]. Another molecular marker for identification of type A spermatogonia is protein gene product 9.5 (PGP9.5) that is originally isolated as a neuron-specific protein. Antibody against this protein has been used for immunohistochemical detection of mouse [29], bovine [30], ram [31], human [32], goat [28] and porcine [33] spermatogonia.
Respecting to the inadequate information regarding to the effect of different growth factors on goat SSCs proliferation and pluripotency characteristics, and considering the potential application of transfected SSCs as an efficient tool in production of transgenic animals, the present study was aimed to introduce the most appropriate culture condition by using different sets of growth factors. Moreover, the possible colonization of xenotransplanted goat SSCs into the mouse seminiferous tubules will be assessed.
Materials and methods
Except where otherwise indicated, all chemicals were obtained from the Sigma (St. Louis, MO, USA).
Collection and evaluation of testes
Following castration of one month old goat, the testes were transported to the lab in transition media (PBS supplemented with 100 IU/ml penicillin and 100 μg/m1 streptomycin) at 37 °C. After macroscopic evaluation of the testes for any pathologic signs, the testes were washed (3 times) with transition media and prepared for tissue digestion.
Cell isolation and preparation
Single cell suspensions were prepared using a protocol previously described [28]. Briefly, after removing the tunica albuginea and visible connective tissues, the testes samples were transferred into the Dulbeco Modified Essential Medium (DMEM) supplemented with NaHCO3 (14 mol/L), Hepes (15 mol/L), NEAA (10 μl/ml), penicillin (50 IU/ml) and streptomycin (50 mg/ml) for 5–8 min. The SSCs were isolated through two step digestion method by collagenase type 1 (1 mg/ml) and trypsin/EDTA (0.25 %/1 mM) respectively. The supernatant was processed by sequential filtration through 60 μm nylon mesh (Small part, F062N-08-C). The filtrate was centrifuged at 500 g for 5 min, and the pellet was then resuspended in DMEM supplemented with 10%FBS (Gibco). In the final cell suspension, total cell number and viability rate were determined by Trypan Blue staining. The different testicular cell types including Sertoli cells and spermatogonia were identified by light and fluorescence microscopes for detection of PGP9.5 and KIT positive cells using immunostaining technique.
Enrichment of goat undifferentiated SSCs by discontinuous percoll density gradient
The spermatogonial stem cells were enriched from cell suspensions using a protocol as previously described [26]. Briefly, percoll was diluted to gradient concentrations of 20 %, 28 %, 30 % and 32 % with PBS. The gradient was made in a sequence that the highest density percoll solution came in bottom and that of the lowest in the top of the tube. The cell suspension was slowly layered on the top of the above gradient and centrifuged at 800 g for 30 min at 18 °C. After washing the collected cells from each gradient, the cell number and viability were determined by Trypan Blue staining. Two immunocytochemical reactions were successively performed using PGP9.5 and KIT primary antibodies for identification and purity detection of SSCs. U373 MG human glioma cells were used as a positive control.
Immunocytochemical staining
Type A spermatogonia was identified through immunocytochemical staining according to the protocol previously described [26]. Briefly after washing the collected cells with PBS-Tween 20 (0.2 % in PBS), approximately 5 × 104 cells were cytospun at 400 rpm for 5 min and fixed in acetone (2 min in −20 °C). Antigen retrieval was performed in citrate buffer (0.01 M, pH 6.0) for 8 min. All sections were exposed to 0.3 % H2O2 for 15 min in dark to inhibit endogenous peroxidase and washed in PBS-Tween. The unspecific sites blocking was done with avidin/biotin and 5 % sheep serum for 10 min. Subsequently, the slides were incubated with unconjugated primary antibodies including rabbit anti-PGP9.5 (Dako, Carpinteria, CA, USA) and rabbit anti KIT (Santa Cruz, CA, USA), each used at 1:400 in PBS with 2.5 % goat serum (PBS-GS), for 1 h at room temperature. After three times washing in PBS-Tween, the sections were exposed to secondary antibody (biotinylated sheep anti-rabbit IgG, Avicenna Research Institute, Iran) for 45 min at room temperature. The sections were exposed to HRP-conjugated streptavidin (Biosource, USA) with 1:250 dilution for 30 min, and then washed with PBS-Tween. At the final step, color was developed by the addition of 3, 3′-Diaminobenzidine (DAB; Roche, Germany) for 10 min. The slides were counterstained with Hematoxylin for 30 s and mounted by glycerol/PBS (50/50). The percentages of PGP9.5 and KIT positive and negative cells were evaluated by two blinded evaluators. Each evaluator inspected at least 5 high-powered fields on each slide for diagnosis of intensity immunohistochemical reactivity with ImageJ 1.37v software (National Institutes of Mental Health, Bethesda, MD, USA).
Culture condition and colony assessment
Freshly collected cells from the gradients with the highest purity of undifferentiated type A spermatogonia (PGP9.5 positive cells) were seeded at a concentration of 2 × 104 cells/cm2 in 12-well cell chamber slide (Falcon, USA). The basic culture system consisted of high glucose DMEM (GibcoBRL) supplemented with either 10 % FBS, 1 % penicillin-streptomycin (GibcoBRL). According to the group’s name, the cells were propagated in culture medium without growth factors (control group) or with any combination of LIF (10 ng/ml), GDNF (20 and 40 ng/ml), EGF (20 ng/ml), or bFGF (10 ng/ml) in ten groups (Table 1). The cells were cultured at 38 °C, at humidified atmosphere with 5 % CO2 for two weeks and refreshed once a week. Seven and 14 days after culture initiation, the appearance, number, size, and shape of SSCs colonies were examined in each group. After 7 days, the cells were evaluated by flow cytometry using polyclonal antibodies against KIT. At the end of second week, the effect of 10 combinations of growth factors on the purification of SSCs was determined by immunocytochemical staining using polyclonal antibodies against PGP9.5 and KIT molecular markers. All experimental groups were evaluated four times.
Table 1.
Colony sizes of goat SSCs after short term culture in the presence of different growth factors
| Treatment | Size (μm) | ||||||
|---|---|---|---|---|---|---|---|
| ≤20 | >20–40< | >40 | P | ||||
| mean ± SE | mean ± SE | mean ± SE | |||||
| Wk1 | Wk2 | Wk1 | Wk2 | Wk1 | Wk2 | ||
| Control | 91.0 ± 4.5 A | 82.9 ± 8.5 a, A | 8.9 ± 4.5 B | 15.4 ± 3.3 a, B | 0.00 ± 0 B | 1.21 ± 0.5 a, c, B | 0.0 |
| LIF | 50.6 ± 22.7 | 53.8 ± 8.1 a, b | 36.3 ± 11.2 | 42.0 ± 8.1 a, b | 13.2 ± 4.8 | 4.22 ± 6.6 a, c | 0.1 |
| FGF | 51.3 ± 19.9 | 46.9 ± 9.6 a, b | 41.1 ± 10.5 | 47.6 ± 5.5 a, b | 7.62 ± 3.0 | 5.4 ± 1.9 a, c | 0.13 |
| EGF | 57.4 ± 12.4 A | 35.6 ± 5.5 b, A | 39.1 ± 10.3 A | 58.8 ± 8.1 b, A | 4.73 ± 1.3 B | 5.61 ± 2.8 a, c, B | 0.02 |
| FGF + EGF | 39.8 ± 19.3 | 29.3 ± 3.9 b | 46.1 ± 9.0 | 67.9 ± 3.8 b, A | 14.0 ± 6.3 B | 2.68 ± 4.8 a, c, B | 0.01 |
| EGF + LIF | 45.7 ± 16.3 | 24.3 ± 5.7 b | 47.3 ± 10.5 | 67.9 ± 4.4 b, A | 6.75 ± 5.8 B | 7.74 ± 1.9 a, d, B | 0.02 |
| FGF + LIF | 58.9 ± 13.8 A | 38.8 ± 5.4 b | 35.7 ± 10.9 | 58.4 ± 7.2 b, A | 5.66 ± 3.4 B | 2.77 ± 3.0 a, c, B | 0.01 |
| FGF + EGF + LIF | 34.4 ± 15.8 | 28.1 ± 6.2 b | 49.5 ± 10.2 | 59.9 ± 3.0 b | 16.1 ± 6.1 | 11.97 ± 1.2 a, b, c, d | 0.09 |
| FGF + EGF + LIF + GDNF (20 ng/ml) | 22.2 ± 2.1 A | 17.9 ± 1.8 b, A | 66.1 ± 3.8 B | 57.8 ± 3.0 b, B | 11.7 ± 5.8 A | 24.2 ± 2.0 b, A | 0.0 |
| FGF + EGF + LIF + GDNF (40 ng/ml) | 25.2 ± 1.6 A | 20.2 ± 2.3 b, A | 57.0 ± 2.5 B | 58.5 ± 4.2 b, B | 17.75 ± 4.3 A | 24.2 ± 2.0 b, A | 0.0 |
| P | 0.3 | 0.0 | 0.2 | 0.002 | 0.4 | 0.0 | |
A, B Numbers with different upper case superscript letters in the same row differ significantly
a-c Numbers with different lower case superscript letters in the same column differ significantly
Flow cytometry
The process of cell staining for flow cytometry was performed at the end of first week in each group. For this purpose, cells were washed with PBS-Tween 20 for 5 min, exposed with avidin/biotin for 10 min and blocked with the blocking buffer (20 % sheep serum) for 30 min at 37 °C. The cells were then incubated with the primary antibody including rabbit anti KIT antibody (Santa Cruz, Santa Cruz, CA, USA) for 1 h at room temperature, washed with PBS-Tween as above and incubated with the secondary antibody (biotinylated sheep anti-rabbit IgG, Avicenna Research Institute, Iran) for 45 min at room temperature. The cells were then exposed to strepavidin FITC for 30 min and analyzed with Flow cytometer Partec PAS (Münster, Germany).
Germ cell transplantation
Adult C57BL/6 mice (14–15 weeks of age) received a single i.p. injection of busulfan (30 mg/kg, Sigma, St. Louis, MO) to deplete endogenous germ cells. The mice were maintained at 20 ± 1 °C with 35–65 % humidity under a light:dark cycle of 12 h:12 h. Six weeks after busulfam treatment, approximately 30 μl of donor testicular suspension (45 × 104 cells/each injection) was transplanted into rete testis using microinjection needle in one of the testes of each of the recipient mice; the other testis served as an internal control. Positive controls were mouse seminiferous tubules injected with donor goat testes cells one week before analysis, at which time donor cells should be abundant. Negative controls were seminiferous tubules not receiving injection of donor testicular cells.
Histological and immunohistochemical evaluations of recipient testes
The testes of busulfan-injected mice were collected 80 days after transplantation and examined for histological and immunohistochemical staining by using an antibody against PGP9.5. For histological evaluation a small sample of testes fixed overnight in Bouin’s solution, washed in 70 % ethanol, and sectioned at 5 μm using standard procedures. The sections were stained with Hematoxylin & Eosin and examined under a light microscope for identification of cell types and their developmental stages. In immunohistochemical staining, the prepared slides were fixed in cold neutral buffered formalin (NBF) for 5 min and immunostained as described above for immunocytochemistry except that the washing was done by Tris-buffered saline containing 1 % bovine serum albumin (TBS/BSA) (three times, 4 min each). The slides were mounted in Entellan (Merck, Germany).
Statistical analysis
The results were expressed as mean ± SE. The statistical significance between the mean values was determined by one-way ANOVA analysis of variance (Tukey Test). A P < 0.05 was defined as statistical significance.
Result
Validation of type A spermatogonia by immunocytochemical evaluation
There was a difference in intensity of PGP9.5 and KIT immunostaining between spermatogonia. Basal (As, Ap) spermatogonia expressed significantly more intensity of PGP9.5 marker (83.82 ± 37.06; strong expression) than aggregated (Aal) spermatogonia (118.48 ± 25.52; moderate expression), while the other cells such as Sertoli cells and committed (A1-A4) spermatogonia were negative (166.79 ± 24.82, 160.93 ± 26.78 respectively; no expression; Fig.1c and d). The expression pattern and color intensity of KIT marker were different, so that the majority of aggregated spermatogonia had a stronger KIT immunoreactivity (62.34 ± 22.65; strong expression) than committed spermatogonia (78.18 ± 19.26; moderate expression). In contrast, no immunoreactivity was observed in Sertoli cells and basal spermatogonia (103.23 ± 26.85 and 113.02 ± 29.29, respectively) (Fig. 1e and f). The immunohistochemical evaluation of seminiferous tubules of one month old and adult goats’ testes was also confirmed the KIT immunoreactivity in testicular cells (Fig. 2).
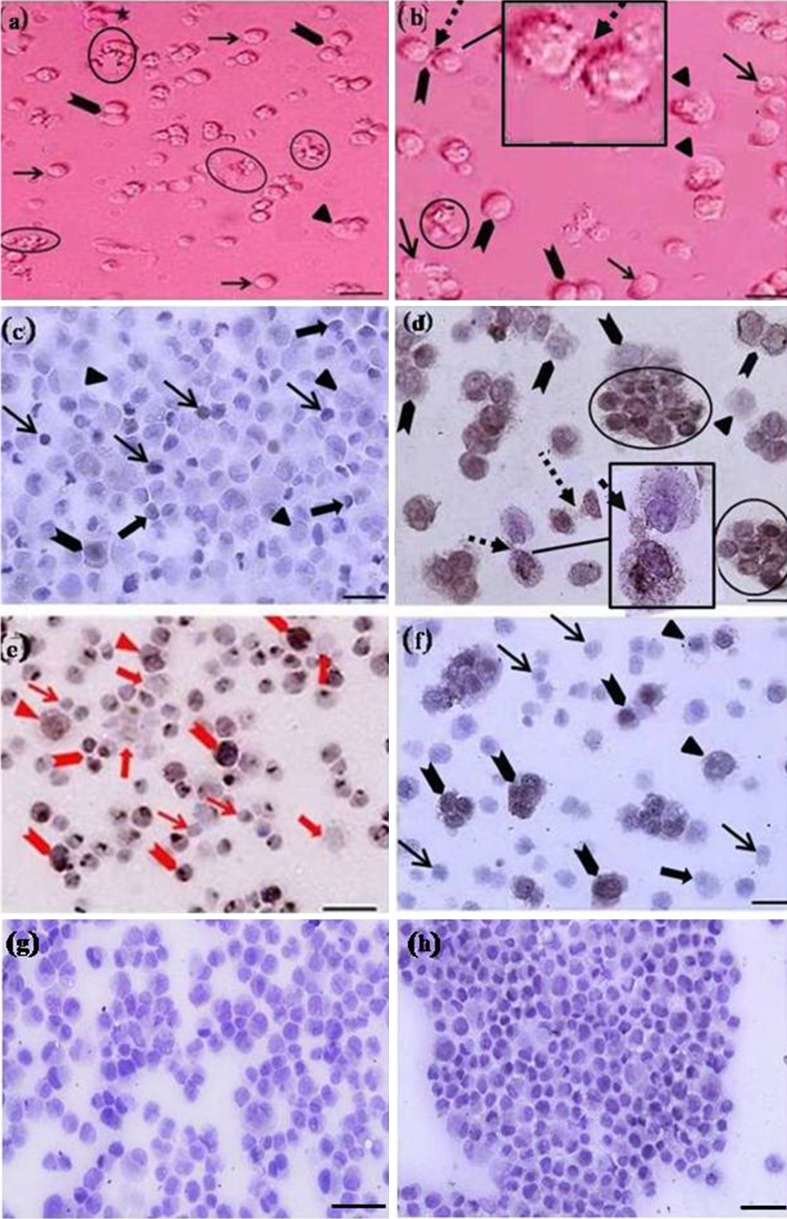
Fig. 1.
Cytological (a and b) and immunocytochemical (c to f) evaluations of 1 month old goat testes before (a, c and e) and after (b, d and f) percoll purification, using antibody against PGP9.5 (c and d) and KIT (e and f). The presence of three groups of spermatogonia with different sizes was determined. Both basal (arrow, c; encircle, d), which positioned in direct contact with each other, and some aggregated (arrowheads, c, d) type A spermatogonia were PGP9.5 positive. Note the strong and weak PGP9.5 immunoreactivity was observed among basal (arrow, c; encircle, d) and some aggregated spermatogonia (arrowheads, c and d), respectively. Committed spermatogonia (triangles, c and d) and surrounding Sertoli cells (encircle, a and b; block arrow, c) were negative for PGP9.5. The difference in the intensity of KIT staining among spermatogonia was noticeable. Strong and weak KIT immunoreactivity was observed among some aggregated (arrowheads, e and f) and committed (triangles, e and f) spermatogonia respectively. Basal spermatogonia (arrows, e and f) and surrounding Sertoli cells (block arrows, e and f) were negative for KIT. Note the cytoplasmic bridges were identified between spermatogonia (dot arrows, b and d). Negative IgG controls for KIT (g) and PGP9.5 (h) were determined. Scale bars represent (a, c, e to h) 50 μm, (b and d) 45 μm
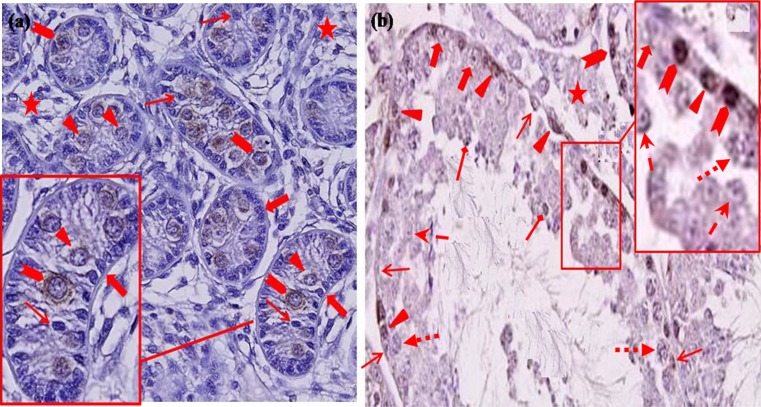
Fig. 2.
The Immunohistochemical identification of type A spermatogonia in one month old (a) and adult (b) goats’ testes using an antibody against KIT. Immunoreactivity was strongly identified in the cytoplasm of gonocytes. Three groups of spermatogonia with different sizes were determined. Basal spermatogonia (arrows, a and b), Primary spermatocytes (square arrows, b), Secondary spermatocyte (dash arrows, b), Spermatid (diamond arrows, b), Sertoli (block arrows, a and b) and leydig cells (Asterisk, a and b) were negative for KIT. Aggregated (arrowhead, a and b) and Committed (triangles, a and b) spermatogonia were KIT positive, Scale bars represent 50 μm
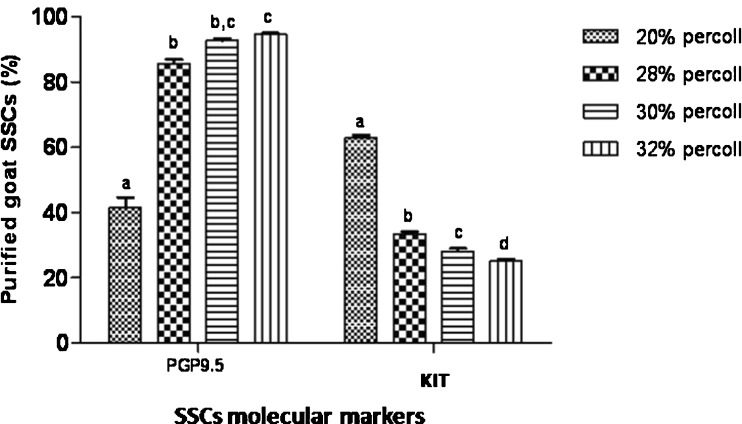
Enrichment of type A spermatogonia by discontinuous percoll gradient
The data was indicated the significant differences in the percentage of type A spermatogonia in cell population across the individual gradients (P < 0.001). Concurrent with the increment of percoll gradient, the number of differentiated type A spermatogonia was decreased (P < 0.001, Fig. 3). The proportion of undifferentiated type A in 32 % gradient (94.6 ± 0.4 %) was significantly higher than 20 and 28 % gradients (41.5 ± 3.1 %, 85.7 ± 1.2 % respectively; P < 0.001; Fig. 3). The highest and lowest number of collected cells, including somatic and stem cells, was achieved at 28 % and 30 % density, respectively (1,147,880 cells/ml vs. 121,660 cells/ml). Though, the maximum number of undifferentiated type A SSCs was obtained at 32 % percoll density (1,286,833 cells/ml). The cell viability in all fractions was found to be almost identical ( > 90 %).
Fig. 3.
Goat SSCs purification using different percoll gradients, confirmed by SSCs molecular markers a to d For each molecular marker, columns with different lowercase letters differ significantly (P < 0.001)
Cell adhesion and morphology of goat SSCs
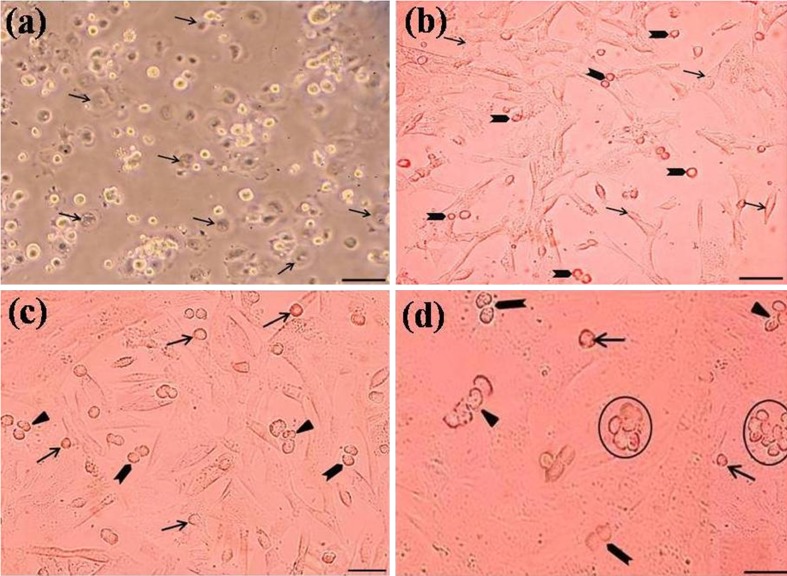
Cell recovery after tissue digestion was about 5 × 106 cells per gram of testis. After enrichment, approximately 14 × 105 cells/ml were collected from the gradients of 30 and 32 % percoll and then cultured. All cultures started with a mixed population of enriched cells including spermatogonia and a low number of Sertoli cells (Figs. 1b and 4). During the process of cultivation, the somatic cells began to stick to the culture plate after 3 to 6 h (Fig. 4a) and were completely stuck to it within 12 h (Fig. 4b). After two days, the somatic cells were constituted a monolayer with 80 % confluency as a feeder layer and the most spermatogonia were attached on its surface (Fig. 4c). Three days after culture initiation, a confluent feeder layer was formed and spermatogonial cells were seen to different forms (Fig. 4d).
Fig. 4.
Typical morphology of somatic and spermatogonial cells during the process of cultivation 4 h (a), 12 h (b), 2d (c) and 4d (d). All cultures started with a mixed population of cells. Somatic cells including Sertoli and leydig cells began to stick to the culture plate after 4 h (arrow, a) and completely stuck to it within 12 h (arrow, b). After two days (c), the somatic cells formed the feeder layer with grown spermatogonia at its surface in three forms; single (arrow, c), paired (arrowhead, c), 3-cell chains (triangle, c). Three days after culture initiation (d), feeder layer was confluent and the spermatogonia were seen to different forms: single (arrow, d), paired (arrowhead, d), multi-cell chains (triangle, d) and small clumps (encircle, d). Scale bars represent (a, b) 50 μm, (c) 45 μm and (d) 40 μm
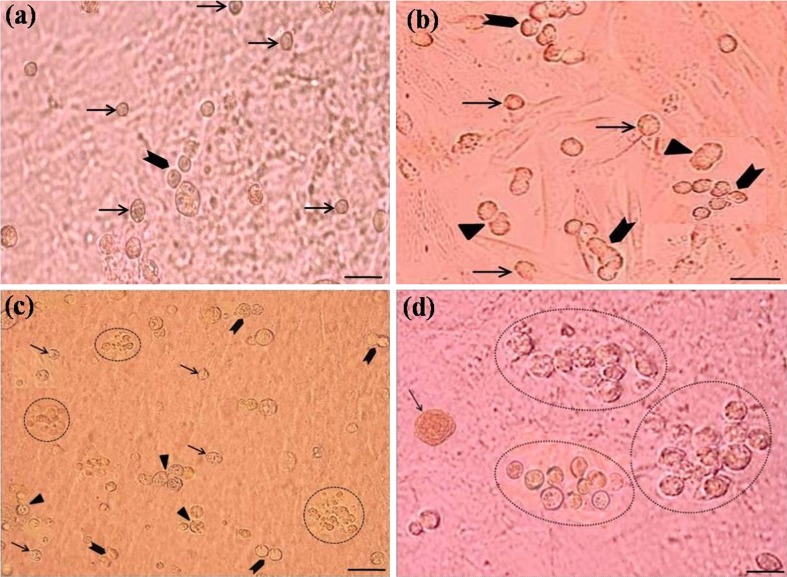
The colony-forming cells, SSCs, in the initial suspension approached each other and became tightly packed locally in some areas of the culture plate. About 90 % SSCs attached to the feeder layer 2–3 days after inoculation. At this time, different forms of type A spermatogonia (mostly single, sometimes paired, multi-cell chains and or small cluster) were observed (Fig. 4c and d). After 4–5 days many small colonies, mostly single, circle to oval, biconvex, and interconnected through cytoplasmic bridges, were formed on the surface of somatic cells monolayer (Fig. 5a). Indeed, when the second spherical colony formed, it commonly remained connected to the first by cytoplasmic elongation of SSCs. After culturing for 6–7 days, some align colonies, with higher than 6-single connected colony, were detected so that the chain length and the number of cells were progressively increased. At this time, the colonies grew bigger and sometimes reached to 50–60 μm (Fig. 5b). One week after cultivation, we observed cell aggregation and grape-like cluster (Fig. 5c and d). A few number of small mulberry-shaped (rosette form) colonies with a distinct boundary from feeder layer was detected after 12 days in control group (Fig. 5d). With increasing the duration of culture to 2 weeks, the cells (somatic and germ cells) and colonies number were increased.
Fig. 5.
Typical development of colonies appearing under the effect of the growth factors after 4d (a), 6d (b), 1w (c) and 2w (d) cultures. After 4 days many small colonies were formed mostly single, circle to oval and biconvex on top of the monolayer (arrows, a). After culturing for 6 days, single (arrow, b), paired (triangle, b) and some align chains with higher than 6-connected colonies (arrowhead, b) were detected. After one week, colonies were observed in different sizes and forms including single (arrow, a, b), paired (arrowhead, c), aligned (triangle, c), grape-like cluster (encircle, c). Clusters with long chains (encircle, d) and rosette form colonies (arrow, d) were observed after 2 weeks culture. Scale bars represent (a, c) 50 μm and (b, d) 40 μm
Effect of growth factors on colonies characteristic
The transferred cells to the culture plate were attached at the bottom of plate but the proliferation of spermatogonia was not observed in control group. The greatest number of somatic cells was observed in EGF group.
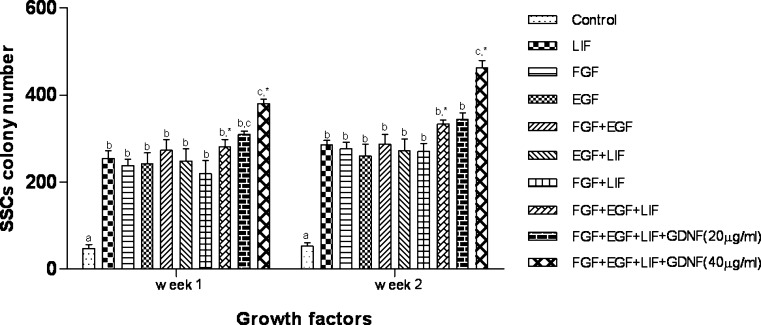
In the presence of growth factors, especially higher concentration of GDNF, more colonies appeared as very large ovoid structures with morphologically normal spermatogonia with a big round nucleus and, usually, 2–3 large nucleolus. Gradually during the first and second weeks of culture, the number of spermatogonia and, therefore, the number and size of colonies were increased (Fig. 6, Table 1). The least and greatest numbers of colonies at the end of each week was observed in control and 40 ng/ml GDNF supplemented groups, respectively (Fig. 6). Concerning to the colony number, the addition of all growth factors to culture medium could increase the size of colonies to 20–40 μm that was associated with increased SSCs proliferation. Addition of GDNF to culture medium, through increasing the SSCs proliferation and inhibition of type A spermatogonia differentiation, was significantly decreased the number of small ( ≤ 20) and large ( ≥ 40) colonies at the end of each weeks (Table 1). And also, higher concentration of GDNF decreased the length of the chains to 4–5 colonies. The highest number of small ( ≤ 20) and rosette form colonies was detected in control group. The most colonies in this group were remained in small size and then gradually degenerated (Tables 1 and 2).
Fig. 6.
Goat SSCs colony numbers after short term culture in the presence of different growth factors a to c Columns with different lower case letters in each week differ significantly (P < 0.001). *Represent significant difference between corresponding columns in weeks 1 and 2
Table 2.
Colony shapes of goat SSCs after short term culture in the presence of different growth factors
| Treatment | Shape | ||||||
|---|---|---|---|---|---|---|---|
| Single | Multiple | Rosette | P | ||||
| mean ± SE | mean ± SE | mean ± SE | |||||
| Wk1 | Wk2 | Wk1 | Wk2 | Wk1 | Wk2 | ||
| Control | 69.41 ± 0.950a | 64.23 ± 2.25a | 3.63 ± 0.76a | 5.94 ± 2.06a | 30.94 ± 1.24b | 35.82 ± 3.04b | 0.000 |
| LIF | 92.00 ± 2.89a | 93.79 ± 3.41a | 7.00 ± 2.31b | 1.65 ± 0.342b | 1.00 ± 0.577b | 4.56 ± 3.18b | 0.000 |
| FGF | 89.60 ± 3.21a | 87.00 ± 4.04a | 9.66 ± 2.96b | 11.65 ± 2.73b | 0.73 ± 0.730b | 1.00 ± 1.00b | 0.000 |
| EGF | 89.33 ± 1.76a | 88.71 ± 2.75a | 9.00 ± 0.577b | 10.01 ± 2.04b | 1.67 ± 1.201b | 1.27 ± 1.27b | 0.000 |
| FGF + EGF | 92.68 ± 3.53a | 85.67 ± 3.93a | 6.65 ± 2.92b | 13.33 ± 4.37b | 0.667 ± 0.667b | 1.00 ± 0.578b | 0.000 |
| EGF + LIF | 93.46 ± 2.92a | 90.56 ± 3.95a | 6.20 ± 2.61b | 7.47 ± 3.52b | 0.333 ± 0.333b | 1.00 ± 0.577b | 0.000 |
| FGF + LIF | 93.79 ± 2.29a | 90.42 ± 3.19a | 5.88 ± 2.39b | 9.24 ± 3.33b | 0.333 ± 0.333b | 0.333 ± 0.333b | 0.000 |
| FGF + EGF + LIF | 90.70 ± 0.306a | 91.28 ± 3.22a | 8.96 ± 0.026b | 7.04 ± 2.92b | 0.330 ± 0.330b | 1.67 ± 0.337b | 0.000 |
| FGF + EGF + LIF + GDNF (20 ng/ml) | 86.41 ± 7.78a | 89.98 ± 2.63b | 12.92 ± 7.12c | 10.02 ± 2.63c | 0.667 ± 0.667c | 0.00 ± .00c | 0.000 |
| FGF + EGF + LIF + GDNF (40 ng/ml) | 85.90 ± 0.675a | 90.83 ± 1.62b | 14.09 ± 0.675c | 8.83 ± 1.66c | 0 ± 0c | 0 ± 0c | 0.000 |
| P | 0.579 | 0.867 | 0.344 | 0.262 | 0.775 | 0.253 | |
a-c Numbers with different lower case superscript letters in the same raw differ significantly
Effect of adding growth factors on enrichment of type A spermatogonia
Evaluation of KIT expression by flow cytometric technique at the end of first week showed that the percentage of differentiated type A spermatogonia in control group was significantly higher than EGF + LIF, triple and all growth factors supplemented groups (Table 3). The addition of growth factors to culture medium was significantly increased the expression of PGP9.5 antigen on the cell surface of spermatogonia after two weeks culture (Table 4). The medium supplemented with all four growth factors, especially higher concentration of GDNF, was superior to the other groups in term of proportion of undifferentiated spermatogonia in culture (P < 0.001).
Table 3.
Flowcytometery of goat SSCs with c-kit after one week culture
| Treatment | c-kit (%) mean ± SE |
|---|---|
| Control | 56.5 ± 2.3 a |
| LIF | 55.5 ± 5.9 a |
| FGF | 39.9 ± 3.8 a, b |
| EGF | 49.3 ± 6 a, c |
| FGF + EGF | 39.3 ± 4.5 a, b |
| EGF + LIF | 31.4 ± 10.6 b, c |
| FGF + LIF | 48.6 ± 10.6 a |
| FGF + EGF + LIF | 34.2 ± 5.5 b, c |
| FGF + EGF + LIF + GDNF (40 ng/ml) | 22.8 ± 2.8 b |
a-c Numbers with different lower case superscript letters in the same column differ significantly (P < 0.001)
Table 4.
Goat SSCs immunocytochemical staining for PGP9.5 and c-kit molecular marker after two weeks culture
| Treatment | Molecular marker | |
|---|---|---|
| PGP9.5 (%) | c-kit (%) | |
| mean ± SE | mean ± SE | |
| Control | 2.40 ± 0.4a | 95.4 ± 1.1a |
| LIF | 60.5 ± 1.4b | 40.2 ± 1.3b |
| FGF | 64.5 ± 2.2 b, e | 29.8 ± 2.1c |
| EGF | 48.1 ± 2.6c | 72.4 ± 2.3d |
| FGF + EGF | 47.7 ± 2.2c | 60.1 ± 1.6e |
| EGF + LIF | 77.5 ± 1.7 d, f | 41.3 ± 2.3b |
| FGF + EGF + LIF | 78.2 ± 1.4f | 35.1 ± 1.3b |
| FGF + EGF + LIF + GDNF (20 ng/ml) | 72.8 ± 1.9 f, e | 26.0 ± 1.6c |
| FGF + EGF + LIF + GDNF (40 ng/ml) | 90.4 ± 0.9g | 7.00 ± 1.8f |
a-g Numbers with different lower case superscript letters in the same column differ significantly (P < 0.001)
Evaluation of SSCs colonization in recipient testes
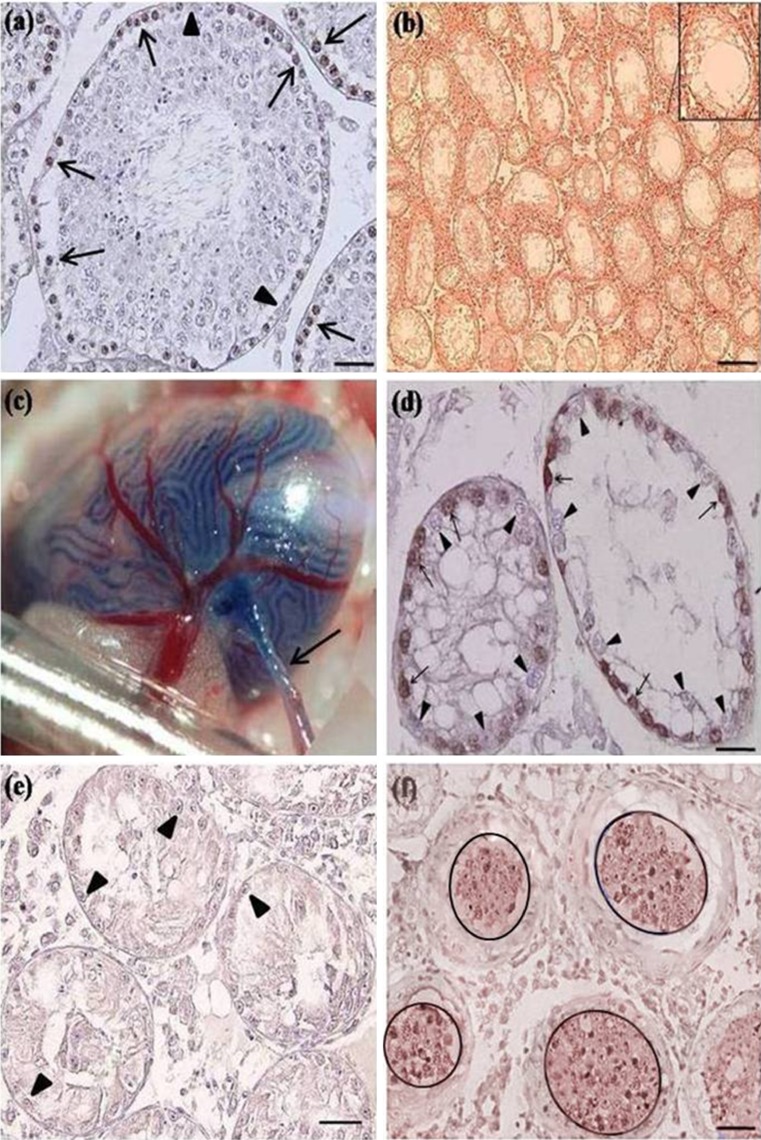
The immunohistochemical examination of adult mouse testis showed that the undifferentiated type A spermatogonia were found round with a spherical nucleus at the base of the seminiferous epithelium (Fig. 7a). Histological and immunohistochemical examinations of the testes in busulfan-treated group revealed that the optimal effect of busulfan on the depletion of spermatogenic cells were seen 6 week after treatment so that the seminiferous epithelium of all tubules was composed of only a single, basal row of Sertoli cells (Fig. 7b and e). Donor goat spermatogonia were able to survive and colonize in depleted recipient’s testis 80 days after transplantation (Fig. 6d). Negative control mouse testes showed no significant cellular staining (Fig. 7e), however an abundance of positively stained cells identified in the positive control group after immunohistochemical procedure (Fig. 7f).
Fig. 7.
Immunohistochemical identification of spermatogonia in mouse seminiferous tubules using an antibody against PGP9.5. In adult mouse testis, immunoreactivity was strongly localized in the nuclear and faintly in the cytoplasm of spermatogonia (arrow, a). Histological characterization of busulfan-treatment mouse testis 6 weeks after busulfan injection (b). Note all seminiferous tubules were devoid of spermatogenic cells and no indication of spermatogenesis was seen in recipient mouse. Injection of goat testicular suspension into mouse rete testis was shown (arrow, c). Busulfan-injected testis 80 days after transplantation (d). Note the maintenance and colonization of goat undifferentiated spermatogonia (arrow, d) in proximity of Sertoli cells (triangle, d) at the base membrane of mouse seminiferous epithelium. Negative (e) and positive (f) controls, receiving no cell injection and goat testicular injection one week before analysis respectively, were demonstrated. The seminiferous epithelium was composed of only a single, basal row of Sertoli cells (triangle, e). Note no significant cellular staining for negative control and the abundance of positively stained cells in positive control (encircle, f). Scale bars represent (a and f) 20 μm, (b) 55 μm, (e and d) 15 μm
Discussion
Improving our knowledge of spermatogenesis and introduction of a practical procedure to enrich the goat undifferentiated SSCs is considered of great importance especially in production of transgenic animals. Therefore, having established an appropriate method for goat SSCs purification and introduction of a proper medium for subsequent enrichment are of important concerns.
Type A spermatogonia are small self-renewing subpopulation of SSCs that have been located in the basal compartment of seminiferous tubules [8]. In present study, after tissue digestion, nearly all large type A spermatogonia were KIT positive in immunocytochemical staining. In contrast, this proportion was much lower in the population of small spermatogonia (As, Ap). This finding confirmed the SSCs classification performed by Wrobel et al. (1995) indicating the smaller spermatogonia are less differentiated than larger spermatogonia [34]. The difference in size of the spermatogonia might be related to either the phase of cell cycle [35] or the differentiation status of the cells [28, 36].
In our study, the viability rate of isolated goat testicular cells, > 90 %, was notably more than what was reported in other species such as prepubertal boar SSCs; 45 % [37], prepubertal mouse germ cells; 30 % [38], pubescent mice spermatogonia; 68 % [39], goat SSCs; 65–70 % [40] and bovine spermatogonia; > 80 % [36]. The higher percentage of viability rate in present study might be related to the type or concentration of used digestive enzymes, applied protocol, as well as the age and species of animals. The maximum type A spermatogonia purification (94.6 ± 0.4 %) was achieved by using 32 % percoll density gradient that was slightly higher than those reported in other species including: rat with 80 ± 6.1 % SSCs purification using 30–32 % gradient [41], pig with 83.62 ± 4.24 % SSCs purification using 30–45 % gradient [42], and humane with 86.7 % SSCs using 27–35 % gradient [43]. The cell viability after percoll enrichment in previous studies ranged between 70 and 95 % [26, 36, 41, 42] which was consistent with our results ( > 90 %). The cultured SSCs of gradients 32 and 30 % tended to congregate and to form the small cell clusters with cytoplasmic bridges between cells. These results were comparable to those reported for the isolation of type A spermatogonia from the testes of 5–7 months calves [36], prepubertal mice [44], rats [45], and pigs [37].
Ideally, to establish the culture condition that could direct a specific type of self-renewal divisions, we use chemically defined media for preservation of stem cell potential in enriched cells populations. As shown, addition of any growth factors to culture condition improved SSCs proliferation and induced the formation of many organized colonies. These clusters gradually continued to grow during their development, evolved to a higher level of organization, and formed many colonies with unique shapes. Our finding was consistent with the previous results indicating that the number of viable bovine, mouse and goat undifferentiated type A spermatogonia were gradually decreased to more than half after 7 days [28, 39, 46, 47] and the most SSCs were differentiated to type B spermatogonia and spermatocytes in the lack of growth factors [14–16, 24, 43, 47–51] . After one week culturing, we demonstrated the distinct cell aggregations and different forms of SSCs colonies (mostly single with multiple shapes) which was consistent with observations in bovine and mouse SSCs colonies [46, 48].
Although the molecular mechanisms controlling SSCs self-renewal is still largely unknown, in vivo and in vitro studies have clearly demonstrated a critical extrinsic mitogenic effect of GDNF on SSCs self-renewal [16, 22, 24, 52]. In present study, we showed that the addition of GDNF, especially at higher concentration, induced logarithmically self-renewing divisions of type A spermatogonia without losing their capacity and stimulated the stem cells to form more, denser and bigger clusters. This was in accordance to other studies in mouse, rabbit and bovine [2, 4, 16, 22]. Aponte et al. (2008) demonstrated that addition of GDNF alone or in combination with other growth factors to bovine SSCs culture medium induced the highest numbers of type A spermatogonia (DBA positive cells) in vitro [2]. In this context, Kanatsu-Shinohara et al. (2003, 2005) reported that GDNF significantly increased the viability rate of mouse SSCs for more than 6 months and that the stem cells could not grow in the absence of this hormone, even when the medium was supplemented with both EGF and bFGF [17, 18]. Oately et al. (2006) demonstrated that the expression of bcl6b and Sox2 in mouse SSCs were significantly reduced (6.8-fold and 5.95-fold, respectively) by withdrawing and replacing GDNF in culture medium so that this expression reduction resulted in decrease in total mouse SSCs numbers and clump size after one week culture [14].
Here, we showed that the addition of higher concentration of GDNF was significantly increased colony number and size and improved the morphology of cells and colonies. This was consistent with the results of other researchers that demonstrated the mouse and bovine SSCs self-renewal potential was reduced due to decreased concentration of GDNF in medium [2, 18]. The ablation of GDNF by gene targeting in heterozygous Gdnf knockout mice progressively induced the differentiation process and resulted in seminiferous tubules without any stem cells [4, 21].
In the present study, GDNF amplified the effect of other growth factors in colony characteristics and total number of undifferentiated spermatogonia after one and two week’s culture. Our finding was consistent with the previous results indicating the supportive effect of GDNF on other growth factors in vitro expansion of SSCs [2, 15, 16, 23, 25].
It has been shown that bFGF besides its positive effects on development and stem cells integrity [14, 48], has a crucial role in maintenance of spermatogenesis [4, 15, 21, 22, 50]. The requirement to this growth factor apparently increases when SSCs form germ cell clusters [15, 51]. In present study, the stimulatory effect of bFGF on goat colonies and total number of undifferentiated type A spermatogonia was in accordance to the reports on bovine [2], rat [48, 50], mouse [15–17, 23, 49], and hamster [24, 35]. We showed the direct impact of EGF on overgrowth of goat Sertoli and spermatogonia that was in accordance to the reports on bovine [2, 53], porcine [54], mouse [16] and rat SSCs [50]. The other Sertoli cell-derived growth factor, LIF, besides to its action on the proliferation of gonocytes [55] and primordial germ cells [56], can also prevent of SSCs differentiation and support spermatogonia propagation in mouse [7], rat [49, 56] and bovine [2].
To confirm the biological activity (re-establishment and colonization) of goat SSCs, we developed a xenotransplantation assay and confirmed that goat SSCs could settle at the niches within the mouse testis 80 day after transplantation. Our finding showed that the mouse testis was capable of supporting the initial steps of goat spermatogonial colonization for periods up to 80 day. However, goat gonocytes most likely did not receive the proper signaling for this conversion within recipient mouse seminiferous tubules because no significant goat spermatogonial development was observed in recipient testes. These results were consistent with those obtained by other researchers as such xenotransplantation of germ cells from rabbits, dogs, pigs, cattle, boar, horses, non-human primates, and humans resulted in colonization of their SSCs in mouse testis but spermatogenesis became arrested at the stage of spermatogonial expansion [57–60].
Conclusions
The best culture condition to maintain the goat SSCs in undifferentiation status, was achieved when growth factors supplementation was complemented by the addition of GDNF, reflected as a higher population of PGP9.5 positive cells. Additionally, the goat male germ cells after xenotransplantion into the mouse testes could maintain their morphologic characteristics after 80 days.
Acknowledgments
The authors would like to thank the Avicenna Research Institute for technical and financial supports, ACECR, Tehran.
Footnotes
Mohammad Sadra Shirazi and Banafsheh Heidari these authors contributed equally to the work.
Capsule Spermatogonial stem cells are the foundation of spermatogenesis. Addition of growth factors to the culture medium could effectively retain the undifferentiation status of goat SSCs. The culture medium containing higher concentration of GDNF was superior to the other groups comprising either the lower concentration of GDNF or other combinations of growth factors. The colonization of goat SSCs within the mouse testis following xenotransplantation, was promising in male fertility preservation and production of transgenic animals.
References
- 1.Franca LR, Becker-Silva SC, Crhiarini-Garcia H. The length of the cycle of seminiferous epithelium in goats (Capra hircus) Tissue Cell. 1999;31:274–280. doi: 10.1054/tice.1999.0044. [DOI] [PubMed] [Google Scholar]
- 2.Aponte PM, Soda T, Teerds KJ, Mizrak SC, van de Kant HJG, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136:543–557. doi: 10.1530/REP-07-0419. [DOI] [PubMed] [Google Scholar]
- 3.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Atmos Ocean Technol. 2000;21:776–798. [PubMed] [Google Scholar]
- 4.Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 5.Dobrinski I. Advances and applications of germ cell transplantation. Hum Fertil. 2006;9:9–14. doi: 10.1080/14647270500440671. [DOI] [PubMed] [Google Scholar]
- 6.Hong Y, Liu T, Zhao H, Xu H, Wang W, Liu R, et al. Establishment of normal medaka fish spermatogonial cell line capable of sperm production in vitro. PNAS. 2004;101:8011–8016. doi: 10.1073/pnas.0308668101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature. 2006;440:1190–1203. doi: 10.1038/nature04697. [DOI] [PubMed] [Google Scholar]
- 8.Han SY, Gupta MK, Uhm SJ, Lee HT. Isolation and in vitro culture of pig spermatogonial stem cell. Asian-Australas J Anim Sci. 2009;22:187–193. doi: 10.5713/ajas.2009.80324. [DOI] [Google Scholar]
- 9.Dobrinski I, Travis AJ. Germ cell transplantation for the propagation of companion animals, non-domestic and endangered species. Reprod Fertil Dev. 2007;19:732–739. doi: 10.1071/RD07036. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida S, Nabeshima Y, Nakagawa T. Stem cell heterogeneity: actual and potential stem cell compartments in mouse spermatogenesis. Ann NY Acad Sci. 2007;1120:47–58. doi: 10.1196/annals.1411.003. [DOI] [PubMed] [Google Scholar]
- 11.Oatley MJ, Racicot KE, Oatley JM. Sertoli cells dictate spermatogonial stem cell niches in the mouse testis. Biol Reprod. 2011;84:639–645. doi: 10.1095/biolreprod.110.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oatley JM, Brinster RL. The germline stem cell nich unit in mammalian testes. Physiol Rev. 2012;92:577–595. doi: 10.1152/physrev.00025.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Barros FRO, Giassetti MI, Visintin JA. Spermatogonial stem cells and animal transgenesis. In: Agbo EC, editor. Innovations in biotechnology. Rijeka: InTech; 2012. pp. 303–318. [Google Scholar]
- 14.Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. PNAS. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. PNAS. 2004;10:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubota H, Avarbock MR, Brinster RL. Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod. 2004;71:722–731. doi: 10.1095/biolreprod.104.029207. [DOI] [PubMed] [Google Scholar]
- 17.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 18.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, et al. Long-term culture of mouse male germline stem cells under serum- or feeder-free conditions. Biol Reprod. 2005;72:985–991. doi: 10.1095/biolreprod.104.036400. [DOI] [PubMed] [Google Scholar]
- 19.Kubota H, Brinster RL. Technology insight: in vitro culture of spermatogonial stem cells and their potential therapeutic uses. Nat Clin Pract Endocrinol Metab. 2006;2:99–108. doi: 10.1038/ncpendmet0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rooij DG, Mizrak SC. Deriving multipotent stem cells from mouse spermatogonial stem cells: a new tool for developmental and clinical research. Development. 2008;135:2207–2213. doi: 10.1242/dev.015453. [DOI] [PubMed] [Google Scholar]
- 21.Meng X, Lindahl M, Hyvönen ME, Parvinen M, de Rooij DG, Hess MW, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- 22.Kubota H, Wu X, Goodyear SM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties. FASEB J. 2011;25:2604–2614. doi: 10.1096/fj.10-175802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci U S A. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanatsu-Shinohara M, Muneto T, Lee J, Takenaka M, Chuma S, Nakatsuji N, et al. Long-term culture of male germline stem cells from hamster testes. Biol Reprod. 2008;78:611–617. doi: 10.1095/biolreprod.107.065615. [DOI] [PubMed] [Google Scholar]
- 25.Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL. Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development. 2009;136:1191–1199. doi: 10.1242/dev.032243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heidari B, Gifani M, Shirazi A, Akhondi MM, Zarnani AH, Behzadi B, et al. Enrichment of undifferentiated type A spermatogonia from goat testis using discontinuous percoll density gradient and differential plating. AJMB. 2014;6:94–103. [PMC free article] [PubMed] [Google Scholar]
- 27.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 28.Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, et al. Isolation, identification, and culture of goat spermatogonial stem cells using c-kit and PGP9.5 markers. J Assist Reprod Genet. 2012;29:1029–1038. doi: 10.1007/s10815-012-9828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuhiro K, Daiji E, Toshihiko I. Expression of protein gene product 9.5, a neuronal ubiquitin C-terminal hydrolase, and its developing change in sertoli cells of mouse testis. Mol Reprod Dev. 1999;54:333–341. doi: 10.1002/(SICI)1098-2795(199912)54:4<333::AID-MRD3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Hill J, Holland M, Kurihara Y, Loveland KL. Bovine sertoli cells colonize and form tubules in murine hosts following transplantation and grafting procedures. J Androl. 2008;29:418–430. doi: 10.2164/jandrol.107.004465. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Sosa JR, Dobson H, Hahnel A. Isolation and transplantation of spermatogonia in sheep. Theriogenology. 2006;66:2091–2103. doi: 10.1016/j.theriogenology.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Von Kopylow K, Kirchhoff C, Jezek D, Schulze W, Feig C, Primig M, et al. Screening for biomarkers of spermatogonia within the human testis: a whole genome approach. Hum Reprod. 2010;25:1104–1112. doi: 10.1093/humrep/deq053. [DOI] [PubMed] [Google Scholar]
- 33.Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, et al. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev. 2009;21:4. doi: 10.1071/RD08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wrobel KH, Bickel D, Kujat R, Schimmel M. Configuration and distribution of bovine spermatogonia. Cell Tissue Res. 1995;279:277–289. doi: 10.1007/BF00318484. [DOI] [PubMed] [Google Scholar]
- 35.Lok D, Weenk D, de Rooij DG. Morphology, proliferation, and differentiation of undifferentiated spermatogonia in the Chinese hamster and the ram. Anat Rec. 1982;203:83–99. doi: 10.1002/ar.1092030109. [DOI] [PubMed] [Google Scholar]
- 36.Izadyar F, Spierenberg G, Creemers L, Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction. 2002;124:85–94. doi: 10.1530/rep.0.1240085. [DOI] [PubMed] [Google Scholar]
- 37.Dirami G, Ravindranath N, Pursel V, Dym M. Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61:225–230. doi: 10.1095/biolreprod61.1.225. [DOI] [PubMed] [Google Scholar]
- 38.Sugiyama N, Obinata M, Matsui Y. BCL-2 inhibits apoptosis of spermatogonia and growth of spermatogenic stem cells in a cell-intrinsic manner. Mol Reprod Dev. 2001; 5830–38. [DOI] [PubMed]
- 39.Creemers LB, Meng X, den Ouden K, van Pelt AM, Izadyar F, Santoro M, et al. Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol Reprod. 2002;66:1579–1584. doi: 10.1095/biolreprod66.6.1579. [DOI] [PubMed] [Google Scholar]
- 40.Kaul G, Kumar S, Kumari S. Enrichment of CD9+ spermatogonial stem cells from goat (Capra aegagrus hircus) testis using magnetic microbeads. Stem Cell Disc. 2012;2:92–99. doi: 10.4236/scd.2012.23014. [DOI] [Google Scholar]
- 41.van Pelt AM, Morena AR, van Dissel-Emiliani FM, Boitani C, Gaemers IC, de Rooij DG, et al. Isolation of the synchronized A spermatogonia from adult vitamin A-deficient rat testes. Biol Reprod. 1996;55:439–444. doi: 10.1095/biolreprod55.2.439. [DOI] [PubMed] [Google Scholar]
- 42.Marret C, Durand P. Culture of porcine spermatogonia: effects of purification of the germ cells, extracellular matrix, and fetal calf serum on their survival and multiplication. J Reprod Dev. 2000;40:305–319. doi: 10.1051/rnd:2000127. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141–150. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellvé AR, Cavicchia JC, Millete CF, O’Brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse. J cell boil. 1977;74:68–85. doi: 10.1083/jcb.74.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morena AR, Boitani C, Pesce M, De Felici M, Stefanini M. Isolation of highly purified type A spermatogonia from prepubertal rat testis. J Atmos Ocean Technol. 1990;17:708–717. [PubMed] [Google Scholar]
- 46.Izadyar F, DenOuden K, Creemers LB, Posthuma G, Parvinen M, de Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68:272–281. doi: 10.1095/biolreprod.102.004986. [DOI] [PubMed] [Google Scholar]
- 47.Aponte PM, Soda T, van de Kant HJ, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. 2006;65:1828–1847. doi: 10.1016/j.theriogenology.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 48.van Dissel-Emiliani FM, De Boer-Brouwer M, De Rooij DG. Effect of fibroblast growth factor-2 on Sertoli cells and gonocytes in coculture during the perinatal period. Endocrinology. 1996;37:647–654. doi: 10.1210/endo.137.2.8593814. [DOI] [PubMed] [Google Scholar]
- 49.Jeong D, McLean DJ, Griswold MD. Long-term culture and transplantation of murine testicular germ cells. J Atmos Ocean Technol. 2003;24:661–669. doi: 10.1002/j.1939-4640.2003.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 50.Wahab-Wahlgren A, Martinelle N, Holst M, Jahnukainen K, Parvinen M, Soder O. EGF stimulates rat spermatogonial DNA synthesis in seminiferous tubule segments in vitro. Mol Cell Endocrinol. 2003;201:39–46. doi: 10.1016/S0303-7207(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 51.Shinohara MK, Inoue M, Takashima S, Takehashi M, Ogonuki N, Morimoto H, et al. Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell. 2011;11:567–578. doi: 10.1016/j.stem.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biol Reprod. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- 53.Kassab M, Abd-Elmaksoud A, Ali MA. Localization of the epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR) in the bovine testis. J Mol Hist. 2007;38:207–214. doi: 10.1007/s10735-007-9089-2. [DOI] [PubMed] [Google Scholar]
- 54.Kuijk EW, Colenbrander B, Roelen BAJ. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138:721–731. doi: 10.1530/REP-09-0138. [DOI] [PubMed] [Google Scholar]
- 55.Matsui Y, Toksoz D, Nishikawa S, Nishikawa S, Williams D, Zsebo K, et al. Effect of steel factor and leukaemia inhibitory factor on murine primordial germ cells in culture. Nature. 1991;353:750–752. doi: 10.1038/353750a0. [DOI] [PubMed] [Google Scholar]
- 56.Dorval-Coiffec I, Delcros JG, Hakovirta H, Toppari J, Jegou B, Piquet-Pellorce C. Identification of the leukemia inhibitory factor cell targets within the rat testis. Biol Reprod. 2005;72:602–611. doi: 10.1095/biolreprod.104.034892. [DOI] [PubMed] [Google Scholar]
- 57.Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61(5):1331–1339. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- 58.Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;5:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 59.Nagano M, McCarrey JR, Brinster RL. Primate spermatogonial stem cells colonize mouse testes. Biol Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- 60.Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78:1225–123. doi: 10.1016/S0015-0282(02)04345-5. [DOI] [PubMed] [Google Scholar]