Abstract
Purpose
In this study, we estimated the effect of blastocoele expansion, ICM and TE quality after warming and culture on the rates of clinical pregnancy, live birth and miscarriage in vitrified-warmed single-blastocyst transfer cycle in a Chinese population.
Methods
A retrospective analysis of 263 cycles of vitrified-warmed single-blastocyst transfers was performed.
Results
The blastocysts with higher TE grade significantly increased the rates of clinical pregnancy (OR = 0.59, 95 % CI, 0.35–0.99, P = 0.045, grade (A + B) vs grade C) and live birth (OR = 0.55, 95 % CI, 0.32–0.94, P = 0.029, grade (A + B) vs grade C). And the association between TE grade and the rate of live birth didn’t change after the number of repeated cycles was adjusted (OR = 0.55, 95 % CI, 0.32–0.95, P = 0.033, grade (A + B) vs grade C). The number of repeated cycles was a confounding factor significantly different between the live birth and no live birth groups. By contrast, neither blastocoele expansion nor inner cell mass was statistically related to the rates of clinical pregnancy, live birth and miscarriage.
Conclusions
Our data firstly provided the evidence that TE grading, but not ICM grading, was significantly associated with the clinical pregnancy rate and live birth rate in vitrified-warmed blastocyst transfer cycles in a Chinese population. TE morphology may help predict outcomes of pregnancy in single-blastocyst transfer.
Keywords: Blastocyst grading, Trophectoderm, Vitrified-warmed single-blastocyst transfer, Clinical pregnancy, Live birth
Introduction
When compared with cleavage-stage embryo transfer, blastocyst culture as an embryo selection tool helps patients to achieve higher rates of implantation, pregnancy and live birth [1–3]. In addition, blastocyst transfer (BT) has been considered to improve synchronicity between embryo development and the uterine environment [4]. Extended culture of surplus embryos to the blastocyst stage for cryopreservation optimizes the clinical outcomes and diminishes the incidence of ovarian hyperstimulation syndrome (OHSS) [5, 6]. A previous study indicated that vitrified-warmed BT cycles resulted in significantly higher clinical pregnancy rate and implantation rate compared with fresh BT cycles [4]. Vitrified-warmed embryo transfer cycles have become an important integral part of IVF treatment.
Meanwhile, the high pregnancy rate obtained with BT increased the use of single embryo transfer and thereby minimized the incidence of multiple pregnancy [7, 8]. Several studies have showed that single embryo transfer policy markedly reduced the incidence of twin gestations while maintaining a high clinical pregnancy rate and live birthrate [9, 10]. Thus, it is very important to select the best blastocyst from a group of sibling embryos for single embryo transfer.
At present, as a relatively easy and noninvasive tool, morphological scoring has been widely used to predict embryo viability. Three morphological parameters, blastocoele expansion, inner cell mass (ICM) and trophectoderm (TE), are used to evaluate blastocyst quality [11]. Many studies have shown that transferring top-scoring blastocysts (high grades for all three parameters) resulted in the highest implantation rates [12–14]. It is necessary to understand each parameter’s contribution to assisted reproductive technology (ART) outcomes of BT. Several investigations have reported trophectoderm morphology, but not the ICM grade, has a positive correlation with pregnancy rate [15, 16]. However, it has also been indicated that there is a positive relationship between ICM and pregnancy rate [17, 18]. Van den Abbeel et al. showed that blastocoele expansion is the most important parameter when selecting a blastocyst for transfer [19].
This discrepancy reflects that the association of each parameter and ART outcomes is still controversial and is not fully understood. Moreover, as far as is known, there is no study examining the relationship between blastocyst parameters and pregnancy outcomes in vitrified-warmed single-blastocyst transfer cycles in a Chinese population. The purpose of our present study was to evaluate the relationship between three post warming and culture blastocyst parameters (blastocoele expansion, ICM and TE) and the rates of clinical pregnancy, live birth and miscarriage in a Chinese population undergoing vitrified-warmed single-blastocyst transfer cycles.
Materials and methods
Patients and embryo culture
This was a retrospective analysis of 263 cycles of vitrified-warmed single-blastocyst transfers in our center from January 2009 to December 2012. The cycles included both elective and nonelective single- blastocysts transfers. For elective single-blastocyst transfers, we only selected one vitrified blastocyst with the best morphology from two or more vitrified blastocysts, and then this selected vitrified blastocyst was warmed and transfer. For nonelective single-blastocyst transfers, the patient has only one vitrified embryo available for warming and transfer or one embryo was available for transfer from two or more vitrified-warmed embryos. The retrospective review and analysis of data collected were approved by the Institutional Review Board.
Cumulus-oocyte complexes (COCs) were identified and picked up from follicular fluid, washed in culture medium (G-IVF; Vitrolife Inc., Gothenburg, Sweden), and then cultured at 37 °C in a 6 % CO2, 5 % O2 and 89 % N2 humidified incubator (APM-30D, ASTEC Inc., Tokyo, Japan). The oocytes were inseminated by IVF or ICSI after collection. After16-18 h, fertilization was confirmed by the presence of two pronuclei and zygotes were transferred into culture medium (G1; Vitrolife) under mineral oil (Ovoil; Vitrolife) for a further 2 days (two- or eight-cell stages). Day-3 embryos were scored by previously published criteria [20], and then embryos were regrouped according to developmental stage and morphologic score. Finally, they were cultured in fresh culture medium (G2; Vitrolife) to the blastocyst stage (Day 5–6).
Blastocyst evaluation
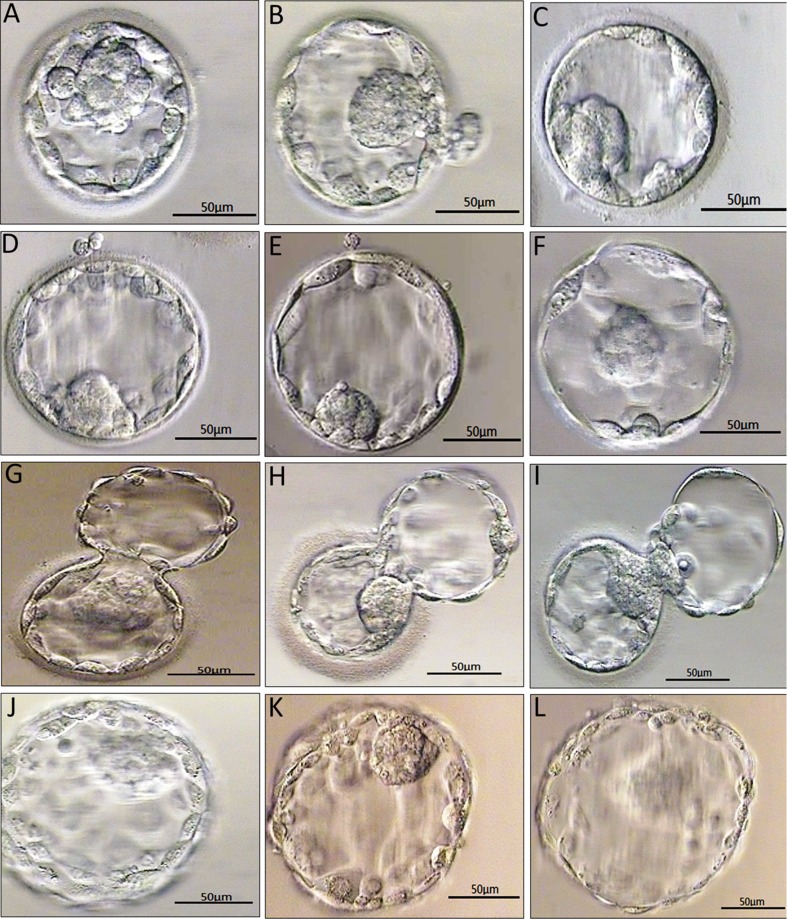
The embryos were re-evaluated after warming and culture. Vitrified-warmed blastocysts were scored and graded according to Gardner and Schoolcraft’s standard [11]. Examples of blastocyst grading are shown in Fig. 1.
Fig. 1.
Examples of blastocyst grading: (a) 3AA blastocyst; (b) 3AB blastocyst; (c) 3BC blastocyst; (d) 4AB blastocyst; (e) 4BB blastocyst; (f) 4BC blastocyst; (g) 5AA blastocyst; (h) 5AB blastocyst; (i) 5BC blastocyst; (j) 6AA blastocyst; (k) 6AB blastocyst; (l) 6BC blastocyst; Details of the blastocoele expansion, ICM and TE. grades were seen in Materials and methods. Bars = 50 μm
Blastocyst vitrification and warming
Blastocysts were vitrified and warmed according to the method previously described [21, 22]. Before vitrification, artificial collapsing of fully expanded blastocysts with a laser was performed. Blastocoele was collapsed before frozen and the shrunken blastocysts were vitrified using the Cryotop device. Vitrification and warming solutions were obtained from Kitazato BioPharma (Kitazato BioPharma Co., Shizuoka, Japan). For vitrification, blastocysts were firstly equilibrated in solution I containing 7.5 % (v/v) ethylene glycol (EG) and 7.5 % (v/v) dimethylsulfoxide (DMSO) at room temperature for 12 to 15 min and then placed into vitrification solution II containing 15 % (v/v) EG, 15 % (v/v) DMSO and 0.5 M sucrose for 1 min. Subsequently, each blastocyst was placed on the film strip of the Cryotop plunged into liquid nitrogen and then covered with the cap, and the sample was stored submerged in liquid nitrogen. This device is an open system. For warming, the film strip of the Cryotop device containing the blastocyst was quickly submerged in 1 ml of warming thaw solution containing 1.0 M sucrose, at 37 °C, for 1 min, followed by transfer of the blastocysts to a second thaw solution containing 0.5 M sucrose and incubation for 3 min. Finally, blastocysts were washed in basic medium at room temperature for 10 min, and then were transferred into 50 μL droplets of culture medium (G2; Vitrolife) under mineral oil.
Vitrified-warmed blastocyst transfer cycle
Vitrified-warmed blastocyst transfers were performed during a natural cycle after spontaneous ovulation or after artificial preparation of endometrium with exogenous steroids [23, 24]. There were 16 natural cycles and 247 artificial preparation cycles. For artificial preparation of endometrium, patients received 3 mg of estradiol valerate (German Remedies Ltd., Mumbai, India) twice daily from day 2 to day 7 of the menstrual cycle. When endometrial thickness exceeded 10 mm after an ultrasound examination, estradiol valerate was administered for a further 2 days, and administration of progesterone for patients began (80 mg IM daily, Shanghai general pharmaceutical co., LTD, China). If the endometrial thickness was < 7 mm, the dose of estradiol valerate was increased to between 4 mg and 6 mg twice daily in individual cases. Blastocysts warming was performed in the afternoon of day 5 after administration of progesterone. After 14 to 16 h, blastocysts transfer was performed under ultrasound guidance. Supplementation of 80 mg progesterone by intramuscular injections was continued at least for the following 2 weeks.
Pregnancy outcomes
The primary study outcomes were clinical pregnancy, live birth and miscarriage. The clinical pregnancy was based on the detection of fetal heartbeats by ultrasound at 5 weeks after embryo transfer. The live birth rate was calculated as the number of live births devided by the total number of transferred blastocysts. Miscarriage was a secondary outcome and was defined as an implanted embryo that failed to result in live birth [15].
Statistical analysis
All data analyses were performed using Stata 9.2 statistical software package (Stata Corp, LP). T test was used to compare the continuous variables; if the variances were far from equal, the Wilcoxon signed rank test was used. The chi-squared test was used to compare the categorical variables. Odds ratios (ORs) with 95 % confidence intervals (CIs) were calculated for pregnancy outcomes in relation to morphology parameters of blastocysts (Expansion, ICM and TE) by logistic regression analysis. All significance tests were two-tailed, and P ≤ 0.05 was considered to be statistically significant.
Results
A total of 263 single blastocyst transfer cycles were investigated retrospectively in this study. Characteristics of patients and cycles from which the frozen blastocysts were obtained are summarized and separated by pregnancy outcomes in Table 1. As shown in Table 1, between patients with clinical pregnancy and no clinical pregnancy, no significant differences were found in all characteristics. The number of repeated cycles were significantly different between the patients with live birth and without live birth. We found no significant differences in all characteristics between patients with miscarriage and no miscarriage.
Table 1.
Patient characteristics divided by pregnancy outcomes
| Characteristic | Clinical pregnancy outcome | Live birth outcome | Miscarriage outcome | |||
|---|---|---|---|---|---|---|
| Clinical pregnancy (n = 95) | No clinical pregnancy (n = 168) | Live birth (n = 78) | No live birth (n = 185) | Miscarriage (n = 14) | No miscarriage (n = 81) | |
| Female age (years, mean ± SD) | 30.69 ± 4.27 | 30.92 ± 4.66 | 30.87 ± 4.28 | 30.82 ± 4.62 | 29.43 ± 4.55 | 30.91 ± 4.20 |
| BMI (mean ± SD)a | 22.17 ± 2.80 | 22 ± 3.02 | 22.17 ± 2.74 | 22.03 ± 3.02 | 22.54 ± 3.19 | 22.11 ± 2.74 |
| The number of repeated cyclesb | 1.13 ± 0.33 | 1.23 ± 0.49 | 1.1 ± 0.31 | 1.22 ± 0.48* | 1.21 ± 0.43 | 1.11 ± 0.32 |
| Method [n (%)] | ||||||
| Standard IVF | 59 (38.82) | 93 (61.18) | 49 (32.24) | 103 (67.76) | 9 (15.25) | 50 (84.75) |
| ICSIc | 36 (32.43) | 75 (67.57) | 29 (26.13) | 82 (73.87) | 5 (13.89) | 31 (86.11) |
| Day of freeze [n (%)] | ||||||
| D5 | 78 (38.61) | 124 (61.39) | 66 (32.67) | 136 (67.33) | 9 (11.54) | 69 (88.46) |
| D6 | 17 (27.87) | 44 (72.13) | 12 (19.67) | 49 (80.33) | 5 (29.41) | 12 (70.59) |
aBMI: kg/m2
bThe number of repeated cycles: the number of IVF/ICSI cycles
cICSI: intracytoplasmic sperm injection
*P ≤ 0.05 for selected characteristics’ distributions between live birth and no live birth groups
As shown in Tables 2, 3 and 4, blastocysts were graded for all three parameters (blastocoele expansion, ICM and TE). Because there were very few patients with a grade A TE, the patients with grade B and grade A were combined. For blastocoele expansion, patients with grades 1 and 2 were combined into one group. When analyzing the effect of all three parameters on pregnancy outcomes, the blastocysts with higher TE grade significantly increased the rates of clinical pregnancy (OR = 0.59, 95 % CI, 0.35–0.99, P = 0.045, grade (A + B) vs grade C) and live birth (OR = 0.55, 95 % CI, 0.32–0.94, P = 0.029, grade (A + B) vs grade C) (Tables 2 to 3). The association between TE grade and the rate of live birth didn’t change after the number of repeated cycles was adjusted (OR = 0.55, 95 % CI, 0.32–0.95, P = 0.033, grade (A + B) vs grade C) (Table 3). The number of repeated cycles was a confounding factor significantly different between the live birth and no live birth groups (Table 1). By contrast, neither blastocoele expansion nor ICM was significantly related to the rates of clinical pregnancy and live birth. There was no significant relationship between blastocoele expansion as well as ICM and the rate of miscarriage (Table 4).
Table 2.
Logistic regression analysis of clinical pregnancy and no clinical pregnancy
| Parameter | Clinical pregnancy, n (%) | No clinical pregnancy, n (%) | OR (95 % CI) | P value | |
|---|---|---|---|---|---|
| Expansion | 1 + 2 | 2 (50) | 2 (50) | 1 | |
| 3 | 1 (11.11) | 8 (88.89) | 0.13 (0.01–2.18) | 0.154 | |
| 4 | 12 (17.39) | 57 (82.61) | 0.21 (0.03–1.65) | 0.138 | |
| 5 | 36 (47.37) | 40 (52.63) | 0.90 (0.12–6.72) | 0.918 | |
| 6 | 44 (41.9) | 61 (58.1) | 0.72 (0.10–5.32) | 0.749 | |
| Inner cell mass | A | 1 (33.33) | 2 (66.67) | 1 | |
| B | 91 (36.25) | 160 (63.75) | 1.14 (0.10–12.72) | 0.917 | |
| C | 1 (20) | 4 (80) | 0.50 (0.02–12.90) | 0.676 | |
| Trophectoderm cells | A + B | 53 (42.06) | 73 (57.94) | 1 | |
| C | 40 (30.08) | 93 (69.92) | 0.59 (0.35–0.99) * | 0.045 |
Expansion: blastocoele expansion; ICM: inner cell mass; TE: trophectoderm
CI: confidence interval
*P ≤ 0.05 was considered statistically significant; “A + B” vs. “C” trophectoderm
Table 3.
Logistic regression analysis of live birth and no live birth
| Parameter | Live birth, n (%) | No live birth, n (%) | Crude | Adjusted | |||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P value | aOR (95%CI) | P value | ||||
| Expansion | 1 + 2 | 2 (50) | 2 (50) | 1.00 | 1 | ||
| 3 | 1 (11.11) | 8 (88.89) | 0.13 (0.01–2.18) | 0.154 | 0.10 (0.01–1.75) | 0.114 | |
| 4 | 11 (15.94) | 58 (84.06) | 0.19 (0.02–1.49) | 0.114 | 0.14 (0.02–1.21) | 0.075 | |
| 5 | 29 (38.16) | 47 (61.84) | 0.62 (0.08–4.62) | 0.638 | 0.45 (0.06–3.62) | 0.455 | |
| 6 | 35 (33.33) | 70 (66.67) | 0.50 (0.07–3.70) | 0.497 | 0.38 (0.05–2.99) | 0.358 | |
| Inner cell mass | A | 1 (33.33) | 2 (66.67) | 1.00 | 1 | ||
| B | 74 (29.48) | 177 (70.52) | 0.84 (0.07–9.36) | 0.885 | 0.96 (0.09–10.75) | 0.972 | |
| C | 1 (20) | 4 (80) | 0.50 (0.19–12.90) | 0.676 | 0.50 (0.02–12.90) | 0.676 | |
| Trophectoderm cells | A + B | 45 (35.71) | 81 (64.29) | 1.00 | 1 | ||
| C | 31 (23.31) | 102 (76.69) | 0.55 (0.32–0.94) * | 0.029 | 0.55 (0.32–0.95) * | 0.033 | |
aOR are adjusted for the number of repeated cycles
Expansion: blastocoele expansion; ICM: inner cell mass; TE: trophectoderm; CI: confidence interval
*P ≤ 0.05 was considered statistically significant; “A + B” vs. “C” trophectoderm
Table 4.
Logistic regression analysis of miscarriage and no miscarriage
| Parameter | Miscarriage, n (%) | No miscarriage, n (%) | OR (95 % CI) | P value | |
|---|---|---|---|---|---|
| Expansion | 1 + 2 + 3 + 4 + 5 | 5 (9.8) | 46 (90.2) | 1 | |
| 6 | 9 (20.45) | 35 (79.55) | 2.37 (0.73–7.69) | 0.152 | |
| Inner cell mass | A | 0 | 1 | N/A | |
| B | 14 | 77 | N/A | ||
| C | 0 | 1 | N/A | ||
| Trophectoderm cells | A + B | 7 (13.21) | 46 (86.79) | 1 | |
| C | 7 (17.5) | 33 (82.5) | 1.39 (0.45–4.35) | 0.568 |
Expansion: blastocoele expansion; ICM: inner cell mass; TE: trophectoderm; CI: confidence interval
Discussion
Our study mainly found that TE cells were the most significant morphological parameter for predicting clinical pregnancy rate and live birth rate after vitrified-warmed single blastocyst transfer. By contrast, blastocoele expansion and ICM were unrelated to the rates of clinical pregnancy, live birth and miscarriage.
Our results were consistent with the recent studies which indicated that characterization of TE was the most important predictor of ART outcomes in both fresh embryo transfer and frozen-thawed embryo transfer (F-TET) cycles. The study of a large sample size, including 3,151 fresh cycles, confirmed that TE morphology could better predict clinical pregnancy and live birth [25]. Hill et al. concluded that TE grading, but not ICM grading, was significantly associated with the rates of implantation and live birth for fresh single-blastocyst transfers [15]. Ahlstrom et al. found that TE was the only statistically significant independent predictor of live birth outcome in F-TET cycles [16]. Honnma et al. indicated that TE morphology was significantly related to the rates of ongoing pregnancy [26]. They also demonstrated that lower TE grade was associated with a higher rate of miscarriage in F-TET cycles, but this association was not seen in our study. However, a recent study found that transfer of a blastocyst with ICM grade A may reduce the risk of early pregnancy loss [19]. Because of the small sample size, we could not evaluate the association of ICM grade and miscarriage. While there appears to be a trend toward a higher miscarriage rate with hatched blastocysts, there was no significant relationship between the degree of expansion and miscarriage. This trend may be due to the bias caused by the small sample size. In the future, a study with a large sample size is needed to clarify the association of the degree of expansion and miscarriage.
As is known, TE cells eventually destine to become the placenta, while ICM cells eventually develop into the fetus. Previous studies have showed that ICM morphology is more important for predicting pregnancy outcomes than TE morphology [18, 17]. However, recent investigations and our data don’t support this, and emphasize the importance of TE morphology in pregnancy outcomes. One possible explanation is that ICM cannot further develop in vivo without TE establishing implantation.
Several possible mechanisms have been proposed to explain the importance of TE morphology in ART outcomes. Firstly, TE has important functions when a blastocyst starts the complex process of implantation, including invasion of the endometrium and communication with the maternal immune system [27–29]. Secondly, it has been demonstrated that TE of higher grades secrete hCG at an earlier time [30]. HCG is critical for implantation and blastocyst-endometrium crosstalk and can induce immunological tolerance [31, 28]. Lastly, Parks et al. examined trophectoderm biopsy samples and revealed that individual blastocyst gene expression profiles were correlated with the success or failure of implantation and pregnancy [32]. Moreover, Alfarawati et al. examined human trophectoderm biopsy samples and found that blastocysts with poor TE morphology had a higher incidence of aneuploidy compared with good TE morphology [33]. Thus, selection of blastocysts with high TE grade may avoid severe chromosomal abnormalities, and thereby decrease miscarriage rate and increase the live birth rate.
Ahlstromm A et al. proposed a three part post-thaw scoring system including degree of blastocoel re-expansion, degree of cell survival and degree of cell contour and combined pre-freeze and post-thaw morphological parameters to predict live birth outcomes after frozen-thawed blastocyst transfer cycles. They found that the pre-freeze blastocele expansion and trophectoderm grade were the most significant morphological predictors of live birth after a frozen-thawed single blastocyst transfer. Degree of re-expansion 2–4 h was the most significant post-thaw predictor of live birth [34]. Unlike their evaluation system, in our study, transferred blastocysts from warming and overnight culture were graded for post-warm morphology by three parameters (blastocoele expansion, ICM and TE). We found that post-warm TE cells were the most significant morphological parameter for predicting clinical pregnancy rate and live birth rate after vitrified-warmed single blastocyst transfer. It is thus clear that no matter pre-freeze or post-thaw TE grade was very important predictor of live birth. Currently, many studies use either the ability of the blastocoele to re-expand after warming and/or the pre-freeze score as good indicators of pregnancy outcomes in vitrified-warmed blastocyst transfer cycles [18, 26, 35]. Further, we should evaluated pre-freeze morphological parameters (blastocoele expansion, ICM and TE) and try to use the new evaluation method (degree of blastocoel re-expansion, degree of cell survival and degree of cell contour) to predict the pregnancy outcomes.
The strength of this study was the use of single blastocyst transfer which could separately interpret the impact of the blastocyst parameters on an individual embryo’s clinical pregnancy and live birth potential. Major limitation of our study was the relatively small sample size. In the future, a study with the large sample size is needed to validate the association of each parameter and pregancy outcomes in vitrified-warmed single-blastocyst transfer cycle.
In conclusion, our data support the results of recent study and firstly provide the evidence that selection of a blastocyst with good TE morphology can increase the clinical pregnancy rate and live birth rate in vitrified-warmed blastocyst transfer cycles in a Chinese population. TE morphology may help predict outcomes of pregnancy in single-blastocyst transfer.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (grant nos. 81100420 and 81270701), the Foundation of Nanjing Medical University (grant no. 2011NJMU210), the Natural Science Foundation of Jiangsu Province (grant no. BK2012520) and Nanjing Medical Science and Technique Development Foundation (2010NJMU030).
Declaration of interest
The authors declare no conflict of interest.
Footnotes
Capsule This study focused on blastocysts morphology and outcomes of pregnancy. Two hundred sixty three cycles of vitrified-warmed single-blastocyst transfers were performed. We found that the blastocysts with higher TE grade increased the rates of clinical pregnancy and live birth.
Xiaojiao Chen and Junqiang Zhang contributed equally.
References
- 1.Cruz JR, Dubey AK, Patel J, Peak D, Hartog B, Gindoff PR. Is blastocyst transfer useful as an alternative treatment for patients with multiple in vitro fertilization failures? Fertil Steril. 1999;72:218–220. doi: 10.1016/S0015-0282(99)00258-7. [DOI] [PubMed] [Google Scholar]
- 2.Papanikolaou EG, Kolibianakis EM, Tournaye H, Venetis CA, Fatemi H, Tarlatzis B, et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A Syst Rev Meta-Analysis Hum Reprod. 2008;23:91–99. doi: 10.1093/humrep/dem339. [DOI] [PubMed] [Google Scholar]
- 3.Zech NH, Lejeune B, Puissant F, Vanderzwalmen S, Zech H, Vanderzwalmen P. Prospective evaluation of the optimal time for selecting a single embryo for transfer: day 3 versus day 5. Fertil Steril. 2007;88:244–246. doi: 10.1016/j.fertnstert.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 4.Zhu D, Zhang J, Cao S, Heng BC, Huang M, Ling X, et al. Vitrified-warmed blastocyst transfer cycles yield higher pregnancy and implantation rates compared with fresh blastocyst transfer cycles–time for a new embryo transfer strategy? Fertil Steril. 2011;95:1691–1695. doi: 10.1016/j.fertnstert.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Xi Q, Zhang H, Li Y, Ai J, Jin L. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod Biomed Online. 2013;27:154–160. doi: 10.1016/j.rbmo.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Endo T, Honnma H, Hayashi T, Chida M, Yamazaki K, Kitajima Y, et al. Continuation of GnRH agonist administration for 1 week, after hCG injection, prevents ovarian hyperstimulation syndrome following elective cryopreservation of all pronucleate embryos. Hum Reprod. 2002;17:2548–2551. doi: 10.1093/humrep/17.10.2548. [DOI] [PubMed] [Google Scholar]
- 7.Veeck LL, Bodine R, Clarke RN, Berrios R, Libraro J, Moschini RM, et al. High pregnancy rates can be achieved after freezing and thawing human blastocysts. Fertil Steril. 2004;82:1418–1427. doi: 10.1016/j.fertnstert.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DK, Vella P, Lane M, Wagley L, Schlenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril. 1998;69:84–88. doi: 10.1016/S0015-0282(97)00438-X. [DOI] [PubMed] [Google Scholar]
- 9.Csokmay JM, Hill MJ, Chason RJ, Hennessy S, James AN, Cohen J, et al. Experience with a patient-friendly, mandatory, single-blastocyst transfer policy: the power of one. Fertil Steril. 2011;96:580–584. doi: 10.1016/j.fertnstert.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kresowik JD, Stegmann BJ, Sparks AE, Ryan GL, van Voorhis BJ. Five-years of a mandatory single-embryo transfer (mSET) policy dramatically reduces twinning rate without lowering pregnancy rates. Fertil Steril. 2011;96:1367–1369. doi: 10.1016/j.fertnstert.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Mortimer JR, editor. Toward Reproductive Certainty: Infertility and Genetics Beyond 1999. Carnforth, UK: Parthenon Press; 1999. pp. 378–388. [Google Scholar]
- 12.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81:551–555. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Balaban B, Yakin K, Urman B. Randomized comparison of two different blastocyst grading systems. Fertil Steril. 2006;85:559–563. doi: 10.1016/j.fertnstert.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 15.Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril. 2013;99:1283–9 e1. doi: 10.1016/j.fertnstert.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Ahlstrom A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod. 2011;26:3289–3296. doi: 10.1093/humrep/der325. [DOI] [PubMed] [Google Scholar]
- 17.Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001;76:1157–1167. doi: 10.1016/S0015-0282(01)02870-9. [DOI] [PubMed] [Google Scholar]
- 18.Goto S, Kadowaki T, Tanaka S, Hashimoto H, Kokeguchi S, Shiotani M. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril. 2011;95:948–952. doi: 10.1016/j.fertnstert.2010.06.067. [DOI] [PubMed] [Google Scholar]
- 19.Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJ, Klein BM, et al. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod Biomed Online. 2013;27:353–361. doi: 10.1016/j.rbmo.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JQ, Li XL, Peng Y, Guo X, Heng BC, Tong GQ. Reduction in exposure of human embryos outside the incubator enhances embryo quality and blastulation rate. Reprod Biomed Online. 2010;20:510–515. doi: 10.1016/j.rbmo.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67:73–80. doi: 10.1016/j.theriogenology.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Mukaida T, Takahashi K, Kasai M. Blastocyst cryopreservation: ultrarapid vitrification using cryoloop technique. Reprod Biomed Online. 2003;6:221–225. doi: 10.1016/S1472-6483(10)61713-0. [DOI] [PubMed] [Google Scholar]
- 23.Sathanandan M, Macnamee MC, Rainsbury P, Wick K, Brinsden P, Edwards RG. Replacement of frozen-thawed embryos in artificial and natural cycles: a prospective semi-randomized study. Hum Reprod. 1991;6:685–687. doi: 10.1093/oxfordjournals.humrep.a137407. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt CL, de Ziegler D, Gagliardi CL, Mellon RW, Taney FH, Kuhar MJ, et al. Transfer of cryopreserved-thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin-releasing hormone agonist and exogenous estradiol and progesterone (GEEP) Fertil Steril. 1989;52:609–616. doi: 10.1016/s0015-0282(16)60973-1. [DOI] [PubMed] [Google Scholar]
- 25.Thompson SM, Onwubalili N, Brown K, Jindal SK, McGovern PG. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET): a national study. J Assist Reprod Genet. 2013;30:1577–1581. doi: 10.1007/s10815-013-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honnma H, Baba T, Sasaki M, Hashiba Y, Ohno H, Fukunaga T, et al. Trophectoderm morphology significantly affects the rates of ongoing pregnancy and miscarriage in frozen-thawed single-blastocyst transfer cycle in vitro fertilization. Fertil Steril. 2012;98:361–367. doi: 10.1016/j.fertnstert.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Licht P, Russu V, Lehmeyer S, Wildt L. Molecular aspects of direct LH/hCG effects on human endometrium–lessons from intrauterine microdialysis in the human female in vivo. Reprod Biol. 2001;1:10–19. [PubMed] [Google Scholar]
- 28.Licht P, Russu V, Wildt L. On the role of human chorionic gonadotropin (hCG) in the embryo-endometrial microenvironment: implications for differentiation and implantation. Semin Reprod Med. 2001;19:37–47. doi: 10.1055/s-2001-13909. [DOI] [PubMed] [Google Scholar]
- 29.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 30.Lopata A. Implantation of the human embryo. Hum Reprod. 1996;11(Suppl 1):175–184. doi: 10.1093/humrep/11.suppl_1.175. [DOI] [PubMed] [Google Scholar]
- 31.Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier D’Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol. 2010;85:93–98. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Parks JC, McCallie BR, Janesch AM, Schoolcraft WB, Katz-Jaffe MG. Blastocyst gene expression correlates with implantation potential. Fertil Steril. 2011;95:1367–1372. doi: 10.1016/j.fertnstert.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Ahlstrom A, Westin C, Wikland M, Hardarson T. Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology. Hum Reprod. 2013;28:1199–1209. doi: 10.1093/humrep/det054. [DOI] [PubMed] [Google Scholar]
- 35.Shu Y, Watt J, Gebhardt J, Dasig J, Appling J, Behr B. The value of fast blastocoele re-expansion in the selection of a viable thawed blastocyst for transfer. Fertil Steril. 2009;91:401–406. doi: 10.1016/j.fertnstert.2007.11.083. [DOI] [PubMed] [Google Scholar]