Abstract
Purpose
Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) play crucial roles in follicular development and oocyte maturation. This study aimed to investigate and compare the expression of these proteins in ovarian tissues of women with and without polycystic ovary syndrome (PCOS).
Methods
Ovarian tissues from 28 patients with PCOS and 26 normal ovulatory women were collected, and the expression of GDF9 and BMP15 in oocytes and granulosa cells was evaluated via immunohistochemical staining.
Results
GDF9 and BMP15 were first expressed in primordial follicles at very low levels, and their expression increased gradually with follicular development, reaching the highest levels in Graafian follicles. However, less GDF9 and BMP15 expression was observed in primordial, primary, and secondary follicles in ovarian tissues of PCOS patients compared with levels in the control tissues (P < 0.05). In Graafian follicles, GDF9 and BMP15 expression reached comparable levels in the PCOS and control groups (P > 0.05).
Conclusions
The expression of GDF9 and BMP15 in ovarian tissues varies among the developmental stages in both oocytes and granulosa cells in human ovarian tissues. The expression of these proteins is reduced and delayed in the early follicular stage in PCOS ovarian tissues, and these differences in expression may be associated with aberrant follicular development in patients with PCOS.
Keywords: Growth differentiation factor 9, Bone morphogenetic protein 15, Polycystic ovary syndrome, Ovarian tissue
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common reproductive endocrinological disorders, and it is characterized by chronic anovulation, infertility, and hyperandrogenism [1]. Moreover, patients with PCOS might be affected by metabolic abnormalities (obesity, impaired glucose tolerance, dyslipidemia, etc.) with their long-term sequelae (metabolic syndrome, type 2 diabetes mellitus, cardiovascular diseases, etc.), psychiatric symptoms, and even gynecological cancers (especially endometrial cancer), which have a detrimental impact on their heath and quality of life [2–4].
Unfortunately, the etiological and pathological mechanisms of PCOS remain unclear. Previous studies demonstrated that endocrinal hormones, such as luteinizing hormone (LH) and testosterone (T), may be involved in abnormal folliculogenesis in ovaries of patients with PCOS. Recently, researchers have investigated the anovulatory condition in women with PCOS and the possible roles of oocyte secreted factors (OSFs) in folliculogenesis [5, 6], specifically growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15). Belonging to transforming growth factor B (TGFB) superfamily, these two factors play crucial roles in follicular development, from recruitment of primordial follicles to ovulation of the dominant follicle as well as in oocyte maturation and subsequent embryo development [7–10].
GDF9 and BMP15 mRNA has been found to be expressed exclusively in human oocytes but not granulosa cells via in situ hybridization, and the expression of GDF9 but not BMP15 was shown to be reduced in oocytes of PCOS patients compared to normal oocytes [5]. Another study showed that GDF9 and BMP15 proteins can be detected both in oocytes and cumulus cells through immunocytochemical staining. The two proteins displayed comparable expression levels in oocytes, whereas GDF9 displayed a lower expression level in cumulus cells of women with PCOS [6]. Taken together, these findings indicate that abnormal expression of OSFs might be involved in the aberrant follicular development associated with PCOS. However, to date, the inconsistent results that have been obtained have made the truth more confusing. Considering the crucial roles of GDF9 and BMP15 in follicular development, it is essential to investigate their expression patterns in unstimulated ovarian tissues from women with and without PCOS in order to explore the mechanisms of abnormal folliculogenesis in PCOS patients.
Materials and methods
Patients and materials
The present study was approved by Institutional Review Board of Sun Yat-sen University, and informed written consent was obtained from all patients before the initiation of the study. From May 2010 to December 2012, ovarian tissues were collected from 28 patients with PCOS (PCOS group) and 26 women with normal ovulatory cycles (control group) who underwent ovarian biopsy with the cold knife technique on day 7–9 of their menstrual cycle. PCOS patients were diagnosed based on the Rotterdam criteria, and patients with other disorders related to androgen excess were excluded [11–13].
In this study, the patients who were found to meet the following criteria were included in the PCOS group: (1) oligo and/or anovulation; (2) clinical and/or biochemical signs of hyperandrogenism; and (3) polycystic ovaries. They were treated by ovarian wedge resection, and ovarian biopsy was performed for pathological diagnosis. The selection criteria for control cases were as follows: regular menstrual cycles (21–35 days), regular ovulation (confirmed by basal body temperature tracking), normal ovarian morphology (confirmed by ultrasonography), and basal follicular stimulating hormone (FSH) <10 IU/L. The control group included 26 patients who were diagnosed with endometrial carcinoma or cervical cancer and underwent ovarian biopsy to exclude ovarian lesions. Only “healthy ovaries” were used in the control group, and all “abnormal ovaries” were excluded. Ovarian pathological biopsy was used to distinguish the two types of ovarian tissues. The exclusion criteria for both groups (PCOS and control) were as follows: premature ovarian failure, endometriosis, thyroid dysfunction, ovulation induction, or use of sexual hormone medication within 3 months.
Immunohistochemical staining
All tissues were fixed in 10 % neutral buffered formalin for immunohistochemical staining. The protocol was as follows: 5-μm paraffin sections from ovarian tissues were deparaffinized, rehydrated, and treated in a pressure cooker for 3 min in 0.01 mM citric acid monohydrate (pH 6.0). Sections were incubated with primary antibodies of rabbit anti-human polyclonal GDF9 (1:40, Abcam, UK) or goat anti-human polyclonal BMP15 (1:40, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight. Sections were washed three times with phosphate-buffered saline (PBS) and incubated with secondary antibodies (1:200) at room temperature. After rinsing with PBS, antigenic sites were stained using 3,3′-diaminobenzidine and rinsed three times in 0.03 % H2O2. All experiments were controlled by incubating parallel sections without primary antibodies. Preliminary experiments were conducted to determine the optimal staining time and antibody titer.
In the ovarian tissues, follicles were classified into four groups: primordial (the oocyte was surrounded by a single layer of squamous granulosa cells); primary (the oocyte was surrounded by a single layer of mixed squamous and cuboidal or a single layer of cuboidal granulosa cells); secondary (the oocyte was surrounded by 2 to 8 layers of granulosa cells but no antrum was present); and small Graafian (the follicle measured 0.5–7 mm in diameter with a fluid-filled antrum).
The background staining of each section was violet blue, and positive staining for the target proteins appeared yellow or brown. The results were scored as follows: negative (−), no yellow signal on the blue background; weakly positive (1+), cells with yellow granules were sparsely distributed with <25 % cells stain positively; moderately positive (2+), cells with yellow particles were densely distributed with 25–50 % of cells stained positively; and strongly positive (3+), cells with dark yellow or brown particles were distributed in clusters with >50 % of cells stained positively. The stained slides were evaluated blindly and independently by two experienced technicians. The average value from the independent evaluations was used as the final results.
Statistical analysis
All statistical analyses were performed using SPSS 11.5 software (SPSS, Chicago, IL, USA). General conditions were compared by Student’s t-test or Mann-Whitney test, whenever these tests were appropriate. The significance of differences in the percentages between two groups were evaluated by chi-squared test. P < 0.05 was considered to indicate statistical significance.
Results
General conditions
The general characteristics of the normal ovulatory women (control group) and PCOS patients (PCOS group) are summarized in Table 1. Mean patient age was greater in the control group, whereas the antral follicle count (AFC) and the levels of AMH, LH, E2 and T were higher in the PCOS group.
Table 1.
Comparison of general conditions of patients with PCOS and controls
| General indicators | Control | PCOS | P value |
|---|---|---|---|
| No. of cases | 26 | 28 | |
| Age (y) | 35.23 ± 3.29 | 29.43 ± 2.23 | <0.05 |
| AFC | 9.19 ± 1.88 | 29.57 ± 4.78 | <0.05 |
| AMH (ng/mL) | 1.98 ± 1.08 | 11.38 ± 5.09 | <0.05 |
| FSH (IU/L) | 5.60 ± 1.10 | 5.96 ± 1.44 | >0.05 |
| LH (IU/L) | 2.81 ± 0.81 | 8.71 ± 4.80 | <0.05 |
| E2 (ng/mL) | 24.73 ± 9.69 | 35.33 ± 20.59 | <0.05 |
| T (nmol/L) | 0.35 ± 0.14 | 0.74 ± 0.89 | <0.05 |
| PRL (μg/L) | 13.10 ± 5.88 | 13.82 ± 9.29 | >0.05 |
The data were expressed as mean ± SD. Student’s t-test and Mann-Whitney test were used for comparisons between the two groups
Expression of GDF9 in oocytes
GDF9 protein was primarily located in the cytoplasm of oocytes (Fig. 1). In oocytes from the control group, low GDF9 expression was found in nearly 75 % of primordial follicles. Then GDF9 expression increased gradually with follicular development. Positive staining for GDF9 was detected in all oocytes of primary and secondary follicles. In Graafian follicles, the expression of GDF9 reached the highest level with 67 % of positively stained oocytes scoring 2+ (moderately positive) and 33 % of positively stained oocytes scoring 3+ (strongly positive; Table 2).
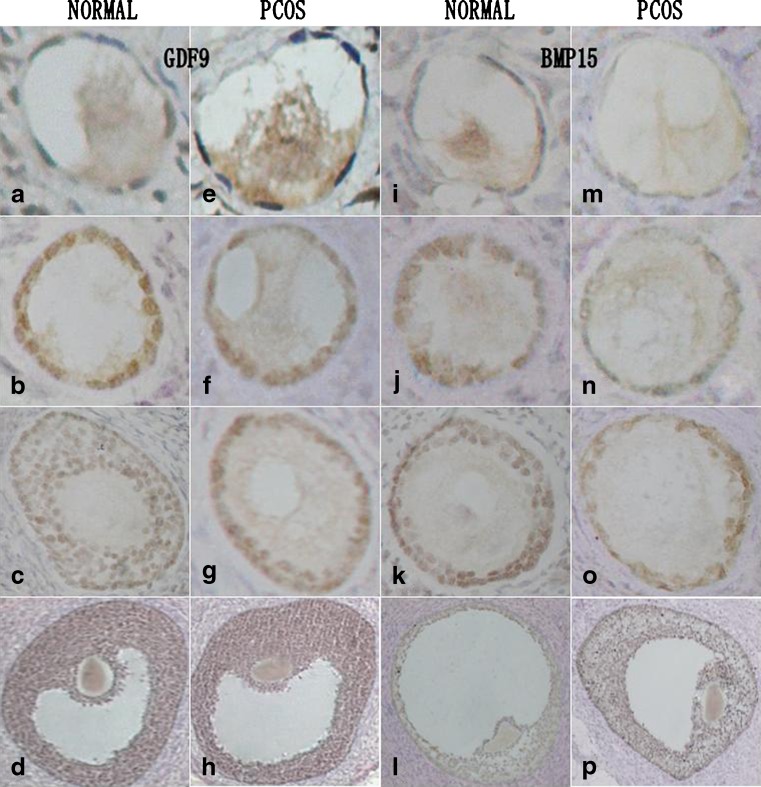
Fig. 1.
Immunohistochemical staining for GDF9 (left two panels) and BMP15 (right two panels) in unstimulated ovarian tissues, including primordial (row 1), primary (row 2), secondary (row 3), and Graafian follicles (row 4) in normal ovarian tissue and that from PCOS patients. The background staining of each section was violet blue, and positive staining for GDF9 or BMP15 expression appeared as yellow or brown
Table 2.
Expression of GDF9 in oocytes
| Follicle type (Follicle No.) | NE % | (1 +) % | (2 +) % | (3 +) % |
|---|---|---|---|---|
| Primordial | ||||
| PCOS (n = 214) a | 57.01 % (122) | 42.99 % (92) | 0 | 0 |
| Control (n = 122) | 26.23 % (32) | 73.77 % (90) | 0 | 0 |
| Primary | ||||
| PCOS (n = 70) b | 31.43 % (22) | 64.29 % (45) | 4.29 % (3) | 0 |
| Control (n = 59) | 0 | 69.49 % (41) | 30.51 % (18) | 0 |
| Secondary | ||||
| PCOS (n = 27)c | 37.04 % (10) | 51.85 % (14) | 11.11 %(3) | 0 |
| Control (n = 22) | 0 | 31.82 % (7) | 54.55 %(12) | 13.64 %(3) |
| Graafian | ||||
| PCOS (n = 11) | 0 | 0 | 72.73 % (8) | 27.27 %(3) |
| Control (n = 9) | 0 | 0 | 66.67 % (6) | 33.33 % (3) |
a, b, c: P < 0.001, respectively. PCOS group compared with the control group at the same developmental stage
In the oocytes of PCOS patients, GDF9 expression was observed in only 43 % of primordial follicles. Then the expression signal increased gradually with follicular development, with about 70 % of the primary and secondary follicles stained positively. Finally, the expression of GDF9 also reached the highest level in Graafian follicles (Table 2).
Compared with the control group, the expression of GDF9 was significantly reduced in primordial, primary, and secondary follicles of the PCOS group (P < 0.001). Similar to the control group, GDF9 protein reached the highest levels in Graafian follicles, and there was no significant difference in GDF9 expression in Graafian follicles between the two groups (P > 0.05).
Expression of GDF9 in granulosa cells
In granulosa cells from the control group, GDF9 protein was observed in nearly half (46 %) of the primordial follicles, with a very low expression score of 1+. Then positive staining could be detected in all primary and secondary follicles, and the intensity increased with follicular development. In Graafian follicles, the expression of GDF9 reached the highest level with 67 % of positively stained oocytes scoring 2+ (moderately positive) and 33 % of positively stained oocytes scoring 3+ (strongly positive; Table 3). This expression pattern was very similar to that in the oocytes.
Table 3.
Expression of GDF9 in granulosa cells
| Follicle type (Follicle No.) | NE % | (1 +) % | (2 +) % | (3 +) % |
|---|---|---|---|---|
| Primordial | ||||
| PCOS (n = 214) a | 78.50 %(168) | 21.50 % (46) | 0 | 0 |
| Control (n = 122) | 54.10 % (66) | 45.90 % (56) | 0 | 0 |
| Primary | ||||
| PCOS (n = 70) b | 25.71 % (18) | 71.43 % (50) | 2.86 % (2) | 0 |
| Control (n = 59) | 0 | 88.14 % (52) | 11.86 % (7) | 0 |
| Secondary | ||||
| PCOS (n = 27)c | 29.63 % (8) | 59.26 % (16) | 11.11 % (3) | 0 |
| Control (n = 22) | 0 | 31.82 %(7) | 54.55 % (12) | 13.64 %(3) |
| Graafian | ||||
| PCOS (n = 11) | 0 | 0 | 72.73 % (8) | 27.27 %(3) |
| Control (n = 9) | 0 | 0 | 66.67 % (6) | 33.33 % (3) |
a, b, c: P <0.001, respectively. PCOS group compared with the control group at the same developmental stage
In granulosa cells from the PCOS group, only a small percentage (22 %) of primordial follicles showed positive staining for GDF9 expression. However, positive signals were observed in about 70 % of primary and secondary follicles. Finally, the expression of GDF9 also reached the highest level with all oocytes stained positively (Table 3). This expression pattern was very similar to that in the oocytes.
Compared with the control group, the expression of GDF9 was significantly reduced in primordial, primary, and secondary follicles of the PCOS group (P < 0.001). Similar to the control group, GDF9 was expressed at the highest levels in Graafian follicles, and there was no significant difference between the two groups (P >0.05).
Expression of BMP15 in oocytes
BMP15 protein was also primarily located in the cytoplasm of the oocytes (Fig. 1). In primordial follicles, less than 20 % of oocytes in the PCOS group stained positively for BMP15 expression, while more than 40 % of control oocytes stained positively for BMP15 expression with a score of 1+. In the primary follicles, positive staining was observed in about 68 % of the control oocytes and 35 % of oocytes in PCOS tissues. In the secondary follicles, all of the oocytes were positive for BMP15 expression both in the control and PCOS groups, with more control oocytes showing a moderately positive signal (scoring 2+). Collectively, the results showed that the expression of BMP15 was weaker in the PCOS group during the early follicular stages (P < 0.01). In Graafian follicles, all of the oocytes both in the control and PCOS groups were positively stained for BMP15 expression at comparable levels (P > 0.05, Table 4).
Table 4.
Expression of BMP15 in oocytes
| Follicle type (Follicle No.) | NE % | (1 +) % | (2 +) % | (3 +) % |
|---|---|---|---|---|
| Primordial | ||||
| PCOS (n = 214) a | 80.84 % (173) | 19.16 %(41) | 0 | 0 |
| Control (n = 122) | 58.20 % (71) | 41.80 %(51) | 0 | 0 |
| Primary | ||||
| PCOS (n = 70) b | 64.29 %(45) | 32.86 % (23) | 2.86 % (2) | 0 |
| Control (n = 59) | 32.20 %(19) | 59.32 %(35) | 8.47 %(5) | 0 |
| Secondary | ||||
| PCOS (n = 27) c | 0 | 88.89 % (24) | 11.11 % (3) | 0 |
| Control (n = 22) | 0 | 72.73 % (16) | 27.27 % (6) | 0 |
| Graafian | ||||
| PCOS (n = 11) | 0 | 0 | 72.73 % (8) | 27.27 %(3) |
| Control (n = 9) | 0 | 0 | 66.67 % (6) | 33.33 % (3) |
a, b: P <0.001, c: P < 0.01, respectively. PCOS group compared with the control group at the same developmental stage
Expression of BMP15 in granulosa cells
In the primordial follicle stage, all of the granulosa cells showed no staining for BMP15 expression in the control and PCOS groups. In the primary follicle stage, positive staining for BMP15 expression was observed in about 66 % of the control oocytes and 33 % of those in the PCOS group. In the secondary follicles, all of the oocytes stained positively for BMP15 expression in control group, whereas only 63 % of those in the PCOS tissues did. Therefore, the expression of BMP15 in granulosa cells was weaker in the PCOS group during the early follicular stages (P < 0.001), very similar to the expression pattern in oocytes. In Graafian follicles, all of the oocytes in both the control and the PCOS groups stained positively for BMP15 expression at the highest level (P > 0.05, Table 5).
Table 5.
Expression of BMP15 in granulosa cells
| Follicle type (Follicle No.) | NE % | (1 +) % | (2 +) % | (3 +) % |
|---|---|---|---|---|
| Primordial | ||||
| PCOS (n = 214) | 100 % (214) | 0 | 0 | 0 |
| Control (n = 122) | 100 % (122) | 0 | 0 | 0 |
| Primary | ||||
| PCOS (n = 70) a | 67.14 % (47) | 30.00 % (21) | 2.86 %(2) | 0 |
| Control (n = 59) | 33.90 % (20) | 61.02 % (36) | 5.08 %(3) | 0 |
| Secondary | ||||
| PCOS (n = 27) b | 37.04 % (10) | 55.56 % (15) | 7.41 % (2) | 0 |
| Control (n = 22) | 0 | 40.91 % (9) | 59.09 % (13) | 0 |
| Graafian | ||||
| PCOS (n = 11) | 0 | 0 | 72.73 % (8) | 27.27 %(3) |
| Control (n = 9) | 0 | 0 | 66.67 % (6) | 33.33 % (3) |
a, b: P <0.001, respectively. PCOS group compared with the control group at the same developmental stage
Discussion
Because oocyte secreted factors play important roles in the regulation of follicular development and oocyte maturation, their abnormal expression may be involved in follicular development-related disorders such as PCOS and premature ovarian failure [5, 14]. In this study, we examined the expression levels of GDF9 and BMP15 proteins in unstimulated ovarian tissues from PCOS patients and normal ovulatory women to explore the molecular mechanisms of aberrant follicular development in PCOS.
The results showed that the expression of GDF9 and BMP15 was stage dependent in oocytes of normal ovaries. Expression of these proteins was first observed in primordial follicles, although at a weak staining intensity, and then increased gradually with follicular development, finally reaching the highest level in Graafian follicles. These results are consistent with those of a previous study that detected the expression of GDF9 and BMP15 mRNA in oocytes of unstimulated ovarian tissues via in situ hybridization [5]. Recently, increasing numbers of studies are focusing on the essential roles of oocyte secreted factors in follicular development, especially related to the activation of primordial follicles. It has been reported that both human recombinant GDF9 and BMP15 can activate the development of human primordial follicles in vitro, with GDF9 offering seemingly more beneficial effects [15]. Studies in animal models demonstrated that GDF9 not only plays important roles in the formation of primordial follicles, but also promotes the activation of primordial follicles in vitro [16, 17]. However, it seems that GDF9 plays more important roles in the activation of primordial follicles, which may partly explain the gradual increase in GDF9 expression.
Our results also showed that GDF9 and BMP15 expression was reduced in oocytes of PCOS patients compared to normal oocytes, which may be associated with aberrant follicular development in PCOS. A large number of studies have demonstrated the essential roles of OSFs, especially GDF9 and BMP15, including the regulation of proliferation, differentiation, apoptosis, luteinization, and metabolism of adjacent granulosa cells [7]. Therefore, the reduced expression of GDF9 and BMP15 in oocytes may have detrimental effects on follicular development in ovaries of PCOS patients. However, the results of the present study conflict with those of a previous study in which immunofluorescence combined with laser scanning confocal microscopy was used to observe that the expression of GDF9 and BMP15 did not differ between oocytes of PCOS patients and normal oocytes [6]. A key difference between that study and ours is that the oocytes used in their study were collected from stimulated ovaries, which may exhibit altered expression levels of OSFs [18].
Moreover, in our present study, we observed positive staining for GDF9 and BMP15 expression in granulosa cells, which indicates that these two factors could also be produced by granulosa cells. Initially, it was thought that these two factors were exclusively produced by oocytes, and thus, were only OSFs [19]. However, now more researches have reported their expression in other cells such as cumulus cells and human breast tissue cells [6, 20]. In our previous study, we also observed the expression of these two factors in granulosa cells using quantitative real-time polymerase chain reaction assays [21]. As shown in the present study, the expression profiles of GDF9 and BMP15 in normal granulosa cells were similar to those in oocytes. It is clear that the bidirectional communication between oocytes and GCs is essential for normal follicular and oocyte development, and OSFs are further demonstrated to be important factors in this process [22–24]. Because the expression of OSFs in granulosa cells may also be essential in follicular development, decreased expression of OSFs in granulosa cells could have adverse effects on follicular development in patients with PCOS.
Another important result in our study is that the expression levels of OSFs in primordial, primary, and secondary follicles were lower in ovaries of PCOS patients than in normal ovaries. This suggests that abnormal folliculogenesis may begin from a very early stage, during which follicular development is primarily controlled by autocrine and paracrine factors, including anti-Müllerian hormone, insulin like growth factors, inhibin, activin, and others [25–28]. These factors interact with each other and regulate follicular development in a coordinated manner. However, the exact mechanisms that underlie this regulation remain unknown. In the present study, we found that GDF9 and BMP15 were expressed at normal levels in the small antral follicles of PCOS ovaries. This suggests that a compensatory increase in the expression of these proteins occurs during follicular development in PCOS ovaries. Endocrine hormones such as FSH and LH are known to regulate the later stages of follicular development, which could lead to the increased expression of OSFs [18]. However, more research is needed to elucidate these pathways.
The present study has some limitations. Immunohistochemical staining is a subjective method, and although the experimental conditions were strictly controlled, we understand that a double immunofluorescence approach can improve the specificity of oocyte staining. We will use this technique to confirm our findings in future studies. Because normal ovarian tissues from healthy women are difficult to obtain, we used ovarian biopsy tissues in which the presence of neoplastic lesions was excluded. There is a possibility of differential protein expression between ovaries from healthy women compared to the ovarian tissues used as controls in this study. Rather than using pathological biopsy to determine the normality of ovarian tissues in cancer patients, using healthy ovary tissue as a control would be more appropriate in future studies. In addition, patients’ genetic predisposition could represent a potential bias in the evaluation of follicular GDF9 and BMP15 expression; although there is little evidence that endometrial carcinoma affects the follicular expression of GDF9 and BMP15.
In conclusion, our study demonstrates that the expression of GDF9 and BMP15 proteins varies among the developmental stages in oocytes and granulosa cells of unstimulated ovaries. Expression of these proteins increases with follicular development and reaches the highest level in Graafian follicles. However, GDF9 and BMP15 expression is reduced and delayed during the early follicular stage in ovaries of patients with PCOS, which may ultimately lead to disrupted follicular development and retarded oocyte maturation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 81200476 and 81200417), the Natural Science Foundation of Guangdong Province (grant numbers S2012040007770 and S2012040007621), the National Doctoral Foundation of China (grant numbers 20120171120122 and 20120171120093), the Medical Science and Technology Research Foundation of Guangdong Province (grant numbers B2012150 and B2012104); the Young Teacher Fund of Sun Yat-sen University (grant number 11ykpy24); the Project of Science and Technology New Star in Zhu Jiang of Guangzhou City; and the Yat-sen Scholarship for Young Scientists.
Footnotes
Capsule
GDF9 and BMP15 expression in human ovarian tissue.
References
- 1.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 2.Chittenden BG, Fullerton G, Maheshwari A, Bhattacharya S. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online. 2009;19:398–405. doi: 10.1016/S1472-6483(10)60175-7. [DOI] [PubMed] [Google Scholar]
- 3.Teede H, Deeks A, Moran L. Polycystc ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;30:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumarapeli V, Seneviratne Rde A, Wijeyaratne C. Health-related quality of life and psychological distress in polycystic ovary syndrome: a hidden facet in South Asian women. BJOG. 2011;118(3):319–28. doi: 10.1111/j.1471-0528.2010.02799.x. [DOI] [PubMed] [Google Scholar]
- 5.Teixeira Filho FL, Baracat EC, Lee TH, Suh CS, Matsui M, Chang RJ, et al. Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1337–44. doi: 10.1210/jcem.87.3.8316. [DOI] [PubMed] [Google Scholar]
- 6.Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94(1):261–7. doi: 10.1016/j.fertnstert.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–77. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 8.Hutt KJ, Albertini DF. An oocentric view of folliculogenesis and embryogenesis. Reprod Biomed Online. 2007;14:758–64. doi: 10.1016/S1472-6483(10)60679-7. [DOI] [PubMed] [Google Scholar]
- 9.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 10.Juengel JL, Mc Natty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update. 2005;11:143–60. doi: 10.1093/humupd/dmh061. [DOI] [PubMed] [Google Scholar]
- 11.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 12.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Endocrine Society. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pall M, Azziz R, Beires J, Pignatelli D. The phenotype of hirsute women: a comparison of polycystic ovary syndrome and 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 2010;94(2):684–9. doi: 10.1016/j.fertnstert.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Dixit H, Rao L, Padmalatha V, Raseswari T, Kapu AK, Panda B, et al. Genes governing premature ovarian failure. Reprod Biomed Online. 2010;20(6):724–40. doi: 10.1016/j.rbmo.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Kedem A, Fisch B, Garor R, Ben-Zaken A, Gizunterman T, Felz C, et al. Growth differentiating factor 9 (GDF9) and bone morphogenetic protein 15 both activate development of human primordial follicles in vitro, with seemingly more beneficial effects of GDF9. J Clin Endocrinol Metab. 2011;96(8):E1246–54. doi: 10.1210/jc.2011-0410. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Roy SK. Expression of growth differentiation factor 9 in the oocytes is essential for the development of primordial follicles in the hamster ovary. Endocrinology. 2006;147(4):1725–34. doi: 10.1210/en.2005-1208. [DOI] [PubMed] [Google Scholar]
- 17.Martins FS, Celestino JJ, Saraiva MV, Matos MH, Bruno JB, Rocha-Junior CM, et al. Growth and differentiation factor-9 stimulates activation of goat primordial follicles in vitro and their progression to secondary follicles. Reprod Fertil Dev. 2008;20(8):916–24. doi: 10.1071/RD08108. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod. 2010;83:514–24. doi: 10.1095/biolreprod.109.083311. [DOI] [PubMed] [Google Scholar]
- 19.Aaltonen J, Laitinen MP, Vuojolainen K, Jaatinen R, Horelli-Kuitunen N, Seppä L, et al. Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J Clin Endocrinol Metab. 1999;84(8):2744–50. doi: 10.1210/jcem.84.8.5921. [DOI] [PubMed] [Google Scholar]
- 20.Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG. The role of growth differentiation factor-9 (GDF-9) and its analog, GDF-9b/BMP-15, in human breast cancer. Ann Surg Oncol. 2007;14(7):2159–66. doi: 10.1245/s10434-007-9397-5. [DOI] [PubMed] [Google Scholar]
- 21.Wei LN, Li LL, Fang C, Huang R, Liang XY. Inhibitory effects of controlled ovarian stimulation on the expression of GDF9 and BMP15 in oocytes from women with PCOS. J Assist Reprod Genet. 2013;30(10):1313–8. doi: 10.1007/s10815-013-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88(4):399–413. doi: 10.1139/Y10-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binelli M, Murphy BD. Coordinated regulation of follicle development by germ and somatic cells. Reprod Fertil Dev. 2010;22(1):1–12. doi: 10.1071/RD09218. [DOI] [PubMed] [Google Scholar]
- 24.Yeo CX, Gilchrist RB, Lane M. Disruption of bidirectional oocyte-cumulus paracrine signaling during in vitro maturation reduces subsequent mouse oocyte developmental competence. Biol Reprod. 2009;80(5):1072–80. doi: 10.1095/biolreprod.108.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pellatt L, Rice S, Mason HD. Anti-Müllerian hormone and polycystic ovary syndrome: a mountain too high? Reproduction. 2010;139(5):825–33. doi: 10.1530/REP-09-0415. [DOI] [PubMed] [Google Scholar]
- 26.Silva JR, Figueiredo JR, van den Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology. 2009;71(8):1193–208. doi: 10.1016/j.theriogenology.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Zhu R, Zhou X, Chen Y, Qiu C, Xu W, Shen Z. Aberrantly increased mRNA expression of betaglycan, an inhibin co-receptor in the ovarian tissues in women with polycystic ovary syndrome. J Obstet Gynaecol Res. 2010;36(1):138–46. doi: 10.1111/j.1447-0756.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- 28.Trombly DJ, Woodruff TK, Mayo KE. Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med. 2009;27(1):14–23. doi: 10.1055/s-0028-1108006. [DOI] [PMC free article] [PubMed] [Google Scholar]