Abstract
Purpose
Environmental influences on reproductive success are recognized. We hypothesized that location of fertility clinics may influence treatment success and explored this hypothesis utilizing donor egg IVF (IVF) embryo transfer (ET) model.
Methods
Publicly accessible national registry data (Society for Assisted Reproductive Technology) on fresh & frozen (FET) ET cycles undertaken at participating clinics across North America (n = 444 IVF centers) for 2007 were utilized. Information on number of donor egg IVF cycles, live birth (LB) rate following fresh and frozen ET(FET), average number (#) of ET and IVF center's location, geographical coordinates (latitude, longitude, altitude), annualized average temperatures and midyear regional ultraviolet B (UVB) radiation intensity were obtained. Multivariable logistic regression analyses assessed relationship between LBR (in tertile and uppermost versus lesser quartiles) following fresh and FET with geographical coordinates (region and altitude of clinic location) and ecological influences (average temperature and midyear UVB intensity), adjusting for #ET and clinic experience with donor egg IVF.
Results
Average number of fresh ET, clinic location (region) and midyear UVB intensity were positive predictors of LBR following fresh ET, whereas altitude and annualized average regional temperature demonstrated an inverse relationship with LBR following fresh ET. For FET cycles, #ET, clinic region and altitude were positive determinants of increasing LBR’s. Annualized regional temperature and midyear UVB failed to demonstrate any relationship with LB following donor egg FET.
Conclusion
Our data suggest that ecological influences may relate to donor egg IVF cycle success. Future studies are needed to better elucidate the mechanisms that could explain the observed associations.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-014-0322-0) contains supplementary material, which is available to authorized users.
Keywords: In vitro fertilization, Donor egg, Ecology, Reproduction, Zip code, UVB
Introduction
Since the eventful birth of Louse Brown in 1978 [1], excess of 3.75 million babies are estimated to have been born worldwide using assisted reproductive technology (ART) [2, 3]. Modifications in ovarian stimulation strategies as well as optimization of in vitro handling of gametes and embryos have all contributed to improving live birth rate (LBR) following ART over the years [4]. While patient characteristics (e.g. age, ovarian reserve, body mass and endometrial receptivity), the experience, and the expertise of fertility clinics are all recognized to impact on cycle outcome, success with ART remains far from assured.
Of all fertility treatment modalities, the donor egg in vitro fertilization (IVF) model offers the most “optimal” likelihood for treatment related reproductive success [2, 5]. Indeed, LBR following transfer of embryos resulting from donor eggs far outweigh those from transfer of autologous embryos. Recognizing that chronology of aging is by far the biggest determinant of ART outcome, success of donor egg IVF can largely be attributed to the selection of healthy young donors. The stringency of egg donor screening processes may however vary between ART centers and across the globe. In the USA, egg donor screening guidelines are clearly outlined by the Center for Disease Control, and requisites for a fertility clinic offering use of donor gametes for third party reproduction are specified. Review of Society of Assisted Reproductive Technology (SART) data for 2009 [6] identified that almost 11 % of ART cycles utilized donor eggs (15,459 donor egg cycles out of total of 142,241 ART cycles). The LBR for donor egg embryo transfer (ET) IVF cycles for 2009 was 55 % for fresh and almost 34 % for frozen ET (FET) IVF cycles.
Despite rigorous screening and selection of “optimal” egg donors, LBR following donor egg IVF is far from “absolute”. While a number of patient specific variables are recognized to adversely impact on the success of donor egg IVF [7–10], contributions of thus far unidentified influences that may modulate the likelihood for procreative success with the use of donor eggs remain a consideration.
Ecological factors have been related to a diverse array of disease processes [11–15], and as well are suggested to have an impact on reproductive success [16, 17]. In a large cross-sectional study, air quality parameters within in clinical laboratories were identified as being of relevance for vitro fertilization (IVF) cycle success [17]. Limited epidemiological data suggest a relationship between environmental light exposure and a number of chronic disorders including diabetes, cardiovascular disease and cancers [18]. Interestingly, a seasonality in population birth rates is also described; an increase in birth rates are reported to follow months of maximal light exposure, by a period equivalent to human gestation in months [19].
While phenomenon that could explain the observed relationship between light exposure and population fecundity are unclear, seasonal variations in the population vitamin D status can be theorized as a plausible mechanism [19–21]. Importance of vitamin D for reproductive success is well described, albeit mostly in non-human animal models [22]; data in humans are sparse. In a cross sectional study of infertile women undergoing IVF, we had previously identified significantly higher levels of 25-hydroxyvitamin D (25OHD), a metabolite that reliably reflects the overall vitamin D status, in the follicular fluid of women achieving clinical pregnancy following IVF [23]. While this latter observation was recently corroborated by others [24], including in donor egg recipients [25], data relating vitamin D status to IVF outcome remain far from unequivocal [26].
Epidemiological data suggest a relationship between geographical location of birth and residence in areas of low ultraviolet B (UVB) exposure with a spectrum of diseases including multiple sclerosis, type I diabetes and cancers [27–35]. Data from the U.S. identify prevalence of most types of cancer, dental caries, and of autism to more closely linked to summertime solar UVB; prevalence of multiple sclerosis in contrast has been related to wintertime UVB and latitude [36].
Vitamin D insufficiency resulting from low levels of UVB exposures is implied to underlie some of the observed geo demographic underpinnings [20, 36]. Given that the intensity of UVB exposure is recognized to vary by geographical coordinates i.e. latitude, longitude, and altitude, and given our earlier observations that suggested that vitamin D status may be of relevance for IVF success, we hypothesized that geographical location of IVF clinics impacts on the IVF success of specific clinics. We explored this hypothesis utilizing the donor egg IVF model.
Materials and methods
Publically accessible IVF outcome data reported to the Society for Assisted Reproductive Technology for year 2007 [6] were manually screened for donor egg IVF-embryo transfer (ET) cycles (both fresh and frozen donor ET cycles). Given the study design, i.e. utilization of data available in the public domain, this work was deemed to not require procurement of any institutional approval. Data variables that are available in the publically accessible format include: annual numbers for all IVF and all donor egg IVF ET (fresh and frozen ET) cycles undertaken at the participating clinics, the average number of donor egg ET and clinic specific donor egg ET related LBR. Each IVF center was identified by its specified zip code. Based on zip code, the participating clinics were segregated into four geographical regions as defined by the US Census Bureau [37] as follows: West (zip codes starting with numerical 8 and 9), Mid-West (zip codes starting with numerical 4, 5 and 6), South (zip codes starting with 2, 3 and 7) and North East (zip codes starting with 0 and 1). Note that the zip code numbers in US regions progressively increase from North East towards the South West. Geographical coordinates for each IVF center (i.e. latitude and altitude) were identified based on the clinic zip code [38]. Additional analyses related live birth rates to the average annualized regional temperature (°F) and midyear UVB values for the month of July (kJ/m2 (kilojoules per meter square) utilizing previously published data for 32 states within North America [29].
Statistics
Data distribution was analyzed. Outcome data (LBR’s) were skewed in distribution (available as proportions) and therefore nonparametric tests (Mann Whitney U, Kruskall Wallis Rank Test, simple and multivariable logistic regression) were employed for data analyses.
Correlation analyses (Spearman’s) assessed the relationship between LBR’s following donor egg ET (fresh and FET) with clinic location (zip code, latitude [°N] and altitude [feet]), with annualized regional temperature (°F) and midyear UVB. Tertiles for altitude and latitude were computed. Kruskall Wallis rank test assessed differences in the specified variables reported by IVF centers across the four regions (as specified by zip code) and across tertiles of data distribution. Given that sunlight at latitudes >37°N is recognized as being insufficient to induce endogenous cutaneous synthesis of vitamin D during the winter months, clinic location was dichotomized based on ≤37°N (low) versus higher latitude [39]; altitudes at and greater than 75th percentile were deemed as “high” and LBR’s were compared between lower versus high altitudes.
Tertiles were computed for clinic reported LBR’s following fresh and FET cycles; Kruskall Wallis Rank Test and simple ordinal logistic regression analyses assessed relationship between geographical (clinic location, altitude) and ecological (average regional annual temperature and midyear UVB) indices with LBR tertiles following fresh and FET. Additional sensitivity analyses assessed predictors of LBR’s in the upper most quartile (>75 % percentile) of all reporting clinics to identify regional and ecological determinants to donor egg IVF success.
Multivariable analyses assessed the relationship between LBR’s following fresh and FET respectively with specified geographical (regional location) and ecological (midyear UVB and average annual regional temperature) parameters after adjusting for the clinic experience with donor egg IVF (reflected by annual number of donor egg IVF cycles) and clinic specified average number of ET (fresh and FET).
Continuous data (normal in distribution) are presented as mean ± standard deviation or median and inter-quartile range (skewed distribution) and categorical data are presented as percentage (%). Odds ratio (OR) with 95 % confidence interval (95 % CI) reflect the strength of associations. Goodness of fit was determined for logistic regression models. Two tailed p < 0.05 was considered of statistical significance; threshold for statistical significance was further adjusted for multiplicity of comparison when indicated and STATA 12.0 (Collage Station, TX) was used for analyses.
Results
In 2007, donor egg IVF cycles were reported by 446 participating IVF clinics within North America with 443 reporting data on fresh ET and 444 on FET donor egg IVF cycles.
Regional differences were observed in the annual number of donor egg IVF cycles, and in the average number of ET per clinic as shown in Table 1. IVF clinic experience with donor egg IVF (i.e. annual donor egg cycles) was positively related to LBR following fresh (r = 0.33, p < 0.001) and FET (r = 0.48, p < 0.001) donor egg cycles.
Table 1.
Regional data on donor egg IVF cycles (2007) undertaken at centers in North America reporting to Society for Assisted Reproductive Technologies (SART)
| Parameters | North Easta (zip 0,1) | Southb (zip 2,3,7) | Mid Westc (zip 4,5,6) | Westd (zip 8,9) | Overall P value |
|---|---|---|---|---|---|
| Regional annual donor egg IVF cycles (n) | 120 | 119 | 103 | 101 | <0.001 |
| Annual fresh donor egg embryo transfer cycles per clinic (n)1 | 14 (4−34) | 9 (3−19) | 8 (2−19) | 18 (8−34) | <0.001 |
| Annual donor egg frozen embryo transfer cycles per clinic (n)1 | 11 (0.5−22) | 4 (1−10.5) | 4 (1−11) | 9 (2−22) | <0.001 |
| Fresh embryos transferred (n)2 | 2.06 ± 0.79 | 1.96 ± 0.69 | 1.82 ± 0.94 | 2.29 ± 0.76 | <0.001 |
| Frozen embryos transferred (n)2 | 1.72 ± 1.09 | 1.89 ± 1.02 | 1.86 ± 1.15 | 2.27 ± 0.98 | <0.001 |
| % Low latitude locations (<=37°N) | 2.5 % | 80 % | 6 % | 61 % | <0.001 |
| % High altitude locations (>75 percentile) | 3 % | 17 % | 48 % | 37 % | <0.001 |
| July – UVB intensity 2,3 | 4.63 ± 0.20 | 7.05 ± 1.03 | 5.06 ± 1.05 | 7.82 ± 1.22 | <0.001 |
| Average Regional Temperature (°F)2,3 | 51.13 ± 1.42 | 62.23 ± 5.67 | 51.15 ± 5.92 | 57.81 ± 9.50 | <0.001 |
| Fresh embryo transfer live birth rate (%)1,4 | 50 (33–61) | 50 ( 33–62) | 49 (22–63) | 57 (45–70) | 0.004 |
| Frozen embryo transfer live birth rate (%)1,5 | 22 (0–29) | 33 (0–46) | 20 (0–33) | 31 (15–44) | 0.001 |
1Median (inter quartile range)
2Mean ± standard deviation
3Data from Grant WB and Garland CF, 2006; average regional temperature data were available for 35/101 IVF centers in the West
4a vs b, p = 0.18; a vs c, p = 0.30; a vs d, p = 0.001*; b vs c, p = 0.08; b vs d, p = 0.02; c vs d, p < 0.001*
5a vs b, p < 0.001*; a vs c, p = 0.31, a vs d, p < 0.001*; b vs c, p = 0.002*; b vs d, p = 0.28; c vs d, p < 0.001*
Statistical significance accounting for multiplicity of comparisons is 0.004
Statistically significant correlations were observed between clinic zip code with latitude (r = −3.0, p < 0.001), longitude (r = 0.67, p < 0.001), and with the altitude of clinic location specified by zip code (r = 0.15, p = 0.001).
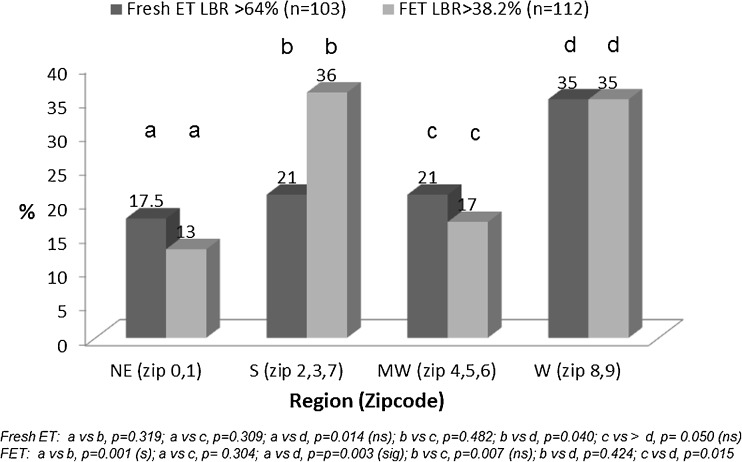
Statistically significant linear correlations were observed between LBR’s following fresh and FET donor egg IVF cycles with clinic location as reflected by zip code (r = 0.14, p = 0.003). Regional success rates were highest for clinics located in the West, compared to clinics in other regions within North America (Table 1). Fertility centers reporting LBR’s in the highest quartile for both fresh and FET differed significantly by region (Fig. 1) with proportion of clinics in the West achieving LBR’s in the highest quartile following both fresh ET compared to IVF centers located in the East and the Mid-West of North America; significantly higher proportion of clinics in the West and South achieved LBR’s in the highest quartile following FET compared to clinics in the East and the Mid-West (Fig. 1).
Fig. 1.
Regional differences in North American IVF clinics reporting donor egg IVF related live birth rates in the highest quartile following fresh and frozen (FET) embryo transfer cycles: SART 2007 data. Footnote: Statistical significance accounting for multiplicity of comparisons is 0.004
Declining, albeit non-significant differences in LBR’s (for both fresh and FET cycles) were seen across increasing tertiles of latitude (i.e. lowest tertile reflecting locations most proximate to the equator, and increasing tertiles being at progressively increasing distance from the equator, and hence reflecting progressively declining UVB exposure. Regional UV Outlook Index, a measure of summertime regional solar UVB (United States Environmental Protection Agency, is included as supplementary figure, [40]. From the lowest to the highest latitude tertiles, LBR’s for fresh ET cycles were 50 % versus 47 % versus 44 % respectively, p = 0.267 and for FET cycles were 30 %, 25 % and 22 % respectively, p = 0.155. Clinics located at low latitudes (≤37°N) reported significantly higher LBR’s following FET (median 29, IQR 0–46 %) compared to centers located at higher latitudes (median 25, IQR 0–34 %, p = 0.036); however, LBR’s following fresh ET were comparable with median LBR of 53 % [IQR 33–64 %] reported by centers located at low latitude sites versus 50 % [IQR 33–63 %] reported by clinics located at higher latitudes, p = 0.281).
Although lower LBR’s following fresh ET were noted across increasing altitude tertiles, these associations were not statistically significant on univariate analyses (50 % versus 47 % versus 45 %, p = 0.346).
Average annual regional temperatures and regional midyear UVB exposure varied across the regions within North America. Statistically significant positive correlations were observed between regional zip codes with midyear UVB intensity (r = 0.53, p < 0.001) and average temperature (r = 0.26, p < 0.001). Predictably, low latitude locations (i.e. ≤37°N) demonstrated significantly higher midyear UVB intensity and higher average regional temperatures compared to higher latitude locales (z = -15, p < 0.001 and z = −15.3, p = 0.001 respectively).
Live birth rates following fresh ET demonstrated statistically significant positive correlation with midyear regional UVB intensity (r = 0.13, p = 0.005); a similar, albeit non-significant relationship was observed between regional UVB exposure and LBR following FET (r = 0.09, p = 0.06). On univariate analyses, average regional temperatures did not demonstrate any relationship with LBR with either fresh (r = 0.03, p = 0.587) or FET (r = 0.07, p = 0.145).
For multivariable analyses, clinic specific LBR following donor egg IVF (fresh and FET) was the outcome and region of clinic location the independent variable of interest; clinic experience with donor egg IVF, average number of ET per clinic, altitude of clinic location (by zip code), midyear UVB (by state) and the annualized average regional temperature were taken as adjustment variables.
Results of ordinal logistic regression analyses, where tertiles of LBR (following fresh and FET) were the specified outcome, are presented in Table 2; region of clinic location, annual number of ET, altitude and annual regional temperatures at clinic location were independent predictors of LBR in the mid to highest compared to the lowest LBR tertile. Relationship between geographical and ecological parameters with the likelihood of achieving LBR in the highest quartile (>75 %) following donor egg ET is presented in Table 3 wherein clinic location, annual number of ET and clinic experience with donor egg IVF were independent predictors of LBR in the highest quartile for donor egg ET cycles. Model specificity for individual outcomes is presented as table footnote for respective analyses.
Table 2.
Ordinal multivariable logistic regression analysis demonstrating predictors of live birth rate (as tertiles) following donor egg fresh and frozen embryo transfer (FET) cycles
| Coefficient | Standard Error | P value | 95 % CI | |
|---|---|---|---|---|
| LBR Tertile- Fresh ET | ||||
| Region a | ||||
| -West | 1.10 | 0.51 | 0.033 | 0.09 to 2.10 |
| -South | 0.40 | 0.36 | 0.268 | −0.31 to 1.12 |
| -Mid West | −0.004 | 0.27 | 0.987 | −0.53 to 0.52 |
| Average # Fresh ETb | 0.82 | 0.15 | <0.001 | 0.53 to 1.11 |
| Annual # Donor Egg IVF Cyclesc | −0.00 | 0.00 | 0.869 | −0.00 to 0.00 |
| Altitude (per 100 ft) | −4.35e-06 | 1.95e-06 | 0.026 | −8.18e-06 to 5.26e-07 |
| Average regional temperature (°F)d | −0.12 | 0.04 | 0.004 | −0.21 to −0.04 |
| Midyear UVBd | 0.62 | 0.24 | 0.001 | 0.14 to 1.10 |
| LBR Tertile- FET | ||||
| Region a | ||||
| -West | 0.76 | 0.49 | 0.125 | −0.21 to 1.72 |
| -South | 1.55 | 0.39 | <0.001 | 0.77 to 2.32 |
| -Mid West | −0.31 | 0.29 | 0.292 | -0.88 to 0.26 |
| Average # FET b | 1.33 | 0.14 | <0.001 | 1.07 to 1.60 |
| Annual # Donor Egg IVF Cycles c | 0.0002 | 0.0003 | 0.550 | −0.0004 to 0.001 |
| Altitude (per 100 ft) | 4.01e-06 | 2.01e-06 | 0.046 | 7.31e-08 to 7.94e-06 |
| Average regional temperature (°F)d | −0.14 | 0.04 | 0.756 | −0.110 to 0.07 |
| Midyear UVBd | −0.27 | 0.26 | 0.286 | −0.78 to 0.23 |
aRegions defined based on zip code (see text for details); referent region: North East
bClinic specific average number of donor egg embryos transferred
cClinic experience with donor egg IVF
dPreviously published data for 32 states within North America (Grant WB and Garland CF, 2006)
Model sensitivity: 68 %
Table 3.
Multivariable logistic regression analyses assessing predictors of clinic performance in the highest quartile for donor egg IVF related live birth rates
| Likelihood of LBR in highest quartile | Unadjusted OR (95 % CI) | P value | Adjusted OR (95 % CI) | P value |
|---|---|---|---|---|
| Fresh ET | ||||
| Region a | ||||
| -West | 2.14 (1.31 to 3.48) | 0.002 | 6.51 (2.08 to 20.35) | 0.001 |
| -South | 0.88 (0.50 to 1.39) | 0.499 | 1.54 (0.61 to 3.85) | 0.359 |
| -Mid West | 0.87 (0.51 to 1.48) | 0.604 | 1.2 (0.59 to 2.42) | 0.618 |
| Average # Fresh ET b | 1.35 (1.01 to 1.81) | 0.041 | 1.50 (1.06 to 2.13) | 0.023 |
| Annual # Donor Egg IVF Cycles c | 0.99 (0.99 to 0.99) | 0.025 | 0.99 (0.98 to 0.999) | 0.022 |
| Altitude (per 100 ft) | 0.99 (0.99 to 1.00) | 0.261 | 0.99 (0.99 to 1.00) | 0.105 |
| Average regional temperature (°F) d | 0.99 (0.97–1.02) | 0.949 | 0.91 (0.81 to 1.01) | 0.080 |
| Midyear UVBd | 1.07 (0.94 to 1.22) | 0.322 | 1.52 (0.83 to 2.76) | 0.173 |
| FET | ||||
| Region a | ||||
| -West | 1.83 (1.13 to 2.96) | 0.014 | 2.50 (0.73 to 8.50) | 0.145 |
| -South | 2.10 (1.33 to 3.3) | 0.002 | 7.12 (2.72 to 18.63) | <0.001 |
| -Mid West | 0.55 (0.31 to 0.95) | 0.035 | 1.11 (0.50 to 2.45) | 0.796 |
| Average # FET b | 2.08 (1.59 to 2.7) | <0.001 | 2.32 (1.65 to 3.25) | <0.001 |
| Annual # Donor Egg IVF Cycles c | 0.99 (0.99–1.00) | 0.659 | 0.99 (0.99-1.00) | 0.696 |
| Altitude (per 100 ft) | 1.00 (0.99–1.00) | 0.418 | 1.00 (0.99-1.00) | 0.148 |
| Average regional temperature (°F)d | 1.03 (1.002 to 1.06) | 0.038 | 1.02 (0.92 to 1.14) | 0.644 |
| Midyear UVBd | 1.23 (1.08 to 1.41) | 0.002 | 0.65 (0.36 to 1.19) | 0.164 |
aRegions defined based on zip code (see text for details); referent region: North East
bClinic specific average number of donor egg embryos transferred
cClinic experience with donor egg IVF
dPreviously published data for 32 states within North America (Grant WB and Garland CF, 2006)
Model sensitivity: 75 %
Discussion
Zip code based analyses, as pursued, are commonly utilized for study of geo-demographic underpinnings of diseases and disorders [41, 42]. Our analyses of donor egg IVF cycles undertaken at ART centers across North America reporting to SART identify meaningful associations between geographical clinic coordinates and ecological parameters with reproductive success following donor egg embryo transfer.
The pattern of the observed regional trends, i.e. lesser donor egg IVF success rates at centers along the North East and improving LBR’s at clinics located towards the West (as reflected by increasing zip code numbers) is reminiscent of associations previously described for disorders that have been related to insufficient UVB exposure [12, 13] [27–35]. Directionality of our observations is consistent with trends noted for breast cancer; the prevalence and mortality from breast cancer in North America is reportedly higher in the North East compared to the West, and lower UVB intensity exposure in the North East is a suggested mechanism for this differential [29, 30] [32–34]. Our primary hypothesis was based on a differential in UVB exposure across the regions; indeed, fresh ET related live birth rates were lower in regions with lesser summertime solar UVB indices, and regional midyear UVB intensity was identified as an independent predictor of increasing fresh ET related LBR’s (from lowest to highest tertile, Table 2).
While presumed differences in population vitamin D status, as hypothesized, may underlie the observed regional differences in LBR’s following donor egg IVF, regional differential in environmental pollutants and toxins cannot be dismissed as plausible contributors to the observed differences in donor egg IVF related LBR’s, as has been previously suggested [17]. Our study design does not allow for evaluation of potential contributory mechanisms that could explain our findings.
The observed geographical differential in determinants of fresh versus FET cycles donor egg IVF cycle success is intriguing; significantly higher LBR following FET were noted for IVF clinics located at a latitude ≤37°N; this observation suggests that higher regional UVB, and by proxy, better vitamin D status, may be facilitatory for endometrial receptivity. Indeed, in vitro exposure of endometrial cells to vitamin D has been shown to modulate the expression of factors that are appreciated to impact on endometrial receptivity [43–45], thus supporting our conjecture. Consistent with our hypothesis, higher midyear UVB intensity was identified as an independent and positive predictor of fresh ET related LBR’s (Table 2).
Focusing on a single mechanism, i.e. higher UVB, to explain the observed regional differences in LBR following donor egg IVF is however simplistic; differences in the macro and microenvironments across the specified latitude and zip codes may impact on ART success, and merit consideration. Both temperature and photoperiod are recognized to affect reproductive physiology of humans as well as nonhuman mammals [19]. A seasonality in birth, for spontaneous as well as assisted conceptions is described [46, 47]. Implications of ambient temperature for procreative success are further suggested and variation in human fertility as a function of both climate and latitude reported [46]. In a large retrospective study (2709 IVF cycles), Wood et al. [47] observed significantly improved ovarian response, implantation and clinical pregnancy rates for IVF cycles undertaken in the summer months (April- September); the authors construed these observations as consequent to increased daylight length and implied a mechanistic role for melatonin. A study of regional birth rates based on data available in World Population Data Sheet [48], explored the relationship between total fertility rates in 187 countries with average latitude and average winter and summer temperatures in the regions of interest. After controlling for parameters reflecting regional economic status, spontaneous birth rates were higher for populations residing at low latitudes, an observation that is recapitulated in our analyses of donor egg IVF cycles. Our findings of lower donor egg IVF related LBR’s at clinics located in warmer regions are also in line with prior observations relating spontaneous birth rates in humans to environmental temperature; Barber N (2006) reported lower spontaneous birth rates for populations residing in regions experiencing warmer winters and our data identify lower donor egg LBR’s following fresh ET for clinics located in higher annual temperature regions. Collectively, these data imply that higher environmental temperatures as potentially detrimental to processes underlying reproductive success through mechanisms that merit further evaluation.
The observed differential in association between altitude and LBR following donor egg fresh versus FET is of interest. While increasing altitude of fertility clinics was predictive of a significantly higher likelihood of LBR’s in the middle to highest tertile following fresh ET, this association was reversed for FET cycle outcome where higher altitude was noted to demonstrate an inverse association with LBR tertile (Table 2). Given the many limitations of our study design, any opinion on plausible mechanism/s for the observed phenomenon can only be conjectural at best. While environmental differences are hypothesized to underlie the observed associations between clinic location and donor egg IVF success, a number of unquantified variables could very well explain the observed directionality of association. Regional practice differences in IVF protocols, such as choice of ovarian suppression strategy, gonadotropin type and dose, and peak estradiol levels prior to ET [49], donor characteristics such as age and sibling donor [50], as well as regional differences in donor egg recipient profiles such as obesity [51], uterine factor [52, 53], presence of hydrosalpinges [54] and even vitamin D deficiency [55] could underlie the observed phenomenon. The publically accessible data reported by SART include cumulative annual data for the reporting clinics; details on individual egg donors and recipients are lacking; similarly, in the absence of information on timing of the undertaken ART cycle/s, any inference regarding site specific UVB values during the time of ART cycle, or effect of seasonality cannot be attained. Recipient characteristics may impact on donor egg IVF outcome, and this information is lacking in our methodology. Despite the identified limitations however, our observations that geographic and ecological variables related to donor egg IVF outcome are nonetheless intriguing, and in line with a paradigm well described in mammalian reproduction. Our findings suggest that success of donor egg IVF, at least in North America, may be influenced by factors that are beyond the expertise and experience of the infertility clinics.
Electronic supplementary material
UV Outlook Index (a measure of summertime solar UVB) across North America (United States Environmental Protection Agency, http://www.epa.gov/sunwise/uvimonth.html [40], last accessed 2/3/14). (JPEG 92 kb)
Acknowledgments
Support
None
Footnotes
Capsule Within North American, geographical location of fertility clinic relates to donor egg IVF success.
These data were presented as an abstract at the 69th annual meeting of American Society for Reproductive Medicine, October 2012, San Diego.
Contributor Information
Lubna Pal, Phone: 203-737 5619, Email: lubna.pal@yale.edu.
Neiha Kidwai, Email: neiha.kidwai@gmail.com.
Jehanzeb Kayani, Email: jehanzeb.kayani@uconn.edu.
William B. Grant, Email: wbgrant@infionline.net
References
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi: 10.1016/S0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.Center for disease control and prevention. Assisted reproductive technology success rates, 2008. National summary and fertility clinic reports [online]. URL: http://www.cdc.gov/art/ART2008/PDF/ART_2008_Full.pdf (Last accessed 25 Nov 2012).
- 3.Ferraretti AP, Goossens V, de Mouzon J, Bhattacharya S, Castilla JA, Korsak V, et al. European IVF-monitoring (EIM); Consortium for European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2008: results generated from European registers by ESHRE. Hum Reprod. 2012;27(9):2571–84. doi: 10.1093/humrep/des255. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Sauer MV. In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag. 2006;2(4):355–64. doi: 10.2147/tcrm.2006.2.4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology. Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril. 2012 Oct 17. [DOI] [PubMed]
- 6.Society for Assisted Reproductive Technology. http://sart.org/find_frm.html. (Last accessed 25 Nov 2012).
- 7.Borini A, Suriano R, Barberi M, Dal Prato L, Bulletti C. Oocyte donation programs: strategy for improving results. Ann N Y Acad Sci. 2011;1221:27–31. doi: 10.1111/j.1749-6632.2010.05934.x. [DOI] [PubMed] [Google Scholar]
- 8.Noyes N, Hampton BS, Berkeley A, Licciardi F, Grifo J, Krey L. Factors useful in predicting the success of oocyte donation: a 3-year retrospective study. Fertil Steril. 2001;76:92–7. doi: 10.1016/S0015-0282(01)01823-4. [DOI] [PubMed] [Google Scholar]
- 9.Soares SR, Velasco JA, Fernandez M, Bosch E, Remohí J, Pellicer A, et al. Clinical factors affecting endometrial receptiveness in oocyte donation cycles. Fertil Steril. 2008;89(3):491–501. doi: 10.1016/j.fertnstert.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 10.Zenke U, Chetkowski RJ. Transfer and uterine factors are the major recipient-related determinants of success with donor eggs. Fertil Steril. 2004;82(4):850–6. doi: 10.1016/j.fertnstert.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 11.Briggs D. Environmental pollution and the global burden of disease. Br Med Bull. 2003;68:1–24. doi: 10.1093/bmb/ldg019. [DOI] [PubMed] [Google Scholar]
- 12.Grant WB. A review of the role of solar ultraviolet-B irradiance and vitamin D in reducing risk of dental caries. Dermatoendocrinol. 2011;3(3):193–8. doi: 10.4161/derm.15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant WB, Cannell JJ. Autism prevalence in the United States with respect to solar ultraviolet-B doses: An ecological study. Dermato-endocrinology. 2013;5:159–64. doi: 10.4161/derm.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillette LJ, Jr, Iguchi T. Ecology. Life in a contaminated world. Science. 2012;337(6102):1614–5. doi: 10.1126/science.1226985. [DOI] [PubMed] [Google Scholar]
- 15.Sloka S, Silva C, Pryse-Phillips W, Patten S, Metz L, Yong VW. A quantitative analysis of suspected environmental causes of MS. Can J Neurol Sci. 2011;38(1):98–105. doi: 10.1017/s0317167100011124. [DOI] [PubMed] [Google Scholar]
- 16.Bronson FH. Mammalian reproduction: an ecological perspective. Biol Reprod. 1985;32(1):1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. Effect of air quality on assisted human reproduction. Hum Reprod. 2010;25(5):1317–24. doi: 10.1093/humrep/deq021. [DOI] [PubMed] [Google Scholar]
- 18.Gillie O. The Scots' Paradox: can sun exposure, or lack of it, explain major paradoxes in epidemiology? Anticancer Res. 2012;32:237–48. [PubMed] [Google Scholar]
- 19.Cummings DR. Human birth seasonality and sunshine. Am J Hum Biol. 2010;22(3):316–24. doi: 10.1002/ajhb.20987. [DOI] [PubMed] [Google Scholar]
- 20.Cummings DR. Seasonal sunshine and vitamin D: a possible explanation for differences in European and United States birth patterns. Biodemography Soc Biol. 2010;56(2):105–22. doi: 10.1080/19485565.2010.524093. [DOI] [PubMed] [Google Scholar]
- 21.Stumpf WE, Denny ME. Vitamin D (soltriol), light, and reproduction. Am J Obstet Gynecol. 1989;161(5):1375–84. doi: 10.1016/0002-9378(89)90699-6. [DOI] [PubMed] [Google Scholar]
- 22.Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod. 2012;27(10):3015–27. doi: 10.1093/humrep/des248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, et al. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril. 2010;94(4):1314–9. doi: 10.1016/j.fertnstert.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod. 2012;27(11):3321–7. doi: 10.1093/humrep/des280. [DOI] [PubMed] [Google Scholar]
- 25.Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril. 2014;101:447–52. doi: 10.1016/j.fertnstert.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Anifandis GM, Dafopoulos K, Messini CI, Chalvatzas N, Liakos N, Pournaras S, et al. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol. 2010;8:91. doi: 10.1186/1477-7827-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canto MT, Anderson WF, Brawley O. Geographic variation in breast cancer mortality for white and black women: 1986-1995. CA Cancer J Clin. 2001;51(6):367–70. doi: 10.3322/canjclin.51.6.367. [DOI] [PubMed] [Google Scholar]
- 28.Elliott JC, Lucas RM, Clements MS, Bambrick HJ. Population density determines the direction of the association between ambient ultraviolet radiation and type 1 diabetes incidence. Pediatr Diabetes. 2010;11(6):394–402. doi: 10.1111/j.1399-5448.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 29.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26(4A):2687–99. [PubMed] [Google Scholar]
- 30.Grant WB. Ecological studies of the UVB-vitamin D-cancer hypothesis. Anticancer Res. 2012;32(1):223–36. [PubMed] [Google Scholar]
- 31.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9(5):A387–94. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51(8):1391–8. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 33.Sariego J. Patterns of breast cancer presentation in the United States: does geography matter? Am Surg. 2009;75(7):545–9. doi: 10.1177/000313480907500703. [DOI] [PubMed] [Google Scholar]
- 34.Sturgeon SR, Schairer C, Gail M, McAdams M, Brinton LA, Hoover RN. Geographic variation in mortality from breast cancer among white women in the United States. J Natl Cancer Inst. 1995;87(24):1846–53. doi: 10.1093/jnci/87.24.1846. [DOI] [PubMed] [Google Scholar]
- 35.Wei-Passanese EX, Han J, Lin W, Li T, Laden F, Qureshi AA. Geographical variation in residence and risk of multiple nonmelanoma skin cancers in US women and men. Photochem Photobiol. 2012;88(2):483–9. doi: 10.1111/j.1751-1097.2012.01077.x. [DOI] [PubMed] [Google Scholar]
- 36.Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005;10(2):94–111. [PubMed] [Google Scholar]
- 37.United States Census Bureau, Census Bureau Regions and Divisions with State FIPC Codes. http://www.census.gov/geo/www/us_regdiv.pdf (last accessed 25 Nov 2012).
- 38.http://www.wunderground.com/wundermap/, (last accessed 25 Nov 2012).
- 39.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6):1678S–88. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 40.United States Environmental Protection Agency, Monthly UV Index. http://www.epa.gov/sunwise/uvimonth.html (last accessed 25 Nov 2012).
- 41.Grubesic TH, Matisziw TC. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. Int J Health Geogr. 2006;5:58. doi: 10.1186/1476-072X-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbert JD, Liese AD, Lawson A, Porter DE, Puett RC, Standiford D, et al. Evaluating geographic imputation approaches for zip code level data: an application to a study of pediatric diabetes. Int J Health Geogr. 2009;8:54. doi: 10.1186/1476-072X-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol. 2005;19:2222–33. doi: 10.1210/me.2004-0336. [DOI] [PubMed] [Google Scholar]
- 44.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, et al. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75(6):816–22. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 45.Tavakoli M, Jeddi-Tehrani M, Salek-Moghaddam A, Rajaei S, Mohammadzadeh A, Sheikhhasani S, et al. Effect of 1,25(OH)(2) vitamin D(3) on cytokine production by endometrial cells of women with repeated implantation failure. Gynecol Endocrinol. 2012;28:906–11. doi: 10.3109/09513590.2012.683062. [DOI] [PubMed] [Google Scholar]
- 46.Lam DA, Miron JA. Seasonality of births in human populations. Soc Biol. 1991;3:51–78. doi: 10.1080/19485565.1991.9988772. [DOI] [PubMed] [Google Scholar]
- 47.Wood S, Quinn A, Troupe S, Kingsland C, Lewis-Jones I. Seasonal variation in assisted conception cycles and the influence of photoperiodism on outcome in in vitro fertilization cycles. Hum Fertil (Camb) 2006;9(4):223–9. doi: 10.1080/14647270600806557. [DOI] [PubMed] [Google Scholar]
- 48.Barber N. On the Relationship Between Fertility and Geographic Latitude: A Cross-National Study. Cross-Cult Res. 2002;36:3–15. doi: 10.1177/1069397102036001001. [DOI] [Google Scholar]
- 49.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344–8. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 50.Rybak EA, Bevilacqua K, Veit CR, Klugman SD, Santoro N. Sibling and self ovum donation for sisters with an intermediate FMR1 mutation: what's a program to do? Fertil Steril. 2009;92:394.e9–394.e12. doi: 10.1016/j.fertnstert.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 51.Bellver J, Pellicer A, García-Velasco JA, Ballesteros A, Remohí J, Meseguer M. Obesity reduces uterine receptivity: clinical experience from 9,587 first cycles of ovum donation with normal weight donors. Fertil Steril. 2013;100:1050–8. doi: 10.1016/j.fertnstert.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Sunkara SK, Khairy M, El-Toukhy T, Khalaf Y, Coomarasamy A. The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Hum Reprod. 2010;25:418–29. doi: 10.1093/humrep/dep396. [DOI] [PubMed] [Google Scholar]
- 53.Zenke U, Chetkowski RJ. Transfer and uterine factors are the major recipient-related determinants of success with donor eggs. Fertil Steril. 2004;82:850–6. doi: 10.1016/j.fertnstert.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 54.Cohen MA, Lindheim SR, Sauer MV. Hydrosalpinges adversely affect implantation in donor oocyte cycles. Hum Reprod. 1999;14:1087–9. doi: 10.1093/humrep/14.4.1087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UV Outlook Index (a measure of summertime solar UVB) across North America (United States Environmental Protection Agency, http://www.epa.gov/sunwise/uvimonth.html [40], last accessed 2/3/14). (JPEG 92 kb)