Abstract
Purpose
To examine the impact on development of derived embryos from smooth endoplasmic reticulum clusters (SERC) in human metaphase II (MII) oocytes.
Methods
Retrospective analysis at Kyono ART Clinic. Comparison of embryological development, pregnancy, live birth and fetal malformation between oocytes with SERC (the SERC(+) group) and those without (the SERC(−) group) in 2,158 patients (3,758 cycles) after ICSI.
Results
Fertilization and implantation rate were significantly lower in SERC(+) MII oocytes than in SERC(−) MII oocytes. After the transfer of fresh and vitrified embryos derived from SERC(+) oocytes, 14 pregnancies resulted in 14 healthy babies, including 2 from fresh embryo transfer (ET) and 12 from vitrified-warmed ET, with no malformations.
Conclusion(s)
The presence of SERC in MII oocytes was associated with significantly lower fertilization rates and implantation rates than seen in SERC(−) MII oocytes within SERC (+) cycles. However, SERC had no impact on post-implantation development as well as neonatal outcome.
Keywords: Smooth endoplasmic reticulum clusters, Malformation, ICSI, Pregnancy, Oocyte morphology
Introduction
Morphological assessment of human oocytes intended for assisted reproduction is important for predictive information regarding implantation and successful pregnancy. Since intracytoplasmic sperm injection (ICSI) has been used for clinical treatment, assessment of oocyte quality has been widely seen as important to successful conception and implantation. Prior to sperm injection, we can use an inverted microscope to rapidly evaluate intracytoplasmic features (refractile bodies, dense cellular granulation, vacuoles, and smooth endoplasmic reticulum clusters (SERC)) and extracytoplasmic features (first polar body morphology, perivitelline space size and granularity, zona pellucida defects, and shape anomalies) of the oocytes available for injection. However, variations among systems used for oocyte and embryo grading make inter-laboratory comparisons extremely difficult; therefore, developing an international consensus on oocyte morphology assessment has been an objective in the field of reproductive medicine. In 2011, the Istanbul Consensus Workshop on embryo assessment [1] led to the publication of a standard for assessing oocytes. However, the value of the majority of oocyte dysmorphisms for predicting the success of in vitro fertilization (IVF) is still under discussion [2].
The presence of SERC is reportedly associated with an increased risk of an abnormal outcome. This conclusion was based on previously reported cases of Beckwith-Wiedemann syndrome [3], diaphragmatic hernia [4], and multiple malformations [5] after transfer of embryos derived from SERC(+) oocytes. Therefore, it was strongly recommended that oocytes with SERC not be used for ICSI [1]. However, Mateizel et al. [6] recently reported that embryos derived from MII oocytes with visible smooth endoplasmic reticulum (SER +MII) can develop normally and may lead to newborns with no major malformations. In the recent publication of systematic mini review, It was reported that total 171 healthy babies have been born from SERC(+) cycles and perinatal complications occurred in 16 pregnancies[7]. Moreover, we have performed ultimately successful ICSI with SERC(+) oocytes that had particular morphological features which resembled pronucleus size translucent vacuoles under the inverted microscope. Therefore, we retrospectively analyzed clinical outcomes of ICSI cycles that involved transfer of fresh or vitrified-warmed embryos from SERC(+) oocytes.
Materials and methods
Ethical considerations
Every woman agreed on our informed consent to use the present data before undergoing any treatment. The procedure and protocol were approved by an Institutional Review Board (IRB) in our clinic.
Patients
We conducted a retrospective analysis of ICSI cycles performed at Kyono ART Clinic from January 2007 through December 2011. During this study period, 2,158 patients underwent 3,578 cycles of ICSI treatment. The mean female age was 38.2 ± 4.7 years. The causes of infertility were male factor (36.5 %), ovarian dysfunction (28.1 %), tubal obstruction or disorder (13.2 %), endometriosis (4.9 %), uterine disorders (3.4 %), and unknown factors (13.9 %).
Ovarian stimulation
Ovarian stimulation involved a combination of gonadotropin-releasing hormone (GnRH) agonist (Nasanyl; Tokyo, Japan), recombinant follicular stimulating hormone (rFSH) (Follistim; Merck, Whitehouse Station, NJ, USA), and human menopausal gonadotropin (hMG) (HMG Teizo; Aska Pharma, Tokyo, Japan) or of GnRH antagonist (Cetrotide; Merck Serono, Geneva, Switzerland), rFSH, hMG, and clomiphene citrate (Clomid; Shionogi, Tokyo, Japan). An injection of 5,000 IU of human chorionic gonadotropin (hCG) (Gonatropin; Aska Pharma) was administered when the dominant follicle reached a mean diameter of 18 mm. Vaginal ultrasound was used to guide follicle puncture after hCG injection or nasal spray administration of a GnRH agonist for 36 h.
Oocyte assessment and embryo culture and assessment
Prior to ICSI, each MII oocyte was evaluated with regard to the presence of SERC.
To evaluate cycle efficiency, we compared SERC(+) cycles and SERC(−) cycles with regard to rates of fertilization, blastulation, implantation, clinical pregnancy, miscarriage, live-birth, and neonatal malformation.
Furthermore, in 252 SERC(+) cycles, 1,557 mature oocytes were divided into 322 SERC(+) MII oocytes and 1,235 SERC(−) MII oocytes and embryological and clinical outcomes were evaluated.
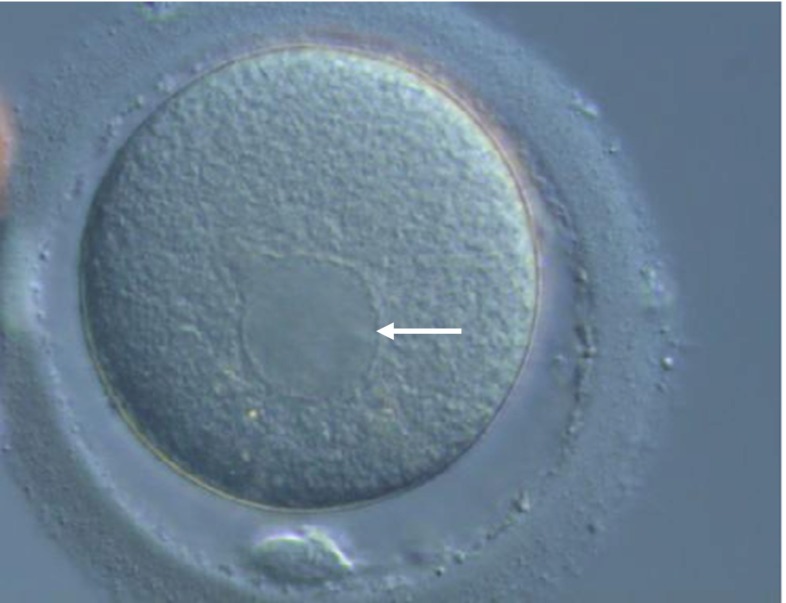
Oocyte denudation and ICSI procedures were carried out as described by Nakajo et al. [8]. At the time of ICSI, an inverted microscope was used to evaluate each oocyte for the presence of cytoplasmic abnormalities, and data were recorded. SERC classification was conducted as described by Otsuki et al. [3] (Fig. 1), We carefully performed ICSI by moving the needle in the cytoplasm so as not to penetrate the SERC, and we avoided the SERC area when depositing sperm in SERC(+) oocytes.
Fig. 1.
Smooth endoplasmic reticulum clusters observed in MII oocyte (arrow)
To monitor the development of two pronuclei (2PN) after ICSI, each oocyte was incubated overnight at 37 °C in an atmosphere of 6 % CO2, 5 % O2, and 89 % N2 under humidified conditions. Early-stage embryo morphological assessment was conducted according to Veeck’s criteria [9]. Fresh embryos were transferred on day 2 or day 3, and supernumerary embryos were subsequently cultured in G-2 series-5 (Vitrolife, Göteborg, Sweden) until they became blastocysts. Good quality blastocysts were vitrified with Cryotop (Kitazato Co., Ltd, Tokyo, Japan) and then immersed and stored in liquid nitrogen (LN2) until the next embryo transfer.
Blastulation rate was expressed as the number of good quality blastocyst embryos per surviving day 5 embryo. Good quality blastocysts were defined as grade 3 or better according to Gardner’s classification [10].
Vitrification and warming
Blastocyst vitrification and warming procedures were conducted as previously described by Nakajo et al. [8]. Briefly, each embryo was put into equilibration solution, then into vitrification solution, and then onto the Cryotop and immediately submerged into LN2. To warm frozen embryos, the Cryotop was removed from the LN2 and instantly immersed into 1.0 M sucrose in medium.
Clinical evaluation
We compared SERC(+) cycles and SERC(−) cycles with regard to rates of fertilization, pregnancy, miscarriage, live-birth, and neonatal malformation to evaluate cycle efficiency. Furthermore, within the SERC(+) cycles, we compared with SERC(+) oocytes and SERC(−) oocytes for clinical results.; In addition, clinical outcome and live birth condition following transfer of vitrified-warmed embryos derived from SERC(+) MII oocytes were analyzed.
To assess neonatal malformation, gynecologists judged the appearance of each baby immediately after delivery.
Assessment of infants
We sent questionnaires to patients and received answers by mail. The questionnaire included items regarding delivery conditions, malformation, sex, weight, length, chest and head girth.
Statistical analysis
Student’s t or chi-square tests were used for statistical analyses. P values of <0.05 were considered statistically significant.
Results
Incidence of patients and cycles with SERC(+) MII oocytes
Of the 2,158 patients and 3,578 ICSI cycles in this study, 212 patients (252 cycles) produced SERC(+) oocytes; specifically, they produced 322 SERC(+) MII oocytes and 1,235 SERC(−) MII oocytes. Additionally, 14,000 SERC(−) MII oocytes from SERC(−) cycles were produced by 1,946 patients who underwent 3,326 ICSI cycles. The mean ages of the patients were 38.2 ± 4.6 years and 38.2 ± 4.7 years for the SERC(+) cycles and SERC(−) cycles, respectively. No significant difference was observed in the mean of female age. In all cases, SERC disappeared at PN check time after the ICSI procedure.
Incidence of SERC(+) MII oocytes was 322/1,557, 20.7 % per cycle for SERC(+) cycles. Among all oocytes, incidence of SERC(+) MII oocytes was 322/15,557 (2.1 %).
The size of SERC observed in the MII oocytes varied, and the average diameter was 25.3 ± 6.6 μm (n = 22).
Clinical outcomes with fresh embryos transfer in SERC(+) and SERC(−) cycles
The embryological outcomes were similar for the two groups, SERC(+) cycles and SERC(−) cycles. Also, fertilization rate (P = 0.47), blastulation rate (P = 0.84), implantation rate (P = 1.00), clinical pregnancy rate per embryo transfer (ET) (P = 0.75), and miscarriage rate (P = 0.72) did not differ significantly between SERC(+) cycles and SERC(−) cycles.
The numbers of fresh embryo transfers on day 2 or 3 were 187 embryos in SERC(+) cycles, and 2,547 embryos in SERC(−) cycles (Table 1). The mean number of transferred embryos per transfer were 1.2 ± 0.5 and 1.2 ± 0.4, respectively. Although we could see SERC at ICSI, SERC was not seen at embryo transfer.
Table 1.
Stimulation, embryological and clinical outcomes of SERC(+) cycles and SERC(−) cycles
| SERC(+) cycles | SERC(−) cycles | P value | |
|---|---|---|---|
| Patients | 212 | 1,946 | |
| Cycles | 252 | 3,326 | |
| Average age | 38.2 ± 4.6 | 38.2 ± 4.7 | NS |
| Basal LH (mIU/ml) | 3.5 ± 2.6 | 3.4 ± 2.3 | NS |
| Basal FSH (mIU/ml) | 6.5 ± 4.0 | 6.2 ± 3.8 | NS |
| Basal estradiol (pg/ml) | 15.7 ± 12.5 | 16.1 ± 12.8 | NS |
| Basal progesterone (ng/ml) | 0.4 ± 0.3 | 0.4 ± 0.3 | NS |
| Initial dose of gonadotrophins (IU/ml) | 264.0 ± 94.6 | 262.6 ± 114.2 | NS |
| Total dose of gonadotrophins (IU/ml) | 2353.5 ± 890.6 | 2079.2 ± 995.2 | P < 0.05 |
| Duration of stimulation (days) | 10.3 ± 2.1 | 9.7 ± 2.6 | P < 0.05 |
| Estradiol levels at hCG-trigger | 1282.2 ± 920.3 | 1059.0 ± 919.7 | P < 0.01 |
| The proportion of used stimulation protocol | |||
| GnRH antagonist | 120 (47.6 %) | 1,606 (48.3 %) | NS |
| Aromatase inhibitor + GnRH antagonist | 40 (15.9 %) | 620 (18.6 %) | NS |
| GnRHa long protocol | 46 (18.3 %) | 449 (13.5 %) | P < 0.05 |
| Ultra long protocol | 3 (1.2 %) | 16 (0.5 %) | NS |
| GnRHa short protocol | 27 (10.7 %) | 306 (9.2 %) | NS |
| Clomiphen citrate | 6 (2.4 %) | 150 (4.5 %) | NS |
| Natural | 8 (3.2 %) | 147 (4.4 %) | NS |
| Other(except of upper) | 2 (0.8 %) | 32 (1.0 %) | NS |
| No. of retrieved COCs | 1833 | 17976 | |
| No. of MII oocytes | 1,557 | 14,000 | |
| Mean no. COC/cycle | 7.3 ± 6.1 | 5.4 ± 5.0 | NS |
| Mean no, MII oocytes /cycle | 6.2 ± 5.0 | 4.2 ± 4.0 | NS |
| Fertilization (%) | 1,109/1,557 (71.2 %) | 9,848/14,000 (70.3 %) | NS |
| Blastulation (%)a | 380/884 (43.0 %) | 2,933/6,880 (42.6 %) | NS |
| Cycles with fresh ET (Day2 or Day3) |
150 | 2,147 | |
| No. of transferred embryos | 187 | 2,547 | |
| Implantation (%) | 31/187 (16.6 %) | 422/2,547 (16.6 %) | NS |
| Clinical pregnancy (%) | 30/150 (20.0 %) | 407/ 2,147 (17.8 %) | NS |
| Miscarriage (%) | 10/30 (30.3 %) | 123/407 (30.2 %) | NS |
| Live-birth rate (%) | 20b/150 (13.3 %) | 284/2,147 (13.2 %)c | NS |
| Malformation rate (%) | 0/20 (0 %) | 7/206 (3.4 %)d | NS |
SERC smooth endoplasmic reticulum clusters. COC cumulus oocyte complex. MII metaphase second meiosis NS not significant
aBlastulation rate is expressed as no. of blastocysts per all developed blastocyst-stage embryos that remained supernumerary embryos after being used for transfer at early stage
bTwo of 20 were produced from SERC(+) MII oocytes (to see Table 3)
c284 is not no. of live-birth babies because it included the no. of ongoing pregnancies
dThe number 206 indicates the no. babies in 284 ongoing or completed pregnancies
Clinical outcome with fresh embryo transfer in SERC(+) MII oocytes and SERC(−) MII oocytes in SERC(+) cycles
We were also interested in analyzing the outcomes of the SERC(+) MII oocytes versus SERC(−) MII oocytes from the SERC(+) cycles. We retrieved 1,833 cumulus-oocyte complexes (COC); they gave rise to 1,557 MII oocytes that included 322 SERC(+) oocytes and 1,235 SERC(−) oocytes. Ultimately, we performed 150 cycles of embryo transfer from this group. This included 36 cycles, 42 embryos with only SERC(+) oocytes; 105 cycles, 127 embryos with only SERC(−) oocytes; and 9 cycles, 18 embryos with mixed SERC(+) and SERC(−) oocytes (Table 2).
Table 2.
Clinical and neonatal outcomes of fresh and vitrified-warmed embryo transfer with SERC(+) and SERC(−) MII oocytes from 252 SERC(+) cycles
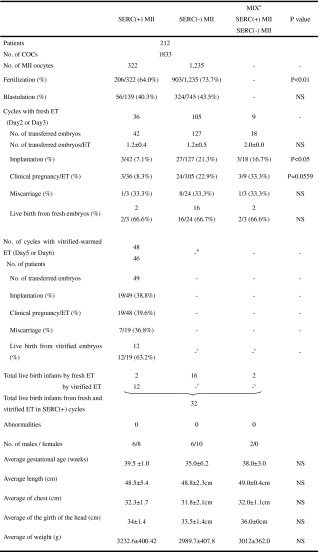
SERC smooth endoplasmic reticulum clusters. COC cumulus oocyte complex. MII metaphase second meiosis
NS not significant. ET embryo transfer
P value expressed statistical significance of differences between SERC(+) MII and SERC(−) MII oocytes
aWhen embryos were transfer, we used two embryos that derived from one SERC(+) MII oocyte and one SERC(−) MII oocyte
bThe total is not yet known. In the SERC(−) MII oocytes, each baby resulted from the transfer of a fresh embryo
cNot yet evaluation
Fertilization rate was significantly lower with SERC(+) MII oocytes than with SERC(−) MII oocytes (P < 0.01). Also, implantation rate was significantly lower with SERC(+) MII oocytes than with SERC(−) MII oocytes (P < 0.05). Pregnancy rate per ET was lower with SERC(+) MII oocytes than with SERC(−) MII oocytes but this result was not statistically significant (P = 0.0559). Blastulation rate, miscarriage rate, or live birth rate did not differ significantly between these two sub-groups. Finally, 32 healthy infants were born from fresh and vitrified-warmed embryos (20 fresh) and (12 vitrified), and no major malformations were observed (Table 2).
Clinical outcome with vitrified embryos transfer in SERC(+) MII oocytes in SERC(+) cycles
Of 206 fertilized SERC(+) MII oocytes, 187 developed to the cleavage stage, and 42 of these embryos were transferred on day 2 or day 3, resulting in two live births. When we transferred fresh embryos at day 2 or day 3, all supernumerary embryos were cultured until the blastocyst stage and then vitrified. There were 48 cycles of vitrified–warmed ET which included 49 with SERC (+) embryos. Therefore, 45 patients (47 cycles) received a single embryo transfer, and one patient (1 cycle) was received a two-embryo transfer (Table 2). However, it does not show the results of vitrified-warmed concerning SERC(−) and mixed oocytes, because this has not yet been evaluated.
The resulting pregnancy rate per ET was 19/48 (39.6 %) with an implantation rate of 19/49 (38.8 %) and a miscarriage rate of 7/19 (36.8 %). Ultimately, 12 babies were born from the vitrified-warmed embryos. Each baby appeared normal at birth (Table 2).
Comparison of ovarian-stimulating protocols
When we compared the proportion of used stimulation protocol between SERC(+) and SERC(−) cycles, the long protocol resulted in significantly more SERC(+) cycles than SERC(−) cycles (P < 0.05) (Table 1); However when we compared between each ovarian-stimulation protocols in SERC(+) cycles, no significant difference was detected (Table 3).
Table 3.
A comparison between each stimulation protocols in SERC(+) cycles and SERC(−) cycles
| SERC(+) cycles | SERC(−) cycles | |
|---|---|---|
| Treatment cycles | 252 | 3,326 |
| Total treatment cycles | 3,578 | |
| GnRH antagonist | 120/1,726 (7.0 %) | l1,606/1,726 (93.0 %) |
| Aromatase inhibitor +GnRH antagonist |
40/660 (6.1 %) | 620/660 (93.9 %) |
| GnRHa long protocol | 46/495 (9.3 %) | 449/495 (90.7 %) |
| Ultra long protocol | 3/19 (15.8 %) | 16/19 (84.2 %) |
| GnRHa short protocol | 27/333 (8.1 %) | 306/333 (91.9 %) |
| Clomiphen citrate | 6/156 (3.8 %) | 150/156 (96.2 %) |
| Natural | 8/155 (5.2 %) | 147/155 (94.8 %) |
| Other(except of upper) | 2/34 (5.9 %) | 32/34 (94.1 %) |
Hormones
Basal hormone levels at the time of ovulation induction showed no differences between SERC(+) cycles and SERC(−) cycles. However, SERC(+) cycles showed significative differences regarding higher doses of total gonadotrophins required (P < 0.05), as well as higher duration of stimulation (P < 0.05) (Table 1).
Serum estradiol concentration following hCG-triggered ovulation was significantly higher in SERC(+) cycles than for SERC(−) cycles, 1282.2 ± 920.3 (pg/ml) and 1059.0 ± 919.7 (pg/ml), respectively (P < 0.01).
Discussion
The aim of the present study was to analyze the capacity of embryos originated from SERC(+) MII oocytes to develop normally and to lead to healthy babies without malformation. Here, we showed that all the babies that derived from SERC(+) MII oocytes were born healthy and normal though the number of infants was small.
Concerning SERC, recently it has been suggested that the presence of SERC in the cytoplasm of an oocyte seems to be related to the level of intracellular calcium released upon activation which is significantly higher and of longer duration than in morphologically normal siblings or counterparts [1]. The most profound disorder in calcium release was detected in oocytes where the SER occurred in a large central aggregate. In these instances, a single, intense flare of fluorescence originated from the SER aggregate [11], and therefore, SERC may have a detrimental effect on embryo development and implantation [3, 4]. Previous investigators concluded that SERC are associated with poorer clinical and newborn outcomes, as well as with imprinting disorders and major malformations [3–5, 11]. Recently, Mateizel et al. [6] reported that embryos derived from MII oocytes with visible SERC(+) have the capacity to develop normally and may lead to babies with no major malformations. Their study included the highest number of SERC(+) cycles analyzed so far. Though our data is not enough, it supports those results because no major malformations were seen in any of the babies derived from SERC(+) oocytes; the transferred embryos derived from these oocytes included fresh and vitrified-warmed embryos. However, the concern is for epigenetic effects that may not manifest as gross congenital malformations at birth but as illness in later life. Therefore, we need more epidemiological studies on the safety of using SERC(+) oocytes. Evaluation of malformation occurrence at the time of birth and during later development is especially necessary.
In the previous studies, the percentage of SERC(+) oocytes/total oocytes from SERC(+) cycles; Mateizel et al., 17.6 % (663/3,759), Otsuki et al., 34.4 % (42/122) and Ebner et al., 25.0 % (56/224). Our data, 20.7 % (322/1,557), is quite consistent with other papers.
The SERC(+) cycles had fertilization rate, pregnancy rate, and other clinical results similar to those with SERC(−) cycles (Table 1). Our embryological data from SERC(+) and SERC(−) cycles are in line with the previous investigations [3, 4, 6], but contradict the findings of Sá et al. [12], who showed lower fertilization, embryo cleavage, and blastocyst formation rate with SERC(+) cycles. Otsuki et al. [3] showed a lower pregnancy rate and a higher biochemical pregnancy rate in SERC(+) cycles. When we evaluated SERC(+) MII oocytes and separate SERC(−) MII oocyte within the SERC(+) cycles, SERC(+) MII oocytes led to lower fertilization rates and implantation rates relative to their sibling SERC(−) MII oocytes (Table 2). However, Mateizel et al. [6] showed similar potency between SERC(+) MII oocytes and SERC(−) MII oocytes. Ebner et al. [4] reported significantly lower fertilization rates with SERC(+) MII oocytes; these findings are similar to our findings. Results with SERC(+) MII oocytes seem to vary among research groups. These differences in outcomes may result from differences in the number of samples, among patients, in the methods of ovarian stimulation, and so on.
As our main purpose was to study the clinical outcomes of ICSI with embryos originally derived from SERC(+) MII oocytes, we divided to the oocyte population into the following three sub-groups: pure SERC(+) MII oocytes, SERC(−) MII oocytes, and both SERC(+) and SERC(−) MII oocytes.
In the sub-group comprising pure SERC(+) MII oocytes, the fertilization rate and implantation rate were significantly lower than with SERC(−) MII oocytes (Table 2). Mateizel et al. [6] and Sá et al. [12] had previously reported sub-group analyses similar to ours. Sá et al. [12] found one baby who was born with malformation; however, Mateizel et al. [6] did not find any malformed babies. Our results, like those of Mateizel et al. [6], indicated that SERC(+) MII oocytes are unrelated to abnormalities in babies.
As with the infant data, we found no significant differences in the birth weight or weeks of gestation between the two sub-groups of SERC(+) cycles or between SERC(+) MII oocytes and SERC(−) MII oocytes (Table 2). Sá et al. [12] reported similar findings on birth weight, but observed a shorter gestational period with the SERC(+) cycles; in contrast, Ebner et al. [4] found a lower birth weight.
The abnormal infant outcomes presented in some of the previous reports failed to demonstrate a clear link between the presence of SERC and abnormal infant outcomes. Otsuki et al. [3] reported a case of Beckwith-Wiedemann syndrome in sibling involving a SERC(+) MII oocyte that resulted in a pregnancy. This imprinting disorder is considered to occur more frequently in children conceived via ART than in children conceived spontaneously [13]. However, it is not clear whether, in this case, there was a direct association between genomic imprinting defects and SERC(+). Akarsu et al. [5] published the first case report presenting three consecutive ICSI cycles for which all oocytes were SERC(+). SERC(+) is strongly related to infant malformation, and it cannot be denied that SERC(+) affects malformation occurrence by mutation at the nucleotide level; however, the reason is not yet clear. It is strange that all reported malformations were different types.
Previous reports included relatively small numbers of samples. Therefore, it is currently difficult to discuss and finally judge whether SERC(+) MII oocytes should never be used for ICSI or other forms of assisted insemination. Further epidemiological studies are needed to reach a definitive conclusion.
On an interesting and related topic, there seems to be a positive correlation between the presence of SERC and serum estradiol concentrations on the day of hCG treatment. Therefore, it was assumed that the duration and dosage of the stimulation may be positively correlated with the incidence of SERC(+) MII oocytes [3, 4]. We also found a higher concentration of estradiol in the SERC(+) cycles, which may support this hypothesis. Moreover It has been suggested that there is a positive correlation between the presence of SERC and high concentration of anti-Mullerian hormone (Ebner et al., 2008), as well as a large number of retrieved oocytes (Mateizel et al., 2013).
In conclusion, we have shown here that the clinical outcomes with SERC(+) cycles are not significantly different than those with SERC(−) cycles; however, within the SERC(+) cycles, the SERC(+) MII oocytes were associated with significantly lower fertilization rates and implantation rates than SERC(−) MII oocytes. Nevertheless, SERC(+) oocytes could lead to the birth of healthy babies.
Despite this conclusion, additional studies on the origin and effects of SERC are needed. These are possibly changes in biological phenomena in oocytes and embryos such as morphological changes, ca-oscillation, chromosome pattern, epigenetic status. Moreover, we need to continue long term follow-up of children derived from SERC-positive oocytes.
Acknowledgments
The author would like to thank Ms. Y. Nakano, Ms. T. Kuzuno and Ms. C. Onuma for assistance in layout.
Footnotes
Capsule To evaluate the impact of smooth endoplasmic reticulum clusters (SERCs) in human metaphase II (MII) oocytes on embryo development, clinical pregnancy rate and miscarriage rate.
References
- 1.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 2.Rienzi L, Vajita G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34–45. doi: 10.1093/humupd/dmq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otsuki J, Okada A, Morimoto K, Nagai Y, Kudo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 4.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. Reprod Biomed Online. 2008;16:113–8. doi: 10.1016/S1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 5.Akarsu C, Cağlar G, Vicdan K, Sözen E, Biberoğlu K. Smooth endoplasmic reticulum aggregations in all retrieved oocytes causing recurrent multiple anomalies: case report. Fertil Steril. 2009;2:1496.e1–e3. doi: 10.1016/j.fertnstert.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 6.Mateizel I, Landuyt LV, Tournaye H, Verheyen G. Deliveries of normal healthy babies from embryos originating from oocytes showing the presence of smooth endoplasmic reticulum aggregates. Hum Reprod. 2013;28:2111–7. doi: 10.1093/humrep/det241. [DOI] [PubMed] [Google Scholar]
- 7.Shaw-Jackson C, Van Beirs N, Thomas AL, Rozenberg S, Autin C. Can healthy babies originate from oocytes with smooth endoplasmic reticulum aggregates? A systematic mini-review. Hum Reprod. 2014;29:1380–6. doi: 10.1093/humrep/deu101. [DOI] [PubMed] [Google Scholar]
- 8.Nakajo Y, Hattori H, Sato Y, Kanto S, Araki Y, Kyono K. Vitrified-warmed and fresh oocytes yield comparable outcomes when fresh testicular sperm is utilized. J Clin Embryol. 2013;16:138–44. [Google Scholar]
- 9.Veeck LL. An Atlas of human gametes and conceptuses: an illustrated reference for assisted reproductive technology. New York: Parthenon Publishing; 1999. [Google Scholar]
- 10.Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: infertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. pp. 378–88. [Google Scholar]
- 11.Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion. 2011;11:797–813. doi: 10.1016/j.mito.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Sá R, Cunha M, Silva J, Luís A, Oliveira C, Teixeira da Silva J, et al. Ultrastructure of smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil Steril. 2011;96:143–9. doi: 10.1016/j.fertnstert.2011.04.088. [DOI] [PubMed] [Google Scholar]
- 13.Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–15. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]