Abstract
We discuss the hypothesis that AMH is an intraovarian regulator that inhibits follicular atresia within the human ovary. Several indirect lines of evidence derived from clinical and basic science studies in a variety of different patient populations and model systems collectively support this hypothesis. Evidence presented herein include 1) timing of onset of menopause in women with polycystic ovary syndrome, 2) site of cellular origin and timing of AMH production, 3) AMH’s influence on other critical growth factors and enzymes involved in folliculogenesis, and 4) AMH’s inhibition of granulosa apoptosis. If this hypothesis is true, it may provide insight for treatment strategies for prevention and treatment of premature ovarian insufficiency, slowing natural ovarian aging, and/or delaying eventual ovarian failure. Such findings may lead to the development of 1) AMH agonists for retarding the onset of menopause and/or as a chemoprotectant prior to cancer therapy and 2) AMH antagonists for the treatment of PCOS.
Keywords: AMH, Atresia, Menopause, PCOS, Ovary, Folliculogenesis, Granulosa, Apoptosis, Vitamin D, Leptin
Introduction
Follicular atresia is the process responsible for the loss of follicles and oocytes from the ovary by means other than ovulation [1, 2]. Initially seen in utero around 6 months of gestation, atresia is a noncyclical, non-gonadotropin dependent, unremitting process which takes place throughout life. This process results in what is believed to be an irreversible attrition of the primordial pool. By the onset of puberty, 95 % (or 1.9 million out of 2 million) of all follicles are lost. Post-pubertal atresia is believed to be an underlying tonic lifelong process upon which cyclical ovulation (400 cycles during a normal reproductive life cycle or 20,000 follicles are disposed) is superimposed. A putative regulator of follicular atresia would likely be regionalized and have an exquisite time sensitive expression which would contribute to the timed progression of normal folliculogenesis. It would likely influence other growth factors and enzymes involved in folliculogenesis. We speculate that AMH is the key regulator which inhibits the default mode of atresia from occurring.
We will discuss the hypothesis that AMH is an intraovarian regulator that controls the atretic process in the human ovary as it is the main regionalized and time sensitive gatekeeper controlling the onset and rate of depletion of the primordial pool. Its influence takes place during the first half of folliculogenesis which is known to be gonadotropin independent. Although there is no single experiment that demonstrates that AMH regulates follicular atresia in women, there are several indirect lines of evidence when examined collectively support such a hypothesis. Such evidence is derived from a variety of clinical and basic science studies. These studies include different patient population (normocyclic, infertile, aging, and PCOS) as well as different basic model systems (knockout mice, human cell, and tissue culture) and collectively support this hypothesis. We will examine different essential aspects of AMH as a putative key regulator of follicular atresia.
- If AMH is responsible for retarding atresia then as AMH levels declines with age, atresia should accelerate. Additionally, adding AMH would retard depletion of primordial pool while removing AMH would result in accelerated menopause. Data extracted from women with or without PCOS will be discussed as evidence for this hypothesis.
- Evidence:
-
PCOS as a model to support the theory: Data regarding the menopausal age in PCOS women are scarce. Small studies indicated that women with PCOS reach menopause at a later age [3–5]. Additionally, preantral follicles of women with PCOS have lower rate of atresia in culture (in vitro) compared to controls [6]. Women with PCOS have elevated serum AMH levels [7, 8] and “later age at menopause” compared to women without PCOS [3–5]. As in normo-ovulatory women without PCOS, AMH levels decline with increasing age in women with PCOS. However, the decline in serum AMH levels is significantly less pronounced from that observed in controls (no PCOS) [9]. A follow-up study investigated this phenomenon in more detail by measuring serum AMH levels in women with or without PCOS on two occasions with a median time interval of 2.6 years. Although AMH levels had declined over time in both groups, the decline was also less prominent in women with PCOS [10]. These results were confirmed by Piltonen et al. [5], who showed that, in contrast to older control women with low to undetectable AMH levels, women with PCOS of the same age had significantly higher AMH levels.AMH is known to inhibit recruitment of primary follicles from primordial pool [11]. Given that women with PCOS have high serum AMH levels and low rates of follicular atresia, the data discussed above suggest that the “slower” ovarian aging process in women with PCOS could be due to the high levels of AMH observed in these women. It needs to be highlighted that despite these data, exhaustion of the primordial follicle pool might occur later in women with PCOS because they might have a larger intrinsic primordial follicle pool [12].
- Women without PCOS: women with lower serum AMH at any age develop menopause earlier than age-matched women with higher AMH levels [13–20]. Curve for reduction in number of oocytes over time parallels the curve for reduction in serum AMH with advancing age. Additionally, the rate of atresia accelerates with age as AMH decreases and/or as the rate of decline of AMH increases [21–23]. Interestingly, addition of AMH can preserve the primordial pool; for instance, recombinant AMH (100 ng/mL) in vitro has an inhibitory effect on early human ovarian follicular development, thus suppressing the initiation of primordial follicle growth [24]. These findings are in agreement with results obtained from mouse follicle cultures where treatment with AMH inhibits early follicular development [11]. Furthermore, AMH knockout mice have accelerated follicular atresia compared to wild type mice [25, 26]. Thus, the fact that AMH is produced by the pool of growing follicles to act as a negative paracrine feedback signal on neighboring primordial follicle initiation strengthen the hypothesis that AMH may function as an anti-atretic agent.
-
-
Hypothesis #2:Factor responsible for regulating atresia would be produced by cells regionalized within the follicle and act to regulate the progression and exquisite timing of folliculogenesis. Additionally, atresia is a gonadotropin-independent process thus a growth factor responsible for regulating atresia needs to be gonadotropin-independent. We present evidence that AMH is both produced within the follicle and is gonadotropin-independent.
- Evidence: AMH is produced by granulosa cells (cumulus more than mural) of small and large preantral and small antral follicles [27]. It inhibits recruitment of primary follicles from primordial pool, prevents selection of follicles by FSH, and inhibits aromatase (an enzyme responsible for a key step in the biosynthesis of estradiol) [28, 29]. AMH is relatively stable throughout the menstrual cycle in normo-ovulatory women, thus it is relatively independent of gonadotropins circulating at physiologic levels [30–34]. Additionally, serum AMH levels are arguably not affected by GnRH agonists or sex steroid contraceptive use [35–37]; pharmacologic states where serum gonadotropins are suppressed. These data taken together support the theory that AMH is a candidate that regulates the gonadotropin-independent process of follicular atresia.
-
Hypothesis #3:A negative regulator of atresia would be produced at high levels in a small follicle then its production would decrease as the follicle grows. This would allow the growing follicle to escape atresia in order to participate in ovulation.
- Evidence: AMH is not detected in primordial follicles and is initially produced in primary follicles then continues to be produced in small and large preantral follicles [38]. AMH expression is greatest in granulosa cells of follicles less than 4 mm diameter found in secondary, preantral, and small antral follicles [38]. AMH is absent in larger antral stage follicles measuring 6–8 mm in diameter hence it is not detected in granulosa cells of preovulatory follicles. Its site of inhibitory action within folliculogenesis is in the recruitment process of primary follicles from the resting primordial pool and in the selection of small antral to large antral/preovulatory follicles by FSH [38, 39].
-
Hypothesis #4:If granulosa cell apoptosis is considered to be the underlying mechanism of follicular atresia then AMH, if it is a negative regulator of atresia, would inhibit granulosa apoptosis.
- Evidence: In women undergoing natural cycle (unstimulated) IVF cycles, there is a negative correlation between granulosa cell apoptosis and follicular AMH levels [40]. Additionally, women with PCOS (known to have high AMH levels) have significantly lower granulosa cell apoptotic rates compared to women without PCOS (those with normal AMH levels) [41]. Compared to granulosa cells obtained from women without PCOS, the lower apoptotic rate of granulosa cells from women with PCOS may be associated with the increase in AMH production on a cell per cell basis of up to 75 times greater [42]. These lower apoptotic rates in PCOS are associated with significantly decreased levels of the apoptotic effector caspase-3 and significantly increased levels of the anti-apoptotic survival factor cellular inhibitor of apoptosis proteins-2 [41]. These data considered together support the hypothesis that AMH represents a granulosa cell anti-apoptotic agent. Whether granulosa cell apoptosis is considered to be the main underlying mechanism of follicular atresia remains to be determined.
-
Hypothesis #5:If AMH is a key mediator of atresia, it would influence other important regulators of folliculogenesis and steroidogenesis.
- Evidence: Indeed, AMH influences enzymes and hormones important in folliculogenesis [43]. Transcriptome analysis of rat ovarian tissue following addition of AMH in-vitro showed that AMH inhibits the stimulatory actions of basic fibroblast growth factor (bFGF), kit ligand (KITL), or keratinocyte growth factor (KGF). Using microarray data in rats, the overall effect of AMH exposure showed a decrease in the expression of stimulatory factors, and an increase in the expression of inhibitory factors, and regulate cellular pathways (e.g. transforming growth factor beta signaling pathway) that result in the inhibition of primordial follicle development [44]. In addition, AMH is a known inhibitor of aromatase, an enzyme important in steroidogeneis [28, 29].
-
Hypothesis #6:AMH signaling is a pathway by which known apoptotic agents, such as vitamin D and leptin, may act by downregulating AMH receptor and AMH signaling.
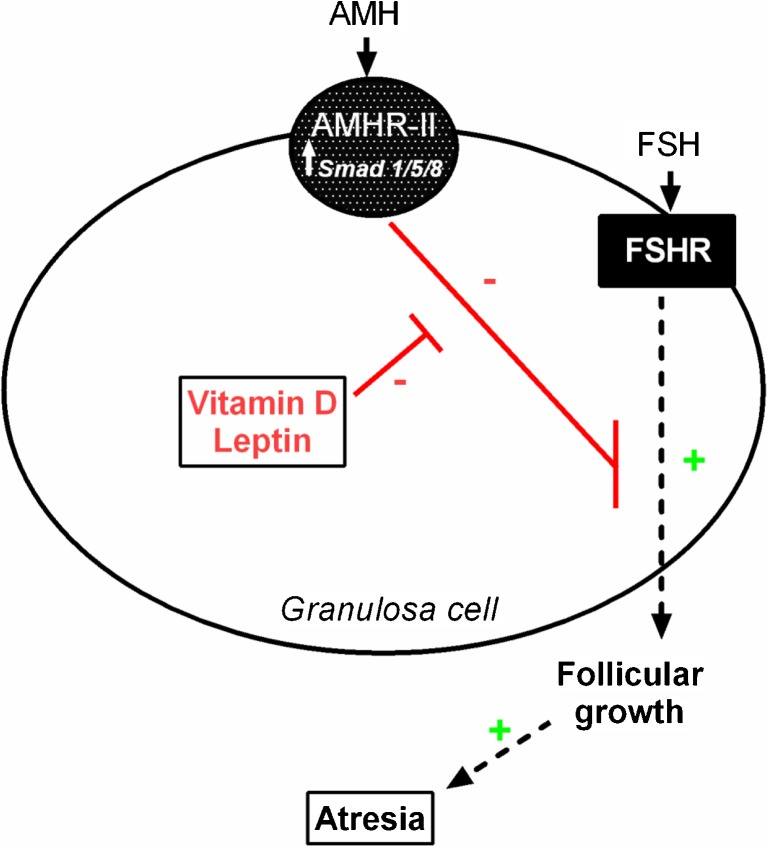
- Evidence: An increasing body of research supports the hypothesis that the active form of vitamin D has significant, protective effects against the development of cancer [45, 46]. The protective effects of vitamin D result from its role as a nuclear transcription factor that regulates apoptosis, cell growth, differentiation, and a wide range of cellular mechanisms [46]. Additionally, in women with PCOS, vitamin D level is inversely correlated with PED/PEA-15 (phosphoprotein enriched in diabetes gene product), an anti-apoptotic protein further supporting the apoptotic action of vitamin D [47]. We have shown that vitamin D in vitro treatment downregulates AMH receptor gene expression and AMH signaling in human granulosa cells via suppressing the phosphorylation and nuclear translocation of Smad 1/5/8 [48]. These data taken together indicate that, in human ovaries, AMH may represent a mechanism by which vitamin D causes a relative increase in apoptosis. This further supports the role of AMH as an anti-apoptotic agent (Fig. 1).
Fig. 1.
Anti-Mullerian hormone (AMH) binds to AMH receptor (AMHR-II) then signals via Smad 1/5/8. AMH has an inhibitory effect on follicle growth by decreasing the sensitivity of ovarian follicles to FSH thus inhibiting the loss of oocyte pool by slowing down growth followed by atresia. In human luteinized granulosa cells, leptin down-regulates AMHR-II and vitamin D down-regulates AMHR-II and Smad 1/5/8 phosphorylation and nuclear translocation
Similar to vitamin D, leptin appears to act as a promoter of apoptosis [49]. For instance, in porcine ovarian granulosa cell, leptin treatment in vitro increased the accumulation of p53 and of apoptosis related (bax) and proliferation-related (PCNA, cyclin B1) substances [49]. This study demonstrated that leptin could be involved in control of porcine ovarian cell proliferation and apoptosis. The similarity of p53 and leptin’s actions on bax and cyclin B1, and the inability of p53 to further promote leptin’s action on this parameter suggest that p53 can be a mediator of leptin’s action on ovarian cell apoptosis. Interestingly, we have shown in human granulosa cells that leptin suppresses AMH action (via JAK2/STAT3 pathway) and its receptor gene expression [27]. Although it needs to be determined as a cause-effect relationship, AMH could represent a pathway by which leptin induces apoptosis, i.e., leptin promotes apoptosis by suppressing the anti-apoptotic action of AMH.
Conclusion
We present herein direct and indirect evidence that AMH is a critical growth factor which inhibits follicular atresia. If this hypothesis is true, it may provide insight for treatment strategies for prevention and treatment of premature ovarian insufficiency, slowing natural ovarian aging or ovarian failure (menopause). These findings support the need to develop 1) AMH agonists for retarding the onset of menopause and/or as a chemoprotectant prior to cancer therapy and 2) AMH antagonists which could be useful in treating PCOS - a state where AMH is abnormally elevated reflecting abnormal follicular development. It needs to be highlighted that although the role for AMH in regulation of follicular atresia may be important, it is quite likely that other hormones/growth factors as well as genetic factors play a role. Further research to quantify the relative contribution of AMH in the regulation of atresia will likely yield a more complete understanding of this fundamental biological process.
Acknowledgments
Grants
American Society for Reproductive Medicine and Ferring Pharamceuticals to Z.M.
Disclosure
D.B.S. received royalties from a licensing agreement between Rutgers Medical School/MGH and Beckman Coulter for the use of AMH in determining ovarian reserve.
Footnotes
Capsule
Evidence suggests that AMH may represent a significant regulator of follicular atresia in the human ovary.
References
- 1.Depalo R, Nappi L, Loverro G, Bettocchi S, Caruso ML, Valentini AM, et al. Evidence of apoptosis in human primordial and primary follicles. Hum Reprod. 2003;18:2678–82. doi: 10.1093/humrep/deg507. [DOI] [PubMed] [Google Scholar]
- 2.Glamoclija V, Vilovic K, Saraga-Babic M, Baranovic A, Sapunar D. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil Steril. 2005;83:426–31. doi: 10.1016/j.fertnstert.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 3.Dahlgren E, Johansson S, Lindstedt G, Knutsson F, Oden A, Janson PO, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. Fertil Steril. 1992;57:505–13. doi: 10.1016/s0015-0282(16)54892-4. [DOI] [PubMed] [Google Scholar]
- 4.Tehrani FR, Solaymani-Dodaran M, Hedayati M, Azizi F. Is polycystic ovary syndrome an exception for reproductive aging? Hum Reprod. 2010;25:1775–81. doi: 10.1093/humrep/deq088. [DOI] [PubMed] [Google Scholar]
- 5.Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-Mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820–6. doi: 10.1093/humrep/deh850. [DOI] [PubMed] [Google Scholar]
- 6.Webber LJ, Stubbs SA, Stark J, Margara RA, Trew GH, Lavery SA, et al. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. 2007;92:1975–8. doi: 10.1210/jc.2006-1422. [DOI] [PubMed] [Google Scholar]
- 7.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzales J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–61. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 8.Catteau-Jonard S, Pigny P, Reyss AC, Decanter C, Poncelet E, Dewailly D. Changes in serum anti-mullerian hormone level during low-dose recombinant follicular-stimulating hormone therapy for anovulation in polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:4138–43. doi: 10.1210/jc.2007-0868. [DOI] [PubMed] [Google Scholar]
- 9.Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC. Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89:318–23. doi: 10.1210/jc.2003-030932. [DOI] [PubMed] [Google Scholar]
- 10.Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC. Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19:2036–42. doi: 10.1093/humrep/deh373. [DOI] [PubMed] [Google Scholar]
- 11.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, et al. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–84. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 12.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, et al. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–21. doi: 10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 13.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 14.Dolleman M, Faddy MJ, van Disseldorp J, van der Schouw YT, Messow CM, Leader B, et al. The relationship between anti-Mullerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab. 2013;98:1946–53. doi: 10.1210/jc.2012-4228. [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97:1673–80. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tehrani FR, Solaymani-Dodaran M, Azizi F. A single test of antimullerian hormone in late reproductive-aged women is a good predictor of menopause. Menopause. 2009;16:797–802. doi: 10.1097/GME.0b013e318193e95d. [DOI] [PubMed] [Google Scholar]
- 17.van Disseldorp J, Faddy MJ, Themmen AP, de Jong FH, Peeters PH, van der Schouw YT, et al. Relationship of serum antimullerian hormone concentration to age at menopause. J Clin Endocrinol Metab. 2008;93:2129–34. doi: 10.1210/jc.2007-2093. [DOI] [PubMed] [Google Scholar]
- 18.van Rooij IA, Tonkelaar I, Broekmans FJ, Looman CW, Scheffer GJ, de Jong FH, et al. Anti-mullerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11:601–6. doi: 10.1097/01.GME.0000123642.76105.6E. [DOI] [PubMed] [Google Scholar]
- 19.Seifer DB, Maclaughlin DT. Mullerian Inhibiting Substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88:539–46. doi: 10.1016/j.fertnstert.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95:747–50. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of antimullerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril. 2012;98:1254–1259. e1251–1252. [DOI] [PMC free article] [PubMed]
- 22.Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-mullerian hormone from conception to menopause. PLoS ONE. 2011;6:e22024. doi: 10.1371/journal.pone.0022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lie Fong S, Visser JA, Welt CK, de Rijke YB, Eijkemans MJ, Broekmans FJ, et al. Serum anti-mullerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97:4650–5. doi: 10.1210/jc.2012-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–7. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 25.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–96. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 26.Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, et al. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Mullerian hormone null mice. Endocrinology. 2007;148:2301–8. doi: 10.1210/en.2006-1265. [DOI] [PubMed] [Google Scholar]
- 27.Merhi Z, Buyuk E, Berger DS, Zapantis A, Israel DD, Chua S, Jr, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28:1661–9. doi: 10.1093/humrep/det072. [DOI] [PubMed] [Google Scholar]
- 28.Grossman MP, Nakajima ST, Fallat ME, Siow Y. Mullerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89:1364–70. doi: 10.1016/j.fertnstert.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Velasco JA, Moreno L, Pacheco A, Guillen A, Duque L, Requena A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82–7. doi: 10.1016/j.fertnstert.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 30.Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91:4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 31.van Disseldorp J, Lambalk CB, Kwee J, Looman CW, Eijkemans MJ, Fauser BC, et al. Comparison of inter- and intra-cycle variability of anti-Mullerian hormone and antral follicle counts. Hum Reprod. 2010;25:221–7. doi: 10.1093/humrep/dep366. [DOI] [PubMed] [Google Scholar]
- 32.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–7. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 33.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22:1837–40. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
- 34.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21:3103–7. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 35.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90:395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Mohamed KA, Davies WA, Lashen H. Antimullerian hormone and pituitary gland activity after prolonged down-regulation with goserelin acetate. Fertil Steril. 2006;86:1515–7. doi: 10.1016/j.fertnstert.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 37.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Mullerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound in obstet & gynecol : the off j of the Int Soc of Ultrasound in Obstet and Gynecol. 2012;39:574–80. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 38.Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10:77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 39.Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98:1602–11. doi: 10.1210/jc.2012-1829. [DOI] [PubMed] [Google Scholar]
- 40.Jancar N, Virant-Klun I, Osredkar J, Vrtacnik Bokal E. Apoptosis, reactive oxygen species and follicular anti-Mullerian hormone in natural versus stimulated cycles. Reprod Biomed Online. 2008;16:640–8. doi: 10.1016/S1472-6483(10)60477-4. [DOI] [PubMed] [Google Scholar]
- 41.Das M, Djahanbakhch O, Hacihanefioglu B, Saridogan E, Ikram M, Ghali L, et al. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:881–7. doi: 10.1210/jc.2007-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellatt L, Rice S, Mason HD. Anti-Mullerian hormone and polycystic ovary syndrome: a mountain too high? Reproduction. 2010;139:825–33. doi: 10.1530/REP-09-0415. [DOI] [PubMed] [Google Scholar]
- 43.Chang HM, Klausen C, Leung PC. Antimullerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013;100: 585–592.e581. [DOI] [PubMed]
- 44.Nilsson E, Rogers N, Skinner MK. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134:209–21. doi: 10.1530/REP-07-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 46.Sintov AC, Yarmolinsky L, Dahan A, Ben-Shabat S. Pharmacological effects of vitamin D and its analogs: recent developments.2014. Drug discovery today. [DOI] [PubMed]
- 47.Savastano S, Valentino R, Di Somma C, Orio F, Pivonello C, Passaretti F, et al. Serum 25-Hydroxyvitamin D Levels, phosphoprotein enriched in diabetes gene product (PED/PEA-15) and leptin-to-adiponectin ratio in women with PCOS. Nutr & metab. 2011;8:84. doi: 10.1186/1743-7075-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merhi Z, Doswell A, Krebs K, Cipolla M. Vitamin d alters genes involved in follicular development and steroidogenesis in human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:E1137–45. doi: 10.1210/jc.2013-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sirotkin AV, Benco A, Tandlmajerova A, Vasicek D. Involvement of transcription factor p53 and leptin in control of porcine ovarian granulosa cell functions. Cell Prolif. 2012;45:9–14. doi: 10.1111/j.1365-2184.2011.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]