Abstract
Purpose
To report another two successful pregnancies and deliveries resulting from autotransplanted cryopreserved ovarian tissue several years after the autotransplantation procedure took place. Further, to review the literature on the treatment history, number of live births and their outcome so far reported worldwide.
Methods
Two women underwent fertility preservation with cryopreservation of their ovarian tissue prior to a potentially sterilizing treatment with bone marrow transplantation. One woman suffered from paroxystic nocturnal hemoglobinuria and one woman from relapse of Hodgkin′s lymphoma. Both suffered from premature ovarian insufficiency after treatment. Because of a pregnancy wish they later had pieces of thawed cortical tissue transplanted to the remaining ovary and the anterior abdominal wall. PubMed was searched for reports of deliveries resulting from cryopreserved ovarian tissue in peer-reviewed papers.
Results
Five years after the autotransplantation the first patient became spontaneously pregnant and delivered a healthy baby boy at term. The second patient became pregnant after undergoing one cycle of in vitro fertilisation five years after the autotransplantation. She delivered a healthy baby boy at gestational week 36. Twenty healthy singletons and two sets of twins have been born according to peer-reviewed publications.
Conclusion
Contrary to most of the published deliveries our latest two cases occurred several years after the autotransplantation procedure took place. This proves that ovarian grafts are capable of functioning for several years after the autotransplantation has occurred. Today, a total of 26 healthy children have been born as a result of cryopreservation of ovarian tissue.
Keywords: cryopreservation, deliveries, fertility preservation, ovary, ovarian tissue
Introduction
To date cryopreservation of ovarian tissue as a means of fertility preservation is still considered experimental, although more and more encouraging reports about the efficacy of this method emerge. [1, 2]. Cancer patients receiving anti-neoplastic treatment, which as a side effect can cause irreversible loss of ovarian function, are the primary group of patients who may benefit from this new treatment [3]. The success of autotransplantation of cryopreserved ovarian tissue has been proven by case reports of births worldwide. One concern, however, is the duration of the fertility potential of the graft. It is unknown how many patients have undergone fertility preservation with autotransplantation, but it is documented that the ovarian reserve in patients transplanted with frozen/thawed ovarian tissue is low and that levels of AMH often are very modest or even immeasurable [4]. It has also been documented that most of the pregnancies are conceived within the first 12 months after the autotransplantation, and that the grafts have a limited duration of function [2]. In general, the lifespan of ovarian grafts is expected to be approximately 4–5 years if follicular density is well preserved, although a longer duration of the grafts has been reported both from orthotopic [5] and heterotopic [6] transplantation sites.
The purpose of this paper is to describe two pregnancies and deliveries both occurring in women several years after autotransplantation of the cryopreserved ovarian tissue. In addition, we want to document all the deliveries resulting from autotransplantation of ovarian tissue on a worldwide basis that have been published in peer-reviewed journals.
Materials and methods
PubMed was searched for deliveries resulting from autotransplanted cryopreserved ovarian tissue from 2004 to May 2014. Terms referring to ‘cryopreservation of ovarian tissue’, ‘autotransplantation of ovarian tissue’, and ‘live births’ were used. We also searched reference lists of identified articles manually for additional references.
Patient 1
In year 2004 at the age of 18 the patient was diagnosed with paroxystic nocturnal heamoglobinuria. As the severity of the disease progressed bone marrow transplantation was planned in year 2005 with the patient′s sister as a donor. Prior to this, the patient was referred for fertility preservation. A vaginal ultrasound revealed two normal ovaries with the expected number of antral follicles. The planned preconditioning protocol of Busulfan and Cyclophosphamide implied a high risk of premature ovarian insufficiency (POI) and a unilateral laparoscopic oophorectomy was performed for fertility preservation. The ovarian tissue was cryopreserved according to the clinic′s standard procedure [7]. After treatment the patient became amenorrhoic. Two years later in 2007 she was referred to the clinic with the aim of autotransplantation of her cryopreserved ovarian tissue as she wished to become pregnant. She had been taking hormonal replacement therapy (HRT) previously but in order to assess her ovarian function correctly she was asked to discontinue the HRT for three months prior to her visit to the clinic. A vaginal ultrasound revealed a small atrophic remaining ovary devoid of antral follicles. Follicle stimulating hormone (FSH) was 96 IU/l and the oestradiol immeasurable (<0.04 nmol/l), consistent with POI. The physician responsible for treating the patient′s haematological disease was contacted and had no objections to the autotransplantation procedure. In April 2008 during a laparoscopically assisted mini-laparotomy seven pieces of cortical tissue were transplanted to the remaining ovary and five pieces to the anterior abdominal wall in a small pocket created underneath the peritoneum. The transplanted tissue comprised of one third of the removed ovary.
Patient 2
In 2001 at the age of 25 the patient was diagnosed with Hodgkin′s lymphoma stage II B for which she received chemotherapy (adriamycin, bleomycin, vincristine and dacarbazine (ABVD) × 6 and mitoguazone, ifosfamid, metothrexate and etoposide (MIME) × 2 and localized radiotherapy. Two years later the patient relapsed and a protocol of dexamethasone, cytarabine and cisplatin (DHAP) followed by a bone marrow transplantation was planned. Prior to this the patient had her left ovary cryopreserved for fertility preservation [7]. After treatment the patient became amenorrhoic. In 2005, due to a pregnancy wish, the patient was again referred to the clinic with the aim of autotransplantation of her cryopreserved ovarian tissue. At the time of referral she complained of hot flushes and other symptoms associated with POI. Her FSH was 42 and her estradiol immeasurable (<0.04 nmol/l). During a laparoscopically assisted mini-laparotomy ten pieces of cortical tissue were transferred to the remaining right ovary just below the cortex. She then underwent seven IVF cycles without becoming pregnant, and in order to increase her chances of a pregnancy the patient requested a second transplantation, which was performed in June 2006. Two longitudinal inscisions were created along the surface of the ovary thus creating two subcortical pockets into which 12 small pieces of cortical tissue were aligned, six in each pocket, making sure the tissue did not overlap. The size of the ovary was big enough to accommodate these pieces. The collective amount of tissue transplanted to this patient comprised 69 % of the removed ovary.
Results
Patient 1
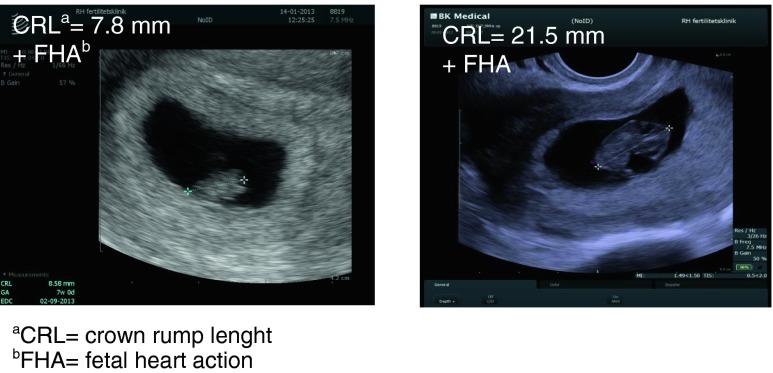
Four months after the autotransplantation in August 2008 the patient had her first menstrual bleeding after the transplantation and her FSH had dropped to 7.5 IU/l. From September 2008 to January 2009 the patient underwent one cycle of ovulation induction, two cycles of intrauterine insemination (IUI) with partner′s sperm and three cycles of in vitro fertilization (IVF) without becoming pregnant. She then split up with her partner and moved to another part of Denmark. All follow-up visits for her haematological condition were normal revealing no signs of recurrence of the disease. In January 2013 five years after the autotransplantation the patient called the clinic to tell that she had become spontaneously pregnant with a new partner. This pregnancy occurred within the first month of trying. She continued having regular menstrual bleedings from August 2008 and until she got pregnant. At no time did she use any hormonal contraception or intra-uterine device (IUD).. Ultrasonography revealed a viable intrauterine pregnancy with a crown-rump length (CRL) of 7.8 mm corresponding to gestational age 7 + 0 weeks and again 14 days later 21.5 mm corresponding to gestational age 9 + 0 (Fig. 1). After an uneventful pregnancy the patient delivered a healthy baby boy vaginally at gestational age 40 + 2 weeks weighing 3351g.
Fig. 1.
Ultrasonography in patient 1 at gestational week 7 and 9 respectively
Patient 2
Five months after the first autotransplantation procedure the patient had a menstrual bleeding.
After the second autotransplantation her FSH level was 16.5 IU/l and her estradiol 200 pg/ml. She was diagnosed with uterine adenomyosis and was operated using the transverse H incision technique. She then had one more cycle of IVF and conceived after transfer of a 4-cell embryo and delivered a healthy baby boy weighing 2,600 g in gestational week 37 by caesarean section (12). After a lactation period of three months the patient had an IUD inserted. She was followed for her cancer with regular visits every 6 months at the haematological department with no signs of recurrence of the disease. Five years later in 2012 the patient returned to the clinic, as she wanted a second child. She still experienced regular menstrual cycles. Her FSH was 6.1 IU/l and her estradiol 0.09 nmol/l on cycle day 3. It was decided to offer IVF again. In the first antagonist cycle the patient was stimulated with rFSH with a starting dose of 200 IU/l increasing to 300 IU/l. After 11 days of stimulation 4 oocytes were aspirated from 4 follicles, ICSI was performed and two embryos were transferred on day 2 but the patient did not become pregnant. In her second antagonist cycle the starting dose of rFSH was 300 IU/L and the patient developed 4 follicles from which 2 oocytes were retrieved, ICSI was performed and both cleaved. The patient conceived after transfer of two embryos (one 4-cell and one 2-cell). The patient developed hypothyroidism during the pregnancy and it was further complicated by a shortening of the cervix causing hospitalization from gestational week 26 and until an elective caesarean section was performed in week 36 + 3. A healthy baby boy weighing 2,600 g was delivered.
Table 1 and 2 gives detailed information on all the deliveries so far published in peer-reviewed journals. Information on the patient′s history before the autotransplantation procedure is given as well as information on the autotransplantation procedure, the results and information on the pregnancies and deliveries.
Table 1.
Live births after transplantation of frozen-thawed ovarian tissue published in peer-reviewed papers / : symbolizes the number of autotransplantations or births
| Reference | Chemotherapy before cryopreservationa | Surgical method | Disease | Chemo./BMTb after cryopreservation | Sign of menopause before transplantationc | Age at cryopre- servation | Age at trans-plantation | Graft site |
|---|---|---|---|---|---|---|---|---|
| [8] | No | 1 | Hodgkin′s lymphoma | MOPP/ABV | Am, FSH 91, LH 85, E2 17 | 25 | 31 | PW |
| [9] | VACOP-B + MINE-ESHAP | 1 | Non-Hodgkin′s lymphoma | BEAM/BMT | Am, FSH and LH 40–104, AMH + inhibin-B immeasurable | 28 | 30 | O |
| [10, 11] | 1xABVD | 2 | Hodgkin′s lymphoma | 5xABVD/3xEVA/CBV BMT | Am, FSH 87, Inhibin B <15 | 24 | 29/31 | 0 + A/ 0 + A |
| [12, case 2] | 6xABVD/ 2xMIME | 2 | Relapse of Hodgkin′s lymphoma | 3xDHAP/BMT | Am, FSH 42, E2 immeasurable | 25 | 28/29 | O/O |
| [12–14] | No | 3 | Ewing′s Sarcoma | 6xVIDE/3xVAI | Am, FSH 80 | 27 | 28 | O |
| [15] | No | 2 | Sickle cell anemia | Busulfan + Cyclophosphamide/BMT | ultrasound: no follicles FSH 98 LH 32 | 20 | 23 | O + PW |
| [16, 17] | No | 4 | POId | No | Am, FSH 82, LH 34 | 25 | 28/30 | O/O |
| [17] | No | 2 | Hodgkin′s lymphoma | ABVD/Cisplastin + Gemcitabine/BMT | Am, FSH 63, LH 34 | 20 | 31 | O |
| [18] | No | 1 | Breast cancer | 3xFEC/3xdocetaxel | Am FSH 36 AMH 1 | 36 | 38 | O |
| [19] | No | 5 | Neuroecto-dermic tumor | BMT | FSH 75 E2 < 10 | 17 | 25 | O |
| [20, 21] | No | 1 | Thalassemia | Fludarabine + Busulfan + Antithymocyticglobulin/ PBSCT | Am, FSH > 30 | 19 | 23/nd/nd | O + BL/O + BL/ BL |
| [22] | No | 6 | PIDf | No | Am | 18 | 26 | BL |
| [23] | 6 × ABVD | 5 | Hodgkin′s lymphoma | BCNU + Etopside + Cytarabine + Melphalan/ BMT | Am, AMH < 0.1 FSH + LH high E2 low | 27 | 32 | BL |
| [24] | No | 6 | POI | No | Am (FSH >40) | 29 | - | PW |
| [25] | No | 1 | Thalassemia | Busulfan + Cyclophosphamide + Cyclosporine/ BMT | FSH 72 LH 32 E2 < 12 | 21 | 29 | O |
| [26e] | No | 7 | Granulosa cell tumor | No | FSH >120 E2 < 10 |
25 | 32/34 | PW + A/ A |
| [27] | No | 6 | Bilateral Dermoid cysts | No | Am FSH >30 | 20 | 30 | BL |
| [21] | 2.55 g cyclophos-phamide | 2 | Microscopic polyangiitis | 7.65 g Cyclophosphamide + 58 g orally | FSH 235 LH 98 | 27 | 35/35 | O + PW/ O + PW |
| (case 1) | No | 2 | Paroxystic noctural haemoglobinuria | Busulfan + Cyclophosphamide | Am FSH 96 E2 immeasurable | 18 | 21 | O + A |
aAll samples are cryopreserved with the slow freezing technique except in reference 20
bbone marrow transplantation
cFSH and LH IU/l; AMH ng/ml; Inhibin B pg/ml.; E2 pg/ml
dPremature ovarian insufficiency
eAfter personal communication with CJ Stern it has been confirmed that the pregnancy resulted in a delivery of twin girls
fpelvic inflammatory disease
Surgical method
1 = Unilateral ovarian biopsy/ Partial left oophorectomy
2 = Unilateral oophorectomy/ Unilateral salpingo-ooforectomy
3 = Unilateral ovarian biopsi. The patient previously had a left oophorectomy because of a dermoid cyst
4 = NA. The patient suffered from idopathic benign POI for many years. She therefore had no surgical procedure prior to transplantation of ovarian tissue from twin sibling
5 = Bilateral ovarian biopsy
6 = Bilateral oophorectomy
7 = Unilateral Oophorectomy. Four years earlier the patient had a left oophorectomy
Chemotherapy
ABVD: doxorubicin, bleomycin , vinblastin, dacarbazine
ALLTO: lapatinib, trastuzumab
BEP: bleomycin, etopside, cisplatin
BCNU: high-dose cyclophosphamide, carmustine
BEAM: carmustine, etopside, cytarabine, melphalan
CBV: cyclophosphamide, carmustine, etopside
EVA: etopside, vinblastine, doxorubicin
FEC: fluorouracil, epirubicin, cyclophosphamide
MIME: Methyl-GAG, ifosfamide, methotrexate, etopside
MINE-ESHAP: mesna, ifosfamide, mitoxantrone, etopside, cytarabine, cisplatin and corticosteroids
MOPP/ABV: mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine
PBSCT: Peripheral blood stem cell transplantation
VACOP-B: etopside, doxorubicin, cyclophosphamide, vincristine, bleomycin and corticosteroids
VIDE: vincristine, iphosphamide, etopside, doxorubicin
Sign of menopause before autotransplantation
Am: amenorrhoeic
Age at transplantation
Nd: not described
Graft site
A: Abdominal wall, O: Ovary, PW: peritoneal window, BL: Broad ligament
Table 2.
Transplantation to pregnancy and pregnancy outcome
| Ref. | Amount transplanted Graft size (cm2) | Recovery of ovarian functiona (in months) E2; FSHb | Pregnancy | Time from transplanta- tion to pregnancy (months) | Live births No. | Sex | Gestational age (weeks) | Birth weight (gram) | Pregnancy complications | Delivery mode |
|---|---|---|---|---|---|---|---|---|---|---|
| [8] | 4,2/1,4 | 5 | SC | 11 | 1 | Girl | 39 | 3,720 | nd | nd |
| [9] | 2,3+ tiny fragments | 8 | IVF | 9 | 1 | Girl | 38 | 3,000 | None | CS |
| [10, 11] | 4,5/ 1 | 3 | SC/SC | 5/33 | 2 | Girl/Girl | 41/39 | 3,130/2,870 | None/nd | nd/nd |
| [12, case 2] | 1,25/3 | 5/5 | IVF/IVF | 19/85 | 2 | Boy/Boy | 37/36 | 2,600/2,600 | Mild preeclampsia + Hypothyroidism + cervical insufficiency | CS/CS |
| [12–14] | 1,5 | 4 | IVF/SC/SC | 5/24/59 | 3 | Girl/Girl/Boy | 38/41/40 | 3,204/3,828/ 4,015 | None/ None/ None | CS/ VD/ VD |
| [15] | nd | 4 | SC | 6 | 1 | Girl | 38 | 3,700 | nd | CS |
| [16, 17] | nd/nd | 4/nd | nd/nd | 5/nd | 2 | nd/nd | nd/nd | nd/nd | nd/nd | nd/nd |
| [17] | nd | 4 | SC | 8 | 1 | Boy | 38 | 3,089 | nd | nd |
| [18] | nd | 2 | IVF | 10–11 | 2 (twins) | Boy + Boy | 33 | 1,650 + 1,830 | None | CS |
| [19] | nd | 4 E2 > 110 FSH + LH <10 | SC | 9 | 1 | Boy | 38 | 2,830 | none | nd |
| [20, 21] | nd/nd/nd | nd/nd/nd | IVF | 10 | 1 | Boy | - | 3,026 | Cholecysto-lithiasis | nd |
| [22] | 1,8 | 5 E2 19 FSH 26 | IVF | 13 | 1 | Boy | 38 | 2,370 | nd | CS |
| [23] | nd | 3 E2 114 FSH 11 LH 4 | SC | 8 | 1 | Boy | 38 | 3,360 | None | CS |
| [24] | nd | nd | IVF | - | 1 | Boy | 37 | 3,254 | nd | nd |
| [25] | 4,3 | 3 E2 79 FSH 46 | SC | 16 | 1 | Girl | 39 | 3,970 | None | CS |
| [26] | 4,8/ 2,4 | 4.5 | IVF | 7 | 2 (twins) | Girl + Girl | 37 | 3,320 3,262 | nd | CS |
| [27] | nd | 5 E2 25 FSH >10 | IVF | 5 | 1 | Boy | 39 | 3,500 | nd | CS |
| [21] | 0,64/ nd | 6 FSH 10 | IVF | 14 | 1 | Girl | 37 | 2,030 | HELLP syndrome | VD |
| (case 1) | 3 | 4 FSH 7,5 | SC | 57 | 1 | Boy | 40 | 3,351 | None | VD |
/: symbolizes the number of autotransplantations or births
aas defined by resumption of spontaneous menstruation
bFSH and LH in IU/l; E2 in pg/ml
cAfter personal communication with CJ Stern it has been confirmed that the pregnancy resulted in a delivery of twin girls
Pregnancy
S: spontaneously conceived
IVF: after in vitro fertilization
ND: Not described
Delivery mode
CS: ceasarean section
VD: vaginal delivery
13 births worldwide from non peer-reviewed papers
Australia: Burmeister L, Kovacs GT and Osianlis T have published a case report of 1 ongoing pregnancy (Burmeister L, Kovacs GT, Osianlis T. First Australian pregnancy after ovarian tissue cryopreservation and subsequent autotransplantation. The Medical journal of Australia. 2,013;198(3):158–9.)
Through the media it is well known that the woman from this case report gave birth to a healthy baby girl in 2013, GA38 +
Sweden: 1 birth of a baby girl, mentioned in a newspaper, see attached link: http://www.dn.se/nyheter/vetenskap/historisk-bebis-ger-hopp/
South Africa: 1 birth of a baby boy, mentioned in a newspaper, see attached link: http://www.iol.co.za/capetimes/medical-method-bears-miracle-baby-1.1570087#.Us1MAZHDN8B
Germany: 3 undocumented births (confirmed from Fertiprotekt)
Israel: 5 undocumented births
Brussels: 2 undocumented births
Table 3 gives information on the babies (gestional age, birthweight and gender).
Table 3.
Gestational age (GA) and birth weight of 26 children, 22 singletons and 2 sets of twins published in peer-reviewed journals
| GA Median (weeks) | GA (weeks) Mean ± SEM (Range) | Birth weight (grams) Median | Birth weight (grams) Mean ± SEM (Range) | Boys N | Girls N | |
|---|---|---|---|---|---|---|
| Singletons | 38 | 38,5 ± 0,3 (36–41) | 3,167 | 3,172 ± 116 (2,030–4,015) | 11 | 9 |
| Twins | 35 | 35 ± 1 (33–37) | 2,546 | 2,516 ± 447 (1,650–3,320) | 2 | 2 |
Discussion
Here we report two new successful Danish pregnancies and deliveries occurring five and six years, respectively, after the autotransplantation procedure. Most of the deliveries described in the literature from autotransplanted ovarian tissue have occurred within the first one to two years after transplantation (Table 1 and 2). However, in the two cases described here the grafts were capable of functioning and providing a normal, intra-ovarian milieu for the oocytes to mature and later fertilize years after grafting: This gives hope to other women with autotransplanted ovarian tissue, who may not become pregnant immediately after autotransplantation. Both of the patients in this study were young, 18 and 24 years of age, when they had their ovarian tissue cryopreserved and thus the grafts were expected to contain many primordial follicles enabling them to function for several years after the autotransplantation. Indeed, apart from two women who were in their mid-thirties when they had their ovarian tissue cryopreserved, all deliveries reported so far have occurred in women who were in their twenties at the time of cryopreservation (Table 1 and 2) supporting the theory that a substantial number of primordial follicles augment the fertility potential of these grafts.
With a mean gestational age at delivery of 38.5 weeks in the singleton pregnancies and a mean birthweight of 3,167 g the uteri of these cancer survivors with autotransplanted ovarian tissue seem to be able to function in a normal way and to provide a healthy milieu to support the growth and development of the fetus (Table 3). What can also be seen from Table 1 and 2 is that despite of the lack of pregnancy complications being described the majority of the deliveries (11/15) were by Caesarean section, probably reflecting that these pregnancies are considered very precious. Another interesting observation is that 11 of the 21 pregnancies in which the history of how the pregnancy was achieved was reported, were naturally conceived and 11 occurred after IVF. This knowledge is very important when counselling the patients before autotransplantation that they may not need IVF in order to conceive and that naturally occurring pregnancies are as likely to happen.
With 24 babies born worldwide we now report two more deliveries thus reaching 26 babies on a worldwide basis and a total of six babies in Denmark. The two latest pregnancies occurred years after the autotransplantation and serve as proof that cryopreservation of ovarian tissue is becoming a method of fertility preservation that may give the patient a chance of motherhood for several years following the autotransplantation procedure.
References
- 1.Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, et al. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy-induced premature ovarian failure: the Danish experience. Fertil Steril. 2011;95:695–701. doi: 10.1016/j.fertnstert.2010.07.1080. [DOI] [PubMed] [Google Scholar]
- 2.Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Serrano MS, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99:1503–13. doi: 10.1016/j.fertnstert.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–31. doi: 10.1016/S0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 4.Greve T, Schmidt KT, Kristensen SG, Andersen CY. Evaluation of the ovarian reserve in women transplanted with frozen and thawed ovarian cortical tissue. Fertil Steril. 2012;97:1394–8. doi: 10.1016/j.fertnstert.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Andersen CY, Silber SJ, Berghold SH, Jørgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod BioMed Online. 2012;25:128–32. doi: 10.1016/j.rbmo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS. Assessment of long term endocrine function after transplantation of frozen-thawed human ovarian tissue to the heterotopic site: 10 year longitudinal follow-up study. J Assist Reprod Genet. 2012;29:489–93. doi: 10.1007/s10815-012-9757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt KLT, Byskov AG, Andersen AN, Muller J, Andersen CY. Density and distribution of primordial follicles in single pieces of cortex from three entire human ovaries. Hum Reprod. 2003;18:1158–64. doi: 10.1093/humrep/deg246. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 9.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–21. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 10.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin′s disease. Oncologist. 2007;12:1437–42. doi: 10.1634/theoncologist.12-12-1437. [DOI] [PubMed] [Google Scholar]
- 11.Demeestere I, Simon P, Moffa F, Delbaere A, Englert Y. Birth of a second healthy girl more than 3 years after cryopreserved ovarian graft. Hum Reprod. 2010;25:1590–1. doi: 10.1093/humrep/deq096. [DOI] [PubMed] [Google Scholar]
- 12.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Oxford, England: Human reproduction; 2008. pp. 2266–72. [DOI] [PubMed] [Google Scholar]
- 13.Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Oxford, England: Human reproduction; 2010. pp. 1280–1. [DOI] [PubMed] [Google Scholar]
- 14.Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod BioMed Online. 2012;25:128–32. doi: 10.1016/j.rbmo.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93(2413):e15–9. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 16.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94:2191–6. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 17.Silber SJ, DeRosa M, Pineda J, Lenahan K, Grenia D, Gorman K, et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Oxford, England: Human reproduction; 2008. pp. 1531–7. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93(268):e11–3. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 19.Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Van Langendonckt A, et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011;95:e1–4. doi: 10.1016/j.fertnstert.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 20.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Oxford, England: Human reproduction; 2011. pp. 1097–103. [DOI] [PubMed] [Google Scholar]
- 21.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Ann Med. 2011;43:437–50. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 22.Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, et al. Live birth after transplantation of frozen-thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril. 2012;98:720–5. doi: 10.1016/j.fertnstert.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–90. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci U S A. 2013;110:17474–9. doi: 10.1073/pnas.1312830110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, et al. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99:227–30. doi: 10.1016/j.fertnstert.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 26.Stern CJ, Gook D, Hale LG, Agresta F, Oldham J, Rozen G, et al. First reported clinical pregnancy following heterotopic grafting of cryopreserved ovarian tissue in a woman after a bilateral oophorectomy. Oxford, England: Human reproduction; 2013. pp. 2996–9. [DOI] [PubMed] [Google Scholar]
- 27.Callejo J, Salvador C, Gonzalez-Nunez S, Almeida L, Rodriguez L, Marques L, et al. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. Journal of ovarian research. 2013;6:33. doi: 10.1186/1757-2215-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]