Abstract
Medial olivocochlear (MOC) influence on cochlear mechanics can be noninvasively, albeit indirectly, explored via the effects of contralateral acoustic stimulation (CAS) on otoacoustic emissions. CAS-mediated effects are particularly pronounced for spontaneous otoacoustic emissions (SOAEs), which are typically reduced in amplitude and shifted upward in frequency by CAS. We investigated whether similar frequency shifts and magnitude reductions were observed behaviorally in the fine structure of pure-tone hearing thresholds, a phenomenon thought to share a common underlying mechanism with SOAEs. In normal-hearing listeners, fine-resolution thresholds were obtained over a narrow frequency range centered on the frequency of an SOAE, both in the absence and presence of 60-dB SPL broadband CAS. While CAS shifted threshold fine structure patterns and SOAEs upward in frequency by a comparable amount, little reduction in the presence or depth of fine structure was observed at frequencies near those of SOAEs. In fact, CAS typically improved thresholds, particularly at threshold minima, and increased fine structure depth when reductions in the amplitude of the associated SOAE were less than 10 dB. Additional measurements made at frequencies distant from SOAEs, or near SOAEs that were more dramatically reduced in amplitude by the CAS, revealed that CAS tended to elevate thresholds and reduce threshold fine structure depth. The results suggest that threshold fine structure is sensitive to MOC-mediated changes in cochlear gain, but that SOAEs complicate the interpretation of threshold measurements at nearby frequencies, perhaps due to masking or other interference effects. Both threshold fine structure and SOAEs may be significant sources of intersubject and intrasubject variability in psychoacoustic investigations of MOC function.
Keywords: threshold fine structure, spontaneous otoacoustic emissions, medial olivocochlear system, contralateral acoustic stimulation
INTRODUCTION
The sensitivity and dynamic range of the inner ear are modulated by an extensive system of efferent neural projections. In particular, the nerve fibers of the medial olivocochlear (MOC) system, originating from the superior olivary complex, provide inhibitory control of cochlear mechanics via direct synapses on the outer hair cells (OHCs) of either the ipsilateral or contralateral cochleae (reviewed in Guinan 2006; Robles and Delano 2008; Guinan 2010). Stimulation of the MOC system reduces basilar membrane motion (Murugasu and Russell 1996; Dolan et al. 1997) and, consequently, auditory nerve activity (Galambos 1956; Wiederhold and Kiang 1970) via modulation of the OHC-mediated feedback mechanism (i.e., the “cochlear amplifier”; Davis 1983). This efferent modulation has been proposed to aid in the detection of signals in background noise (Nieder and Nieder 1970a, b; Winslow and Sachs 1987; Kawase and Liberman 1993; Kawase et al. 1993), protect against acoustic trauma (Cody and Johnstone 1982; Rajan and Johnstone 1988; Kujawa and Liberman 1997; Maison et al. 2013), and mediate selective attention (Scharf et al. 1997; Delano et al. 2007), although its primary functional role is still the subject of speculation.
MOC influence on cochlear mechanics may be noninvasively, though indirectly, explored via measurement of otoacoustic emissions (OAEs) (Mountain 1980; Siegel and Kim 1982). OAEs are sounds recorded in the ear canal (Kemp 1978) that originate as a by-product of cochlear amplification and are sensitive indicators of OHC function. While MOC modulation of OAEs evoked by clicks or tones is typically quite small, with amplitude reductions on the order of a few decibels or less, more pronounced effects are observed for spontaneous OAEs (SOAEs), narrowband cochlear signals measured in the absence of an evoking stimulus. Activation of the MOC system via contralateral acoustic stimulation (CAS) reduces SOAE amplitudes by as much as 15 dB and produces small upward shifts in SOAE frequencies (Mott et al. 1989; Harrison and Burns 1993; Zhao and Dhar 2010). SOAE frequency shifts may be related to the phase leads observed in evoked OAEs (Francis and Guinan 2010) and in direct measurements of basilar membrane motion (Cooper and Guinan 2003) with MOC activation.
Here, we examine whether CAS-mediated magnitude reductions and frequency shifts are also evident for a behavioral phenomenon termed threshold fine structure, i.e., quasiperiodic fluctuations in hearing sensitivity across frequency (Elliott 1958). This fine structure is thought to be intrinsically related to OAE generation, particularly to the mechanisms underlying SOAE generation. More specifically, threshold fine structure is proposed to arise via a cochlear resonance phenomenon, whereby a portion of the OAE initiated by the test tone undergoes multiple reflections between its generation site and the middle ear boundary (Kemp 1979a; Talmadge et al. 1998; Epp et al. 2010). Cochlear responses are enhanced at frequencies for which the round-trip delay of the forward and reverse traveling OAE energy is an integer number of cycles, producing local peaks in sensitivity (i.e., threshold minima). Provided sufficient round-trip amplification, self-sustaining standing waves are generated at these same frequencies, which may be detected in the ear canal as SOAEs (Kemp 1979b; Wilson 1980; Zwicker and Schloth 1984). The frequency correspondence between SOAEs and threshold minima, as well as the common dependence of these phenomena on cochlear amplification, has been demonstrated experimentally (Long and Tubis 1988a, b; Furst et al. 1992).
While CAS is known to elevate behavioral hearing thresholds (e.g., Wegel and Lane 1924; Ingham 1957; Zwislocki et al. 1967; Kawase et al. 2003), the effects of CAS on threshold fine structure have not been explored. Such effects would provide a behavioral confirmation of the magnitude and frequency shifts observed for SOAEs, as well as a potentially sensitive behavioral measure of MOC-induced changes in cochlear function. Conversely, such shifts could contribute significant variability in psychoacoustic measures of MOC effects either across frequency or across individuals. Just as MOC effects on OAEs are known to fluctuate with frequency (e.g., Siegel and Kim 1982; Guinan 1986; Sun 2008; Abdala et al. 2009), the effects of CAS on behavioral thresholds may also exhibit fine structure. Understanding such complexities may aid in refining attempts to relate MOC-mediated changes in OAEs with performance on psychoacoustic tasks (e.g., Micheyl et al. 1995a, b; Garinis et al. 2011).
In the present study, we examined the effects of CAS on pure-tone thresholds measured at frequencies immediately near those of SOAEs in normal-hearing participants. Although threshold fine structure is potentially measurable in the absence of SOAEs (Zwicker and Schloth 1984), we focused our measurements on SOAE frequencies so as to reliably locate threshold minima and to make precise comparisons between magnitude and frequency shifts in the SOAE and behavioral measures. However, this approach was complicated by the perceptual effects associated with SOAEs. SOAEs interact with external tones at nearby frequencies to produce perceptions of beating and roughness (Wilson 1980; Long and Tubis 1988a; Long 1998) and may also cause masking or adaptation (Long and Tubis 1988a, b; Smurzynski and Probst 1998). Indeed, we found that the presence of SOAEs produced unanticipated CAS-mediated effects on both threshold levels and fine structure magnitude. Therefore, additional threshold measurements were made at frequencies distant from SOAEs for comparison.
METHODS
Subjects
Twenty-two normal-hearing individuals without history of ear pathology were recruited for participation. All had pure-tone thresholds less than or equal to 20-dB hearing level (HL) at octave frequencies from 0.25 to 8 kHz, as well as at 3 and 6 kHz, measured using an Interacoustics Audio Traveller AA220 (Interacoustics, Assens, Denmark). Normal middle ear function was verified using a GSI Tympstar Middle Ear Analyzer (Grason Stadler Inc., Eden Prairie, MN).
Of the initial recruits, four did not complete the experiment, and two were excluded from analysis due to excessive variability in either the SOAE or threshold measurements. Data from 16 subjects (14 female, 2 male) between 20 and 45 years old (mean = 25 years) are presented here. Three of these subjects were associated with the laboratory (including first and second authors) and volunteered their time. The remaining subjects provided written, informed consent and were compensated monetarily. All procedures were approved by the Institutional Review Board at Northwestern University.
Instrumentation and Calibration
All measurements were made in a sound-treated audiometric booth. Signals were generated and recorded using custom software written in C++ run from an Apple Macintosh computer, with digital-to-analog and analog-to-digital conversion performed by a MOTU 828mkII FireWire interface (Mark of the Unicorn, Cambridge, MA) using a 44.1-kHz sampling rate and 24-bit resolution. Outgoing signals were amplified (Etymotic Research ER H4C, Elk Grove Village, IL) and presented via MB Quart 13.01 HX drivers (Maxxsonics, Chicago, IL). The drivers were coupled to the ear canal using either an Etymotic Research ER-10B+ probe assembly (for the test ear) or with plastic tubing connected to an ER3-14A foam ear tip (for the contralateral ear). SOAE recordings were obtained from the test ear using the ER-10B+ microphone and preamplifier (20-dB gain), with signals digitized by the MOTU and saved for offline analysis.
An in situ calibration procedure was used to compensate for the effects of probe insertion depth and the frequency response of the transducers, ensuring that stimuli were delivered to the eardrum at a uniform level (Lee et al. 2012). At the beginning of each test session, the depth of probe insertion was determined from the half-wave resonance peak of the ear canal response to a slow chirp (0.2–20 kHz), normalized to the chirp response in a long lossy tube (50 ft long, 0.375 in outside diameter, copper tubing). Chirp responses previously obtained at various probe insertion depths in a Brüel & Kjær (Nærum, Denmark) 4157 ear simulator (IEC 60318–4) were then used to derive frequency-specific compensation factors appropriate for the depth of the probe in the subject’s ear canal. These factors were applied to all stimuli subsequently presented to the test ear. Stimuli presented to the contralateral ear (i.e., broadband noise) were uncompensated.
Protocol
Pure-tone thresholds and SOAEs were measured for two conditions: (1) in quiet and (2) with 60-dB SPL broadband (0.1–10 kHz) noise presented to the contralateral ear (CAS condition). For each condition, thresholds were measured in a continuous 20–30-min block, with SOAE measurements made immediately before and after the threshold measurement block. All measurements were repeated in a second test session, with sessions typically separated by no more than 1 week. Condition order (quiet-CAS or CAS-quiet) was alternated across sessions.
SOAE-Specific Measurements
To provide detailed comparisons of CAS-mediated changes in SOAEs and thresholds, twelve female subjects completed “SOAE-specific” threshold measurements. For each subject, an SOAE that could be reliably measured in quiet and with CAS was selected for more detailed investigation. Thresholds were subsequently obtained at 25 frequencies spanning a 0.136-octave range centered on the selected SOAE frequency, both in quiet and with CAS. This frequency span was chosen in order to characterize the threshold minimum (presumably occurring at or near the SOAE frequency), as well as the two adjacent threshold maxima. The frequency spacing between adjacent test points was 1/500th of an octave for the first four test points on either side of the center frequency, 1/200th of an octave for the next four points, and 1/100th of an octave for the last four points. This graded frequency resolution was chosen to more precisely estimate the frequency and level of the threshold minimum, where threshold level changes rapidly with frequency, while also providing a reasonable estimate of threshold levels at the maxima, where the rate of change in threshold levels is more gradual.
Due to the variability in the frequency of an individual SOAE between the quiet and CAS conditions, as well as across sessions, the center frequency used for the threshold measurements was always based on the SOAE frequency estimate obtained immediately prior to the threshold measurement. Thus, thresholds were rarely obtained at precisely the same frequencies between conditions or sessions, although the shift in center frequency was usually less than 10 Hz. Since all SOAE-specific measurements maintained the same relative frequency spacing (on a logarithmic frequency scale), threshold curves could still be compared between conditions and averaged across sessions. The assumption that the threshold fine structure pattern would shift in frequency with the SOAE was supported by preliminary measurements as well as the final data presented here. No attempt was made to control for potential changes in the fine structure pattern due to changes in SOAE level across days.
SOAEs were selected so as to survey a wide range of frequencies and levels, as well as CAS-mediated effects. The frequencies and levels of all twelve selected SOAEs, both in quiet and with CAS, are shown in panel B of Figure 1. For clarity, changes in SOAE frequency and level with CAS are shown in panel C. Ear canal spectra in quiet and CAS for one subject are also shown in panel A, demonstrating the typical CAS-mediated reduction in SOAE amplitude and upward shift in SOAE frequency. These changes were representative of the effects observed on the total population of SOAEs from which the selected SOAEs were taken and are consistent with previous reports of the effects of CAS on SOAEs (Mott et al. 1989; Harrison and Burns 1993; Zhao and Dhar 2010, 2011, 2012).
FIG. 1.

A Ear canal spectra showing the selected SOAE for one subject in quiet and with CAS. B Frequency and level of the twelve SOAEs chosen for study in the SOAE-specific measurements in quiet (blue circles) and with CAS (red squares). Solid lines connect the frequencies/levels of the same SOAE in the two conditions. C CAS-mediated changes in the frequency (as a percentage of the frequency in quiet) and level of each SOAE.
While we were principally interested in relating changes in thresholds with the behavior of only the selected SOAE, subjects often had multiple SOAEs near the frequency range of interest. To minimize the relative influence of adjacent SOAEs, the selected SOAE was required to have a spectral peak at least 10 dB above those of any SOAEs within a fifth-octave span. Of the seven such adjacent SOAEs detected (in six subjects), all had spectral levels of less than −10 dB SPL.
Non-SOAE-Specific Measurements
To demonstrate the effects of CAS on thresholds across a wider range of frequencies, both near and far from those of SOAEs, additional measurements were completed in six subjects (two male, four female). Two subjects (04MR and 10FR) had no measurable SOAEs, one subject (11MR) had several small (−16 to −20 dB SPL) SOAEs between 3.5 and 5 kHz, and the remaining three subjects (08FR, 15FR, and 17FL) had multiple SOAEs. Of these, 08FR and 15FR also completed an SOAE-specific threshold measurement.
For each test session, thresholds were measured at 25 frequencies in 1/100th-octave steps, covering a quarter-octave range. All measurements were repeated in a second test session, with condition order alternated between sessions, as in the SOAE-specific measurements. For three subjects (04MR, 08FR, 17FL), this process was repeated until average threshold curves had been obtained for a two-octave range between 1 and 4 kHz (requiring approximately 20 test sessions each). Frequency lists for adjacent frequency regions overlapped by five test points (1/20th of an octave) in order to create smooth transitions between individual quarter-octave test regions in the final threshold curve. For the three other subjects, thresholds were obtained in an exploratory fashion for separate quarter-octave ranges. Representative and relevant portions of this data are presented here.
Threshold Measurements
Thresholds were obtained using a modified fixed-frequency Békésy tracking procedure (Lee et al. 2012). Stimuli were pulsed tones (250-ms duration, 25-ms rise and fall times) presented twice per second. Subjects were instructed to press a button as long as the stimulus was audible and to release the button when it was not. Presentation level was decreased if the button was pressed and increased if the button was not pressed, with each press or release of the button considered a “reversal.” Starting at 30 dB SPL, presentation level was changed at the rate of 6 dB per stimulus (12 dB/s). After the first two reversals, presentation level was changed at the rate of 2 dB per stimulus (4 dB/s), and the presentation levels at the midpoints between reversals were calculated for each subsequent ascending run. This adaptive procedure continued for a minimum of four more ascending runs until the standard error of the mean midpoint value fell below 1 dB. Threshold was then defined to be the mean midpoint level.
To obtain the most accurate threshold measures, no maximum number of reversals was set. If a subject reported a lapse in attention or was responding inconsistently, he or she was reinstructed and the threshold tracking procedure was started again. Most subjects were able to complete the threshold measurements at all frequencies without restarting. Test frequencies were presented in random order, so as to minimize any systematic influence of fatigue or inattention. Although the tracking procedure does not eliminate subject response bias, which may fluctuate with attention, more rigorous measurement procedures (such as a three-alternative forced choice paradigm) would be prohibitively time-consuming given the number of test frequencies for each condition.
SOAE Measurements
Two-minute ear canal recordings were obtained immediately prior to and after each block of threshold measurements. For each recording, a fast Fourier transform (FFT) of every second was calculated to produce 120 amplitude spectra. The median amplitude at each frequency bin was then used to generate the final ear canal spectrum. SOAE level and frequency were taken to be those of the 1-Hz-wide bin with the maximum spectral amplitude nearest the expected frequency.
For the first SOAE measurement in each condition, recordings commenced after 1 min of quiet or CAS. This provided time for SOAEs to settle or for the effects of CAS to plateau. SOAE measurements were intended to estimate the state of the SOAE during the block of threshold tracking, although the accuracy of this estimation was not explicitly tested. Incorporating additional SOAE measurements throughout the threshold tracking procedure was avoided, as this would have added a considerable amount of time and potentially compromised the comfort or concentration of the subject during threshold measurements. As such, any drift or variation in SOAE frequency and amplitude over time may have influenced the measurements.
Middle Ear Muscle Reflex Test
A middle ear muscle reflex (MEMR) in response to the CAS could potentially confound both the threshold and SOAE measurements by altering forward and reverse middle ear transmission. Thus, during each test session, absence of significant MEMR in response to the 60-dB SPL CAS was verified using a procedure described in Zhao and Dhar (2010) and Deeter et al. (2009). Briefly, a 602-Hz, 60-dB SPL probe tone was presented to the test ear for 4 s, with a 500-ms burst of CAS presented after the first second. The level of the CAS ranged from 50 to 80 dB SPL in 10-dB steps. Responses to five repetitions of each CAS level were averaged, and the pressure at the probe frequency was estimated as a function of time using a least-squares fit analysis of the averaged waveform (Long and Talmadge 1997). MEMR threshold was defined as the lowest CAS level producing a change in probe tone level and/or phase that was time-locked to the presentation of the CAS. MEMR thresholds were collected during an initial screening session, as well as during all subsequent test sessions, with the exception of two occasions (for two different subjects), when time limitations precluded obtaining MEMR thresholds during the test session. However, across all screening and test sessions, at least two MEMR threshold estimates were obtained for each subject. All MEMR thresholds were 70 dB SPL or higher.
Analysis
For the SOAE-specific measurements, threshold curves were parameterized in order to calculate CAS-mediated changes in (1) threshold minimum frequency, (2) threshold minimum level, (3) threshold maxima level, and (4) fine structure depth. First, each threshold curve was smoothed using a three-point moving average. The frequencies and levels of the low- and high-frequency threshold maxima were then identified as those corresponding to the highest smoothed threshold level below and above the center frequency, respectively. Lastly, the frequency and level of the threshold minimum were taken as those corresponding to the lowest smoothed threshold between the two maxima frequencies. Fine structure depth was calculated as the difference between the smoothed threshold level at the minimum and the average smoothed threshold levels at the two maxima. The parameters for the individual threshold curves were averaged across sessions for each condition, and CAS-mediated effects were quantified as the difference between these averaged values.
Visual inspection confirmed that the minima and maxima identified by this automatic process were similar to those estimated by subjective judgments for all but one case. For this subject, all threshold curves exhibited additional, symmetric threshold dips (minima) below and above what was subjectively judged to be the threshold minimum. Threshold measurements were centered on the frequency of a particularly large (∼13 dB SPL) SOAE, and this symmetric pattern of dips around the center frequency may have resulted from complex perceptual effects (e.g., the perception of beating or distortion) due to the presence of the emission (Long 1998). Although this pattern was repeatable, the lowest smoothed threshold level occurred at different minima across conditions and sessions, producing an inconsistent estimate of the frequency shift in the threshold curve. This was corrected manually by selecting the minimum frequency to be that of the lowest smoothed threshold level near the center minimum (i.e., the middle of the three minima) for each curve.
CAS-mediated changes in SOAEs were calculated as the difference between the average SOAE frequency and level estimates for the CAS and quiet conditions. Averages for each condition were based on four SOAE measurements (two per session, across two sessions). Linear regression fits and/or Spearman’s rank correlations were used to relate CAS-mediated changes in threshold minimum frequency and SOAE frequency and to relate changes in SOAE level with changes in (1) threshold level averaged across frequency, (2) threshold minimum level, (3) threshold maxima level, and (4) fine structure depth. Paired t tests (two-tailed) were also used to compare parameters between conditions. P values <0.05 were considered statistically significant. All statistical analysis was performed using MATLAB (The MathWorks Inc., Natick, MA).
RESULTS
CAS Elevates Thresholds at Frequencies Distant from Those of SOAEs
The primary purpose of the study was to characterize the effects of CAS on thresholds and threshold fine structure at frequencies near those of SOAEs. However, to establish a baseline with which to compare these effects, we first consider cases where neither SOAEs nor prominent threshold fine structure are present. As shown in Figure 2, threshold measurements at frequencies distant from those of SOAEs confirm that the basic effect of the broadband 60-dB SPL CAS is an elevation of thresholds. Threshold measurements across a quarter-octave range are shown for each of two test sessions (“S1” and “S2”) with thin solid and dashed traces, respectively, along with their average (thick trace), for three subjects (panels A–C). Representative ear canal spectra demonstrate the lack of measurable SOAE activity above the noise floor within the frequency region tested. Subjects 04MR and 10FR had no measurable SOAEs outside of the displayed frequency range, and subject 11MR had two small SOAEs more than two octaves higher.
FIG. 2.
A–C Thresholds measured in quiet (blue) and with CAS (red) for three subjects with no measurable SOAEs within the test range. Representative ear canal spectra are shown for each condition (light blue and red traces). Thresholds measured in each of two sessions (“S1” and “S2”, thin solid and dashed traces, respectively) are shown along with their average (thick solid trace). D–F CAS-mediated changes in threshold (CAS-quiet) for each of the individual sessions (thin and solid dashed traces, as above) and the average change (thick trace) for each subject.
Although the absence of measurable SOAEs does not guarantee the absence of threshold fine structure, thresholds for each subject were relatively flat across this narrow frequency range, both in quiet and with CAS. Likewise, CAS-mediated threshold changes (shown in panels D–F of Fig. 2) were fairly stable across frequency. CAS typically elevated thresholds by ∼2–6 dB relative to those in quiet, with a mean elevation of 4.68 dB across all frequencies and subjects. CAS-mediated threshold elevations of ∼5 dB are consistent with those previously obtained under similar conditions (e.g., Kawase et al. 2003).
CAS Improves Thresholds at Frequencies Near Those of SOAEs
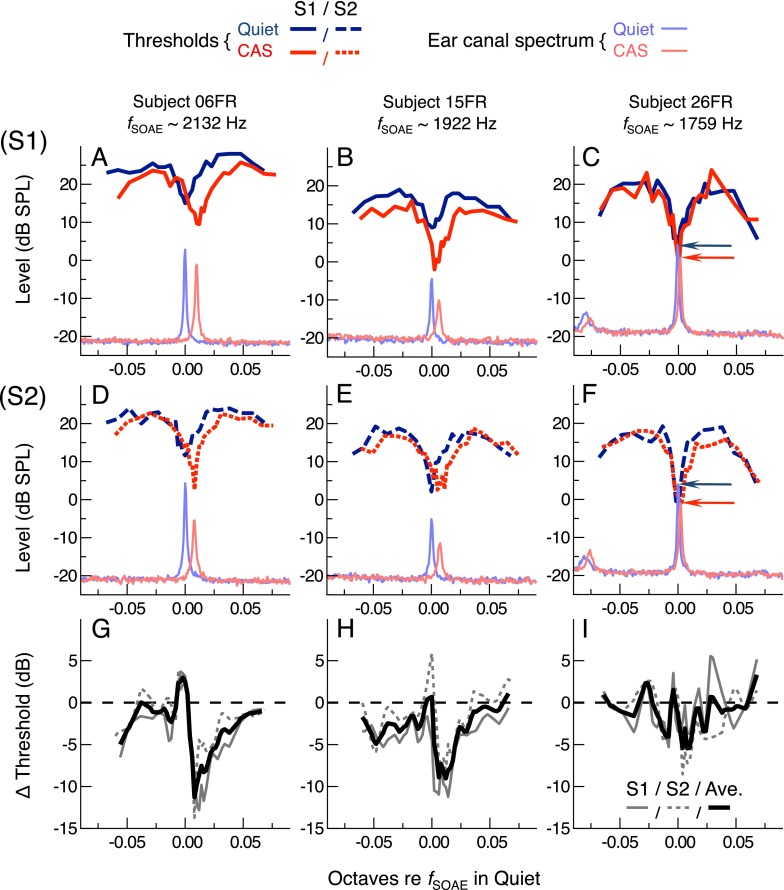
To examine the effects of CAS on threshold fine structure, thresholds were obtained for a narrow frequency range centered on an SOAE frequency for each of twelve subjects. Figure 3 shows SOAE-specific measurements for three representative subjects (06FR, 15FR, 26FR), which illustrate that the effects of CAS on thresholds near SOAE frequencies were rather complex. As can be appreciated in the data from the individual test sessions (top two panels of each column, i.e., panels A–C and D–F), threshold minima were reliably observed at frequencies near those of the SOAEs. CAS shifted SOAEs and threshold minima upward in frequency by a comparable amount, with larger shifts (∼7–12 Hz) seen for subjects 06FR and 15FR and a smaller shift (∼3 Hz) seen for subject 26FR. In contrast, reductions in SOAE level were not accompanied by comparable threshold elevations or reductions in the depth of the fine structure. Instead, thresholds were often improved with CAS, particularly near threshold minima, with improvements exceeding 10 dB at certain frequencies. Of the three subjects shown, threshold improvements were more evident for 06FR and 15FR than those for 26FR, although they could also be rather variable across sessions (for 15FR in particular). For clarity, SOAE levels in quiet and with CAS for subject 26FR are indicated by the blue and red arrows, respectively.
FIG. 3.
SOAE-specific measurements in quiet and with CAS for three representative subjects. Each column contains data from a single subject plotted versus frequency distance (in octaves) from the SOAE frequency measured in quiet. The nominal SOAE frequency, f SOAE, is indicated at the top of the column. Threshold curves and representative ear canal spectra from the two individual test sessions are shown separately in panels A–C and D–F, respectively. For clarity, blue and red arrows indicate the SOAE amplitude in quiet and with CAS for subject 26FR (panels C, F). Changes in threshold (CAS-quiet) for each of the two sessions (thin solid and dashed gray lines) and their average (thick black line) are shown in panels G–I.
For each subject, the effect of CAS on thresholds varied widely across frequency. This was due to both the upward frequency shift in fine structure pattern and the modulation (typically improvement) of overall threshold sensitivity. In the bottom panel of each column of Figure 3, CAS-mediated threshold changes are plotted as a function of the octave distance relative to the SOAE frequency in the quiet condition. Threshold changes for each session (solid and dashed gray lines for S1 and S2, respectively) are shown along with their average (thick black line). For subjects 06FR and 15FR, CAS-mediated changes in thresholds oscillated from slight reductions to elevations at frequencies just lower than that of the SOAE in quiet. Larger reductions (10–12 dB) were observed at frequencies just higher than the SOAE frequency, with small or no changes at the highest frequencies tested. For subject 26FR (third column), threshold changes fluctuated around 0 dB, although the general trend was for an improvement in thresholds with CAS. Note that thresholds in quiet and CAS were usually not obtained at identical frequencies, as the measurement spans were centered on the SOAE frequency in that condition (quiet or CAS). Linear interpolation of the threshold curves at the same frequencies was therefore required prior to calculation of the difference curves.
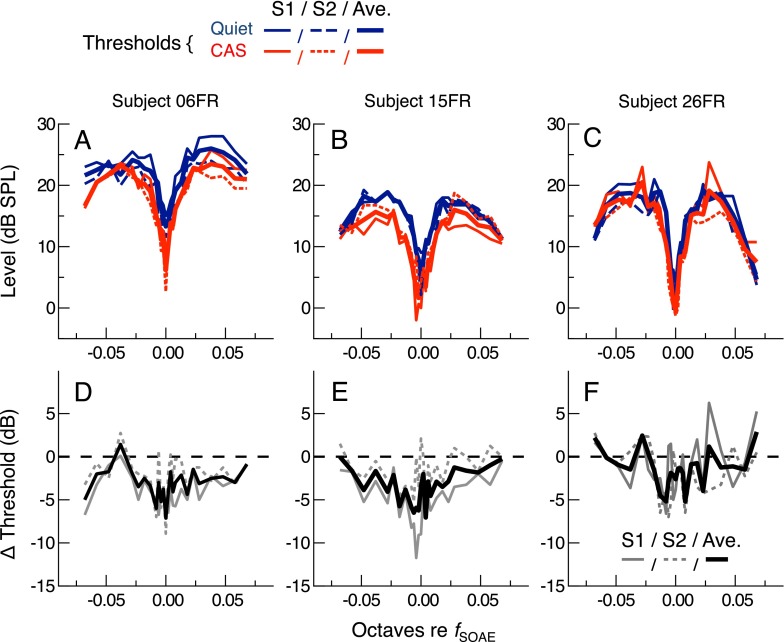
Reductions in thresholds with CAS were not solely a consequence of the upward frequency shift in the fine structure pattern. Thresholds in quiet and with CAS are replotted as a function of the frequency distance from the SOAE measured in the respective condition (i.e., quiet or CAS) in panels A–C of Figure 4. Plotting the data this way essentially aligns all threshold minima in frequency. Changes in thresholds calculated from the difference between the two uninterpolated, aligned curves are shown in panels D–F. Thresholds were still somewhat improved with CAS, even after eliminating the influence of any frequency shifts in the fine structure pattern. Threshold improvements of up to 5–10 dB occurred at or near threshold minima, with smaller changes at more distant frequencies (i.e., at threshold maxima). Consequently, CAS actually increased the depth of the threshold fine structure pattern in these subjects.
FIG. 4.
Threshold curves from the three subjects in Figure 3 replotted to minimize the effect of the frequency shift in the fine structure pattern. A–C Thresholds for each session (thin and dashed lines) and the two-session average (thick lines) are plotted as a function of the frequency distance from the SOAE frequency measured in the same condition (quiet or CAS), such that all curves are aligned relative to their minima. D–F CAS-mediated changes (CAS-quiet) in the aligned threshold curves are shown for each session (thin and dashed lines) along with their average (thick lines).
Individual and average CAS-mediated changes in thresholds are shown for all twelve subjects in Figure 5. In panel A, threshold changes were calculated at frequencies relative to those of the SOAE in quiet (as in Fig. 3), thus preserving the effect of the upward frequency shift in the fine structure pattern. Threshold changes ranged from 14.4-dB improvements to 7.3-dB elevations and varied in magnitude by as much as 18 dB with only a 1/100th-octave change in test frequency for some subjects. In panel B, CAS-mediated changes were calculated after first aligning the threshold curves with respect to their minima (as in Fig. 4). The effects of CAS were smaller and less variable when minimizing the effect of the frequency shift, although the majority of subjects still demonstrated consistent threshold improvements. In some subjects, sensitivity improvements were observed throughout the entire 0.136-octave range, although they were still largest at the minimum, approaching 10 dB in several cases. When averaged across all frequencies, CAS-mediated threshold changes were positive for only two of the twelve subjects, with average threshold elevations of 0.33 and 1.21 dB, respectively. In no case was a uniform elevation in threshold observed.
FIG. 5.
Individual (thin gray lines) and average (thick black line) CAS-mediated changes in thresholds for all twelve SOAE-specific measurements. In A, threshold changes were calculated at frequencies relative to the SOAE frequency in quiet, thus preserving the effects of the frequency shift in the fine structure patterns (as in Fig. 3). In B, changes were calculated after first aligning the threshold curves with respect to their minima, i.e., by plotting thresholds as a function of frequency distance from the SOAE in the same condition (as in Fig. 4).
Although qualitative appreciation of the data may be most appropriate due to the limited sample size, two-tailed paired t tests were performed for threshold parameters that were deemed to follow a normal distribution (via the one-sample Kolmogorov-Smirnov test implemented in MATLAB). These analyses are largely consistent with the effects that are visible in the individual and group data: CAS significantly improved thresholds when averaged across frequency (mean = 1.66 dB, t11 = 3.61, P = 0.0041), with larger improvements at threshold minima (mean = 2.36 dB, t11 = 3.12, P = 0.0097), and smaller, nonsignificant improvements at threshold maxima (mean = 0.82 dB, t11 = 1.87, P = 0.089). Fine structure depth also increased significantly with CAS (mean = 1.54 dB, t11 = 2.26, P = 0.045).
CAS Shifts SOAEs and Threshold Minima Upward in Frequency
As expected, there was a close correspondence between the frequency of each threshold minimum (fMin) and the frequency of the associated SOAE (fSOAE), both in quiet and with CAS. The difference between fMin and fSOAE for each subject is plotted in Hz and as a percentage of fSOAE in panels A–B, respectively, of Figure 6. There was a tendency for threshold minima to occur slightly lower in frequency than the SOAE. On average, this frequency difference was quite small: −1.41 Hz or −0.07 % in quiet and −2.29 Hz or −0.14 % with CAS. The frequency differences observed in the CAS condition were statistically significant according to two-tailed, paired t tests (in Hz t11 = −2.48, P = 0.031; percentage t11 = −2.73, P = 0.020).
FIG. 6.
A–B The frequency of each threshold minimum (f Min) corresponded closely with the associated SOAE frequency (f SOAE), both in quiet and CAS. The frequency difference (f Min re f SOAE) is shown in Hz (A) and as a percentage (B) for each subject (open symbols) along with the average (solid symbols, +/− one standard deviation). Threshold minima tended to occur slightly lower in frequency than the associated SOAE. Asterisks indicate that this frequency difference was significant at the P < 0.05 level according to two-tailed, paired t tests for the CAS condition. C–D CAS-mediated changes in SOAE frequency (Δ f SOAE) were significantly linearly correlated with changes in the threshold minimum frequency (Δ f Min), whether expressed in Hz (C) or as a percentage (D). Adjusted r 2 values are shown in each panel with asterisks indicating significance at the P < 0.05 level. Solid black lines indicate the linear regression fits, and gray dashed lines are unity.
CAS-mediated shifts in fSOAE and fMin were typically small, ranging from 1.5 to 15 Hz (or 0.06–1.3 %). Consistent with the close frequency correspondence described above, shifts in fSOAE and fMin were significantly linearly correlated (in Hz adjusted r210 = 0.67, P = 0.00037; percentage adjusted r210 = 0.52, P = 0.0027), as shown in panels C–D of Figure 6. The slopes of the linear regression fits (black lines) fell short of unity (dashed gray lines), perhaps due to drift in the SOAE frequency over time or due to the small number of subjects with larger frequency shifts.
Reductions in SOAE Amplitude Are Associated with Threshold Improvements
Changes in SOAE level are plotted versus changes in threshold levels averaged across all frequencies (TAve), threshold minimum level (TMin), threshold maxima level (TMax, averaged across both maxima), and fine structure depth (i.e., TMax–TMin) in Figure 7 (panels A–D, respectively). While no significant Spearman’s rank correlations were found when all of the data were included, larger SOAE level reductions were typically associated with greater threshold improvements, particularly at threshold minima. These trends were mainly apparent for SOAE level reductions of less than 10 dB (points to the right of the dashed vertical line in each panel), with threshold improvements saturating or reversing for the two cases with larger SOAE reductions. It is not clear if these two points are outliers or if the relationships are actually non-monotonic.
FIG. 7.
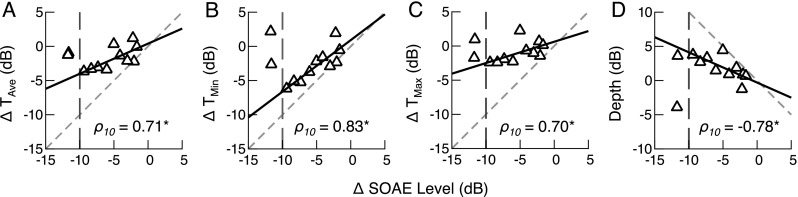
A–D Relationships between CAS-mediated changes in SOAE level and changes in average threshold level (T Ave), threshold minimum level (T Min), threshold maxima level (T Max), and threshold fine structure depth. At least for SOAE level reductions less than 10 dB (the ten points to the right of the dashed vertical lines), larger SOAE level reductions were associated with greater threshold improvements and increases in fine structure depth. Spearman’s rank correlation coefficients calculated for these ten data points are shown in each panel, with asterisks indicating significance at the P < 0.05 level. For reference, solid black lines are the linear regression fits computed for these data, and dashed gray lines are y = x in A–C or y = −x in D. Data from the two subjects with SOAE reductions larger than 10 dB indicate that threshold improvements may saturate or reverse with increasing CAS effects.
When considering only SOAE level reductions of less than 10 dB, Spearman’s rank correlations indicated that larger reductions in SOAE level were associated with larger improvements in thresholds averaged across frequency (ρ10 = 0.71, P = 0.028), threshold minimum level (ρ10 = 0.83, P = 0.0056), and threshold maxima level (ρ10 = 0.70, P = 0.031). Larger SOAE level reductions were also associated with larger increases in fine structure depth (ρ10 = −0.78, P = 0.012), due to greater threshold improvements at minima than at maxima. Linear regression fits for these ten data points (black lines) and unity (dashed gray lines) are shown for reference. These relationships are opposite to those anticipated for a simple codependence of SOAEs and threshold fine structure on cochlear gain and indicate that the presence of SOAEs complicates interpretation of the threshold measurements.
SOAEs Influence the Effects of CAS on Threshold Levels and Fine Structure
To provide a more comprehensive view of how CAS affects threshold levels and fine structure at frequencies both near and far from those of SOAEs, additional threshold measurements were obtained in 1/100th-octave steps across a two-octave range for three subjects with different SOAE and threshold fine structure profiles. Thresholds for each subject (04MR, 08FR, 17FL) are shown in Figure 8, and the CAS-mediated changes in thresholds are plotted together in Figure 9.
FIG. 8.
Thresholds and representative ear canal spectra for three subjects with distinct SOAE profiles and CAS-mediated threshold changes. Each subject completed threshold measurements from 1 to 4 kHz in 1/100th-octave steps over the course of approximately 20 test sessions. Threshold measurements were repeated twice, with frequency lists between sessions overlapped by five points, so that all thresholds represent the average of two to four measurements. For subjects 08FR (B) and 17FL (C), black triangles and brackets indicate frequency regions where thresholds were improved by CAS. Blue and red arrows in subject 17FL’s plot indicate the peak spectral level of an SOAE measured in quiet and with CAS, respectively. See text for further discussion.
FIG. 9.
CAS-mediated changes in thresholds across frequency for the three subjects in Figure 8.
Subject 04MR (Fig. 8A; black trace in Fig. 9) had no measurable SOAEs and minimal threshold fine structure. CAS elevated thresholds across the entire frequency range, with an average elevation of 3.11 dB, consistent with the results shown for subjects with similar SOAE profiles in Figure 2 (note that the first quarter-octave span of 04MR’s data is also shown in Fig. 2). Threshold elevations tended to be larger at lower frequencies (1–2-kHz average = 3.91 dB; 2–4-kHz average = 2.31 dB). Although thresholds generally varied little across frequency, a few shallow (<5 dB), periodic ripples were observed near 2 and 4 kHz. With CAS, this fine structure appeared to be somewhat preserved near 2 kHz but reduced at 4 kHz. Interpretation of these changes is limited by the small magnitude of the threshold fluctuations and the coarseness of the measurements. The overall elevation in thresholds, however, is consistent with an MOC-induced reduction in cochlear gain.
In contrast, subject 08FR (Fig. 8B; dashed red trace in Fig. 9) possessed multiple SOAEs and pronounced fine structure within the test range. Changes in SOAE level with CAS were variable across SOAEs and between test sessions but typically ranged from 3 to 5 dB. Thresholds were decreased with CAS by an average of 1.40 dB across the entire two-octave range, with the most substantial threshold reductions occurring at frequencies nearest those of SOAEs (from 1 to 1.8 kHz and 2.8 to 3.2 kHz, indicated by the black triangles and brackets in Fig. 8B). Within these frequency regions, CAS consistently improved thresholds at both maxima and minima, including those not associated with an SOAE. The depth of the fine structure either remained unchanged or increased slightly. An exception to this was observed for the threshold minimum near 2.8 kHz (corresponding in frequency with a −20-dB SPL SOAE), which was elevated in level with CAS, despite the adjacent maxima being reduced in level. There was therefore a decrease in fine structure depth of ∼6 dB at this minimum.
Curiously, CAS did not appear to change thresholds at frequencies more distant from those of SOAEs (see region between 1.8 and 2.8 kHz). Considering that CAS modulated SOAEs at lower and higher frequencies, it seems likely that the MOC system also inhibited OHC responses to the frequencies in between. That thresholds were unaffected may indicate that any reduction in cochlear gain was balanced by the apparent benefit of reducing nearby SOAE amplitudes. The influence of SOAEs on thresholds may therefore extend across a considerable frequency range.
Subject 17FL (Fig. 8C; blue trace in Fig. 9) had prominent threshold fine structure but fewer SOAEs and greater CAS-mediated reductions in SOAE level than subject 08FR. The higher-level emission near 1.2 kHz was typically reduced by 15 dB (see red and blue arrows indicating SOAE level for clarity in Fig. 8C), and the next-largest emission near 1.5 kHz was reduced from 0 dB SPL to the noise floor. Data from this subject illustrate that both threshold improvements and elevations with CAS can be observed in the same ear. More importantly, this subject provides evidence that CAS does reduce threshold fine structure depth but primarily at frequencies distant from those of SOAEs, or when nearby SOAEs are largely reduced in level.
CAS improved subject 17FL’s thresholds only within a narrow frequency range near the larger SOAE (marked by the black triangle and brackets in Fig. 8C). Improvements primarily occurred near the two adjacent threshold maxima with little or no change at the minimum, resulting in a decrease in fine structure depth of 3.5 dB. At all other frequencies, thresholds were elevated by approximately 2–5 dB. This was the case at frequencies near the second-largest SOAE, as well as those near the several smaller SOAEs that were barely visible above the noise floor (see small peaks near 2 and 4 kHz in the ear canal spectrum in quiet). Threshold elevations were always larger at threshold minima than at maxima, producing a reduction in fine structure depth of 1.3–6 dB (3.38 dB on average). Larger CAS-mediated reductions in fine structure depth tended to occur at lower frequencies and when the fine structure depth in quiet was large.
Data from subject 17FL provide further evidence that threshold improvements associated with reductions in SOAE level may saturate and reverse (i.e., become elevations) with larger SOAE level reductions. Threshold changes for the two “outlying” subjects in Figure 7 (whose SOAE level reductions exceeded 10 dB) previously suggested this trend. The relationships between changes in SOAE level and threshold levels illustrated in Figure 7 may in fact be strongly curved. However, the effects of CAS on minima and maxima were rather variable for these subjects and subject 17FL. Whether thresholds are improved or elevated overall may not just depend on the change in SOAE level, but also on the absolute SOAE level in either quiet and/or CAS. For instance, despite sizable (>15 dB) reductions in the levels of both of subject 17FL’s two largest SOAEs, thresholds were only elevated when the associated SOAE was reduced to the noise floor. Thus, the presence, amplitude, and frequency proximity of SOAEs, in addition to their behavior with CAS, may all influence how CAS modulates threshold levels and fine structure within a given frequency region.
DISCUSSION
Overview
We examined the effects of CAS on behavioral pure-tone thresholds at frequencies near and far from those of SOAEs, with the aims of describing MOC-mediated modulation of threshold fine structure and, conversely, the influence of this fine structure on behaviorally measured MOC effects. The primary findings were that (1) CAS produced comparable upward shifts in the frequencies of SOAEs and threshold minima; (2) CAS elevated thresholds and reduced threshold fine structure depth at frequencies distant from those of SOAEs, or when nearby SOAEs were reduced in amplitude by a large amount (>10 dB); and (3) CAS improved thresholds and increased threshold fine structure depth when nearby SOAEs were reduced in amplitude by a smaller amount. CAS-mediated changes in thresholds therefore varied widely, though somewhat predictably, across frequency and subjects.
The results suggest that threshold fine structure is sensitive to changes in cochlear gain but that this sensitivity is complicated by the presence of, and CAS-mediated effects on, SOAEs. As suggested previously (Long et al. 1988a, b; Smurzynski and Probst 1998), SOAEs may mask or otherwise interfere with detection of tones at nearby frequencies, such that the effects of CAS at these frequencies are dominated by a release from masking. CAS-mediated changes in threshold may therefore be determined by both the primary effect of MOC-mediated inhibition of the cochlear amplifier and the secondary effects of this inhibition on the mechanisms underlying SOAEs and threshold fine structure. These secondary effects can be as large or larger than the primary effect and may be of the opposite sign when SOAEs are present.
Pathways of the CAS Effect
CAS is predominantly known to elevate behavioral thresholds. The degree of elevation depends on the amplitude, frequency content, and temporal characteristics of the CAS relative to the test tone, though is typically less than 10 dB (e.g., Dirks and Malmquist 1965; Zwislocki et al. 1967). Consistent with previous investigations using moderate-level broadband CAS (e.g., Kawase et al. 2003; see also Smith et al. 2000 for comparable effects in Japanese macaque), we observed CAS-mediated threshold elevations ranging from 2 to 5 dB, but mainly in ears with no measurable SOAEs or prominent threshold fine structure in the frequency range tested. In contrast, we also observed unprecedented threshold improvements (approaching 15 dB) and variability in CAS effects when measured at frequencies associated with SOAEs and/or fine structure. A survey of the contralateral masking literature reveals only a few isolated instances of CAS improving thresholds (by up to 3 dB), primarily when contralateral tones were presented at higher or lower frequencies than the ipsilateral test tone (e.g., Figs. 2 and 3 of Ingham 1959; Fig. 5 in Zwislocki et al. 1967).
Contralateral effects have been attributed to a variety of peripheral and central mechanisms, including interaural crosstalk via air- or bone-conducted sound (Wegel and Lane 1924; Zwislocki 1953), middle ear muscle contraction (Ward 1961; Gjaevenes and Vigran 1967; Youngblood and Martin 1981), MOC inhibition of OHC activity (Kawase et al. 2003; Smith et al. 2000), and binaural interactions occurring more centrally in the auditory system (i.e., “central masking”; e.g., Zwislocki 1972). The moderate level of broadband CAS used in the present study was presumed to primarily elicit MOC activity, as it was insufficient to produce suprathreshold crosstalk and was also below the MEMR threshold for all subjects. While we cannot eliminate the possible influence of binaural interactions at higher auditory centers, the modulation of SOAEs by CAS confirmed MOC activity in our subjects, and the contralateral effects we observed appear to be driven by peripheral modulation of OHC activity and OAE behavior.
Frequency Shifts in Threshold Minima and SOAEs
Variation in CAS-mediated threshold changes across frequency was partially due to a small, upward frequency shift in the threshold fine structure pattern. This shift was anticipated based on previously observed effects of CAS on SOAEs (Mott et al. 1989; Harrison and Burns 1993; Zhao and Dhar 2010, 2011, 2012) and the close frequency correspondence between threshold minima and SOAEs (e.g., Wilson 1980; Schloth 1983; Zwicker and Schloth 1984). We confirmed that threshold minima occurred very near in frequency to each selected SOAE, with frequency discrepancies typically less than a few Hz (or 0.1 %), both in quiet and with CAS. Correspondingly, CAS-mediated shifts in the frequencies of SOAEs and threshold minima were very similar. Frequency shifts were reliably positive, though typically less than 1 %, and diminished percentage-wise with increasing frequency, consistent with previous reports of CAS effects on SOAEs (see Fig. 3 of Zhao and Dhar 2011).
CAS-mediated frequency shifts can be interpreted within the cochlear resonance framework initially proposed by Kemp (1979a, b). According to this framework, threshold fine structure and SOAEs arise via multiple reflections of OAE energy between the site of OAE generation and the middle ear boundary, leading to resonance at frequencies where the round-trip phase accumulation is an integer number of cycles. The frequencies of threshold minima and SOAEs are therefore determined by the phase delays inherent in the OAE generation mechanism and the reflectance at the stapes. Since the generated OAE occurs at the frequency of stimulation (whether initiated by external sound or internal physiological noise) and may be thought of as a stimulus-frequency OAE (SFOAE), the frequencies of cochlear resonance should be related to SFOAE phase. Consistent with this, SOAEs occur with a frequency spacing that is associated with approximately one cycle of SFOAE phase accumulation (Shera 2003; Bergevin et al. 2012). Changes in SFOAE phase should therefore produce shifts in the frequencies of SOAEs and threshold minima.
Indeed, activation of the MOC system with CAS causes a small lead in SFOAE phase (Francis and Guinan 2010), theoretically shifting SOAEs and threshold minima upward in frequency. Phase leads have also been observed in direct measures of basilar membrane motion during electrical stimulation of the MOC system (Cooper and Guinan 2003), although whether these effects are related to changes in SFOAEs is unknown. While we verified absence of MEMR to the CAS, note that alterations of middle ear impedance would produce similar upward frequency shifts (e.g., Kemp 1979a; Wilson and Sutton 1981; Schloth and Zwicker 1983) due to a change in the phase of the stapes reflectance (Shera 2003).
Interestingly, non-MOC-related alterations of cochlear function produce frequency shifts in the opposite direction. SOAE and threshold minima frequencies shift downward by 1–2 % following aspirin use (Long and Tubis 1988a, b) and intense noise exposure (Furst et al. 1992; see also Kemp 1981; Norton et al. 1989 for examination of the effects on SOAEs alone), and by approximately 0.25 % per year of life (Burns 2009). These frequency shifts presumably all arise via phase changes in the SFOAE generating mechanism following reductions in the efficiency of the cochlear amplifier. MOC-mediated effects on the cochlear amplifier therefore differ in this respect from other more dramatic or damaging changes in OHC function.
Interactions Among Cochlear Gain, SOAEs, and Thresholds
The magnitude and direction of CAS-mediated effects on threshold levels and fine structure were largely influenced by the presence of SOAEs and CAS-mediated changes in SOAE level. In the absence of SOAEs, CAS elevated thresholds overall, with larger changes at threshold minima than those at maxima (subject 17FL in Fig. 8C). This is consistent with a reduction in cochlear gain (i.e., an upward shift in the threshold curve) combined with a reduction in the fine structure depth (i.e., an upward shift in thresholds at minima and a downward shift at maxima). The former effect presumably leads to the latter via inhibition of the OAE evoked by the test tone. This inhibition would reduce both constructive and destructive interference caused by intracochlear reflections of the evoked OAE. Similar upward threshold shifts and reductions in fine structure depth have been observed following noise exposure (Furst et al. 1992) and sustained (∼3 days) consumption of aspirin (Long and Tubis 1988a, b), which is known to inhibit OHC function (Shehata et al. 1991; Dieler et al. 1991).
The presence of an SOAE typically reversed the aforementioned effects, with CAS improving thresholds, particularly at threshold minima, and increasing fine structure depth. In these cases, the benefit of reducing the SOAE level appeared to dominate the total effect, at least when the level reduction was small. For larger SOAE level reductions, which presumably indicate greater MOC inhibition, the changes in threshold levels and fine structure at nearby frequencies were smaller and/or less consistent (Fig. 7). In one subject (17FL in Fig. 8C), these changes were similar to those observed in the absence of SOAEs (i.e., threshold elevation and reduction in fine structure depth). These cases suggest that when MOC inhibition is strong, any benefit of reducing the SOAE level may be balanced or outweighed by the effects of gain reduction (i.e., elevated thresholds) and diminished intracochlear reflections (i.e., reduced fine structure depth).
Long and Tubis (1988a, b) previously observed this transition from threshold improvements to elevations (at frequencies near those of SOAEs) with increasing aspirin-mediated reductions in cochlear gain. They noted that thresholds were typically improved within the first 8–24 h of aspirin intake, during which SOAEs (but not evoked OAEs) were greatly reduced in amplitude. However, in contrast to the majority of our measurements, thresholds were primarily improved at maxima, with less change at minima, resulting in slight reductions in fine structure depth. This difference may be due to the larger reductions in SOAE levels with aspirin than with CAS, and perhaps the relative number and levels of the SOAEs in the subjects tested. Furst et al. (1992) also observed that noise exposure slightly improved thresholds at frequencies near that of an SOAE (which was reduced in amplitude by ∼5 dB) for one subject. However, data from this subject appeared to be an exception to the general trend.
Masking by SOAEs
While SOAEs are associated with local improvements in sensitivity (i.e., threshold minima) and better sensitivity across a wider frequency range (McFadden and Mishra 1993), our results (and those of Long and Tubis 1988a, b) suggest that reducing the amplitude of an SOAE improves signal detection at nearby frequencies. Smurzynski and Probst (1998) demonstrated that the converse is also true, noting in one subject that the ∼20-dB growth of an SOAE over the course of a test session was accompanied by threshold elevations at nearby frequencies. Thresholds were elevated by ∼10 dB at the associated threshold minimum, with less change observed at the adjacent maxima. These effects essentially mirror those observed in the present study and further suggest that SOAEs cause some form of masking or perceptual interference.
SOAE-related masking may arise via multiple mechanisms. SOAEs are associated with spontaneous basilar membrane vibrations (in guinea pig; Nuttall et al. 2004), as well as elevated spontaneous firing rates in auditory nerve fibers (in chinchilla; Powers et al. 1995). This background activity could modulate responses to external stimuli via mechanical two-tone suppression and neural adaptation or inhibition. These interactions may not be negligible, as SOAE-related vibrations have been estimated to be as much as 30 dB larger than those evoked by tones of equivalent ear canal SPL (Powers et al. 1995). The fact that such vibrations are often not perceived unless perturbed (Zurek 1981; Schloth and Zwicker 1983) suggests that some degree of neural adaptation or inhibition has occurred more centrally. Reducing SOAE-related background activity would reduce the aforementioned effects and increase the signal-to-noise ratio of neural responses to external sounds, thus improving signal detection.
Threshold levels and fine structure morphology may also be influenced by acoustic interactions between the test tone and the SOAE. A reduction in SOAE amplitude would decrease the stimulus level required to entrain the SOAE (resulting in the perception of a pure tone) and alter the range of stimulus levels over which beating or distortions are perceived, as shown during aspirin consumption (Long et al. 1991). Although these interactions were not a focus of the present study, some subjects reported that perceptions of beating and roughness during the threshold measurements were greatly reduced with CAS. It is unclear whether such interactions aided or hindered threshold detection.
Extension to Evoked OAEs
We focused our measurements on SOAE frequencies so as to reliably locate threshold minima and measure a constant fraction of a threshold fine structure cycle. This facilitated comparison of the measurements across conditions, sessions, and subjects. However, threshold minima are also known to align in frequency with peaks in the spectra of OAEs evoked by low-level transients, even in the absence of measurable SOAEs (Zwicker and Schloth 1984). Long and Tubis (1988a, b) noted that aspirin-induced changes in threshold fine structure tended to parallel changes in the amplitudes of OAEs evoked by low-level transients and tones. These observations are consistent with notion that the fine structure is related to the OAE evoked by the test tone. While more time-consuming than our SOAE recordings, measurement of the CAS-mediated changes in the OAEs evoked at each test frequency may have clarified the interrelationships among threshold fine structure, SOAEs, and evoked OAEs.
Implications for Psychoacoustics
The fine structure of the CAS-mediated effects on pure-tone thresholds may extend to, and therefore complicate, suprathreshold behavioral investigations of MOC activity. Threshold fine structure has been shown to influence measures of temporal integration (Cohen 1982), loudness judgments (Kemp 1979a; Mauermann et al. 2004), psychophysical tuning (Long 1984), and amplitude modulation detection (Zwicker 1986; Heise et al. 2009a, b). Previous assessments of MOC influence on loudness perception (Micheyl et al. 1995a, 1997; Morand-Villeneuve et al. 2002) or psychophysical tuning (Quaranta et al. 2005; Vinay 2008) may therefore have been influenced by threshold fine structure. Future psychophysical investigations of MOC function may consider using test stimuli with broader frequency content and/or shorter durations (as in Kawase et al. 2000; Jennings and Strickland 2012; Aguilar et al. 2013), since thresholds and other perceptual judgments for these types of stimuli exhibit less fine structure (Cohen 1982; Long 1984; Lee and Long 2012). However, it is not clear if these stimulus types would also reduce the influence of SOAEs, as Smurzynski and Probst (1998) found that thresholds for both long (310 ms) and short (10 ms) test stimuli were elevated by the growth in level of an SOAE at a nearby frequency.
These considerations may be particularly important when investigating relationships between CAS-mediated changes in OAEs and perception. While individuals with large evoked OAEs are ideal for achieving better signal-to-noise ratios in OAE-based MOC assays, they may also be more likely to have threshold fine structure and SOAEs (Moulin et al. 1993; Kulawiec and Orlando 1995; Kapadia and Lutman 1999). Variability in psychoacoustic measures due to MOC modulation of SOAEs or threshold fine structure may be likely in these individuals. As we have illustrated in the present report, the effects of CAS on behavioral measures can vary to a large degree with small changes in test frequency and may be of the opposite sign relative to changes in OAEs.
Acknowledgments
The authors thank Rachael Baiduc, Glenis Long, Gayla Poling, Jonathan Siegel, and Wei Zhao for helpful discussions of the data. A portion of this work was presented at the 161st meeting of the Acoustical Society of America, May 2011, in Seattle, WA. This research was partially supported by NIDCD grants R01 DC008420 and T32 DC009399.
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
James B. Dewey, Phone: +1-847-4671787, Email: jbdewey@u.northwestern.edu
Jungmee Lee, Email: jmlee6@northwestern.edu.
Sumitrajit Dhar, Email: s-dhar@northwestern.edu.
References
- Abdala C, Mishra SK, Williams TL. Considering distortion product otoacoustic emission fine structure in measurements of the medial olivocochlear reflex. J Acoust Soc Am. 2009;125:1584–1594. doi: 10.1121/1.3068442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar E, Eustaquio-Martín A, Lopez-Poveda EA. Contralateral efferent reflex effects on threshold and suprathreshold psychoacoustical tuning curves at low and high frequencies. J Assoc Res Otolaryngol. 2013;14:341–357. doi: 10.1007/s10162-013-0373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergevin C, Fulcher A, Richmond S, Velenovsky D, Lee J. Interrelationships between spontaneous and low-level stimulus-frequency otoacoustic emissions in humans. Hear Res. 2012;285:20–28. doi: 10.1016/j.heares.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Burns EM. Long-term stability of spontaneous otoacoustic emissions. J Acoust Soc Am. 2009;125:3166–3176. doi: 10.1121/1.3097768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Temporary threshold shift modified by binaural acoustic stimulation. Hear Res. 1982;6:199–205. doi: 10.1016/0378-5955(82)90054-5. [DOI] [PubMed] [Google Scholar]
- Cohen MF. Detection threshold microstructure and its effect on temporal integration data. J Acoust Soc Am. 1982;71:405–409. doi: 10.1121/1.387442. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ. Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Phsyiol. 2003;548:307–312. doi: 10.1113/jphysiol.2003.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Deeter R, Abel R, Calandruccio L, Dhar S. Contralateral acoustic stimulation alters the magnitude and phase of distortion product otoacoustic emissions. J Acoust Soc Am. 2009;126:2413–2424. doi: 10.1121/1.3224716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci. 2007;27:4146–4153. doi: 10.1523/JNEUROSCI.3702-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieler R, Shehata-Dieler WE, Brownell WE. Concomitant salicylate-induced alterations of outer hair cell subsurface cisternae and electromotility. J Neurocytol. 1991;20:637–653. doi: 10.1007/BF01187066. [DOI] [PubMed] [Google Scholar]
- Dirks D, Malmquist C. Shifts in air-conduction thresholds produced by pulsed and continuous contralateral masking. J Acoust Soc Am. 1965;37:631–637. doi: 10.1121/1.1909383. [DOI] [PubMed] [Google Scholar]
- Dolan DF, Guo MH, Nuttall AL. Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation. J Acoust Soc Am. 1997;102:3587–3596. doi: 10.1121/1.421008. [DOI] [PubMed] [Google Scholar]
- Elliott E. A ripple effect in the audiogram. Nature. 1958;181:1076. doi: 10.1038/1811076a0. [DOI] [PubMed] [Google Scholar]
- Epp B, Verhey JL, Mauermann M. Modeling cochlear dynamics: interrelation between cochlea mechanics and psychoacoustics. J Acoust Soc Am. 2010;128:1870–1883. doi: 10.1121/1.3479755. [DOI] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ. Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: implications for cochlear filter bandwidths. Hear Res. 2010;267:36–45. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst M, Reshef I, Attias J. Manifestations of intense noise stimulation on spontaneous otoacoustic emission and threshold microstructure: experiment and model. J Acoust Soc Am. 1992;91:1003–1014. doi: 10.1121/1.402626. [DOI] [PubMed] [Google Scholar]
- Galambos R. Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J Neurophysiol. 1956;19:424–437. doi: 10.1152/jn.1956.19.5.424. [DOI] [PubMed] [Google Scholar]
- Garinis A, Werner L, Abdala C. The relationship between MOC reflex and masked threshold. Hear Res. 2011;282:128–137. doi: 10.1016/j.heares.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjaevenes K, Vigran E. Contralateral masking: an attempt to determine the role of the aural reflex. J Acoust Soc Am. 1967;42:580–585. doi: 10.1121/1.1910625. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Effect of efferent neural activity on cochlear mechanics. Scand Audiol Suppl. 1986;25:53–62. [PubMed] [Google Scholar]
- Guinan JJ. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010;18:447–453. doi: 10.1097/MOO.0b013e32833e05d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison WA, Burns EM. Effects of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Acoust Soc Am. 1993;94:2649–2658. doi: 10.1121/1.407349. [DOI] [PubMed] [Google Scholar]
- Heise SJ, Mauermann M, Verhey JL. Threshold fine structure affects amplitude modulation perception. J Acoust Soc Am. 2009;125:EL33–EL38. doi: 10.1121/1.3040031. [DOI] [PubMed] [Google Scholar]
- Heise SJ, Mauermann M, Verhey JL. Investigating possible mechanisms behind the effect of threshold fine structure on amplitude modulation perception. J Acoust Soc Am. 2009;126:2490–2500. doi: 10.1121/1.3224731. [DOI] [PubMed] [Google Scholar]
- Ingham JG. The effect upon monaural sensitivity of continuous stimulation of the opposite ear. Q J Exp Psychol. 1957;9:52–60. doi: 10.1080/17470215708416219. [DOI] [Google Scholar]
- Ingham JG. Variations in cross-masking with frequency. J Exp Psychol. 1959;58:199–205. doi: 10.1037/h0041226. [DOI] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA. Evaluating the effects of olivocochlear feedback on psychophysical measures of frequency selectivity. J Acoust Soc Am. 2012;132:2483–2496. doi: 10.1121/1.4742723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia S, Lutman ME. Reduced ‘audiogram ripple’ in normally-hearing subjects with weak otoacoustic emissions. Audiology. 1999;38:257–261. doi: 10.3109/00206099909073031. [DOI] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. Antimasking effects of the olivocochlear reflex. I. Enhancement of compound action potentials to masked tones. J Neurophysiol. 1993;70:2519–2532. doi: 10.1152/jn.1993.70.6.2519. [DOI] [PubMed] [Google Scholar]
- Kawase T, Delgutte B, Liberman MC. Antimasking effects of the olivocochlear reflex. II. Enhancement of auditory-nerve response to masked tones. J Neurophysiol. 1993;70:2533–2549. doi: 10.1152/jn.1993.70.6.2533. [DOI] [PubMed] [Google Scholar]
- Kawase T, Ogura M, Hidaka H, Sasaki N, Suzuki Y, Takasaka T. Effects of contralateral noise on measurement of the psychophysical tuning curve. Hear Res. 2000;142:63–70. doi: 10.1016/S0378-5955(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Kawase T, Ogura M, Sato T, Kobayashi T, Suzuki Y. Effects of contralateral noise on the measurement of auditory threshold. Tohoku J Exp Med. 2003;200:129–135. doi: 10.1620/tjem.200.129. [DOI] [PubMed] [Google Scholar]
- Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386–1391. doi: 10.1121/1.382104. [DOI] [PubMed] [Google Scholar]
- Kemp DT (1979a) The evoked cochlear mechanical response and the auditory microstructure—evidence for a new element in cochlear mechanics. Scand Audiol Suppl:35–47 [PubMed]
- Kemp DT. Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Arch Otorhinolaryngol. 1979;224:37–45. doi: 10.1007/BF00455222. [DOI] [PubMed] [Google Scholar]
- Kemp DT (1981) Physiologically active cochlear micromechanics-one source of tinnitus. In: Evered D, Lawrenson G (eds) Tinnitus - Ciba Foundation Symposium 85. Pitman, London, pp 54–81 [DOI] [PubMed]
- Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol. 1997;78:3095–3106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- Kulawiec JT, Orlando MS. The contribution of spontaneous otoacoustic emissions to the click evoked otoacoustic emissions. Ear Hear. 1995;16:515–520. doi: 10.1097/00003446-199510000-00008. [DOI] [PubMed] [Google Scholar]
- Lee J, Long G. Stimulus characteristics which lessen the impact of threshold fine structure on estimates of hearing status. Hear Res. 2012;283:24–32. doi: 10.1016/j.heares.2011.11.011. [DOI] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, Banakis R, Grolley E, Lee J, Zecker S, Siegel J. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear. 2012;33:315–329. doi: 10.1097/AUD.0b013e31823d7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GR. The microstructure of quiet and masked thresholds. Hear Res. 1984;15:73–87. doi: 10.1016/0378-5955(84)90227-2. [DOI] [PubMed] [Google Scholar]
- Long G. Perceptual consequences of the interactions between spontaneous otoacoustic emissions and external tones. I. Monaural diplacusis and aftertones. Hear Res. 1998;119:49–60. doi: 10.1016/S0378-5955(98)00032-X. [DOI] [PubMed] [Google Scholar]
- Long GR, Talmadge CL. Spontaneous otoacoustic emission frequency is modulated by heartbeat. J Acoust Soc Am. 1997;102:2831–2848. doi: 10.1121/1.420339. [DOI] [PubMed] [Google Scholar]
- Long G, Tubis A. Investigations into the nature of the association between threshold microstructure and otoacoustic emissions. Hear Res. 1988;36:125–138. doi: 10.1016/0378-5955(88)90055-X. [DOI] [PubMed] [Google Scholar]
- Long G, Tubis A. Modification of spontaneous and evoked otoacoustic emissions and associated psychoacoustic microstructure by aspirin consumption. J Acoust Soc Am. 1988;84:1343–1353. doi: 10.1121/1.396633. [DOI] [PubMed] [Google Scholar]
- Long GR, Tubis A, Jones KL. Modeling synchronization and suppression of spontaneous otoacoustic emissions using Van der Pol oscillators: effects of aspirin administration. J Acoust Soc Am. 1991;89:1201–1212. doi: 10.1121/1.400651. [DOI] [PubMed] [Google Scholar]
- Maison SF, Usubuchi H, Liberman MC. Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci. 2013;33:5542–5552. doi: 10.1523/JNEUROSCI.5027-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauermann M, Long GR, Kollmeier B. Fine structure of hearing threshold and loudness perception. J Acoust Soc Am. 2004;116:1066–1080. doi: 10.1121/1.1760106. [DOI] [PubMed] [Google Scholar]
- McFadden D, Mishra R. On the relation between hearing sensitivity and otoacoustic emissions. Hear Res. 1993;71:208–213. doi: 10.1016/0378-5955(93)90036-Z. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Carbonnel O, Collet L. Medial olivocochlear system and loudness adaptation: differences between musicians and non-musicians. Brain Cogn. 1995;29:127–136. doi: 10.1006/brcg.1995.1272. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Morlet T, Giraud AL, Collet L, Morgon A. Contralateral suppression of evoked otoacoustic emissions and detection of a multi-tone complex in noise. Acta Otolaryngol. 1995;115:178–182. doi: 10.3109/00016489509139286. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Perrot X, Collet L. Relationship between auditory intensity discrimination in noise and olivocochlear efferent system activity in humans. Behav Neurosci. 1997;111:801–807. doi: 10.1037/0735-7044.111.4.801. [DOI] [PubMed] [Google Scholar]
- Morand-Villeneuve N, Garnier S, Grimault N, Veuillet E, Collet L, Micheyl C. Medial olivocochlear bundle activation and perceived auditory intensity in humans. Physiol Behav. 2002;77:311–320. doi: 10.1016/S0031-9384(02)00855-7. [DOI] [PubMed] [Google Scholar]
- Mott JB, Norton SJ, Neely ST, Warr WB. Changes in spontaneous otoacoustic emissions produced by acoustic stimulation of the contralateral ear. Hear Res. 1989;38:229–242. doi: 10.1016/0378-5955(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Moulin A, Collet L, Veuillet E, Morgon A. Interrelations between transiently evoked otoacoustic emissions, spontaneous otoacoustic emissions and acoustic distortion products in normally hearing subjects. Hear Res. 1993;65:216–233. doi: 10.1016/0378-5955(93)90215-M. [DOI] [PubMed] [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980;210:71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder P, Nieder I. Stimulation of efferent olivocochlear bundle causes release from low level masking. Nature. 1970;227:184–185. doi: 10.1038/227184a0. [DOI] [PubMed] [Google Scholar]
- Nieder P, Nieder I. Antimasking effect of crossed olivocochlear bundle stimulation with loud clicks in guinea pig. Exp Neurol. 1970;28:179–188. doi: 10.1016/0014-4886(70)90172-X. [DOI] [PubMed] [Google Scholar]
- Norton SJ, Mott JB, Champlin CA. Behavior of spontaneous otoacoustic emissions following intense ipsilateral acoustic stimulation. Hear Res. 1989;38:243–258. doi: 10.1016/0378-5955(89)90069-5. [DOI] [PubMed] [Google Scholar]
- Nuttall AL, Grosh K, Zheng J, Boer E, Zou Y, Ren T. Spontaneous basilar membrane oscillation and otoacoustic emission at 15 kHz in a guinea pig. J Assoc Res Otolaryngol. 2004;5:337–348. doi: 10.1007/s10162-004-4045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers NL, Salvi RJ, Wang J, Spongr V, Qiu CX. Elevation of auditory thresholds by spontaneous cochlear oscillations. Nature. 1995;375:585–587. doi: 10.1038/375585a0. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Scaringi A, Nahum S, Quaranta A. Effects of efferent acoustic reflex activation on psychoacoustical tuning curves in humans. Acta Otolaryngol. 2005;125:520–523. doi: 10.1080/00016480510026214. [DOI] [PubMed] [Google Scholar]
- Rajan R, Johnstone BM. Electrical stimulation of cochlear efferents at the round window reduces auditory desensitization in guinea pigs. I. Dependence on electrical stimulation parameters. Hear Res. 1988;36:53–73. doi: 10.1016/0378-5955(88)90137-2. [DOI] [PubMed] [Google Scholar]
- Robles L, Delano PH. Efferent system. In: Dallos P, Oertel D, editors. The senses: a comprehensive reference. London: Academic Press; 2008. pp. 413–445. [Google Scholar]
- Scharf B, Magnan J, Chays A. On the role of the olivocochlear bundle in hearing: 16 case studies. Hear Res. 1997;103:101–122. doi: 10.1016/S0378-5955(96)00168-2. [DOI] [PubMed] [Google Scholar]
- Schloth E. Relation between spectral composition of spontaneous otoacoustic emissions and fine-structure of threshold in quiet. Acustica. 1983;53:250–256. [Google Scholar]
- Schloth E, Zwicker E. Mechanical and acoustical influences on spontaneous oto-acoustic emissions. Hear Res. 1983;11:285–293. doi: 10.1016/0378-5955(83)90063-1. [DOI] [PubMed] [Google Scholar]
- Shehata WE, Brownell WE, Dieler R. Effects of salicylate on shape, electromotility and membrane characteristics of isolated outer hair cells from guinea pig cochlea. Acta Otolaryngol. 1991;111:707–718. doi: 10.3109/00016489109138403. [DOI] [PubMed] [Google Scholar]
- Shera CA. Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves. J Acoust Soc Am. 2003;114:244–262. doi: 10.1121/1.1575750. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Kim DO. Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hear Res. 1982;6:171–182. doi: 10.1016/0378-5955(82)90052-1. [DOI] [PubMed] [Google Scholar]
- Smith DW, Turner DA, Henson MM. Psychophysical correlates of contralateral efferent suppression. I. The role of the medial olivocochlear system in “central masking” in nonhuman primates. J Acoust Soc Am. 2000;107:933–941. doi: 10.1121/1.428274. [DOI] [PubMed] [Google Scholar]
- Smurzynski J, Probst R. The influence of disappearing and reappearing spontaneous otoacoustic emissions on one subject’s threshold microstructure. Hear Res. 1998;115:197–205. doi: 10.1016/S0378-5955(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Sun XM. Distortion product otoacoustic emission fine structure is responsible for variability of distortion product otoacoustic emission contralateral suppression. J Acoust Soc Am. 2008;123:4310–4320. doi: 10.1121/1.2912434. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Tubis A, Long GR, Piskorski P. Modeling otoacoustic emission and hearing threshold fine structures. J Acoust Soc Am. 1998;104:1517–1543. doi: 10.1121/1.424364. [DOI] [PubMed] [Google Scholar]
- Vinay MBCJ. Effects of activation of the efferent system on psychophysical tuning curves as a function of signal frequency. Hear Res. 2008;240:93–101. doi: 10.1016/j.heares.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Ward WD. Studies on the aural reflex. I. Contralateral remote masking as an indicator of reflex activity. J Acoust Soc Am. 1961;33:1034–1045. doi: 10.1121/1.1908886. [DOI] [Google Scholar]
- Wegel RL, Lane CE. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Phys Rev. 1924;23:266–285. doi: 10.1103/PhysRev.23.266. [DOI] [Google Scholar]
- Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Wilson JP. Evidence for a cochlear origin for acoustic re-emissions, threshold fine-structure and tonal tinnitus. Hear Res. 1980;2:233–252. doi: 10.1016/0378-5955(80)90060-X. [DOI] [PubMed] [Google Scholar]
- Wilson JP, Sutton GJ (1981) Acoustic correlates of tonal tinnitus. In: Evered D, Lawrenson G (eds) Tinnitus - Ciba Foundation Symposium 85. Pitman, London, pp 82–107 [DOI] [PubMed]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol. 1987;57:1002–1021. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Youngblood J, Martin FN. On the relationship of stapedial contraction to central masking. J Aud Res. 1981;21:45–49. [PubMed] [Google Scholar]
- Zhao W, Dhar S. The effect of contralateral acoustic stimulation on spontaneous otoacoustic emissions. J Assoc Res Otolaryngol. 2010;11:53–67. doi: 10.1007/s10162-009-0189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Dhar S. Fast and slow effects of medial olivocochlear efferent activity in humans. PLoS One. 2011;6:e18725. doi: 10.1371/journal.pone.0018725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Dhar S. Frequency tuning of the contralateral medial olivocochlear reflex in humans. J Neurophysiol. 2012;108:25–30. doi: 10.1152/jn.00051.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurek PM. Spontaneous narrowband acoustic signals emitted by human ears. J Acoust Soc Am. 1981;69:514–523. doi: 10.1121/1.385481. [DOI] [PubMed] [Google Scholar]
- Zwicker E. Spontaneous oto-acoustic emissions, threshold in quiet, and just noticeable amplitude modulation at low levels. In: Moore BCJ, Patterson RD, editors. Auditory frequency selectivity. New York: Plenum; 1986. pp. 49–59. [Google Scholar]
- Zwicker E, Schloth E. Interrelation of different oto-acoustic emissions. J Acoust Soc Am. 1984;75:1148–1154. doi: 10.1121/1.390763. [DOI] [PubMed] [Google Scholar]
- Zwislocki J. Acoustic attenuation between the ears. J Acoust Soc Am. 1953;25:752–759. doi: 10.1121/1.1907171. [DOI] [Google Scholar]
- Zwislocki JJ. A theory of central auditory masking and its partial validation. J Acoust Soc Am. 1972;52:644–659. doi: 10.1121/1.1913154. [DOI] [Google Scholar]
- Zwislocki JJ, Damianopoulos EN, Buining E, Glantz J. Central masking: some steady-state and transient effects. Percept Psychophys. 1967;2:59–64. doi: 10.3758/BF03212462. [DOI] [Google Scholar]