Abstract
This study describes the long-term effects of sound-induced cochlear trauma on spontaneous discharge rates in the central nucleus of the inferior colliculus (ICC). As in previous studies, single-unit recordings in Sprague–Dawley rats revealed pervasive increases in spontaneous discharge rates. Based on differences in their sources of input, it was hypothesized that physiologically defined neural populations of the auditory midbrain would reveal the brainstem sources that dictate ICC hyperactivity. Abnormal spontaneous activity was restricted to target neurons of the ventral cochlear nucleus. Nearly identical patterns of hyperactivity were observed in the contralateral and ipsilateral ICC. The elevation in spontaneous activity extended to frequencies well below and above the region of maximum threshold shift. This lack of frequency organization suggests that ICC hyperactivity may be influenced by regions of the brainstem that are not tonotopically organized. Sound-induced hyperactivity is often observed in animals with behavioral signs of tinnitus. Prior to electrophysiological recording, rats were screened for tinnitus by measuring gap pre-pulse inhibition of the acoustic startle reflex (GPIASR). Rats with positive phenotypes did not exhibit unique patterns of ICC hyperactivity. This ambiguity raises concerns regarding animal behavioral models of tinnitus. If our screening procedures were valid, ICC hyperactivity is observed in animals without behavioral indications of the disorder. Alternatively, if the perception of tinnitus is strictly linked to ongoing ICC hyperactivity, our current behavioral approach failed to provide a reliable assessment of tinnitus state.
Keywords: tinnitus, hyperactivity, neuropathology, hearing loss, sound damage
INTRODUCTION
According to the hyperactivity model of tinnitus, neurons in the central auditory system respond to the weakened input of a sound-damaged cochlea by down-regulating its inhibitory connections (Moller 2007; Middleton et al. 2011; Wang et al. 2011) and enhancing membrane excitability (Dong et al. 2010; Pilati et al. 2012; Koehler and Shore 2013; Li et al. 2013). This “rebalancing” may raise spontaneous activity (i.e., activity in the absence of sound) to levels that impart a false perception of sound stimulation (Chen and Jastreboff 1995; Kaltenbach and Afman 2000; Ma et al. 2006).
While there is considerable evidence that acoustic damage elevates spontaneous rates throughout the central auditory system (Eggermont 2003; Manzoor et al. 2013), a causal relationship between hyperactivity and tinnitus remains tenuous. One impediment to the hyperactivity model has been the reliable assessment of an animal’s tinnitus state (Jastreboff and Sasaki 1994).
Gap pre-pulse inhibition of the acoustic startle reflex (GPIASR; Turner et al. 2006) has gained increasing popularity as a simple behavioral assay for confirming tinnitus in laboratory animals. Because the screening procedure is based on an unlearned auditory reflex, it is well suited for neurophysiological studies that require efficient screening methods. GPIASR testing assumes that gap inhibition of the acoustic startle reflex is attenuated in animals with tinnitus because tinnitus “fills the gap.” This assumption has been criticized by recent studies showing inconsistent relationships between the gap stimulus and the startle response (Fournier and Hebert 2013; Lobarinas et al. 2013; Hickox and Liberman 2014).
The characterization of tinnitus-related hyperactivity is further complicated by the physiological diversity of central auditory neurons. Because different neuron types are uniquely affected by acoustic trauma (Cai et al. 2009), hyperactivity may be expressed in one output pathway of the cochlear nuclei, but not another. The dorsal cochlear nucleus (DCN) has received notable attention as a source of tinnitus-related hyperactivity (Zhang and Kaltenbach 1998; Brozoski et al. 2002), although similar patterns of neuropathology have been confirmed in the ventral cochlear nucleus (VCN) (Vogler et al. 2011).
The central nucleus of the inferior colliculus (ICC) is an ideal structure for studying how different subdivisions of the cochlear nucleus influence higher levels of auditory processing because it receives direct or indirect inputs from every major projection of the auditory brainstem (Brunso-Bechtold et al. 1981; Oliver 1987; Zook and Casseday 1987). These parallel ascending pathways are transformed within the ICC into unique response types (Ramachandran et al. 1999; Ehret et al. 2003; Palmer et al. 2013), thus creating a potential method for identifying remote sources of local hyperactivity. Because ascending projections from the DCN are known to modulate the spontaneous rates of ICC type O units (Davis 2002; Imig and Durham 2005), sound-induced hyperactivity in the DCN is expected to produce a selective elevation of the spontaneous activity of that unit type. Alternatively, VCN hyperactivity is expected to be reflected in the physiological characteristics of ICC type V and I units. If it is assumed that hyperactivity is a specific correlate of tinnitus, the neuropathology should only be observed in subjects that exhibit tinnitus behaviors.
METHODS
The effects of acoustic trauma on spontaneous activity in the ICC were evaluated by treating adult male Sprague–Dawley rats with a unilateral sound exposure, screening them for tinnitus with a behavioral procedure, and then recording the spontaneous activity of physiologically characterized units. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University.
Unilateral Sound Exposure
The inner ear was damaged with a single unilateral sound exposure. During the exposure, the unanesthetized rat was held in a slowly rotating hardware cloth cage at the center of a sound-attenuating chamber. Two high-output tweeters (Pyramid TW57) on the ceiling of the chamber delivered a continuous 16-kHz tone at a peak level of 116-dB sound pressure level (SPL). An automated program gradually raised the tone presentation level to its peak level during the initial 60 s of the exposure. Once attained, the peak level was maintained for 2 h.
A unilateral exposure was achieved by exposing one ear to the damaging sound while occluding the other ear with an acoustic foam plug (EAR, 3 M). The manufacturer rates the attenuation of the plugs at 41.2 dB at 6 kHz. Our own ABR-based measures indicate at least 50-dB attenuation at 16 kHz, which is the frequency of sound exposure. The damaged ear was expected to trigger the physiological changes that ultimately induce tinnitus. The undamaged ear ensured that subjects could hear the stimuli used in behavioral testing, thus avoiding a confound between hearing impairment and tinnitus.
The rats were housed in the laboratory’s vivarium for 1–4 months after the sound exposure. The ambient noise spectrum level in this limited access facility was 32-dB SPL. Institutional housing was avoided to minimize uncontrolled sources of auditory stimulation during the recovery period (Lauer et al. 2009).
Behavioral screening was conducted two to three times a week during the recovery period. Subjects that developed stable tinnitus behaviors were advanced to single-unit recording experiments immediately. Subjects that produced negative test results were given a minimum of 2 months to develop the disorder.
Auditory Brainstem Responses
Auditory brainstem responses (ABRs) were measured immediately before the sound exposure to verify normal hearing and repeated 1 week after the exposure to assess acoustic damage. During the recordings, the rat was lightly anesthetized with ketamine and xylazine (40:10 mg/kg) and placed on a regulated heating pad to maintain a core temperature of 37 °C. Platinum subcutaneous electrodes were inserted over the bulla of the test ear (active), on the vertex (reference), and in the ipsilateral leg (ground).
The rat was placed inside an electrically shielded sound-attenuating chamber with the head approximately 45 cm from a wide-range tweeter (Fostex FT28D). The test ear was directed toward the speaker. The opposite ear was plugged and oriented toward the foam-lined floor of the chamber. Control experiments confirmed that a threshold shift of 55 dB could be reliably measured at frequencies above 10 kHz before the plugged protected ear contaminated the ABR of the sound-exposed ear.
Sound-evoked brainstem activity was amplified (300,000×), filtered (300–3,000 Hz), and digitally sampled (10-kHz sampling rate). The digitized waveforms were collected at a rate of 30 waveforms/s and averaged over 300 repetitions. Input–output functions were derived by varying the presentation level of pure tones at frequencies between 2.5 and 40.0 kHz (5-ms duration, 0.5-ms rise/fall times). Hearing thresholds were determined by a statistical analysis of the peak-to-peak voltages of ABR signals (stimulus-on intervals) relative to recording noise (stimulus-off intervals).
Figure 1 presents the hearing thresholds of sound-exposed rats in the form of ABR audiograms. This plotting format depicts the ABR threshold at each tone frequency in terms of a shift relative to the average threshold of unexposed Sprague–Dawley rats in our laboratory (N = 174). All thresholds for the protected ear remained within ±15 dB of normal hearing levels (upper panel). Exposed ears exhibited variable patterns of acoustic damage (middle panel) but always featured a sharp transition from normal to impaired thresholds. The “EDGE” frequency of hearing loss was defined as the frequency that showed the steepest transition. This value ranged from 10.6 to 26.8 kHz (median = 18.9 kHz).
FIG. 1.
Auditory brainstem response (ABR) thresholds after unilateral sound exposure. Audiograms are shown for the protected ear (top) and exposed ear (middle). Neural data were aligned across subjects by reporting best frequencies in terms of octaves re EDGE. The EDGE of threshold shift was defined as the steepest threshold transition in the audiograms of exposed ears (bottom).
Variations in EDGE frequency complicate statistical comparisons that combine neurophysiological measures across subjects. This source of error was minimized by aligning the EDGE of exposed audiograms (lower panel) and then reporting neural frequency tuning characteristics in terms of octaves re EDGE. The best frequency (BF, most sensitive frequency) of an exposed unit was referenced to the EDGE of the subject in which it was recorded. The BFs of units in unexposed rats were referenced to the median EDGE of units in exposed rats (18.9 kHz).
Tinnitus Screening
Sound-exposed rats were behaviorally screened for tinnitus by measuring gap pre-pulse inhibition of the acoustic startle reflex (GPIASR). A detailed description of this protocol is provided by Turner et al. (2006). GPIASR is based on pre-pulse inhibition (PPI), which is the reduction in startle magnitude that is observed when a transient sound (i.e., pre-pulse) precedes a startle-eliciting stimulus. The GPIASR procedure achieves a similar result by replacing the pre-pulse with a transient gap in an otherwise continuous background sound. The background is usually a pure-tone or narrow band of noise. It is assumed that subjects with tinnitus will show less GPIASR when the background sound approximates the tinnitus pitch because tinnitus “fills the gap.” Thus, tinnitus is operationally defined as a frequency-dependent loss of GPIASR.
Acoustic startle reflexes were measured by confining rats in a hardware cloth cage that was fitted with a piezoelectric sensor. The force profile of the reflex was transduced by the sensor, amplified, and digitally recorded at a 10-kHz sampling rate. The absolute magnitude of the reflex was quantified in terms of the peak-to-peak voltage of the digitized waveform. The absolute magnitude of startle reflexes may vary between subjects, as well as within subjects before and after acoustic trauma or over the course of prolonged testing. Consequently, GPIASR is usually reported as a relative startle magnitude which is calculated as the ratio of response magnitude on pre-pulse trials with a gap and control trials without a gap. A small relative startle magnitude indicates normal gap detection, and a value approaching 1 indicates the presence of tinnitus.
GPIASR testing was conducted with 1/4-octave noise backgrounds. The center frequency of the noise varied between 7,071; 10,000; 14,142; and 20,000 Hz. The level of the noise was fixed at 50-dB SPL. The noise was turned on for a variable interval (5–30 s), turned off for 30 ms (i.e., the gap), and then turned on again for 50 ms before the presentation of the startle-eliciting stimulus. The startle stimulus was a 20-ms burst of broadband noise with a presentation level of 110-dB SPL.
Each session was organized into five sequential blocks of 12 trials. Blocks contained one gap trial and two control trials for each of the four noise frequencies. The order of stimulus presentation was randomized within each block. Relative startle magnitudes were calculated for gap and control trials with the same noise background and then averaged across blocks.
Rats were classified as tinnitus positive if there was a reliable elevation of relative startle magnitudes. To be considered reliable, the loss of gap inhibition had to be frequency dependent, at least 0.35 in relative magnitude, and replicated across three consecutive sessions. The magnitude of the elevation was measured in relation to the most inhibited gap frequency produced by the same rat during the same testing sessions. Thus, each rat served as its own control.
Surgical Preparation and Single-Unit Recording
Because the spontaneous rates of ICC neurons are suppressed by barbiturate anesthesia (e.g., sodium pentobarbital), an areflexic state was induced with a single intramuscular injection of ketamine and acepromazine (75:2.5 mg/kg). This method of anesthesia was selected because it has been reported to have minimal effects on spontaneous activity in the ICC (Duque and Malmierca 2014). The spontaneous rates (SRs) of well-isolated units were monitored before, during, and after independent injections of ketamine and acepromazine to confirm those findings. Anesthesia was maintained during electrophysiological recordings with supplemental doses of ketamine (25–40 mg/kg). Mucous secretions were reduced with an intraperitoneal injection of atropine sulfate (0.1 ml). The site of the surgical incision was desensitized with subcutaneous lidocaine (1 ml, 100 mg/ml). A core temperature of 37 °C was maintained with a regulated heating pad. The rat was euthanized at the end of the experiment with an intraperitoneal injection of barbiturate (Euthasol, to effect).
The rat was mounted in standard stereotaxic coordinates using ear bars and a nose adaptor (Paxinos et al. 1985). A midline scalp incision was made to expose the dorsal surface of the skull. A head post was attached to the anterior skull with dental acrylic and anchoring screws. The posterior skull was opened to access the recording site. For most experiments, the recording site was the ICC contralateral to the sound-damaged ear.
At the completion of surgical preparation, the head post was secured to a manipulator arm, and the stereotaxic rail and ear bar were removed from the contralateral ear to allow monaural acoustic stimulation of the target ICC. A tungsten microelectrode (5 MΩ, A-M Systems) was advanced through the brain with a hydraulic micromanipulator. The electrode signal was amplified (1,000×), filtered (0.2–4 kHz), and passed to a voltage discriminator. The digitized spike times of isolated action potentials were recorded with a National Instruments digital interface board (model PCI6602).
Entry into the ICC was indicated by the synchronized sound-driven activity of neural units (i.e., hash). The BF of the electrode site was determined by manually sweeping the frequency and level of pure tones. Electrode tracks were considered to be in the ICC if there was a progressive increase in the BF of multiunit activity as the electrode advanced and isolated units exhibited physiological characteristics that are associated with the central nucleus (Ramachandran et al. 1999). These properties were retained by sound-exposed rats at frequencies below the EDGE of acoustic trauma. The frequency response of the acoustic system limited physiological characterization to units with BFs between 1 and 45 kHz.
When a unit with well-isolated action potentials was encountered along the electrode track, its BF and threshold were determined. Next, a frequency response map (FRM) was constructed from responses to tone pulses that swept in frequency across the unit’s receptive field. The frequency sweeps were presented at multiple sound levels to characterize level-dependent changes in the unit’s excitatory/inhibitory responses. If the unit had discernible spontaneous activity, action potentials were recorded during 10 min of silence. Units with no spontaneous activity were documented with a 1-min recording period.
Previous electrophysiological studies have described FRM-based classification systems for unit activity in the ICC (Ramachandran et al. 1999; Ehret et al. 2003; Palmer et al. 2013). The present analysis is based on the system of Ramachandran et al. with three response types. Examples of the response types are shown in Figure 2.
FIG. 2.
Representative frequency response maps for physiologically classified units. Type V, I, and O units are commonly encountered in the ICC of unexposed rats. Tail units are only observed in sound-exposed rats. Black fill indicates excitatory responses. Gray fill indicates inhibitory responses.
Type V units have a V-shaped excitatory area centered on BF (similar to the type V and perhaps VN units of Palmer et al.). Type I units have narrower, I-shaped excitatory tuning and lateral inhibition (similar to the N and perhaps VN units of Palmer et al.). Type O units have a closed region of excitation centered on BF, with inhibitory responses to BF tones at higher sound levels (similar to the C and perhaps TD and TU units of Palmer et al.). A small percentage of neurons (5.4 %) could not be classified in unexposed rats. They are called type X units in this report.
Differences in the inhibitory receptive fields of FRMs have been used to infer the dominant inputs to ICC unit types (Davis et al. 1999; Ramachandran et al. 1999). Type V and I units are assumed to receive direct or indirect inputs from the VCN, while type O units receive direct inputs from the DCN (Davis 2002; Ramachandran and May 2002). In the context of existing neurophysiological models, DCN-based hyperactivity is expected to target type O units (Kaltenbach 2006; Robertson et al. 2013). Conversely, VCN-based hyperactivity is directed toward type V and I units (Vogler et al. 2011).
Sound exposure radically altered the FRM shapes of a subset of ICC neurons. As a result, these neurons no longer fit the canonical definitions of type V, I, or O units. As shown in Figure 2, units with highly elevated thresholds and abnormally broad frequency tuning are classified as “tail” units in the present description of postexposure response patterns. Tail units have been observed in the DCN after sound exposure (Ma and Young 2006).
RESULTS
Sound-induced hyperactivity was characterized by recording well-isolated action potentials in the ICC of rats with unilateral acoustic trauma (232 units, 16 rats). Normal baselines of spontaneous activity were determined by recording single-unit responses in unexposed control rats (149 units, 22 rats). To minimize the contribution of uneven sampling at outlying frequencies, the analysis of units in sound-exposed rats was limited to BFs that fell within ±2 octaves of the EDGE frequency of acoustic trauma. BFs in unexposed rats were similarly limited to ±2 octaves of the median EDGE frequency of sound-exposed rats (18.9 kHz). Recordings in sound-exposed rats were collected from the contralateral (179 units, 12 rats) or ipsilateral ICC (53 units, six rats), relative to the damaged ear. Bilateral recordings were obtained from two rats. Slightly less than half of the sound-exposures produced behaviorally defined tinnitus (88 units, seven rats).
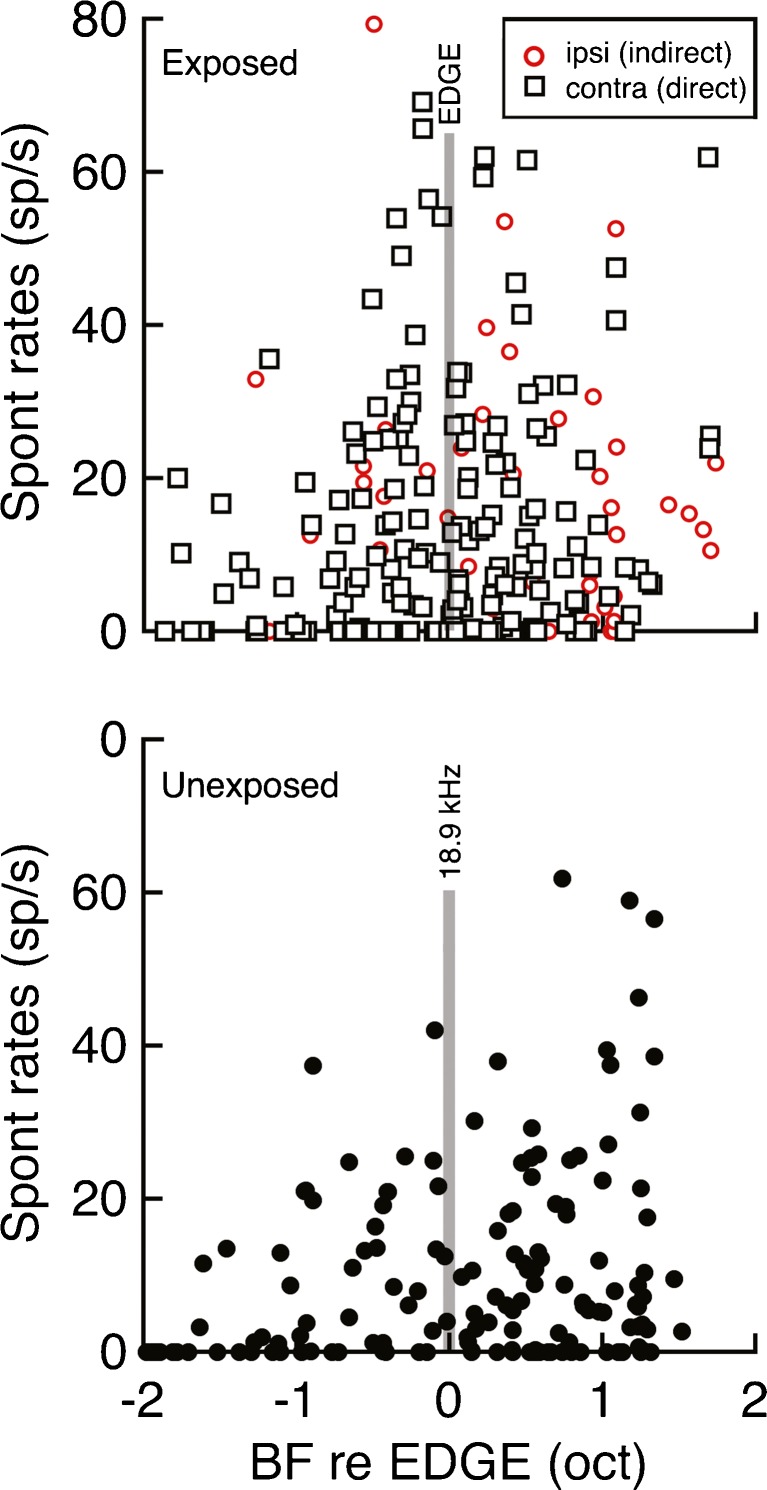
Figure 3 plots SRs in relation to unit BFs. Across all frequencies, SRs showed extensive overlap between unexposed and exposed rats and considerable diversity within each treatment. Many units exhibited no SR (i.e., zero-SR neurons).
FIG. 3.
Effects of acoustic trauma on spontaneous activity in the central nucleus of the inferior colliculus. Symbols indicate the spontaneous rates of individual units that were recorded in exposed rats (top) or unexposed rats (bottom). Units in exposed rats are plotted in relation to the EDGE region of threshold shift (vertical line). Units in the ipsilateral and contralateral pathways of the exposed ear are identified by symbol type and color. Units in unexposed rats are plotted in relation to the median EDGE frequency of exposed rats (18.4 kHz).
Zero-SR neurons were equally prevalent in control and exposed rats (24.2 and 25 %, Table 1). Thus, it seems unlikely that significant numbers of neurons shifted from non-spontaneous to spontaneous activity after sound exposure. Neurons that do not fire action potentials in the absence of sound stimulation are not expected to contribute to a tinnitus percept. Consequently, zero-SR neurons are not included in subsequent analyses of hyperactivity.
TABLE 1.
Comparison of the number, BF range, median spontaneous rate (SR), and percentage of zero-SR neurons in unexposed and exposed rats
| Type | Unexposed | Exposed | ||||||
|---|---|---|---|---|---|---|---|---|
| N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | |
| All | 149 | −4.2, 1.5 | 10.4 | 24.2 | 232 | −3.7, 1.7 | 14.1 | 25.0 |
| V | 42 | −4.2, 1.3 | 1.7 | 57.1 | 62 | −3.5, 1.7 | 7.7 | 46.8 |
| I | 59 | −3.8, 1.3 | 8.0 | 15.3 | 57 | −3.7, 1.7 | 10.5 | 26.3 |
| O | 40 | −2.4, 1.3 | 18.4 | 7.5 | 53 | −3.4, 1.7 | 19.5 | 5.7 |
| X | 8 | −1.9, 1.5 | 19.0 | 12.5 | 27 | −3.7, 1.7 | 15.0 | 3.7 |
| Tail | 0 | 33 | −3.5, 1.1 | 11.1 | 30.3 | |||
Number of units (N) indicates the total number of units in each sample. BF range is expressed in octaves re EDGE. Median SR is based only on spontaneously active units with BFs within two octaves of the EDGE
The median SR of all spontaneously active neurons was 10.4 spikes/s in unexposed rats and 14.1 spikes/s in sound-exposed rats (Table 1). The following sections describe how SR was distributed by unit type, anatomic region, and tinnitus status.
Analysis by Unit Type
The three ICC unit types show different ranges of baseline spontaneous activity in unexposed rats. These differences potentially confound statistical comparisons with exposed rats. For example, there could be a difference in the proportion of units of a high SR type due to sampling error, causing the ICC to appear hyperactive. Such biases were avoided by employing a two-factor statistical design that compared changes in spontaneous activity between the two treatment groups within the same unit type.
Table 1 separates ICC activity by unit type. In unexposed rats, type V units showed the lowest median SR for active units (1.7 spikes/s) and the highest percentage of inactive units (57.1 %). In exposed rats, type V units showed a higher median SR (7.7 spikes/s) and fewer inactive units (46.8 %). Both of these outcomes support current models of tinnitus-related hyperactivity (Ma et al. 2006; Manzoor et al. 2012).
Type I units displayed intermediate rates of spontaneous activity (unexposed = 8.0 spikes/s, exposed = 10.5 spikes/s) and the largest change in the percentage of zero-SR units (unexposed = 15.3 %, exposed = 26.3 %). The near doubling of inactive units in exposed rats is counter-intuitive from the perspective of tinnitus-related hyperactivity.
Type O units in unexposed rats exhibited the highest rates of spontaneous activity (18.4 spikes/s) and the fewest inactive units (7.5 %). These baselines changed only slightly following sound exposure (19.5 spikes/s, 5.7 %).
Box plots in the left column of Figure 4 contrast the unexposed and exposed SR distributions of the major ICC unit types. The lower and upper limits of the boxes span the middle 50 % of the two distributions (interquartile range (IQR). Each box is subdivided by the median score. Vertical “whiskers” extend to the lowest and highest data points within ±1.5 IQR. Data beyond these limits are plotted as outliers (open symbols). Hyperactivity is indicated by an elevation of the exposed distribution relative to unexposed baselines.
FIG. 4.
Effects of acoustic trauma on three major physiological response types in the central nucleus. For each unit type, box plots (left) compare the distributions of spontaneous rates that were recorded in exposed and unexposed rats. Quantile–quantile plots (right) contrast matching quantile scores from the two distributions. Hyperactivity is indicated by an increase in the interquartile slope (dashed line) relative to unity (solid line). Filled symbols highlight 0.75 quantile scores.
The quantile–quantile (Q-Q) plots in the right column of Figure 4 provide pairwise comparisons of the quantile scores of the unexposed and exposed distributions. To illustrate this plotting format, points representing the 0.75 quantile have been highlighted with filled symbols. Note that the 0.75 quantile is equivalent to the upper limits of the boxes in corresponding box plots. When data points in the Q-Q plot fall above the unity line (solid line), the exposed ICC is hyperactive.
Type V units were hyperactive in sound-exposed rats (upper row, Fig. 4). The box plot of the exposed SR distribution is shifted upward and elongated, suggesting an overall increase in spontaneous activity and more extreme SRs. The corresponding Q-Q plot contrasts the same two sets of SR at points of rank-order equality within their respective cumulative distributions. The resulting points are located exclusively above the unity line, recapitulating the upward shift that was observed for the box plot of the exposed SR distribution. Following Q-Q plotting conventions, a line has been fit to all points within the IQR (dashed line). Linearity within this range suggests that the two distributions have the same shape. Deviation from a unity slope indicates that the exposed SR distribution is not simply shifted upward; it has been scaled by a factor of 2.8. In other words, SRs in the middle 50 % of the exposed distribution are approximately three times higher than SRs in the unexposed distribution.
Type I units also show signs of hyperactivity (middle row, Fig. 4). The rate increases are smaller than those observed for type V units. Box plots show less elevation of the exposed IQR. The Q-Q plot indicates a doubling of SR rates within the IQR.
Type O units were not hyperactive (lower row, Fig. 4). This unit type produced the highest SRs of all unit types, but the distribution was not significantly altered by sound exposure. Box plots for unexposed and exposed rats show largely overlapping distributions. Data points in the Q-Q plot fall close to the unity line.
A two-way ANOVA (MATLAB, anovan) indicated statistically significant differences for the main effects of sound exposure (F1,230 = 6.94, P < 0.01) and unit type (F2,230 = 13.59, P < 0.001). These results confirm expected patterns of hyperactivity and general differences in SR between the three response classifications. A post hoc analysis of group means (MATLAB, multcompare with corrections for multiple comparisons) indicated significant effects of sound exposure on the SRs of type V and I units, but not type O units. For these comparisons, the sample of unexposed type V units (N = 18) was constrained by the exclusion of a relatively high percentage of zero-SR units (57.1 %).
Regionalized Hyperactivity
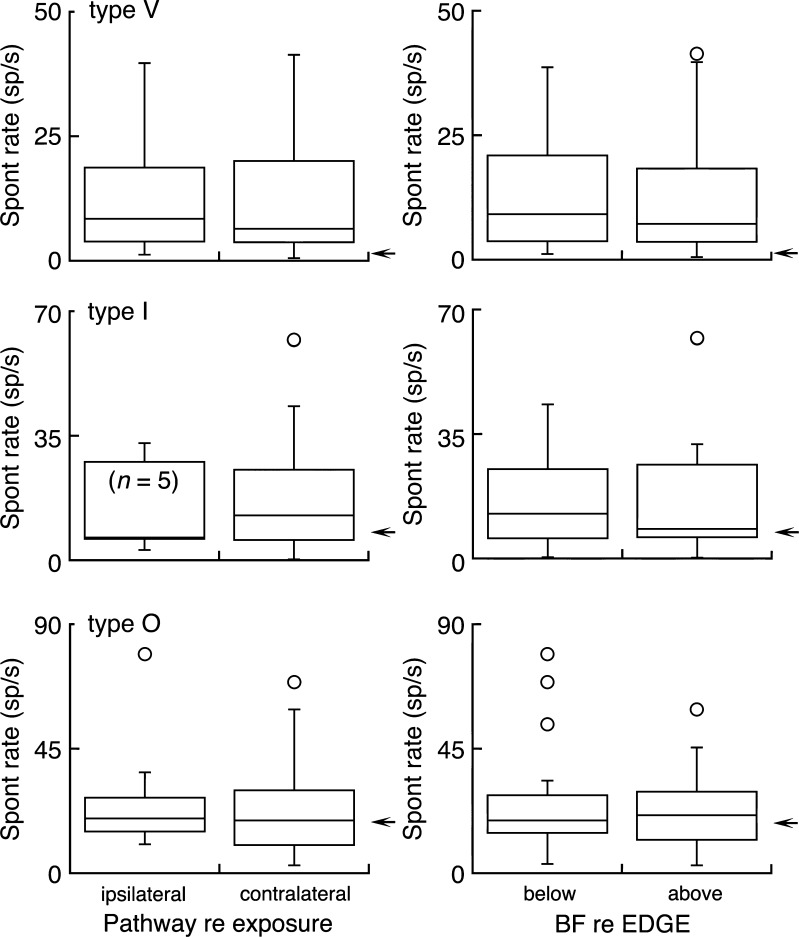
If the sources of sound-induced ICC hyperactivity originate in the cochlear nucleus, the DCN is expected to project its neuropathology to the contralateral ICC. The VCN will distribute its influences bilaterally. Thus, the relative contribution of the two nuclei can be assessed by comparing the lateralization of hyperactivity.
The SR distributions of the ipsilateral and contralateral ICC are compared in the left column of Figure 5. As expected, contralateral recordings replicated the global patterns of hyperactivity for each unit type (Fig. 4). The IQR of every ipsilateral distribution closely matched its contralateral counterpart, indicating identical patterns of hyperactivity in the ipsilateral ICC. A two-way ANOVA found no difference in SRs between the contralateral and ipsilateral ICC (F1,124 < 0.001, P = 0.98). Differences between unit types remained statistically significant (F2,124 = 4.49, P < 0.05). These results implicate the bilateral projections of the VCN as the primary pathway for chronic ICC hyperactivity
FIG. 5.
Regionalized effects of acoustic trauma. Box plots compare spontaneous activity in the ipsilateral and contralateral inferior colliculus of sound exposed rats (left) and at frequency regions below and above the EDGE of threshold shift (right). Arrows indicate median SR of unexposed units from the same response type.
The EDGE frequency of acoustic trauma represents the transition from normal hearing thresholds (below the EDGE) to profound hearing loss (above the EDGE). It was hypothesized that units with BFs above the EDGE would exhibit hyperactivity because their ascending brainstem inputs suffer the most severe cochlear processing deficits (Mulders et al. 2011).
The SR distributions of below and above EDGE units are compared in the right column of Figure 5. This analysis failed to confirm regionalized patterns of hyperactivity. A two-way ANOVA indicated that SRs did not differ significantly between below EDGE and above EDGE units (F1,124 = 0.04, P = 0.85), although SR differences between unit types were maintained (F2,124 = 4.67, P < 0.05). As illustrated in Figure 3, these results suggest that the frequency organization of hyperactivity is symmetrical. SRs peak near the EDGE of acoustic trauma and decrease at a similar rate as BFs move to lower or higher frequencies.
GPIASR Screening
Sound-exposed rats were screened with the GPIASR procedure to identify the patterns of ICC hyperactivity that were specific to tinnitus. Behavioral assessments were conducted before sound exposure and at various delays after sound exposure. Each rat was given a postexposure recovery period of at least 8 weeks in which to develop behavioral signs of tinnitus. If a rat showed stable indications of tinnitus during this period, the subject was advanced immediately to physiological evaluations. In many cases, behavioral assessments were prolonged to explore the possibility of delayed induction. The postexposure recovery period ranged from 5 to 21 weeks in tinnitus-positive rats and from 9 to 22 weeks in tinnitus-negative rats. In all cases, the final behavioral assessment was performed within 1 week of physiological recording sessions.
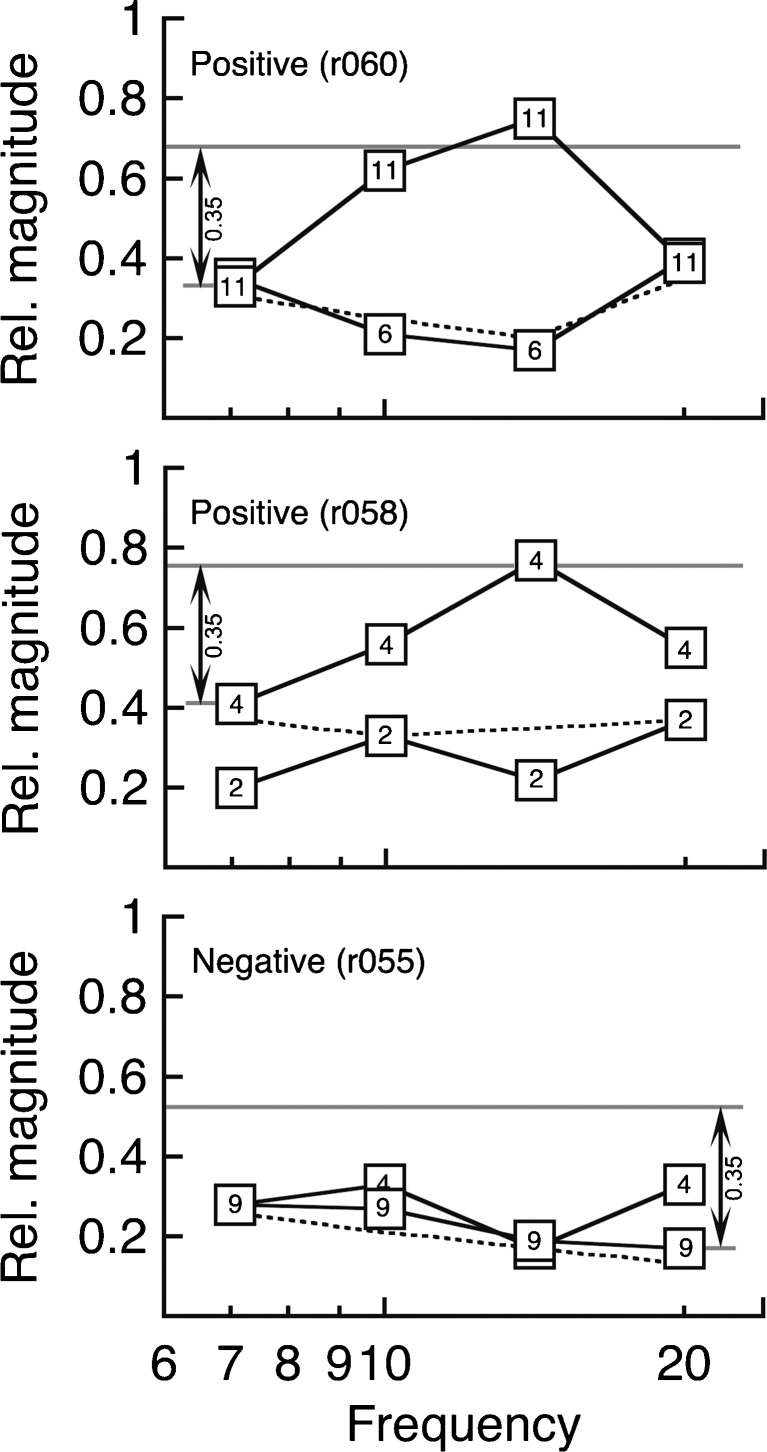
Examples of typical screening results are presented in Figure 6. Each plot displays relative startle magnitudes at four frequencies. Responses at each frequency have been averaged over three consecutive testing sessions. Numerical labels indicate the week of testing relative to the date of sound exposure.
FIG. 6.
Effects of acoustic trauma on gap pre-pulse inhibition of the acoustic startle reflex (GPIASR). Response profiles represent positive (top, middle) and negative tinnitus tests (bottom). Numerical labels indicate the week that the tests were performed relative to the sound exposure. Dashed lines are response profiles that were measured prior to the sound exposure. Arrows indicate the 0.35 shift that was used as the criterion for a positive tinnitus test.
The two rats in the upper and middle panels of Figure 6 produced positive screening results. In both instances, GPIASR scores returned to normal baselines in the weeks immediately following sound exposure. These results confirm that unilateral threshold shifts did not strongly influence gap detection. During subsequent testing, the rats developed a loss of startle inhibition at frequencies near 14 kHz. The specificity of the behavioral impairment implies that gap detection was disrupted by tinnitus with a similar pitch. One rat required 11 weeks to develop tinnitus, while the other rat displayed a loss of startle inhibition after only 4 weeks. The rat in the lower panel of Figure 6 produced negative test results. The GPIASR profile did not deviate from pre-exposure baselines when the rat was tested 4 and 9 weeks after sound exposure.
It was hypothesized that rats with positive tinnitus tests would show higher SRs than rats with negative tests or changes in the SR distributions of particular physiological response types. These distributions are compared in Figure 7. Type I and O units produced similar distributions for the two test outcomes. Type V units showed lower SRs in rats with positive tinnitus tests, but activity levels remained higher than unexposed baselines (arrow). Although this potential difference appears to decouple ICC hyperactivity from tinnitus behavior, the comparison is weakened by the small number (N = 7) of type V units in the tinnitus-positive group. Once again, the sample of type V units was constrained by a high percentage of zero-SR units (60.0 %; Table 2). An additional three units were eliminated because their BFs fell outside frequency criteria.
FIG. 7.
Correlations between spontaneous activity and behavioral screening procedures. Box plots contrast the distribution of spontaneous activity in rats that showed negative or positive GPIASR results. Arrows indicate median SR of unexposed units from the same response type.
TABLE 2.
Sample details for major statistical comparisons
| Type | Contralateral | Ipsilateral | ||||||
| N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | |
| V | 48 | −3.5, 0.9 | 6.5 | 50.0 | 26 | −3.2, 1.7 | 8.5 | 46.2 |
| I | 54 | −3.7, 1.7 | 12.7 | 29.6 | 7 | −1.3, 0.9 | 6.5 | 28.6 |
| O | 35 | −1.8, 0.6 | 19.2 | 2.9 | 16 | −3.4, 1.7 | 19.9 | 10.5 |
| Type | Below EDGE | Above EDGE | ||||||
| N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | |
| V | 35 | −3.5, −0.1 | 9.2 | 60.0 | 39 | 0.0, 1.7 | 7.2 | 38.5 |
| I | 31 | −3.7, −0.1 | 12.7 | 41.9 | 30 | 0.0, 1.7 | 8.3 | 16.7 |
| O | 30 | −3.4, −0.0 | 19.2 | 10.0 | 24 | 0.0, 1.7 | 21.0 | 0.0 |
| Type | Tinnitus positive | Tinnitus negative | ||||||
| N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | N | BF range (min, max) | Median SR (spikes/s) | Zero-SR (%) | |
| V | 25 | −3.2, 0.8 | 4.9 | 60.0 | 49 | −3.5, 1.7 | 9.2 | 42.9 |
| I | 18 | −3.3, 0.4 | 7.1 | 27.8 | 43 | −3.5, 1.7 | 12.7 | 30.2 |
| O | 25 | −1.8, 0.6 | 20.0 | 0.0 | 29 | −3.4, 1.7 | 19.5 | 10.3 |
Number of units (N) indicates the total number of units in each sample. BF range is expressed in octaves re EDGE. Median SR is based only on spontaneously active units with BFs within two octaves of the EDGE
A two-way ANOVA failed to reveal statistically significant differences between SRs in tinnitus-positive and tinnitus-negative rats (F1,131 = 0.04, P = 0.85). Differences between units were significant (F1,131 = 5.46, P < 0.01). The absence of unique patterns of hyperactivity among tinnitus-positive rats leads to opposing interpretations. Hyperactivity may be a general consequence of sound exposure, or GPIASR screening may fail to identify all subjects with tinnitus. Potential problems with GPAISR testing will be discussed later.
Type X and Tail Units
The sample of non-canonical unit types is summarized in the upper panel of Figure 8. Units assigned to the type X classification increased from 5.4 % of the total sample in unexposed rats to 11.6 % in exposed rats. The median SR of type X units declined slightly from 19.0 spikes/s in unexposed rats to 15.0 spikes/s in exposed rats (Table 1). The number of zero-SR units decreased from 12.5 to 3.7 %
FIG. 8.
Effects of acoustic trauma on the distribution of physiological response types. Histograms (top) compare the unit type percentages of exposed and unexposed rats. In addition to the three canonical response types, type X and tail units were defined by abnormally shaped frequency response maps. A sharp decrease in type I units (arrow) may partially explain the increase in non-canonical unit types after sound exposure. Box plots (bottom) show the SR distributions of type X and tail units. The combined distribution of V, I, and O units (VIO) and the individual distribution of type I units (I) are presented for comparison.
Tail units were only observed in exposed rats where they comprised 14.2 % of the total sample. The median SR of tail units most closely approximated type I units (11.1 versus 10.5 spikes/s), as did the percentage of zero-SR units (30.3 % versus 26.3 %). Given the sharp decline in the number of type I units (Fig. 8), this response type may contribute to the sample of tail units.
The box plots in the lower panel of Figure 8 show the SR distributions of type X and tail units in sound-exposed rats. In addition, the individual SR distribution of type I units and the combined distributions of V, I, and O units are presented to estimate the statistical properties of non-canonical units that may have been derived solely from type I units or from the mixing of all canonical classifications. The main effect of the addition of type V and O units is the overall elevation of the type I distribution, presumably because of the high SR of type O units. The lower median SR of tail units closely matches the type I distribution, while higher median SR of type X units resembles the combined distribution.
DISCUSSION
The sound-induced hyperactivity of the ICC has been repeatedly demonstrated. Neurons may show increased SRs (Imig and Durham 2005; Ma et al. 2006; Bauer et al. 2008; Longenecker and Galazyuk 2011; Manzoor et al. 2012), greater synchronization or bursting activity (Wang et al. 2011), decreased inhibition (Milbrandt et al. 2000; Basta and Ernst 2004; Wang et al. 2011), and increased excitability (Salvi et al. 1990). The present study used physiological criteria to infer the relative contributions of brainstem sources for this hyperactivity.
The functional taxonomy of the ICC was originally derived from frequency response maps (FRMs) that were observed in decerebrate cats (Davis et al. 1999; Ramachandran et al. 1999). These definitive FRM shapes are conserved in laboratory rats. Similar response patterns have been confirmed in a number of other studies (LeBeau et al. 2001; Yu and Young 2013).
Type O units produce an FRM with a circumscribed O-shaped island of excitation at threshold (Ramachandran et al. 1999). Other combinations of frequency and level tend to evoke inhibitory responses. Type O units are assumed to receive dominant inputs from the DCN based on similarities of their FRMs, their weak binaural properties, and the effects of pharmacological manipulations of ascending projections from the DCN (Davis 2002; Ramachandran and May 2002).
Relative to type O units, type V units fall at the opposite extreme of the inhibitory–excitatory continuum. The FRM is largely excitatory, preserving the basic V-shaped tuning that is established by mechanical properties of the basilar membrane (Ramachandran et al. 1999). Type V units are binaurally excited (EE) and exhibit the “peak” binaural delay that is typically observed in the medial superior olive (MSO) (Davis et al. 1999; Ramachandran and May 2002). Because spherical bushy cells (SBCs) in the anterior subdivision of the VCN project bilaterally to the MSO (Cant and Casseday 1986; Smith et al. 1993), the dominant ascending inputs of type V units originate in the VCN.
Type I units display strong lateral inhibition. As a result, the central excitatory region of the FRM is reduced to a narrow I-shape (Ramachandran et al. 1999). Type I units are excited by contralateral sounds and inhibited by ipsilateral sounds (EI) (Davis et al. 1999). They exhibit “trough” delay functions that suggest that their binaural responses are dictated by inputs from the lateral superior olive (LSO) (Ramachandran and May 2002). The LSO receives excitatory inputs from SBCs in the ipsilateral VCN (Cant and Casseday 1986; Cant and Benson 2003). Inhibitory inputs originate in the ipsilateral medial nucleus of the trapezoid body (MNTB) which are driven by globular bushy cells (GBCs) in the contralateral VCN (Kuwabara et al. 1991; Smith et al. 1991).
Physiological analysis of ICC hyperactivity failed to support the DCN as a primary tinnitus generator site (Fig. 4). Type O units did not show evidence of hyperactivity. Instead, an expanded role for the VCN was advocated by significant increases in the SRs of type V and I units. This observation is consistent with direct studies of DCN in cat (Ma and Young 2007) and rat (Li and Young personal communication) in which spontaneous discharge rates were not elevated after an extended period of postexposure recovery.
It is important to point out that the diminished role of the DCN was noted at least 2 months after sound exposure. Although sound-induced hyperactivity has been repeatedly demonstrated in the DCN (for a review, see Kaltenbach 2006), recent studies from our laboratory using the same method of tinnitus induction suggest that the SRs of DCN type IV units (i.e., fusiform cells) return to normal baselines after a recovery period of 1–2 months (median = 41 days) (Ma and Young 2006).
It is intriguing that ablation of the dorsal acoustic stria reduces spontaneous activity in the ICC (Manzoor et al. 2012) and prevents the development of tinnitus behavior (Brozoski et al. 2012). To be effective, the procedure must be performed soon after a damaging sound exposure. The same lesioning strategy does not reverse established tinnitus behaviors when it is performed months later (Brozoski and Bauer 2005). Thus, the transient pathophysiology of the DCN may be necessary for tinnitus induction, while the persistent neural reorganization of the VCN is sufficient for tinnitus maintenance.
Role of the VCN
SRs were bilaterally elevated after a unilateral sound exposure. This phenomenon was previously described in the ICC of guinea pigs (Dong et al. 2010). In that study, the contralateral nucleus showed more pronounced short-term hyperactivity than the ipsilateral nucleus, and the magnitude of the contralateral effect declined 4 weeks after sound exposure. This dynamic asymmetry may be driven by a transient reorganization of neurotransmitter systems in the DCN.
Dong et al. (2010) noted pervasive long-term changes in the expression of genes related to both excitatory (glutamate) and inhibitory neurotransmission (GABA and glycine) in the cochlear nuclei, although the analysis was not applied separately to the DCN and VCN subdivisions. Significant changes in gene expression were also observed bilaterally in the ICC. These observations compliment studies that explicitly link hyperactivity and tinnitus behavior to inhibitory changes in the DCN (Middleton et al. 2011) and ICC (Bauer et al. 2000). Consequently, the hyperactivity of ICC neurons may reflect both ascending and local influences that interact with independent time courses.
Recent studies have described hyperactivity in the ICC of guinea pigs using alternative physiological classifications (Vogler et al. 2014). Neurons with monaural rate–level functions showed a statistically significant increase in spontaneous activity after sound exposure. Because monotonic units are functionally equivalent to the strictly excitatory type V units, our present findings replicate sound-induced hyperactivity in that response classification. Conversely, units with non-monotonic rate–level functions exhibited lower than normal SRs after sound exposure. Non-monotonic units are identified by on-BF inhibition, which is a characteristic of all type O units. The slightly negative slope of the Q-Q plot for type O units provides subtle evidence for this hypoactivity (Fig. 4). An important source of ambiguity is the assignment of type I units to both monotonic and non-monotonic classifications based on variations in the strength of on-BF inhibition. If only monotonic units are hyperactive, as suggested by the results of Vogler et al., the selective analysis of monotonic type I units may raise hyperactivity beyond levels that are reported in the present study.
The bilateral hyperactivity of type V and I units implicates the VCN as a primary tinnitus generator site (Fig. 5). Electrophysiological studies of the VCN have confirmed significant increases in spontaneous activity after acoustic trauma or mechanical lesions of the cochlea (Vogler et al. 2011). Unit classifications based on the shape of peri-stimulus time histograms (PSTHs) linked the largest rate increases to primary-like units, which is the physiological counterpart of SBCs. The SRs of onset units (octopus cells) and chopper units (stellate cells) were elevated to a lesser degree. Related ABR studies in human tinnitus patients have reported an enhancement of wave III and V potentials (Gu et al. 2012), which is also interpreted as increased excitability in the SBC pathways. This pathology may be relayed to the ICC through the indirect binaural projections of the VCN. Although a single unit analysis has not been applied to the binaural brainstem, local hyperactivity is implied by chronic changes in the expression of excitatory and inhibitory neurotransmitters (Potashner et al. 1997; Suneja et al. 1998).
Recent studies from our laboratory suggest that the SRs of DCN type IV units (i.e., fusiform cells) are not significantly different from normal baselines after a 2-month recovery period (Ma and Young 2006) (Li and Young, personal communication). The potentially reduced role of the DCN as a primary tinnitus generator site contradicts independent observations that surgical ablations of the dorsal acoustic stria (DAS) eliminate sound-induced ICC hyperactivity (Manzoor et al. 2012) and prevent tinnitus behavior in sound-exposed animals (Brozoski et al. 2012). In both of those studies, the DAS was lesioned soon after acoustic trauma. The same lesioning strategy does not reverse tinnitus behaviors once they have been established (Brozoski and Bauer 2005). Thus, the transient pathophysiology of the DCN may be necessary for tinnitus induction, while an alternative persistent neural reorganization is sufficient for tinnitus maintenance. Because those long-term changes are selectively associated with type V and I units in the present study, they may involve either remote or local changes in the neurotransmitter systems that link the VCN and ICC.
Adequacy of Behavioral Screening
Tinnitus-positive rats did not exhibit unique patterns of hyperactivity (Fig. 7). These findings place in conflict the most influential behavioral and physiological models of tinnitus. If we accept the behavioral model that assumes rats with tinnitus show a frequency-dependent loss of GPIASR (Turner et al. 2006), we must reject the physiological model that assumes hyperactivity is a specific correlate of tinnitus (Kaltenbach 2006). Conversely, if we accept hyperactivity as an essential physiological correlate of tinnitus, we must reject the loss of GPIASR as a behavioral assay.
When animals are trained to respond to gaps in an ongoing background sound, tinnitus-positive subjects are expected to show performance deficits because tinnitus renders silence less detectable (Turner et al. 2006; Longenecker and Galazyuk 2011; Lobarinas et al. 2013). These deficits are exacerbated when the background sound closely matches the tinnitus percept. In the context of GPIASR, the deleterious effects of tinnitus on gap detection are revealed by a frequency-dependent loss of PPI (Fig. 6).
Existing behavioral approaches are challenged by their need to match an objective sound to an unknown tinnitus experience (Campolo et al. 2013). When the stimulus space is repeatedly sampled to achieve this goal, behavioral subjects learn to discriminate sound from silence (tinnitus) based on increasing small differences in pitch, loudness, location, or timbre. Sharpening the sound/tinnitus dichotomy further restricts the range of acoustic variation that is able to support a positive tinnitus test.
Undesirable learning effects can reduce the magnitude of positive test results over repeated sessions (Heffner and Harrington 2002; Kaltenbach et al. 2004) and ultimately will lead to false negative test results. That is, a subset of rats with tinnitus may display normal GPIASR because they have learned effective listening strategies for gap detection. The contamination of the tinnitus-negative group with misclassified tinnitus-positive rats may have contributed to the lack of tinnitus-specific hyperactivity in the present study.
Considerations for Future Studies
Approximately, one quarter of all units in the unexposed ICC showed no spontaneous activity (Table 1). Sound exposure did not change the proportion of zero-SR units. Because these units do not fire action potentials in the absence of sound stimulation, they are an unlikely source of tinnitus-related neural activity. Inclusion of inactive, stable units in the analysis of spontaneously active units may substantially reduce the statistical significance of sound-induced hyperactivity (Fig. 4).
In structures with multiple physiological response types such as the ICC, unit classification is an essential feature of the statistical analysis. Type V and I units showed the lowest SRs in normal rats and the highest rate elevation in sound-exposed rats. Type O units showed the highest baseline rates and no elevation. If ICC neurons are treated as one generic response class, sampling biases may lead to critical errors in statistical interpretation. For example, sampling more type V units in the unexposed ICC, or more type O units in the exposed ICC, would produce false hyperactivity. The opposite sampling bias would diminish actual hyperactivity.
A dilemma with any system of physiological classification is the proliferation of unusual response types after sound exposure (Ma and Young 2006; Wang et al. 2013). Tail units express the most profound consequences of cochlear damage (Fig. 8) and therefore may represent a key element in the underlying pathophysiology of tinnitus. At the present time, the physiological characteristics of tail units cannot be directly linked to the appropriate baselines of canonical ICC response classes. Although this study presents indirect evidence for the transformation of type I units into tail units, future studies may gain important insights into the mechanisms of tinnitus induction from a more detailed functional segregation that is based on empirical confirmation of their brainstem inputs. Based on the results of Vogler et al. (2014), further refinements in the classification of type I units are also warranted.
Physiological descriptions of sound-induced hyperactivity are typically organized according to the relationship between unit BFs and the exposure frequency. Acoustic damage is surprisingly heterogeneous, even under well-controlled laboratory conditions (Fig. 1). When single-unit data must be combined across experimental animals, this variability should be managed by aligning the data in terms of individualized patterns of hearing loss. ABR-based analyses such as the EDGE frequency are one method for achieving standardization.
A broad survey of existing laboratory studies reveals an almost complete lack of consensus regarding procedures for tinnitus induction and verification. The exposure stimulus may be a loud tone that is presented briefly or a moderate broadband noise that is presented for several hours. Sound-exposed animals may be tested immediately or allowed to recover for several months. The recovery process may advance in a controlled laboratory environment or in the uncontrolled, potentially disruptive sound levels of animal facilities. The behavioral protocols that ultimately screen sound-exposed animals for tinnitus also vary in approach and interpretation. Subjects may experience lengthy training with operant procedures, or they may be tested without explicit training using methods that are based on acoustic startle reflexes. A positive test result may be based on categorical behaviors, or statistical trends. Until a systematic analysis of these critical variables promotes a unified behavioral strategy that can be applied with reasonable certainty to individual animals, researchers investigating the neural reorganization of the sound-damaged auditory system will continue to struggle with the fundamental question, does this animal have tinnitus?
References
- Basta D, Ernst A. Noise-induced changes of neuronal spontaneous activity in mice inferior colliculus brain slices. Neurosci Lett. 2004;368:297–302. doi: 10.1016/j.neulet.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147:175–182. doi: 10.1016/S0378-5955(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206:227–236. doi: 10.1016/j.heares.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Wisner KW, Sybert LT, Bauer CA. Bilateral dorsal cochlear nucleus lesions prevent acoustic-trauma induced tinnitus in an animal model. J Assoc Res Otolaryngol. 2012;13:55–66. doi: 10.1007/s10162-011-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- Cai S, Ma WL, Young ED. Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment. J Assoc Res Otolaryngol. 2009;10:5–22. doi: 10.1007/s10162-008-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolo J, Lobarinas E, Salvi R. Does tinnitus “fill in” the silent gaps? Noise Health. 2013;15:398–405. doi: 10.4103/1463-1741.121232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/S0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cant NB, Casseday JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol. 1986;247:457–476. doi: 10.1002/cne.902470406. [DOI] [PubMed] [Google Scholar]
- Chen GD, Jastreboff PJ. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear Res. 1995;82:158–178. doi: 10.1016/0378-5955(94)00174-O. [DOI] [PubMed] [Google Scholar]
- Davis KA. Evidence of a functionally segregated pathway from dorsal cochlear nucleus to inferior colliculus. J Neurophysiol. 2002;87:1824–1835. doi: 10.1152/jn.00769.2001. [DOI] [PubMed] [Google Scholar]
- Davis KA, Ramachandran R, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. II. Sensitivity to interaural level differences. J Neurophysiol. 1999;82:164–175. doi: 10.1152/jn.1999.82.1.164. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci. 2010;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Duque D, Malmierca MS (2014) Stimulus-specific adaptation in the inferior colliculus of the mouse: anesthesia and spontaneous activity effects. Brain Struct Funct. doi:10.1007/s00429-014-0862-1 [DOI] [PubMed]
- Eggermont JJ. Central tinnitus (2003) Auris Nasus Larynx 30 Suppl: S7–12 [DOI] [PubMed]
- Ehret G, Egorova M, Hage SR, Muller BA. Spatial map of frequency tuning-curve shapes in the mouse inferior colliculus. Neuroreport. 2003;14:1365–1369. doi: 10.1097/01.wnr.0000078545.07662.85. [DOI] [PubMed] [Google Scholar]
- Fournier P, Hebert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res. 2013;295:16–23. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, Melcher JR. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol. 2012;13:819–833. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Harrington IA. Tinnitus in hamsters following exposure to intense sound. Hear Res. 2002;170:83–95. doi: 10.1016/S0378-5955(02)00343-X. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig TJ, Durham D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J Comp Neurol. 2005;490:391–413. doi: 10.1002/cne.20674. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. An animal model of tinnitus: a decade of development. Am J Otol. 1994;15:19–27. [PubMed] [Google Scholar]
- Kaltenbach JA (2006) Summary of evidence pointing to a role of the dorsal cochlear nucleus in the etiology of tinnitus. Acta Otolaryngol Suppl: 20–26 [DOI] [PubMed]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/S0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013;33:19647–19656. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara N, DiCaprio RA, Zook JM. Afferents to the medial nucleus of the trapezoid body and their collateral projections. J Comp Neurol. 1991;314:684–706. doi: 10.1002/cne.903140405. [DOI] [PubMed] [Google Scholar]
- Lauer AM, May BJ, Hao ZJ, Watson J. Analysis of environmental sound levels in modern rodent housing rooms. Lab Anim (NY) 2009;38:154–160. doi: 10.1038/laban0509-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau FE, Malmierca MS, Rees A. Iontophoresis in vivo demonstrates a key role for GABA(A) and glycinergic inhibition in shaping frequency response areas in the inferior colliculus of guinea pig. J Neurosci. 2001;21:7303–7312. doi: 10.1523/JNEUROSCI.21-18-07303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Choi V, Tzounopoulos T. Pathogenic plasticity of Kv7.2/3 channel activity is essential for the induction of tinnitus. Proc Natl Acad Sci U S A. 2013;110:9980–9985. doi: 10.1073/pnas.1302770110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL. The gap-startle paradigm for tinnitus screening in animal models: limitations and optimization. Hear Res. 2013;295:150–160. doi: 10.1016/j.heares.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Young ED. Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity. Hear Res. 2006;216–217:176–188. doi: 10.1016/j.heares.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, Kaltenbach JA. Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol. 2012;108:976–988. doi: 10.1152/jn.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor NF, Gao Y, Licari F, Kaltenbach JA. Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear Res. 2013;295:114–123. doi: 10.1016/j.heares.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–260. doi: 10.1016/S0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Moller AR. The role of neural plasticity in tinnitus. Prog Brain Res. 2007;166:37–45. doi: 10.1016/S0079-6123(07)66003-8. [DOI] [PubMed] [Google Scholar]
- Mulders WH, Ding D, Salvi R, Robertson D. Relationship between auditory thresholds, central spontaneous activity, and hair cell loss after acoustic trauma. J Comp Neurol. 2011;519:2637–2647. doi: 10.1002/cne.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DL. Projections to the inferior colliculus from the anteroventral cochlear nucleus in the cat: possible substrates for binaural interaction. J Comp Neurol. 1987;264:24–46. doi: 10.1002/cne.902640104. [DOI] [PubMed] [Google Scholar]
- Palmer AR, Shackleton TM, Sumner CJ, Zobay O, Rees A. Classification of frequency response areas in the inferior colliculus reveals continua not discrete classes. J Physiol. 2013;591:4003–4025. doi: 10.1113/jphysiol.2013.255943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Pilati N, Ison MJ, Barker M, Mulheran M, Large CH, Forsythe ID, Matthias J, Hamann M. Mechanisms contributing to central excitability changes during hearing loss. Proc Natl Acad Sci U S A. 2012;109:8292–8297. doi: 10.1073/pnas.1116981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1997;148:222–235. doi: 10.1006/exnr.1997.6641. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, May BJ. Functional segregation of ITD sensitivity in the inferior colliculus of decerebrate cats. J Neurophysiol. 2002;88:2251–2261. doi: 10.1152/jn.00356.2002. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Davis KA, May BJ. Single-unit responses in the inferior colliculus of decerebrate cats. I. Classification based on frequency response maps. J Neurophysiol. 1999;82:152–163. doi: 10.1152/jn.1999.82.1.152. [DOI] [PubMed] [Google Scholar]
- Robertson D, Bester C, Vogler D, Mulders WH. Spontaneous hyperactivity in the auditory midbrain: relationship to afferent input. Hear Res. 2013;295:124–129. doi: 10.1016/j.heares.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–257. doi: 10.1016/0378-5955(90)90049-U. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Carney LH, Yin TC. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol. 1991;304:387–407. doi: 10.1002/cne.903040305. [DOI] [PubMed] [Google Scholar]
- Smith PH, Joris PX, Yin TC. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol. 1993;331:245–260. doi: 10.1002/cne.903310208. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. Plastic changes in glycine and GABA release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol. 1998;151:273–288. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Hyperactivity in the ventral cochlear nucleus after cochlear trauma. J Neurosci. 2011;31:6639–6645. doi: 10.1523/JNEUROSCI.6538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler DP, Robertson D, Mulders WH. Hyperactivity following unilateral hearing loss in characterized cells in the inferior colliculus. Neuroscience. 2014;265:28–36. doi: 10.1016/j.neuroscience.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res. 2011;279:111–117. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zuo L, Hong B, Han D, Range EM, Zhao L, Sui Y, Guo W, Liu L. Tonotopic reorganization and spontaneous firing in inferior colliculus during both short and long recovery periods after noise overexposure. J Biomed Sci. 2013;20:91. doi: 10.1186/1423-0127-20-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JJ, Young ED. Frequency response areas in the inferior colliculus: nonlinearity and binaural interaction. Front Neural Circ. 2013;7:90. doi: 10.3389/fncir.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250:197–200. doi: 10.1016/S0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH. Convergence of ascending pathways at the inferior colliculus of the mustache bat, Pteronotus parnellii. J Comp Neurol. 1987;261:347–361. doi: 10.1002/cne.902610303. [DOI] [PubMed] [Google Scholar]