Abstract
Evidence has accumulated that hematopoietic stem progenitor cells (HSPCs) share several markers with the germline, a connection supported by reports that prolactin, androgens, and estrogens stimulate hematopoiesis. To address this issue more directly, we tested the expression of receptors for pituitary-derived hormones, such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH), on purified murine bone marrow (BM) cells enriched for HSPCs and tested the functionality of these receptors in ex vivo signal transduction studies and in vitro clonogenic assays. We also tested whether administration of pituitary- and gonad-derived sex hormones (SexHs) increases incorporation of bromodeoxyuridine (BrdU) into HSPCs and expansion of hematopoietic clonogenic progenitors in mice and promotes recovery of blood counts in sublethally irradiated animals. We report for the first time that HSPCs express functional FSH and LH receptors and that both proliferate in vivo and in vitro in response to stimulation by pituitary SexHs. Furthermore, based on our observations that at least some of CD45− very small embryonic-like stem cells (VSELs) may become specified into CD45+ HSPCs, we also evaluated the expression of pituitary and gonadal SexHs receptors on these cells and tested whether these quiescent cells may expand in vivo in response to SexHs administration. We found that VSELs express SexHs receptors and respond in vivo to SexHs stimulation, as evidenced by BrdU accumulation. Since at least some VSELs share several markers characteristic of migrating primordial germ cells and can be specified into HSPCs, this observation sheds new light on the BM stem cell hierarchy.
Introduction
Hematopoietic stem progenitor cells (HSPCs) are exposed in bone marrow (BM) to several growth factors, cytokines, chemokines, and bioactive lipids. It has been also reported that they respond by clonogenic growth in vitro to certain sex hormones (SexHs), such as prolactin (PRL), androgens, and estrogens [1–3]. In further support of this notion, androgens (eg, danazol) are currently employed to treat aplastic anemia patients [4]. Similarly, the pro-hematopoietic activity of estrogens and progesterone play a role during pregnancy, so that HSPCs can respond to increased oxygen consumption and produce more erythrocytes [1].
Furthermore, the recent heated debate about the existence of developmentally early stem cells with broader specification in murine BM has challenged the established hierarchy within the stem cell compartment [5,6]. The responsiveness of HSPCs to SexHs may support the challenging concept of a developmental link between primordial germ cells (PGCs) and hematopoiesis [5–11]. Specifically, as proposed by some investigators, HSPCs could become specified from a population of migrating PGCs during embryogenesis [7]. In support of this intriguing possibility, HSPCs and PGCs are highly migratory cells, and specification of the first primitive HSPCs in yolk sac blood islands as well as the origin of definitive HSPCs in the aorta-gonad-mesonephros (AGM) region is chronologically and anatomically correlated with the developmental migration of PGCs in extra- and intra-embryonic tissues [5,6,11]. Furthermore, several papers have described the sharing of chromosomal aberrations between germline tumors and leukemias or lymphomas, which suggests their shared clonal origin [12–15]. Moreover, as recently reported, germline-derived cells share with HSPCs a functional erythropoietin receptor (EpoR) [16].
However, the exact mechanism of action of SexHs secreted by the gonads and, in particular, those secreted by the pituitary gland on hematopoiesis is not well understood. To address this important issue, we performed a complex series of experiments to address the influence of pituitary SexHs, such as follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin (PRL), as well as gonadal SexHs, such as androgen (danazol), estrogen (estradiol), and progesterone. Because the levels of the two latter SexHs rapidly fluctuate in mice during their very short (just 4-day-long) menstrual cycle, estradiol and progesterone were tested in ovariectomized female mice.
We tested the expression of SexHs receptors on murine BM-purified Sca-1+ cells enriched for HSPCs and, importantly, the functionality of these receptors was tested in clonogenic assays in vitro in the presence of suboptimal doses of hematopoiesis-stimulating cytokines and growth factors as well as by signal transduction studies. We also administered SexHs into mice and evaluated the incorporation of bromodeoxyuridine (BrdU) into BM-residing Sca-1+Lin−CD45+ HSPCs, the expansion of BM clonogenic progenitors, and the recovery of peripheral blood (PB) counts in sublethally irradiated mice.
We observed that HSPCs express functional SexHs receptors, for both pituitary and gonadal SexHs, and proliferate in response to SexHs stimulation. Furthermore, based on our observations that population of BM-residing CD45− very small embryonic-like stem cells (VSELs) express several markers shared with migratory PGCs [11], and may become specified into CD45+ HSPCs [17,18], we also evaluated the expression of SexH receptors on these cells at mRNA and protein level and tested whether these quiescent cells can proliferate and accumulate BrdU if stimulated by SexHs. We found that VSELs express SexHs receptors and respond in vivo to SexHs stimulation similar to HSPCs, as evidenced by BrdU accumulation. This observation may shed new light on the developmental origin of HSPCs and support our previous observations of a potential developmental link between PGCs, some CD45−VSELs, and CD45+ HSPCs.
Materials and Methods
Mice
We employed in our experiments pathogen-free, 4–6 week-old C57BL/6 mice (Jackson Laboratory). In some of the experiments, ovariectomized C57BL/6 mice were purchased from National Cancer Institute. Animal procedures were approved by the Local Ethics Committee and performed in accordance with guidelines for laboratory animal care. All efforts were made to minimize animal suffering and the number of animals used.
Isolation of murine HSPCs and VSELs
Isolated by flushing bones, BM cell suspensions were lysed in BD lysing buffer (BD Biosciences, San Jose, CA) for 15 min at room temperature and washed twice in phosphate-buffered saline (PBS). VSELs (Sca-1+Lin−CD45−) and HSPCs (Sca-1+Lin−CD45+) were isolated by multi-parameter, live-cell sorting (Influx, Beckton Dickinson) and analyzed by flow cytometry (Navios, Beckman Coulter), as described in our previous studies [5,19].
Isolation of Sca-1+ cells from BM
BM cells were obtained from the femurs and tibias of C57BL/6 mice, and mononuclear cells were separated by Ficoll–Paque density gradient (Ficoll-Paque PLUS; GE Healthcare Bio-Sciences). To obtain Sca-1-positive cells, magnetic-activated cell sorting of Sca-1-stained cells was performed according to the manufacturer's protocol (Miltenyi Biotec, Inc.). In some of the experiments, murine Sca-1+Kit+Lin− (SKL) cells were isolated by MoFlo™ XDP (Beckman Coulter, Inc.) [18].
Hormon treatment of mice in vivo
Normal 2-month-old C57BL/6 mice were exposed to daily injections of FSH (5 IU/mice/day), LH (5 IU/mice/day), PRL (1 μg/mice/1 day), danazol (4 mg/kg/day), and estrogen (20 mg/mouse/day). Hormone treatment was performed for 10 days.
FACS analysis
The following mAbs were employed to stain Lin−/Sca-1+/CD45− and Lin−/Sca-1+/CD45+ cells: biotin-conjugated rat anti-mouse Ly-6A/E (Sca-1, clone E13-161.7), streptavidin–PE–Cy5 conjugate, anti-CD45–APC–Cy7 (clone 30-F11), anti-CD45R/B220–PE (clone RA3-6B2), anti-Gr-1–PE (clone RB6-8C5), anti-TCRαβ–PE (clone H57-597), anti-TCRγζ–PE (clone GL3), anti-CD11b–PE (clone M1/70), and anti-Ter-119–PE (clone TER-119). All mAbs were added at saturating concentrations, and the cells were incubated for 30 min on ice, washed twice, and then resuspended for sorting in cell-sorting medium containing 1× Hank's Balanced Salt Solution without phenol red (GIBCO), 2% heat-inactivated fetal calf serum (GIBCO), 10 mM HEPES buffer (GIBCO), and 30 U/mL gentamicin (GIBCO) at a concentration of 5×106 cells/mL. Sca-1+Lin−CD45− cells (VSELs) and Sca-1+Lin−CD45+ cells (HSCs) were isolated according to the gating and sorting strategy described by us [5,19]. Proliferation events in BM-derived VSEL and HSC populations were examined by BrdU incorporation followed by flow cytometry.
In vivo BrdU treatment
Adult C57BL/6 mice (4–8 weeks old; Jackson Laboratory) were intraperitoneally (i.p.) were injected daily with 1 mg of BrdU solution to evaluate whether SexHs affect proliferation of stem cells, and the final injection of BrdU was performed 1 h before sacrificing the animals [20]. MNCs were subsequently isolated from BM after lysis of erythrocytes and immunostained for expression of Sca-1, CD45, and Lin markers, as previously reported [19], as well as for the presence of BrdU (FITC BrdU Flow Kit; BD Pharmingen). Samples were analyzed with a Navios flow cytometer (Beckman Coulter).
Clonogenic assays in vivo
BM-derived (2×105) or PB-derived (4×105) cells or FACS-sorted SKL (2×103) cells were resuspended in 0.4 mL of RPMI-1640 medium and mixed with 1.8 mL of MethoCult HCC-4230 methylcellulose medium (Stem Cell Technologies, Inc.), supplemented with L-glutamine and antibiotics. Specific murine recombinant growth factors (all purchased from R&D Systems) were added at suboptimal doses. Specifically, to stimulate granulocyte-macrophage colony-forming units (CFU-GM), IL-3 (10 ng/mL) and GM-CSF (25 ng/mL) were used, while EPO (5 U/mL) was used to stimulate erythrocyte burst-forming units (BFU-E), and CFU-Megs were stimulated by thrombopoietin (50 ng/mL). The colonies were counted under an inverted microscope after 7–10 days of culture. Each clonogenic assay was performed in quadruplicate.
Signal transduction studies
Cells were kept in RPMI medium containing 0.5% BSA overnight in an incubator to achieve quiescence; then stimulated with FSH (5 IU), LH (5 IU), danazol (4 mg/mL), PRL (1 μg/mL), estradiol (0.1 μM), progesterone (0.1 μM), or vehicle only (0.9% sodium chloride diluted in medium with 0.5% BSA) for 5 or 10 min at 37°C; and finally lysed for 20 min on ice with RIPA Lysis Buffer System (Santa Cruz Biotechnology) containing protease and phosphatase inhibitors (Santa Cruz Biotechnology). The concentrations of extracted proteins were measured with the BCA Protein Assay Kit, according to the manufacturer's instructions (Thermo Scientific), and equal amounts of protein were separated and analyzed for phosphorylation of MAPKp44/42 and AKT (Ser473). Loading of the lanes was evaluated by stripping the blots and reprobing with antibodies against MAPKp44/42 and AKT. All phosphospecific antibodies were purchased from Cell Signaling Technology, Inc. The membranes were developed with an Amersham ECL Western Blotting Detection Reagents kit and exposed to Amersham Hyperfilm (GE Healthcare).
Real-time polymerase chain reaction for SexH receptor expression
For analysis of SexH receptor expression at the mRNA level, total mRNA was isolated from cells with the RNeasy Mini Kit (Qiagen, Inc.), and mRNA was reverse transcribed with TaqMan Reverse Transcription Reagents (Applied Biosystems). Detection of target genes and β2-microglobulin mRNA levels was performed by real time polymerase chain reaction (RT-PCR) using an ABI PRISM® 7500 Sequence Detection System (ABI). A 25-μL reaction mixture contained 12.5 μL SYBR Green PCR Master Mix, 10 ng of cDNA template, and forward and reverse primers. Primers were designed with Primer Express software (Table 1). The threshold cycle (Ct), that is, the cycle number at which the amount of amplified gene of interest reached a fixed threshold, was subsequently determined. The relative quantitation of target gene mRNA expression was calculated with the comparative Ct method. The relative quantitative value of the target, normalized to an endogenous β2-microglobulin gene control and relative to a calibrator, is expressed as 2−ΔΔCt (fold difference), whereas ΔCt=Ct of target genes—Ct of an endogenous control gene (β2-microglobulin), and ΔΔCt=ΔCt of samples measuring the target gene—ΔCt of samples measuring the calibrator for the target gene. To avoid the possibility of amplifying contaminating DNA (i) all of the primers for real-time RT-PCR were designed to contain a DNA intron sequence for specific cDNA amplification, (ii) reactions were performed with appropriate negative controls (template-free controls), (iii) uniform amplification of the products was rechecked by analyzing the melting curves of the amplified products (dissociation graphs), (iv) the melting temperature (Tm) was in the range 57°C–60°C, with the product Tm at least 10°C higher than the primer Tm, and v) gel electrophoresis was performed to confirm the correct size of the amplified products and the absence of nonspecific bands.
Table 1.
Primers Employed to Detect Expression of SexH Receptors
| FSH-R | |
| Forward | tcaacggaacccagctagatg |
| Reverse | gtctaaaacgactggcccagag |
| LH-R | |
| Forward | atctgtaacacaggcatccgg |
| Reverse | cgttccctggtatggtggttat |
| Prolactin receptor | |
| Forward | tgcttgctgggaagtacgg |
| Reverse | ggtgacggagatagttgggg |
| Androgen-R | |
| Forward | gactgcatgtacgcgtcgc |
| Reverse | ggcgtaacctcccttgaaagag |
| Esr1 | |
| Forward | gccaaggagactcgctactgtg |
| Reverse | tgtcaatggtgcattggtttgt |
| Esr2 | |
| Forward | taccccttggctaccgcaa |
| Reverse | gcatcaggaggttggccag |
| Progesterone-R | |
| Forward | aatggaagggcagcacaact |
| Reverse | gcggattttatcaacgatgca |
Esr1, estrogen receptor 1 alpha; Esr2, estrogen receptor 2 beta; FSH-R, follicle-stimulating hormone receptors; LH-R, luteinizing hormone/choriogonadotropin receptor.
Fluorescent staining of the sorted cells
BM-derived Lin−/Sca-1+/CD45− (VSELs) and Lin−/Sca-1+/CD45+ (HSPCs) cells were fixed in 3.5% paraformaldehyde for 20 min, permeabilized by employing 0.1% Triton X100 for 5 min, washed in PBS, preblocked with 2.5% BSA, and subsequently stained with antibodies to follicle-stimulating hormone receptor (FSH-R, 1:200, rabbit polyclonal antibody; Santa Cruz), luteinizing hormone/choriogonadotropin receptor (LH-R, 1:200, rabbit polyclonal antibody; Santa Cruz), androgen receptor (1:200, rabbit polyclonal antibody; Thermo Scientific), and estrogen receptor (1:200, mouse monoclonal IgG antibody; Thermo Scientific). Staining was performed for 1 h 15 min. at 37°C. Antibodies were diluted in 2.5% BSA in PBS. Appropriate secondary Texas Red (1:400; Vector Labs) were used (Texas Red Goat Anti-Rabbit IgG and Texas Red Horse Anti-Mouse IgG) for staining for 1 h 15 min. at 37°C. In control experiments, cells were stained with secondary antibodies only. The nuclei were labeled with DAPI. The fluorescence images were collected with a confocal laser scanning microscope, FV1000 (Olympus).
PB counts
Fifty microliters of PB was taken from the retro-orbital plexus of the mice and collected into microvette EDTA-coated tubes (Sarstedt, Inc.). Samples were run within 2 h of collection on a Hemavet 950 hematology analyzer (Drew Scientific, Inc.) as described [21].
White blood cell, platelet, and hematocrit recovery
Mice were irradiated with a sublethal dose of 650 cGy and then injected for 10 days with vehicle control (Thermo Scientific), FSH (5 IU/day), LH (5 IU/day), PRL (1 μg per mouse per day), or danazol (4 mg/kg/day; Sigma Aldrich). Blood was collected on days 0, 3, 7, 14, 21, and 28, and white blood cell (WBC), platelet, red blood cells (RBC), and hematocrit were measured on a Hemavet instrument (HV950FS; Drew Scientific Group) as described [21]. Experiments were performed twice (each time, n=5).
Statistical analysis
The data were analyzed using Student's t-test or one-way analysis of variance (ANOVA) with the Bonferroni post-hoc test. We used the GraphPad Prism 5.0 program (GraphPad Software, Inc.), and P values <0.05 were considered to indicate statistically significant differences. Data from murine HSPCs and VSELs proliferation both in vivo and in vitro after stimulation by SexHs are expressed as mean±SEM. Differences were analyzed using ANOVA (one way or multiple comparisons) as appropriate. Post hoc multiple-comparison procedures were performed using two-sided Dunnett or Dunn tests as appropriate with control samples as the control category. The significance level throughout the analyses was chosen to be 0.05. All statistical analyses were performed using the GraphPad Prism (version 6) statistical software.
Results
Murine BM-purified HSPCs and VSELs express mRNA for several pituitary and gonadal SexHs
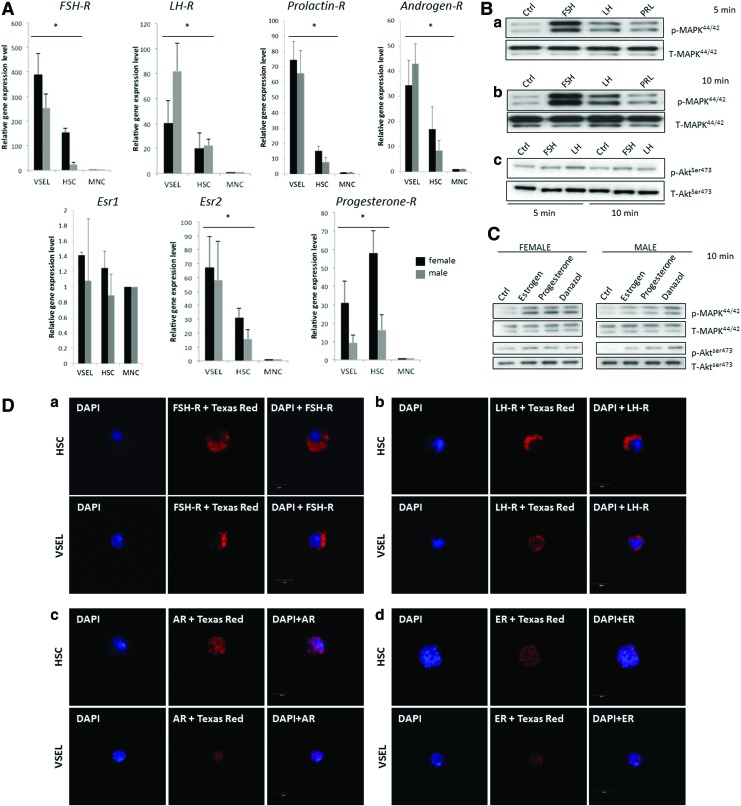
To shed more light on the role of SexHs in hematopoiesis and to address a potential developmental link between hematopoiesis and the germline, we analyzed the expression of receptors for pituitary (FSH, LH, PRL) and gonadal (androgen, estrogen, and progesterone) SexHs in Sca-1+Lin−CD45+ cells (HSPCs) as well as small Sca-1+Lin−CD45− cells (VSELs) purified from BM and compared this expression with normal BM MNCs (Fig. 1A). We observed that, except for estrogen receptor 1 alpha (Esr1), all receptors were expressed by both HSPCs and VSELs. However, there were some differences in expression between female and male mice; in particular, the progesterone receptor was expressed at much higher levels in VSELs and HSPCs of females than of males, in contrast to LH-R, which was expressed at higher levels in male VSELs. Overall, VSELs tend to express mRNA for SexHs at higher levels than HSPCs, with the exception of the progesterone receptor, which was highly expressed in female HSPCs.
FIG. 1.
Murine HSPCs and VSELs express functional SexHs receptors. (A) Real time polymerase chain reaction (RQ-PCR) results for SexHs receptor expression in purified murine HSPCs and VSELs isolated from female (black bars) and male (gray bars) mice. The relative expression level is represented as the fold difference to the value of MNCs and shown as the mean±SD from at least three independent experiments on different samples of sorted VSELs, HSPCs, and MNCs. *P<0.05. (B) The effect of pituitary SexHs on phosphorylation of p42/44 MAPK (a, b) and AKT (c) in murine BM-purified Sca-1+ cells. Cells were stimulated by SexHs for 5 min (a) or 10 min (b, c). One representative blot out of two is shown. (C) The effect of gonadal SexHs on phosphorylation of p42/44 MAPK and AKT in murine BM-purified Sca-1+ cells from ovariectomized females (left) and normal males (right). One set of representative blots out of two is shown. (D) Expression of pituitary SexHs receptors—FSH-R (a) and LH-R (b) and gonadal SexHs receptors Androgen-R (c) and Estrogen-R (d) has been detected on purified by FACS from BM murine HSPCs and VSELs by immunohistochemical staining as described in the “Materials and Methods” section. Experiment has been repeated twice on sorted cells with similar results, and representative pictures are shown. An example of control staining has been shown in Supplementary Fig. S2. BM, bone marrow; FSH-R, follicle-stimulating hormone receptors; HSPCs, hematopoietic stem progenitor cells; LH-R, luteinizing hormone/choriogonadotropin receptor; SexHs, sex hormones; VSELs, very small embryonic-like stem cells. Color images available online at www.liebertpub.com/scd
Subsequently, to test whether these receptors are functional, we sorted Sca-1+ BMMNCs using immunomagnetic beads and evaluated their responsiveness to SexH stimulation by western blot analysis of MAPKp42/44 and AKT phospohorylation (Fig. 1B). We found that all pituitary SexHs tested in our studies stimulated MAPKp42/44 phosphorylation in purified Sca-1+ BMMNCs. The strongest stimulation of MAPKp42/44 after 5 and 10 min was observed after administration of FSH, followed by LH and PRL. The activation of MAPKp42/44 was similar in male and female mice (not shown).
We also evaluated the responsiveness of sorted Sca-1+ BMMNCs using immunomagnetic beads to gonadal SexHs (Fig. 1C). Because of the short 4-day estrus cycle in mice and rapid fluctuation in the level of estrogens circulating in the blood, we employed ovariectomized females in this experiment. As shown, gonadal SexHs stimulated MAPKp42/44 both in female and in male mouse-derived Sca-1+ BMMNCs. Male mice also responded to gonadal SexH stimulation by phosphorylation of AKT.
Final evidence for the expression of SexHs receptors by murine BM-derived HSCPs and VSELs was performed by immunofluorescence staining. As shown in Fig. 1D, both FACS sorted from BM murine HSPCs and VSELs express pituitary SexHs receptors (FSH-R and LH-R) as well as receptors for intracellular gonadal SexHs (Androgen-R and Estrogen-R).
Effect of in vivo administration of SexHs on the proliferation of murine BM-residing HSPCs and VSELs
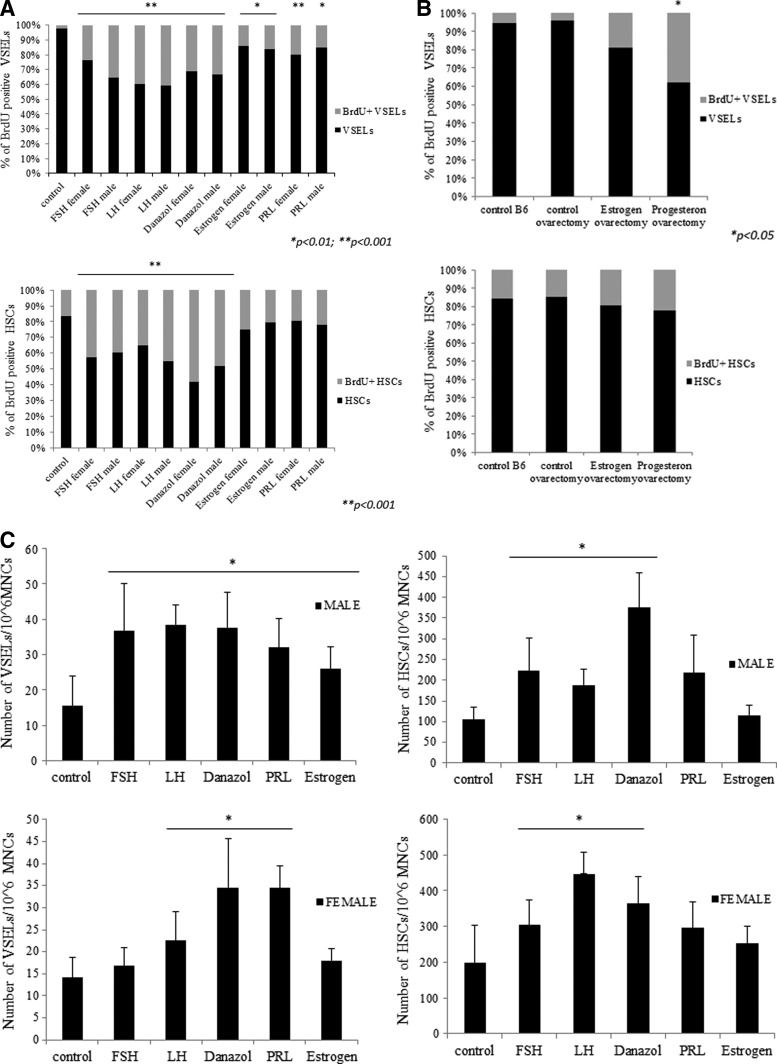
Since HSPCs and VSELs express SexHs receptors (Fig. 1A), we asked whether they respond to these hormones in vivo. This is an especially important question for BM-residing VSELs, which are reportedly a highly quiescent population of stem cells [22] but under appropriate micro-environmental conditions give rise to HSPCs [17,18]. To address this issue, female and male mice were exposed to daily s.c. injection for 10 days of pituitary (FSH, LH, PRL) and gonadal (androgen, estrogen, and danazol) SexHs. In parallel with hormone therapy, mice were administered BrdU i.p. Control mice received vehicle instead of SexHs but were also administered BrdU.
We found that 10-day administration of each of the SexHs evaluated in this experiment directly stimulated proliferation of VSELs and HSPCs in vivo, as evaluated by the percentage of cells that incorporated BrdU. Specifically, the percentage of quiescent BrdU+Sca-1+Lin−CD45− cells increased from ∼2% to ∼15%–40% (Fig. 2A). The highest response was observed for LH, FSH, and danazol for both female and male cells and for PRL for female cells. We also observed an increase in the percentage of BrdU+Sca-1+Lin−CD45+ cells from ∼20% to 25%–60%, with the highest increases after injections of danazol, LH, and FSH (Fig. 2A).
FIG. 2.
Murine HSPCs and VSELs proliferate both in vivo and in vitro after stimulation by SexHs. (A) Incorporation of BrdU into HSPCs and VSELs in murine BM (shown in gray) in response to 10-day administration of SexHs in vivo (see Materials and Methods). The percentage of cells that showed proliferative activity and incorporated BrdU is shown in light gray. The percentage of cells that did not proliferate (BrdU-negative) is shown in black. *P<0.01; **P<0.001 compared with control group. (B) Because of the short estrus cycle in mice, the experiment was repeated with ovariectomized females. The percentage of cells that showed proliferative activity and incorporated BrdU is shown in light gray. The percentage of cells that did not proliferate (BrdU-negative) is shown in black. *P<0.05 compared with control group. (C) Changes in the total number of BM VSELs and HSPCs after prolonged administration of SexHs for 10 days. Experiments were repeated thrice with similar results. *P<0.05 compared with control. BrdU, bromodeoxyuridine.
Because of the short estrus cycle, the effect of estradiol and progesterone on BrdU accumulation by BM-residing VSELs and HSPCs was evaluated in ovariectomized female mice. Figure 2B shows that progesterone enhanced BrdU incorporation, particularly in small Sca-1+Lin−CD45−VSELs. However, simultaneously, we observed a small increase in BrdU incorporation into the population of Sca1+Lin−CD45+ HSPCs.
We also evaluated whether SexHs increase the total number of both VSELs and HSPCs in BM, as measured by FACS (Fig. 2C). The total number of VSELs/106 BMMNCs increased in male BM by ∼2.5× after 10-day administration of FSH, LH, PRL, danazol, and estrogen. In female mice, the most effective SexHs were PRL, danazol, and LH. Simultaneously, we observed an increase in BM HSPCs/106 BMMNCs, with the highest stimulatory effect for danazol, LH, and FSH in both male and female mice.
The observed differences between the number of cells accumulating BrdU (Fig. 2B) and the increase in the total number of cells (Fig. 2C) most likely depends on differences in proliferation kinetics between the tested cells.
In vitro and in vivo effect of SexHs on the clonogenicity of murine CFU-GM, BFU-E, and CFU-Megs
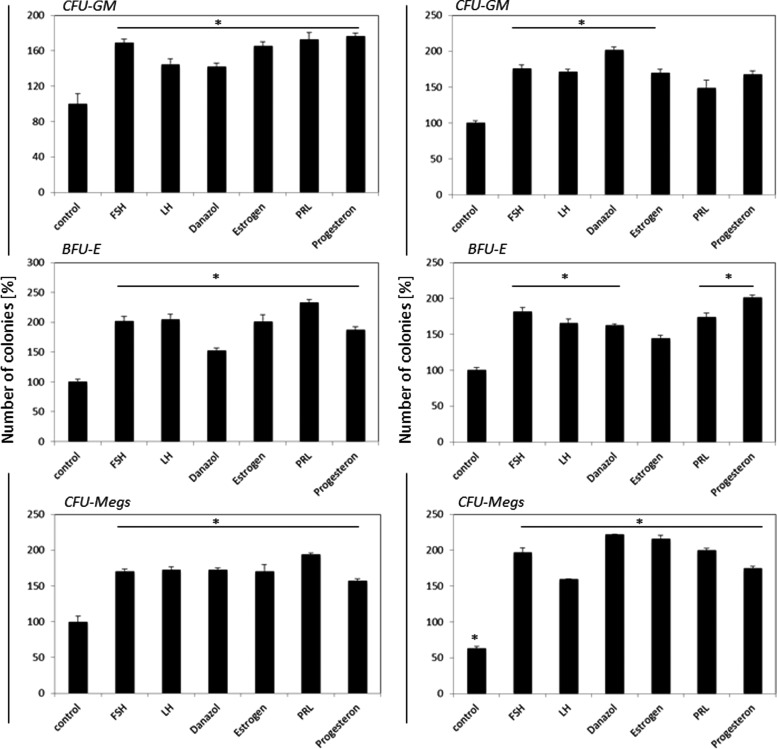
Finally, to assess the effect of SexHs on clonogenic growth of murine HSPCs, we performed in vitro clonogenic assays on Sca-1+Kit+Lin− (SKL) BMMNCs stimulated to grow CFU-GM, BFU-E, and CFU-Meg colonies in the presence or absence of SexHs. To better assess the effect of SexHs on clonogenic growth of hematopoietic progenitors, the appropriate growth factors and cytokines were employed at 1/10 of their optimal dose (Fig. 3).
FIG. 3.
SexHs co-stimulate in vitro proliferation of murine Sca-1+Kit+Lin− (SKL) cells if added with suboptimal (1/10) concentrations of colony-stimulating cytokines and growth factors. Left panels: female Sca-1+ cell—derived CFU-GM, BFU-E, and CFU-Meg colonies; right panels: male Sca-1+ cells—derived CFU-GM, BFU-E, and CFU-Meg colonies. Number of colonies formed in absence of SexH was assumed to be 100%. Data are combined from four independent experiments. *P<0.05 compared with control group. BFU-E, erythrocyte burst-forming units; CFU-GM, granulocyte-macrophage colony-forming units.
We observed that SexHs enhanced clonogenic growth of CFU-GM, BFU-E, and CFU-Meg colonies, for female- as well as for male-derived cells. Similar data we obtained for less purified Sca-1+ cells (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd).
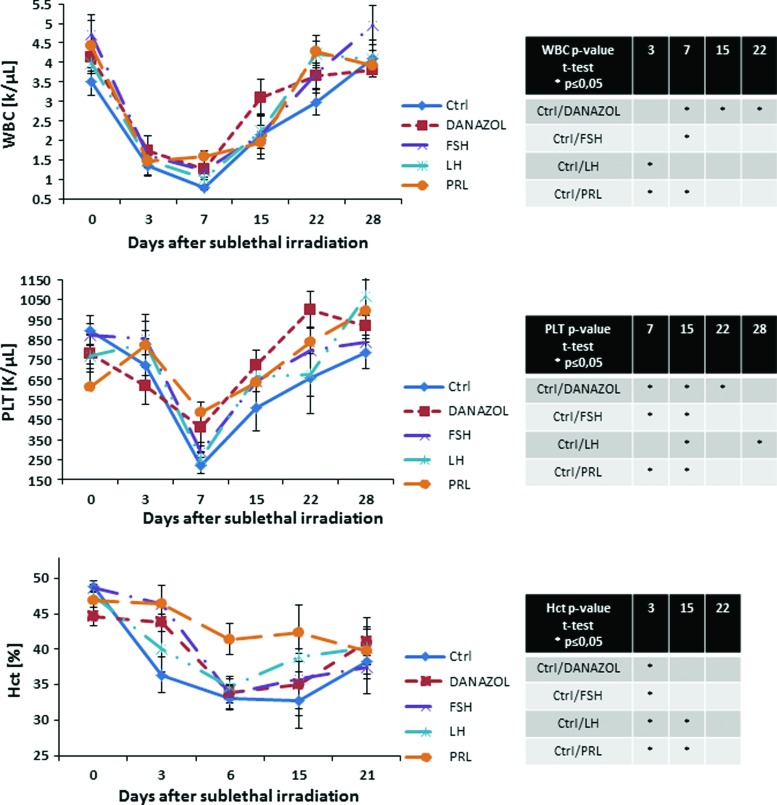
Finally, we evaluated the recovery of PB counts in mice that were sublethally irradiated and subjected to injections of SexHs (Fig. 4). We observed a particularly statistically significant increase in leucocyte counts after administration of danazol and PRL and platelets counts on day 15 after administration of all tested SexHs.
FIG. 4.
SexHs accelerate hematopoietic recovery in subletally irradiated mice. Mice were irradiated with a sublethal dose of gamma irradiation (650 cGy) and subsequently injected for 10 days with: control vehicle, FSH (5 IU), LH (5 IU), PRL (1 μg), or danazol (4 mg/kg per day). White blood cells (WBC) and platelets were counted at intervals (0, 3, 7, 15, 22, and 28 days after irradiation). Hematocrit (Hct) was measured at 0, 3, 6, 15, and 21 days after irradiation. Results are combined from two independent experiments (5 mice per group, n=10). *P<0.05—results of statistical analysis are shown in right panel tables. PRL, prolactin. Color images available online at www.liebertpub.com/scd
Discussion
The salient observation of this paper is that HSPCs express functional receptors not only for gonadal SexHs, as reported [1–3] but also, as we show here for the first time, for pituitary FSH and LH. Another important observation is that also some BM-residing cells enriched for VSELs also express functional SexHs receptors. The expression of pituitary and gonadal SexHs receptors by these cells has been demonstrated at both mRNA and protein levels. This intriguing observation somehow supports a potential developmental link between germ line, some VSELs, and HSPCs.
The development of HSPCs is still an intriguing phenomenon, because evidence indicates that the first primitive HSPCs emerge at the 7th day post coitus (d.p.c.) in extra-embryonic tissues in the yolk sac blood islands [23]. Cells that initiate primitive hematopoiesis in this location are called hemangioblasts and give rise to endothelial cells and primitive RBC that enter the circulation as nucleated erythrocytes [23]. The question of what happens with hemangioblasts after the early stages of embryogenesis is still open. Do they contribute to the wave of definitive hematopoiesis, or are they eliminated at the time when definitive hematopoiesis emerges? Interestingly, it has been reported that hemangioblasts can still be recovered from adult BM [24].
In contrast to primitive hematopoiesis, definitive hematopoiesis is initiated a few days later at the 11th d.p.c. in the hemogenic endothelium of the dorsal aorta in the so-called aorta–gonad–mesonephros (AGM) region. The origin of HSPCs in this region [25] as well as the role of the initiation of circulation in the embryo [26], the influence of shear forces [27] in the appearance of the first definitive HSPCs in the AGM, and the role of other locations of putative hemogenic endothelium, for example, the placenta vasculature [28], are still under debate.
Moreover, recent reports on the existence of developmentally early stem cells with broader specification potential in BM [5,11] pose two of the most important questions in developmental biology: What is the hierarchy within the stem cell compartment in murine BM, and are there remnants of embryonic development found in adult BM [5,29–36]?
While some investigators propose that primitive and definitive hematopoiesis are independent events in embryonic development and that a primary source of HSPCs is hemogenic endothelium in the dorsal aorta [26], parallel evidence has accumulated that HSPCs can become specified from a population of migrating PGCs during embryogenesis [5,7]. PGCs are the first stem cells to be specified as a separate population in the proximal part of the embryo proper at ∼7 d.p.c., and right after specification these cells leave the embryo proper. Accordingly, shortly before the beginning of gastrulation, they migrate into the extra-embryonic endoderm close to the yolk sac at the bottom of the allantois, where the first hemangioblasts and hematopoietic islands emerge, and subsequently return to the embryo proper and migrate into the genital ridges through the primitive streak, crossing the AGM region at around 11 d.p.c., as mentioned earlier [37]. During migration, PGCs amplify in number and change expression of some genes, so that, based on their molecular signature, we can distinguish early-migrating, migrating, and post-migrating PGCs. These latter cells are the precursors of gametes. This migratory route of PGCs and the appearance of primitive and definitive hematopoiesis raise the possibility that HSPCs could be derived from early PGCs (hemangioblasts in the yolk sac) or late-migratory PGCs (in hemogenic endothelium in the AGM region). In fact, it has been demonstrated in some species that migrating PGCs may go astray during their migration to the developing gonads and settle in other tissues [38].
In support of the intriguing concept of a developmental link between PGCs and HSPCs, it has been demonstrated that murine PGCs isolated from embryos [7], stem cells isolated from murine testes [8], and teratocarcinoma cell lines [39] can be specified into HSPCs. We have also recently reported that functional EpoR is expressed on murine and human teratocarcinoma and gonadal tumor cell lines [16].
VSELs are small, quiescent, Sca-1+Lin−CD45− stem cells identified in adult murine BM and as CD133+Lin−CD45− cells in human BM and umbilical cord blood [40,41]. These cells sorted based on surface markers and small size seem to be still somehow heterogeneous as revealed by gene expression analysis performed on single sorted murine VSELs [42]. However, what is important, they show multilineage differentiation potential into all three germ layers [34–36,40] and, under appropriate co-culture conditions with OP-9 stromal cells, some of them can be specified into HSPCs [17,18]. They express embryonic stem cell markers such as Oct-4 and Nanog in their nuclei, and some murine VSELs express several markers, including Stella, Prdm14, Fragilis, Blimp1, Nanos3, and Dnd1, which are shared with migratory PGCs [11]. As mentioned earlier, cDNA studies performed on single sorted VSELs [42] revealed differences in Stella expression among these cells, and further studies are needed to better address the developmental origin of these cells. Populations sorted from murine BM VSELs also reportedly express EpoR [16] and highly express the hemangioblast marker, Flk-1 receptor. Based on these observations, we have proposed a potential developmental link between PGCs, hemangioblasts, VSELs, and HSPCs [11].
Our data presented in this paper support the challenging, alternative concept that at least some VSELs and HSPCs can be specified during development from epiblast/migrating PGCs and that VSELs are the most primitive precursors of HSPCs in BM. Moreover, in spite of the expression of pluripotent stem cell markers, changes in the epigenetic signature of imprinted genes (eg, by erasure of imprinting at the Igf-2–H19 locus) in VSELs are involved in the resistance of these cells to Igf-1/Igf-2 signaling, which keeps these cells quiescent in adult tissues and prevents teratoma formation [22]. In fact, we have reported that VSELs correspond to long-term repopulating hematopoietic stem cells (LT-HSCs), which, similar to PGCs, express a maternal type of imprinting (erasure) at the Igf-2–H19 locus [22,43], and such epigenetic changes in LT-HSCs were recently confirmed by another group [44].
While there have been a few reports indicating a responsiveness of HSPCs to gonadal SexHs and PRL [1–4], here we demonstrate that HSPCs respond similar to VSELs to pituitary-secreted FSH and LH. This is an interesting observation, taking into consideration that the serum level of FSH increases with age at a time when the function of the gonads decreases. Thus, one could speculate that the increase in FSH may contribute to development of leukemia in older patients. While this possibility requires further study, our preliminary data indicate that human leukemic cells lines express functional SexHs receptors (manuscript in preparation).
In conclusion, our data support the concept of a potential developmental link between the germline and hematopoiesis, and the presence of VSELs in adult BM and the responsiveness of HSPCs to SexHs lend support to this hypothesis. Further studies are needed to see whether it is a common feature of all small cells that are sorted as VSELs population, or, for example, only their subset that expresses PGCs marker Stella [42]. Moreover, another silent observation is the positive effect of SexHs in sublethally irradiated mice demonstrated in this study, which opens up the new possibility that, besides androgens, some other SexHs could be employed in certain situations to stimulate hematopoietic recovery in the clinic [45].
Supplementary Material
Acknowledgments
This work was supported by NIH grants 2R01 DK074720, R01HL112788, the Stella and Henry Endowment, and Maestro grant 2011/02/A/NZ4/00035 to MZR and UK COBRE Early Career Program (P20 GM103527) and NIH grant R56 HL124266 to AL.
Author Disclosure Statement
University of Louisville as owner of VSELs patent and some areas of VSELs technology are licensed to Neostem, Inc. None of the authors have any stocks in Neostem or other biotechnological stem cell companies.
References
- 1.Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR. and Morrison SJ. (2014). Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature 505:555–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C. and Kovats S. (2008). Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol 180:727–738 [DOI] [PubMed] [Google Scholar]
- 3.Maggio M, Snyder PJ, Ceda GP, Milaneschi Y, Luci M, Cattabiani C, Masoni S, Vignali A, Volpi R, et al. (2013). Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology 1:24–28 [DOI] [PubMed] [Google Scholar]
- 4.Selleri C, Catalano L, De Rosa G, Fontana R, Notaro R. and Rotoli B. (1991). Danazol: in vitro effects on human hemopoiesis and in vivo activity in hypoplastic and myelodysplastic disorders. Eur J Haematol 47:197–203 [DOI] [PubMed] [Google Scholar]
- 5.Suszynska M, Zuba-Surma EK, Maj M, Mierzejewska K, Ratajczak J, Kucia M. and Ratajczak MZ. (2014). The proper criteria for identification and sorting of very small embryonic-like stem cells and some nomenclature issues. Stem Cells Dev 23:702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ratajczak MZ, Zuba-Surma E, Wojakowski W, Suszynska M, Mierzejewska K, Liu R, Ratajczak J, Shin DM. and Kucia M. (2014). Very small embryonic-like stem cells (VSELs) represent a real challenge in stem cell biology: recent pros and cons in the midst of a lively debate. Leukemia 28:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich IN. (1995). Primordial germ cells are capable of producing cells of the hematopoietic system in vitro. Blood 86:463–472 [PubMed] [Google Scholar]
- 8.Yoshimoto M, Heike T, Chang H, Kanatsu-Shinohara M, Baba S, Varnau JT, Shinohara T, Yoder MC. and Nakahata T. (2009). Bone marrow engraftment but limited expansion of hematopoietic cells from multipotent germline stem cells derived from neonatal mouse testis. Exp Hematol 37:1400–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohtaka T, Matsui Y. and Obinata M. (1999). Hematopoietic development of primordial germ cell-derived mouse embryonic germ cells in culture. Biochem Biophys Res Commun 260:475–482 [DOI] [PubMed] [Google Scholar]
- 10.Kritzenberger M. and Wrobel KH. (2004). Histochemical in situ identification of bovine embryonic blood cells reveals differences to the adult haematopoietic system and suggests a close relationship between haematopoietic stem cells and primordial germ cells. Histochem Cell Biol 121:273–289 [DOI] [PubMed] [Google Scholar]
- 11.Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M. and Ratajczak MZ. (2010). Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia 24:1450–1461 [DOI] [PubMed] [Google Scholar]
- 12.Woodruff K, Wang N, May W, Adrone E, Denny C. and Feig SA. (1995). The clonal nature of mediastinal germ cell tumors and acute myelogenous leukemia. A case report and review of the literature. Cancer Genet Cytogenet 79:25–31 [DOI] [PubMed] [Google Scholar]
- 13.Chaganti RS, Ladanyi M, Samaniego F, Offit K, Reuter VE, Jhanwar SC. and Bosl GJ. (1989). Leukemic differentiation of a mediastinal germ cell tumor. Genes Chromosomes Cancer 1:83–87 [DOI] [PubMed] [Google Scholar]
- 14.Nichols CR, Hoffman R, Einhorn LH, Williams SD, Wheeler LA. and Garnick MB. (1985). Hematologic malignancies associated with primary mediastinal germ-cell tumors. Ann Intern Med 102:603–609 [DOI] [PubMed] [Google Scholar]
- 15.De Miguel MP, Arnalich Montiel F, Lopez Iglesias P, Blazquez Martinez A. and Nistal M. (2009). Epiblast-derived stem cells in embryonic and adult tissues. Int J Dev Biol 53:1529–1540 [DOI] [PubMed] [Google Scholar]
- 16.Suszynska M, Poniewierska-Baran A, Gunjal P, Ratajczak J, Marycz K, Kakar SS, Kucia M. and Ratajczak MZ. (2014). Expression of the erythropoietin receptor by germline-derived cells—further support for a potential developmental link between the germline and hematopoiesis. J Ovarian Res 7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratajczak J, Wysoczynski M, Zuba-Surma E, Wan W, Kucia M, Yoder MC. and Ratajczak MZ. (2011). Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol 39:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratajczak J, Zuba-Surma E, Klich I, Liu R, Wysoczynski M, Greco N, Kucia M, Laughlin MJ. and Ratajczak MZ. (2011). Hematopoietic differentiation of umbilical cord blood-derived very small embryonic/epiblast-like stem cells. Leukemia 25:1278–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuba-Surma EK. and Ratajczak MZ. (2010). Overview of very small embryonic-like stem cells (VSELs) and methodology of their identification and isolation by flow cytometric methods. Curr Protoc Cytom 9:9.29. [DOI] [PubMed] [Google Scholar]
- 20.Grymula K, Tarnowski M, Piotrowska K, Suszynska M, Mierzejewska K, Borkowska S, Fiedorowicz K, Kucia M. and Ratajczak MZ. (2014). Evidence that the population of quiescent bone marrow-residing very small embryonic/epiblast-like stem cells (VSELs) expands in response to neurotoxic treatment. J Cell Mol Med 18:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borkowska S, Suszynska M, Mierzejewska K, Ismail A, Budkowska M, Salata D, Dolegowska B, Kucia M, Ratajczak J. and Ratajczak MZ. (2014). Novel evidence that crosstalk between the complement, coagulation and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs). Leukemia 28:2148–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DM, Zuba-Surma EK, Wu W, Ratajczak J, Wysoczynski M, Ratajczak MZ. and Kucia M. (2009). Novel epigenetic mechanisms that control pluripotency and quiescence of adult bone marrow-derived Oct4(+) very small embryonic-like stem cells. Leukemia 23:2042–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palis J. (2014). Primitive and definitive erythropoiesis in mammals. Front Physiol 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guthrie SM, Curtis LM, Mames RN, Simon GG, Grant MB. and Scott EW. (2005). The nitric oxide pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood 105:1916–1922 [DOI] [PubMed] [Google Scholar]
- 25.Ivanovs A, Rybtsov S, Welch L, Anderson RA, Turner ML. and Medvinsky A. (2011). Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med 208:2417–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J. and Yoder MC. (2008). All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 111:3435–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, et al. (2009). Biomechanical forces promote embryonic haematopoiesis. Nature 459:1131–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee LK, Ueno M, Van Handel B. and Mikkola HK. (2010). Placenta as a newly identified source of hematopoietic stem cells. Curr Opin Hematol 17:313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R, Gromoll J. and Engel W. (2006). Derivation of male germ cells from bone marrow stem cells. Lab Invest 86:654–663 [DOI] [PubMed] [Google Scholar]
- 30.Bhartiya D, Shaikh A, Nagvenkar P, Kasiviswanathan S, Pethe P, Pawani H, Mohanty S, Rao SG, Zaveri K. and Hinduja I. (2012). Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev 21:1–6 [DOI] [PubMed] [Google Scholar]
- 31.McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R. and Denner L. (2005). Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif 38:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M. and Verfaillie CM. (2002). Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle and brain. Exp Hematol 30:896–904 [DOI] [PubMed] [Google Scholar]
- 33.D'Ippolito G, Diabira S, Howard GA, Menei P, Roos BA. and Schiller PC. (2004). Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci 117:2971–2981 [DOI] [PubMed] [Google Scholar]
- 34.Havens AM, Shiozawa Y, Jung Y, Sun H, Wang J, McGee S, Mishra A, Taichman LS, Danciu T, et al. (2013). Human very small embryonic-like cells generate skeletal structures, in vivo. Stem Cells Dev 22:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kassmer SH, Jin H, Zhang PX, Bruscia EM, Heydari K, Lee JH, Kim CF, Kassmer SH. and Krouse D. (2013). Very small embryonic-like stem cells from the murine bone marrow differentiate into epithelial cells of the lung. Stem Cells 31:2759–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Havens AM, Sun H, Shiozawa Y, Jung Y, Wang J, Mishra A, Jiang Y, O'Neill DW, Krebsbach PH, Rodgerson DO. and Taichman RS. (2014). Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells Dev 23:689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leitch HG, Tang WW. and Surani MA. (2013). Primordial germ-cell development and epigenetic reprogramming in mammals. Curr Top Dev Biol 104:149–187 [DOI] [PubMed] [Google Scholar]
- 38.Jordan HE. (1917). The history of the primordial germ cells in the loggerhead turtle embryo. Proc Natl Acad Sci U S A 3:271–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miwa Y, Atsumi T, Imai N. and Ikawa Y. (1991). Primitive erythropoiesis of mouse teratocarcinoma stem cells PCC3/A/1 in serum-free medium. Development 111:543–549 [DOI] [PubMed] [Google Scholar]
- 40.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J. and Ratajczak MZ. (2006). A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia 20:857–869 [DOI] [PubMed] [Google Scholar]
- 41.Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B. and Ratajczak MZ. (2007). Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia 21:297–303 [DOI] [PubMed] [Google Scholar]
- 42.Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ. and Kucia M. (2012). Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev 21:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratajczak MZ, Shin DM, Schneider G, Ratajczak J. and Kucia M. (2013). Parental imprinting regulates insulin-like growth factor signaling: a Rosetta Stone for understanding the biology of pluripotent stem cells, aging and cancerogenesis. Leukemia 27:773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venkatraman A, He XC, Thorvaldsen JL, Sugimura R, Perry JM, Tao F, Zhao M, Christenson MK, Sanchez R, et al. (2013). Maternal imprinting at the H19-Igf2 locus maintains adult haematopoietic stem cell quiescence. Nature 500:345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratajczak MZ. (2015). A novel view of the adult bone marrow stem cell hierarchy and stem cell trafficking. Leukemia [Epub ahead of print]; DOI: 10.1038/leu.2014.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.