Abstract
There is a growing body of literature focusing on the somatotropic axis and regulation of aging and longevity. Many of these reports derive data from multiple endocrine mutants, those that exhibit both elevated growth hormone (GH) and insulin-like growth factor I (IGF-1) or deficiencies in one or both of these hormones. In general, both spontaneous and genetically engineered GH and IGF-1 deficiencies have lead to small body size, delayed development of sexual maturation and age-related pathology, and life span extension. In contrast, characteristics of high circulating GH included larger body sizes, early puberty and reproductive senescence, increased cancer incidence and reduced life span when compared to wild-type animals with normal plasma hormone concentrations. This information, along with that found in multiple other species, implicates this anabolic pathway as the major regulator of longevity in animals.
Keywords: Growth hormone, IGF-1, Stress resistance, Aging
1. Introduction
It has been more than 40 years since Everitt and Cavanagh (1965) removed the pituitary gland of rats and observed fewer age-related changes in tail tendon collagen fibers and a delayed onset of proteinuria compared to intact rats. Food restriction (CR) studies of rodents which predate this work showed that the secretion of most pituitary hormones was inhibited by this experimental manipulation (Mulinos and Pomerantz, 1940; Everitt and Porter, 1976). Food restriction also appeared to delay the onset of age-related disease and extend lifespan. Melding this evidence at the time, it was postulated that there were anti-aging factors produced by the pituitary gland. Everitt et al. (1980) then showed that in rats, both hypophysectomy and CR similarly retarded collagen aging and abolished proteinuria. Hypophysectomy and CR also delayed the onset of several age-related pathologies such as total tumor incidence, hindlimb paralysis, aortic wall thickening and cardiac and kidney enlargement. Based on these studies, it was believed that pituitary hormones played a significant role in lifespan regulation. More recently, hypophysectomy performed in adult mice was also shown to significantly increase lifespan (Powers et al., 2006).
The anterior pituitary produces and secretes multiple hormones including growth hormone (GH), thyroid stimulating hormone (TSH), prolactin, follicle-stimulating hormone (FSH), luteinizing hormone (LH) and adrenocorticotropic hormone (ACTH). With regard to aging and longevity, by far the most well-studied of these hormones is GH. As such, this review will focus on the somatotropic axis in rodents as it relates to aging processes and lifespan.
The somatotropic axis consists of growth hormone, upstream hypothalamic hormones, the insulin-like growth factors (IGFs) and downstream signaling molecules. Growth hormone production and release from the anterior pituitary is controlled by two hypothalamic factors, growth hormone-releasing hormone (GHRH) and somatostatin (SS). It is the balance of these two that determines the rate of GH secretion. Plasma GH directly stimulates IGF-1 production and secretion by the liver in addition to exerting direct effects on other tissues. Local tissue production of GH or IGF-1 also occurs, suggesting the importance of autocrine and paracrine actions of these hormones. Growth hormone and IGF-1 have both somatic effects stimulating the growth of tissues and metabolic effects that play a role in protein, carbohydrate and lipid metabolism. Alterations in these interrelated pathways can thus lead to both growth retardation or tissue proliferation and a variety of metabolic disturbances.
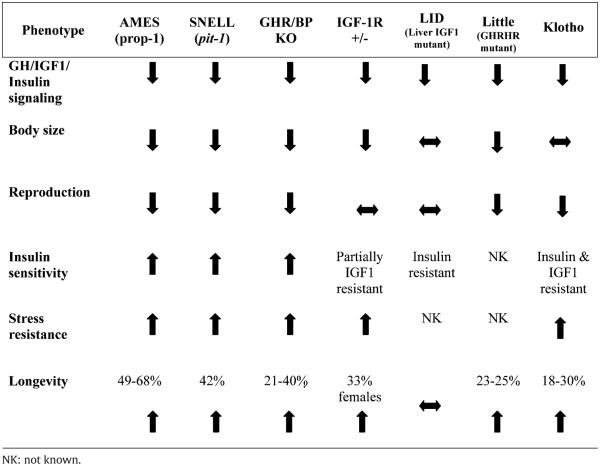
Endocrine mutants have provided remarkable insight into the role of GH and IGF-1 in aging (Table 1). Several spontaneous mutants have been discovered and genetically engineered mice created that exhibit altered GH/IGF-1 signaling. These animals have been studied to varying degrees by investigators in the aging field, and each mutant strain has provided clues that contribute to our current understanding of the role these endocrine pathways play in lifespan regulation. These animals will be reviewed in the context of physiological actions of GH and IGF-1, including growth, metabolism, reproduction, resistance to stress and lifespan regulation.
Table 1.
Phenotypic characteristics of GH/IGF-1 long-living mutant mice
|
2. GH and lifespan
To date, probably the best-characterized long-living rodent is the Ames dwarf mouse (reviewed in Bartke and Brown-Borg, 2004). Ames dwarf mice are diminutive in size, exhibit infantile facial features and delayed puberty (Schaible and Gowen, 1961; Bartke, 1964). A point mutation in the Prop-1 gene was identified and shown to be responsible for the inappropriate differentiation of specific pituitary gland cell types (somatotropes, lactotropes, thyrotropes; Bartke, 1979; Sornson et al., 1996). Dwarf mice thus lack circulating GH, prolactin and thyrotropin, resulting in the phenotype described above. Significantly, female Ames mice live 68% longer than wild-type females while male dwarfs live 49% longer than their wild-type counterparts (Brown-Borg et al., 1996). The lifespan data in Ames mice is derived from animals of a heterogenous background produced in a closed colony (>30 years; Bartke, personal communication). Phenotypically identical Snell dwarf mice also exhibit deficiencies in plasma GH, prolactin and thyrotropin concentrations and live longer than normal littermates (Bartke, 1964; Flurkey et al., 2001). A mutation in the Pit-1 gene (downstream of Prop-1) was identified as the causal factor in this type of dwarfism (Li et al., 1990). Lifespan data for the Snell dwarf is derived from animals where the dwarf gene mutation was studied on different backgrounds. Flurkey et al. (2001) reported no difference in lifespan of the dw/dw mutation on DW/J × C3H or DW/J × C3H/HeJ backgrounds (42% increase). In addition, longevity does not differ between female and male Snell dwarf mice (Flurkey et al., 2001; Vergara et al., 2004). The GH deficiency in both Ames and Snell dwarf mice results in undetectable plasma levels of IGF-1. Studies have been conducted examining hormone replacement in dwarf mice. While 11 weeks of GH and thyroxine replacement did not affect the longevity of Snell dwarfs, thyroxine administration throughout adult life did shorten lifespan significantly (Vergara et al., 2004). Moreover, hypothyroid rats (pharmacologically induced) only live a few months longer than euthyroid animals (Ooka et al., 1983). Replacing prolactin in dwarf mice shortened lifespan in one study but had no affect on longevity in another laboratory (Bartke, unpublished observations; Flurkey et al., 2001). Therefore, the deficiencies in GH and IGF-1 are proposed as the main mediators of the lifespan extension in these mice; additional evidence for this hypothesis is outlined below.
Consistent with the lack of GH and IGF-1 actions in Ames and Snell dwarf mice, the growth hormone receptor/binding protein knockout mice (GHR/BP KO; Laron dwarf) also live significantly longer than wild-type siblings (Zhou et al., 1997; Coschigano et al., 2000). The original GHR KO animals were generated on a OLA/Balb/cJ background with females living 21% longer than gender-matched wild-type mice and males living 40% longer than their counterparts (Coschigano et al., 2000). When the GHR KO mutation was expressed on a C57Bl/6 background, female and male knockout animals lived 16 and 26% longer than wild-type control mice (although the difference in females was not significant; Coschigano et al., 2003). These mice have high plasma GH but very low plasma IGF-1 levels because of the lack of GH receptor binding in target tissues. These mice are smaller than normal mice and exhibit delayed puberty. Another mutant, the ‘Little’ mouse, was first described by Eicher and Beamer (1976). These animals express a mutant GHRH receptor, resulting in low plasma GH, small body size and 23 and 25% increases in lifespan compared to female and male wild-type controls, respectively, when fed a low-fat diet (C57Bl/6 background; Flurkey et al., 2001). Studies describing attempts to knock out IGF-1 or the IGF-1 receptor report significantly reduced viability in the knockout animals along with valiant efforts to maintain live mice, therefore, no lifespan data are available (Liu et al., 1993; Powell-Braxton et al., 1996; Liu and LeRoith, 2001). However, the liver-specific IGF-1 ablated mouse (LID) exhibits serum IGF-1 deficiency (75% reduction) yet similar longevity compared to wild-type counterparts (Yakar et al., 1999). Importantly, a 50% reduction in IGF-1 receptors (IGF1R knockdown) results in 33 and 16% longer lifespans in females and males, respectively, when compared to normal littermates (difference between mutant and wild-type males was not significant; 129/Sv background; Holzenberger et al., 2003). Null animals (IGF1R knockout) were not viable. Mice that overexpress the protein Klotho on a C3H background represent yet another mouse strain with impaired IGF-1 signaling that lives longer (females 19% and males 25%) than normal mice (Kurosu et al., 2005). Finally, mice created to express a GH antagonist (GHa) that competes with endogenous GH exhibit a significant reduction in GH-induced intracellular signaling (Chen et al., 1990, 1991). These GHa animals are smaller than wild-type controls and tend to live longer than wild-type animals (especially the females) but the differences are not significant (C57Bl/6 background; Coschigano et al., 2003).
One complicating factor with the lifespan studies of mutant mice is that the mutations are expressed on a variety of background mouse strains and it is known that genetic backgrounds contribute to lifespan. The C57Bl/6 background is considered one of the longerlived strains while the 129/Sv background is a much shorter living strain. With the GHR KO mutation in particular, expression of the mutant gene on a C57Bl/6 background still results in significant longevity compared to wild-type control animals (Coschigano et al., 2003). Holzenberger et al. (2003) confirmed their findings of lifespan extension when the IGF1R mutation was expressed on a hybrid 129/Sv × C57Bl/6 background.
The evidence in rodents is overwhelming and clearly demonstrates that reductions in GH/IGF-1 signaling via reductions in plasma hormone levels, or altered hormone receptor interactions, extend lifespan. Dwarf rats expressing an antisense GH transgene have moderately suppressed GH levels and live longer than normal rats (Shimokawa et al., 2002). However, dwarf rats (dw/dw) expressing specific and limited GH and IGF-1 (40% reduction) in adulthood do not exhibit lifespan extension (Sonntag et al., 2005). Problematic is that the dw/dw mutation is thought to disturb somatotroph differentiation, resulting in an increase in prolactin accompanying the GH deficiency, a highly unusual event that could alter lifespan (Tierney and Robinson, 2002). High pituitary prolactin, in this case, may compensate for the GH deficiency as it has been shown that prolactin overexpression increases body size (Greenman et al., 1998; Byatt et al., 1993). Overall, it appears that the degree of lifespan extension is associated with both the degree of hormone deficiency and background strain, among other factors.
Several other noteworthy lines of study support the evidence that the somatotropic axis regulates of lifespan. First, mice expressing GH transgenes are characterized by high plasma GH levels, increased body weights and lengths, and lifespans half that of wild-type siblings (12 months; Steger et al., 1993). Second, overexpression of IGF-2 in smooth muscle cells of mice shortens lifespan significantly, especially in males (Zaina et al., 2003). Third, high-dose GH treatment in rats has also proved to be toxic (Groesbeck et al., 1987). Although the majority of reports indicate that GH regulates lifespan, one study showed that low to moderate doses of GH in mice either slightly increased or had no effect on lifespan (Khansari and Gustad, 1991). Human GH was administered in this study, leading to the question of specificity because human GH has both lactotrophic and somatotropic activity in rodents. Finally, significant evolutionary evidence also supports the role of these pathways in lifespan regulation in yeast, worms and flies (Fabrizio et al., 2001; Kenyon et al., 1993; Tatar et al., 2001). Reductions in GH/IGF-1 signaling lead to major lifespan extensions in these diverse species.
3. GH, growth and body size
Somatic growth is driven by components of these pathways; therefore, body size differences are obvious in many of the mutants. Those mutants with reduced signaling of this pathway are significantly smaller than wild-type controls. The range of differences is most likely related to the degree of suppression of the IGF-1 pathway. IGF-1 receptor knockdown mice are slightly smaller (8% in males) than wild-type mice while Ames and Snell dwarf mice are 66% smaller (one third the size of wild-type; Holzenberger et al., 2003; Bartke, 1964). The GHR/BP knockout mice are 40% the size of wild-type siblings as adults (Coschigano et al., 2000). Mice with a mutated GHRH receptor gene (Little) are 33% smaller than normal body size, while strains of dwarf rats are 25% (antisense GH transgenic) and 40% (dw/dw at 3 months of age) smaller than their wild-type counterparts (Eicher and Beamer, 1976; Shimokawa et al., 2002; Charlton et al., 1988). GHa mice are 61% the size of wild-type control mice (Coschigano et al., 2003). Despite a significant reduction (75–80%) in serum IGF-1 levels, LID mice do not differ in size from wild-type mice and parameters of growth and development are normal (Yakar et al., 1999). In fact, when LID mice are crossed with mice overexpressing a GH antagonist to inactivate GH, the LID × GHa mice are 44% smaller than LID mice and 30% smaller than control mice (Yakar et al., 2004). Together, these data suggest that GH is the most important mediator of postnatal growth. Moreover, the fact that small body size is significantly associated with longevity also holds true for domestic dogs and plausibly for humans (Patronek et al., 1997; Samaras et al., 2003). Further support for a relationship between small body size and longevity is derived from reports showing that mice selected for reduced body sizes live longer (Roberts, 1961). In contrast to GH or IGF-1 deficiencies, animals overexpressing GH are significantly larger (30–60%) than wild-type controls (Shea et al., 1987; Wanke et al., 1992; Rollo et al., 1999). de Magalhães et al. (2005) used Gompertz analysis to show that GH and the GH receptor statistically influence aging, while IGF1R and the insulin receptor (IR) do not. Overall, it appears that growth negatively influences lifespan in mammals (Rollo, 2002) and that GH is the major player.
In addition to reductions in body size, changes in body composition are observed in GH mutant mice. Ames mice exhibit the expected declines in lean muscle mass and bone mineral densities (BMDs) concurrent with a lower percent body fat compared to age-matched wild-type mice (Heiman et al., 2003). Less body fat may appear paradoxical in a GH-deficient state as GH is a known lipolytic factor. However, the low insulin levels in the Ames mice may counter this effect by decreasing storage of this fuel. In contrast to Ames dwarf mice, GHR/BP KO mice showed an increased percentage of body fat, yet were similar to the Ames mice in reduced total-body bone mineral density, bone mineral content and bone area in comparison to wild-type control mice (Bonkowski et al., 2006). Body composition changes have been noted in other mutants and include an elevation in the percentage of body fat in Little mice with a significant reduction in protein in comparison to normal animals, perhaps reflecting the lack of GH and IGF-1, respectively (Donahue and Beamer, 1993). Similarly, whole body fat was increased twofold in GH antagonist transgenic and LID × GHa mice over that of controls (Yakar et al., 2004), reflecting the absence of GH lipolytic activity. In GH transgenic mice, components of body composition differ between studies. Eckstein et al. (2002) demonstrated that body composition of transgenic mice is relatively normal when normalized to body weight. However, other studies report that the overexpression of GH leads to relatively lean mice with a potential resistance to diet-induced obesity (Olsson et al., 2005; Berryman et al., 2006). Evidence in GH mutant mice suggests that this hormone plays an important role in energy utilization and regulation further contributing to differences in lifespan among mutants and their wild-type counterparts.
4. GH and metabolism
Reduced insulin secretion and enhanced insulin sensitivity are hallmarks of longevity in mutant mice. Insulin sensitivity declines with age and is related to visceral fat stores in that sensitivity increases when visceral fat decreases (Barzilai et al., 1998; Barzilai and Gupta, 1999). GH is classified as a diabetogenic factor for it opposes the actions of insulin. Maintenance of blood glucose concentrations and lipid levels represent two key systems that are modulated by GH. While insulin is the only hormone that lowers blood glucose, GH is one of several factors present that serves to increase plasma glucose concentrations. GH stimulates gluconeogenesis and glycogenolysis and inhibits glucose uptake at the tissue level. These actions are apparent in GH transgenic mice. The overexpression of GH leads to hyperglycemia, hyperinsulinemia and insulin resistance in these mice (Balbis et al., 1996; Dominici et al., 1998). In contrast, the plasma of GH-deficient (Ames and Snell) and GH-resistant (Laron dwarf) mice is low in both glucose and insulin and the animals are very insulin sensitive (Borg et al., 1995; Dominici et al., 2000a,b, 2003; Hauck et al., 2001). In addition, it was shown that the enhanced insulin sensitivity in dwarf mice, in part, results from elevated liver insulin receptors in GHR/BP KO mice and elevations in IR, IRS-1 and IRS-2 (downstream effectors of IR) in Ames mice (Dominici et al., 2000a,b, 2002). Supportive evidence is found in a recent report showing that glucose utilization, gluconeogenesis and glycogenolysis are significantly decreased in Snell dwarf mice (Brooks et al., 2007). Lower pancreatic islet numbers in Ames mice lends credence to data indicating that insulin secretion is altered via significantly reduced glucose disposal following acute glucose challenge (Parsons et al., 1995; Coschigano et al., 2000). In male IGF1R knockdown mice, glucose tolerance is reduced while insulin secretion is intact (Holzenberger et al., 2003), suggesting the existence of peripheral insulin resistance. Serum insulin levels (fasting) in the GHa mice do not differ from controls (Coschigano et al., 2003). Liver-specific IGF-1 deficient mice are hyperinsulinemic and insulin resistant despite low plasma IGF-1 and normal blood glucose levels and clearance (Yakar et al., 2001; Yu et al., 2003). This insulin resistance results from GH hypersecretion as the actions of GH are inactivated in the LID × GHa animals, and these animals are insulin sensitive (Yakar et al., 2004). The Klotho transgenic mice exhibit higher blood insulin levels with normal glucose levels, along with other data suggesting that these animals are insulin resistant (Kurosu et al., 2005). Thus, IGF-1 plays a smaller role metaboli-cally, likely fine-tuning GH and insulin activities, some of which may occur via IGF-1 binding to insulin receptors and direct effects on adipose tissue. Low plasma glucose throughout life may slow aging via decreasing the accumulation and detrimental processes associated with glycation end products, slowing metabolism and reducing the associated ROS generation (Reiser, 1998; Baynes and Monnier, 1988). The effects of GH and IGF-1 on metabolism are quite apparent in mutant rodents and provide additional clues as to the role of these hormones in aging and longevity.
5. GH and reproduction
Both growth hormone and IGF-1 exert significant control over reproductive competence in rodents. Reproductive organ development and function are impaired as well as their neuroendocrine function is dysregulated when plasma GH or IGF-1 levels are abnormally low or high (extensively reviewed in Chandrashekar and Bartke, 2003). The GHR/BP knockout mice exhibit delays in sexual maturation but most animals are fertile. Puberty in Ames and Snell dwarf mice is significantly delayed. Female Ames and Snell dwarf mice are infertile while males are considered subfertile, although the degree of gonadal function is dependent upon background strain (Bartke, 2000). Mice that overexpress the Klotho gene resulting in IGF-1 resistance, also exhibit reduced fecundity (Kurosu et al., 2005). In contrast, the IGF-1-deficient LID mice are fertile, and females have normal litter sizes (8–10 pups) and appear to nurse pups normally (Yakar et al., 1999). The IGF1R heterozygous (knockdown) mice also do not differ from wild-type animals with regards to puberty and fertility although male mice have not been examined as thoroughly as females (Holzenberger et al., 2003). The fecundity of Little mice was reported by Chubb (1987). Female mice exhibit delayed sexual maturation and males are considered subfertile due to defects in sexual behavior. In dw/dw rats, fertility is also considered subnormal, with the males exhibiting small testes and impaired sperm motility (Gravance et al., 1997; Vickers et al., 1999). When plasma GH levels are significantly elevated as seen in GH transgenic mice, both sexual maturation and reproductive senescence occur earlier (Chandrashekar et al., 1988; Bartke et al., 1994; Cecim et al., 1993). In one line of GH transgenic mice (MT-bGH), fertility appears normal (Naar et al., 1991). However, in PEPCK-bGH mice, a line that produces very high levels of bovine GH, pregnancy failure is high, yet the ovulation rate is elevated compared to wild-type animals (Cecim et al., 1995; Danilovich et al., 2000). Excess bGH seems to have little effect on male fertility although senescence does occur earlier (Bartke et al., 1994). Most of the GH effects on the reproductive system are due to alterations in IGF-1 concentrations and actions (Chandrashekar et al., 2004), leading to the reproductive anomalies described in GH/IGF1 mutants. This idea is exemplified by findings in the IGF-1 null mouse. Males IGF-1 mutants exhibit reduced spermatogenesis, low testosterone and an absence of mating behavior, while females lack antral follicles and fail to ovulate, resulting in the sterility of both sexes (Baker et al., 1996; Wang et al., 2003).
6. GH and stress resistance
Growth hormone and IGF-1 also affect a more diffuse mechanism of lifespan extension, that of stress resistance. The components of this system are many and include heat shock proteins, cellular repair factors, metal chelators, phase II detoxification proteins, antioxidants and factors that prevent or suppress tumor growth. Resistance to oxidative stress has been evaluated in several GH/IGF-1 mutants, for it is one of the primary physiological effects that is strongly associated with longevity in multiple species. Accordingly, this topic will receive substantial coverage in this review.
It has been proposed that endogenously generated reactive oxygen species (ROS) cause aging via damage to DNA, proteins and lipids (Harman, 1988). The metabolic effects of GH and IGF-1 on the oxidative pathway and oxidative damage have been documented in numerous reports. GH is an anabolic factor that increases cellular metabolism. Increased metabolic activity (glucose oxidation and oxygen consumption) leads to increased oxidative phosphorylation and increased production of ROS as byproducts of metabolism. Growth hormone overexpression in mice increases superoxide radicals and oxidative damage to membrane lipids (Rollo et al., 1996). Tissues from mice with high plasma GH exhibit significantly reduced levels of antioxidative enzymes including manganese superoxide dismutase (MnSOD), copper-zinc SOD, catalase and glutathione peroxidase (GPX; Brown-Borg et al., 1999; Brown-Borg and Rakoczy, 2000; Hauck and Bartke, 2001). More directly, effects of GH and IGF-1 in vitro strongly support the in vivo data showing that these two hormones downregulate catalase, GPX and MnSOD in hepatocytes from normal mice (Brown-Borg et al., 2002).
In contrast to the suppression observed in GH excess, when a deficiency is present, enhanced antioxidative defense capacity is observed in GH/IGF-1 mutants. Numerous tissues of the Ames dwarf exhibit elevated levels of catalase, GPX and SOD (Brown-Borg et al., 1999; Brown-Borg and Rakoczy, 2000; Hauck and Bartke, 2000; Brown-Borg, unpublished data). GPX activity is preserved in muscle tissues of dwarf mice following exercise while that from wild-type mice declines with age (Romanick et al., 2004). GH replacement in dwarf mice downregulates catalase, GPX and MnSOD in both young and adult animals (Brown-Borg and Rakoczy, 2003). The thiol-containing proteins, metallothionein and glutathione (GSH) both exhibit ROS scavenging abilities, and levels of these are significantly increased in tissues of Ames dwarf mice (Meyer et al., 2003; Brown-Borg et al., 2001b). The amino acid methionine, whose metabolic pathway feeds cysteine residues into the GSH pathway, is also highly upregulated in the Ames mouse (Brown-Borg et al., 2005; Uthus and Brown-Borg, 2003, 2006). Gene expression analysis supports evidence for enhanced stress resistance in Ames dwarf mice. Several genes involved in both Phases I and II xenobiotic metabolism were found to be elevated in dwarf mice (Tsuchiya et al., 2004). These findings indicate that the Ames dwarf mouse exhibits characteristics that lead to an enhanced ability to counter genotoxic and metabolic insults.
Snell dwarf mice follow similarly with regard to antioxidative defense although the means of demonstration have been somewhat different. Skin-derived fibroblasts from Snell mice are more resistant to multiple forms of cellular stress including percent increases in LD50 values following exposure to UV light (45%), H2O2 (147%), paraquat (53%), cadmium (180%) and heat (102%) (Murakami et al., 2003). These studies indicate an overall increase in stress resistance to both oxidative and non-oxidative challenges. Further studies in Snell mice showed that enhanced antioxidative defense was not solely responsible for the resistance observed (Salmon et al., 2005). Madsen et al. (2004) challenged dwarf mice with an inhibitor of succinate dehydrogenase (mitochondrial complex II), which causes free radical generation in tissues (Coles et al., 1979; Fu et al., 1995) and presented evidence suggesting altered management of oxidative stress in the long-lived dwarfs compared to the wild-type control mice. Although antioxidative defense enzymes have not been systematically evaluated in the Snell dwarf, an overall enhancement of this system is likely responsible for increased resistance to oxidative insult as found in phenotypically identical Ames dwarf mice. Ames mice showed similar resistance to these cellular stressors (UV: 43%; H2O2: 79%; cadmium: 95%) as did GHR/BP knockout mice (UV: 194%; H2O2: 108%; paraquat: 47%; Salmon et al., 2005). The IGF1R knockdown mice are also resistant to oxidative stress (Holzenberger et al., 2003) as are mice that overexpress the protein, Klotho (Kurosu et al., 2005).
The GHR/BP knockout animals differ from Ames mice with regard to antioxidative defense. Hauck et al. (2002) showed that these dwarf animals had higher GPX activity levels in kidney tissues but levels of GPX and catalase were lower in liver tissue compared to wild-type mice. Preliminary data on tissue metallothionein levels indicates an overall increase compared to wild-type animals (similar to Ames mice; Swinscoe et al., 2006). Studies are underway in our laboratory to discern whether glutathione metabolism is similar. Minimal differences in hepatic gene expression were observed between GHR/BP knockouts and wild-type mice with the exception of a 50% increase in SOD2 in the knockouts (Al-Regaiey et al., 2005).
Mitochondrial oxidant production (liver H2O2) is significantly lower in dwarf mice, possibly indicating decreased metabolic activity in the absence of GH and thyroid hormone (Brown-Borg et al., 2001a). Isoprostanes are thought to reflect the level of oxidative stress due to lipid peroxidation (Roberts and Morrow, 2000). A recent report by Choksi et al. (2007) showed that Ames mice have lower levels of isoprostanes in both serum and liver at multiple ages, suggestive of lower oxidative stress. The reduced ROS and elevated antioxidative capacity of dwarf mice leads to lower nuclear DNA, mitochondrial DNA, and protein oxidative damage in several tissues (Brown-Borg et al., 2001a,b; Sanz et al., 2002). Functionally, Ames mice out-survive their GH sufficient counterparts following paraquat administration (systemic oxidative stressor; Bartke et al., 2000). The IGF-1 receptor knockdown mice challenged with paraquat also lived longer than mice with normal levels of IGF-1 receptors (wild-type; Holzenberger et al., 2003). In addition, mean survival following paraquat challenge was significantly reduced in the GHR/BP knockout males while female knockout and wild-type mice did not differ. Taken together, these studies suggest that low to normal plasma GH is consistent with resistance but that high levels suppress mechanisms that counter oxidative stress.
Overall, the reported studies provide significant support for the concept that GH and IGF-1 signaling pathways are intimately involved in the modulation of oxidative stress. The suppressive effect of GH on multiple components of the antioxidant system and consequent oxidative damage may be a mechanistic reason that levels of this hormone decline with aging.
GH and IGF-1 are anabolic hormones that support cell proliferation and prevent apoptosis and as such, deficiencies lead to a decreased propensity to develop tumors. Dwarf mice and rats have been shown to resist cancer development following administration of chemical carcinogen (Bielschowsky and Bielschowsky, 1961; Ramsey et al., 2002) and exhibit a reduction in growth of transplanted tumors (Rennels et al., 1965). Spontaneous tumor incidence is delayed and the severity reduced in the dwarf mice (Flurkey et al., 2001; Ikeno et al., 2003). Additionally, in IGF-1-deficient mice, tumor growth is reduced relative to control mice (Yang et al., 1996). Ames mice also exhibit greater heat shock protein levels and elevated levels of methionine sulfoxide reductase, an enzyme involved in protein repair (Brown-Borg, 2005). It has been proposed that stress resistance is coordinately upregulated (heat shock proteins, antioxidants, detoxification systems, metal chelators and repair systems) and this increase results in multi-stress resistance to different stressors (Rollo, 2002; Jazwinski, 1996; Martin et al., 1996). Indeed, studies by Murakami et al. (2003) and Salmon et al. (2005) show that cells from long-living mice are resistant to multiple forms of cellular stress.
7. Delayed and premature aging
Growth hormone and IGF-1 exert significant control over physiological processes that, in turn, affect aging. High circulating GH levels in rodents are strongly associated with signs of both premature and accelerated aging. An early onset of age-related kidney pathology is found in the form of glomerulonephritis and glomerulosclerosis in GH transgenic mice (Doi et al., 1988; Quaife et al., 1989; Wanke et al., 1992). The increased severity of this pathology along with the earlier expression in comparison to normal mice likely leads to the earlier demise of the transgenic animals. In addition, these mice develop significant mammary (those expressing human GH) and liver tumors earlier in their life in comparison to wild-type animals (Cecim et al., 1994; Bartke and Ikeno, unpublished). Relatively young mice (6–8 months of age) begin to show physical signs of aging including weight loss, scoliosis and coat deterioration. Other characteristics of aged normal animals are also found at much younger ages in GH transgenic mice including reduced hypothalamic neurotransmitter turnover, astrogliosis and an increase in age-related plasma corticosterone levels (Steger et al., 1993; Miller et al., 1995). In addition, stress-induced elevations in plasma corticosterone levels persist longer in animals with high plasma GH than in normal mice (Bartke, 2003). As mentioned previously, reproductive senescence occurs earlier in transgenic mice with infertility appearing at 5–7 months of age (Bartke et al., 1994). Declines in cognitive function have also been observed at much younger ages in GH transgenic mice (Meliska et al., 1997; Rollo et al., 1999). Finally, evidence that cells from GH transgenic mice exhibit a reduced capacity to replicate is also consistent with accelerated aging (Pendergrass et al., 1993). These data suggest that pharmacological levels of circulating GH lead to early onset of age-related pathologies in rodents. Some of the pathology observed may also be related to the overactivation of the somatotropic axis and its effects on insulin release and action as GH-transgenic mice are hyperinsulinemic and severely insulin-resistant (Quaife et al., 1989; Dominici et al., 1999a,b). The metabolic effects of high GH and insulin are costly; these include increased ROS, reduced antioxidative defense and increased oxidative damage. In addition, animals with high GH levels have been shown to partition energy differently, favoring rapid growth at the expense of reproduction and defense and repair processes (Kajiura and Rollo, 1994; Brown-Borg and Rakoczy, 2000; Hauck and Bartke, 2001). IGF-1 transgenic mice do not experience such severe pathological changes as GH transgenic mice (Doi et al., 1988), suggesting that the main effector is GH.
An abundance of evidence suggests that GH deficiency is consistent with delayed or decelerated aging in rodents. GH/IGF-1 mutant mice exhibit delays in sexual maturation, delayed tumor development, reduced tumor incidence and resistance to tumor growth. In addition, dwarf mice develop significantly less osteoarthritis than wild-type mice (Silberberg, 1972). Enhanced antioxidative defenses, lower ROS generation and less oxidative damage also contribute to delays in age-related pathology. Significant delays in the occurrence of both age-dependent collagen cross-linking and multiple indices of age-sensitive immune system status have been reported in dwarf mice (Flurkey et al., 2001). Age-dependent splenomegaly is also prevented in GH deficiency (Flurkey and Harrison, 1990). Low GH and IGF-1 signaling results in low circulating glucose and insulin levels as well as significantly enhanced insulin sensitivity. Finally, there is compelling evidence suggesting that GH deficiency is associated with maintenance of cognitive function. Ames dwarf mice do not exhibit the age-related decline in cognitive function (including memory) and behavior that is observed in wild-type control mice (Kinney et al., 2001a). Similarly, GHR/BP knockout mice show no decline in cognitive function when age-matched wild-type littermates are significantly impaired (Kinney et al., 2001b), suggesting that the absence of GH action enhances memory retention. Other than hypophysectomy, which decreases brain IGF-1 mRNA, direct evidence of brain dysfunction in GH/IGF-1-deficient animal models is lacking (Hynes et al., 1987). Correlative data are available and show that deficits in the IGF-1 axis occur in aged brain and that IGF-1 levels are associated with cognitive function (Sonntag et al., 2000; van Dam and Aleman, 2004). Enhanced neurogenesis and elevated hippocampal IGF-1 may explain the maintenance of cognitive activity in GH-deficient dwarf mice (Sun et al., 2005).
8. Conclusion
The natural age-related decline in plasma GH levels and the concomitant decrease in IGF-1 that occurs in mammals is likely a protective mechanism to decrease metabolic activity and cellular division. Elevated levels of either of these hormones throughout life contribute to the pathological changes associated with aging such as increased collagen cross-linking, osteoarthritis, immune system dysfunction, insulin resistance, oxidative damage, sensitivity to stress and cancer. There is a wealth of literature on hormones and aging in other species including nematodes and flies, with most, if not all, directly supporting that found in mammals. It has been proposed that during evolution, the common GH/IGF/insulin pathway diverged into two: one to regulate cell division and growth and the other to control metabolism and partitioning of energy resources (Guarente and Kenyon, 2000; Finch and Ruvkun, 2001). In nematodes, flies and mammals, these metabolic pathways regulate energy, reproductive activity, and stress responses so that when food is abundant, growth, sexual maturation and reproduction dominate. When food is scarce, resources are used to favor survival and directed away from growth and reproduction to increase stress resistance and repair processes leading to delayed aging and longevity. Overall, there is clearly very strong evolutionary evidence implicating the endocrine system and the somatotropic axis in particular, as a major regulator of aging and lifespan.
References
- Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor 1/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Balbis A, Bartke A, Turyn D. Overexpression of bovine growth hormone in transgenic mice is associated with changes in hepatic insulin receptors and in their kinase activity. Life Sci. 1996;59:1362–1371. doi: 10.1016/0024-3205(96)00462-6. [DOI] [PubMed] [Google Scholar]
- Bartke A. Histology of the anterior hypophysis, thyroid and gonads of two types of dwarf mice. Anat Rec. 1964;149:225–235. doi: 10.1002/ar.1091490206. [DOI] [PubMed] [Google Scholar]
- Bartke A. Genetic models in the study of anterior pituitary hormones. In: Shire JGM, editor. Genetic Variation in Hormone Systems. CRC Press; Boca Raton: 1979. [Google Scholar]
- Bartke A. Effects of growth hormone on male reproductive functions. J. Androl. 2000;21:181–188. [PubMed] [Google Scholar]
- Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H, Kinney B, Mattison J, Wright C, Hauck S, Coschigano K, Kopchick J. Growth hormone and aging. J. Am. Aging Assoc. 2000;23:219–225. doi: 10.1007/s11357-000-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg HM. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bartke A, Cecim M, Tang K, Steger RW, Chandrashekar V, Turyn D. Neuroendocrine and reproductive consequences of overexpression of growth hormone in transgenic mice. Proc. Soc. Exp. Biol. Med. 1994;206:345–359. doi: 10.3181/00379727-206-43771. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Che W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J. Clin. Invest. 1998;201:1353–1361. doi: 10.1172/JCI485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Gupta G. Revisiting the role of fat mass in the life extension induced by caloric restriction. J. Gerontol. Biol. Sci. 1999;54:B89–B96. doi: 10.1093/gerona/54.3.b89. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Monnier VM. In: The Maillard Reaction in Aging, Diabetes and Nutrition. Liss AR, editor. New York: 1988. [Google Scholar]
- Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]
- Bielschowsky F, Bielschowsky M. Carcinogenesis in the pituitary of dwarf mouse. The response to dimethylbenzanthracene applied to the skin. Br. J. Cancer. 1961;15:257–262. doi: 10.1038/bjc.1961.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J. Gerontol. Biol. Sci. 2006;61A:562–567. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- Borg KE, Brown-Borg HM, Bartke A. Assessment of the primary adrenal cortical and pancreatic hormone basal levels in relation to plasma glucose and age in the unstressed Ames dwarf mouse. Proc. Soc. Exp. Bio. Med. 1995;210:126–133. doi: 10.3181/00379727-210-43931. [DOI] [PubMed] [Google Scholar]
- Brooks NL, Trent CM, Raetzsch CF, Flurkey K, Boysen G, Perfetti MT, Jeong Y-C, Klebanov S, Patel KB, Khodush VR, Kupper LL, Carling D, Swenberg JA, Harrison DE, Combs TP. Low utilization of circulation glucose after food withdrawal in Snell dwarf mice. J. Biol. Chem. 2007;282:35069–35077. doi: 10.1074/jbc.M700484200. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Longevity in mice: Is stress resistance a common factor? Age. 2005;28:145–162. doi: 10.1007/s11357-006-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Growth hormone administration to long-living dwarf mice alters multiple components of the antioxidative defense system. Mech. Ageing Dev. 2003;124:1013–1024. doi: 10.1016/j.mad.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Johnson WT, Rakoczy SG, Kennedy MA, Romanick MA. Mitochondrial oxidant production and oxidative damage in Ames dwarf mice. J. Am. Aging Assoc. 2001a;24:85–96. doi: 10.1007/s11357-001-0012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy S, Kennedy MA, Romanick MA. Relationship between plasma growth hormone, antioxidants and oxidative damage in premature and delayed aging mice. 83rd Annual Meeting of Endocrine Society.2001b. p. 237. [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG. Catalase expression in delayed and premature aging mouse models. Exp. Gerontol. 2000;35:199–212. doi: 10.1016/s0531-5565(00)00079-6. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Bode AM, Bartke A. Antioxidative mechanisms and plasma growth hormone levels: potential relationship in the aging process. Endocrine. 1999;11:41–48. doi: 10.1385/ENDO:11:1:41. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Romanick MA, Kennedy MA. Effects of growth hormone and insulin like growth factor-1 on hepatocyte antioxidative enzymes. Exp. Biol. Med. 2002;227:94–104. doi: 10.1177/153537020222700203. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Rakoczy SG, Uthus EO. Growth hormone alters methionine and glutathione metabolism in Ames dwarf mice. Mech. Ageing Dev. 2005;126:389–398. doi: 10.1016/j.mad.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Byatt JC, Staten NR, Salsgiver WJ, Kostelc JG, Collier RJ. Stimulation of food intake and weight gain in mature female rats by bovine prolactin and bovine growth hormone. Am. J. Physiol. 1993;264:E986–E992. doi: 10.1152/ajpendo.1993.264.6.E986. [DOI] [PubMed] [Google Scholar]
- Cecim M, Bartke A, Yun JS, Wagner TE. Growth allometry of transgenic mice expressing the mouse metallothionein-I/bovine growth hormone gene. Transgenics. 1993;1:125–132. [Google Scholar]
- Cecim M, Bartke A, Yun YS, Wagner TE. Expression of human, but not bovine growth hormone genes promotes development of mammary tumors in transgenic mice. Transgenics. 1994;1:431–437. [Google Scholar]
- Cecim M, Fadden C, Kerr J, Steger R, Bartke A. Infertility in transgenic mice overexpressing the bovine growth hormone gene: disruption of the neuroendocrine control of prolactin secretion during pregnancy. Biol. Reprod. 1995;52:1187–1192. doi: 10.1095/biolreprod52.5.1187. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A, Wagner TE. Endogenous human growth hormone (GH) modulates the effect of gonadotropin-releasing hormone on pituitary function and the gonadotropin response to the negative feedback effect of testosterone in adult male transgenic mice bearing human GH gene. Endocrinology. 1988;123:2717–2722. doi: 10.1210/endo-123-6-2717. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A. The role of insulin-like growth factor-I in neuroendocrine function and the subsequent effects on sexual maturation: inferences from animal models. Reprod. Biol. 2003;3:7–28. [PubMed] [Google Scholar]
- Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol. Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060. [DOI] [PubMed] [Google Scholar]
- Charlton HM, Clark RG, Robinson IC, Goff AE, Cox BS, Bugnon C, Bloch BA. Growth hormone-deficient dwarfism in the rat: a new mutation. J. Endocrinol. 1988;119:51–58. doi: 10.1677/joe.0.1190051. [DOI] [PubMed] [Google Scholar]
- Chen WY, White ME, Wagner TE, Kopchick JJ. Functional antagonism between endogenous mouse growth hormone (GH) and a GH analog results in dwarf transgenic mice. Endocrinology. 1991;129:1402–1408. doi: 10.1210/endo-129-3-1402. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone genes suppresses growth of transgenic mice. Proc Natl Acad Sci USA. 1990;87:5061–5065. doi: 10.1073/pnas.87.13.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi KB, Roberts LJ, 2nd, DeFord JH, Rabek JP, Papaconstantinou J. Lower levels of F2-isoprostanes in serum and livers of long-lived Ames dwarf mice. Biochem. Biophys. Res. Commun. 2007;364:761–764. doi: 10.1016/j.bbrc.2007.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb C. Sexual behavior and fertility of little mice. Biol. Reprod. 1987;37:564–569. doi: 10.1095/biolreprod37.3.564. [DOI] [PubMed] [Google Scholar]
- Coles CJ, Edmonson DE, Singer TP. Inactivation of succinate-dehydrogenase by 3-nitropropionate. J. Biol. Chem. 1979;254:5161–5167. [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Danilovich NA, Bartke A, Winters TA. Ovarian follicle apoptosis in bovine growth hormone transgenic mice. Biol. Reprod. 2000;62:103–107. doi: 10.1095/biolreprod62.1.103. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Cabral JA, Magalhães D. The influence of genes on the aging process of mice: a statistical assessment of the genetics of aging. Genetics. 2005;169:265–274. doi: 10.1534/genetics.104.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Striker LJ, Quaife C, Conti FG, Palmiter R, Behringer R, Brinster R, Striker GE. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulin-like growth factor-1. Am. J. Pathol. 1988;131:398–403. [PMC free article] [PubMed] [Google Scholar]
- Dominici FP, Cifone D, Bartke A, Turyn D. Alterations in the early steps of the insulin-signaling system in skeletal muscle of GH-transgenic mice. Am. J. Physiol. 1999a;277:E447–E454. doi: 10.1152/ajpendo.1999.277.3.E447. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Cifone D, Bartke A, Turyn D. Loss of sensitivity to insulin at early events of the insulin signaling pathway in the liver of growth hormone-transgenic mice. J. Endocrinol. 1999b;161:383–392. doi: 10.1677/joe.0.1610383. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J. Endocrinol. 2000a;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Arostegui Diaz G, Kopchick JJ, Bartke A, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J. Endocrinol. 2000b;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Balbis A, Bartke A, Turyn D. Role of hyperinsulinemia on hepatic insulin binding and insulin receptor autophosphorylation in the presence of high growth hormone (GH) levels in transgenic mice expressing GH gene. J. Endocrinol. 1998;159:1–25. doi: 10.1677/joe.0.1590015. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate IRS-1 and IRS-2 in liver of Ames dwarf mice. J. Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Argentino DP, Bartke A, Turyn D. The dwarf mutation decreases high dose insulin responses in skeletal muscle, the opposite of effects in liver. Mech. Ageing Dev. 2003;124:819–827. doi: 10.1016/s0047-6374(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Donahue LR, Beamer WG. Growth hormone deficiency in ‘little’ mice results in aberrant body composition, reduced insulin-like growth factor-I and insulin-like growth factor-binding protein-3 (IGFBP-3), but does not affect IGFBP-2, -1 or -4. J. Endocrinol. 1993;136:91–104. doi: 10.1677/joe.0.1360091. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Lochmuller E-M, Koller B, Wehr U, Weusten A, RAmbeck W, Hoeflich A, Wolf E. Body composition, bone mass and microstructural analysis in GH-transgenic mice reveals that skeletal changes are specific to bone compartment and gender. Growth Horm. IGF Res. 2002;12:116–125. doi: 10.1054/ghir.2002.0272. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Beamer WG. Inherited ateliotic dwarfism in mice. Characteristics of mutation, little, on chromosome 6. J. Hered. 1976;67:87–91. doi: 10.1093/oxfordjournals.jhered.a108682. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Cavanagh LM. The ageing process in the hypophysectiomised rat. Gerontologia. 1965;11:198–207. doi: 10.1159/000211493. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Porter B. Nutrition and aging. In: Everitt AV, Burgess JA, editors. Hypothalamus, Pituitary and Aging. Charles C. Thomoas; Springfield, IL: 1976. p. 570. [Google Scholar]
- Everitt AV, Seedsman NJ, Jones F. The effects of hypophysectomy and continuous food restriction, begun at ages 70 and 400 days, on collagen aging, proteinuria, incidence of pathology and longevity in the male rat. Mech. Ageing Dev. 1980;12:161–172. doi: 10.1016/0047-6374(80)90092-5. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Finch CE, Ruvkun G. The genetics of aging. Ann. Rev. Genom. Hum. Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- Flurkey KF, Harrison DE. Use of genetic models to investigate the hypophyseal regulation of senescence. In: Harrison DE, editor. Genetic Effects on Aging II. The Telford Press; Caldwell, NJ: 1990. pp. 435–456. [Google Scholar]
- Flurkey K, Papconstantinou J, Miller RA, Harrison DA. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YT, He FS, Zhang SL, Zhang JS. Lipid-peroxidation in rats intoxicated with 3-nitropropionic acid. Toxicology. 1995;33:327–331. doi: 10.1016/0041-0101(94)00173-6. [DOI] [PubMed] [Google Scholar]
- Gravance CG, Breier BH, Vickers MH, Casey PJ. Impaired sperm characteristics in postpubertal growth-hormone-deficient dwarf (dw/dw) rats. Anim. Reprod. Sci. 1997;49:71–76. doi: 10.1016/s0378-4320(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Greenman Y, Tordjman K, Stern N. Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clin. Endocrinol. 1998;48:547–553. doi: 10.1046/j.1365-2265.1998.00403.x. [DOI] [PubMed] [Google Scholar]
- Groesbeck MD, Parlow AF, Daughaday WH. Stimulation of supranormal growth in pubertal, adult plateaued, and hypophysectomized female rats by large doses of rat growth hormone: physiological effects and adverse consequences. Endocrinology. 1987;120:1963–1975. doi: 10.1210/endo-120-5-1963. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radicals in aging. Mol. Cell. Biochem. 1988;84:155–161. doi: 10.1007/BF00421050. [DOI] [PubMed] [Google Scholar]
- Hauck SJ, Hunter WS, Danilovich N, Kopchick JJ, Bartke A. Reduced levels of thyroid hormones, insulin, and glucose, and lower body core temperature in the growth hormone receptor/binding protein knockout mouse. Exp. Biol. Med. 2001;226:552–558. doi: 10.1177/153537020122600607. [DOI] [PubMed] [Google Scholar]
- Hauck S, Bartke A. Free radical defenses in the liver and kidney of human growth hormone transgenic mice: possible mechanisms of early mortality. J. Gerontol. Biol. Sci. 2001;56A:B153–162. doi: 10.1093/gerona/56.4.b153. [DOI] [PubMed] [Google Scholar]
- Hauck S, Aaron JM, Wright C, Kopchick JJ, Bartke A. Antioxidant enzymes, free-radical damage, and response to paraquat in liver and kidney of long-living growth hormone receptor/binding protein gene-disrupted mice. Horm. Metab. Res. 2002;34:481–486. doi: 10.1055/s-2002-34787. [DOI] [PubMed] [Google Scholar]
- Hauck S, Bartke A. Effects of growth hormone on hypothalamic catalase and CuZn superoxide dismutase. Free Radic. Biol. Med. 2000;28:970–978. doi: 10.1016/s0891-5849(00)00186-6. [DOI] [PubMed] [Google Scholar]
- Heiman ML, Tinsley FC, Mattison JA, Hauck S, Bartke A. Body composition of prolactin-, growth hormone, and thyrotropin-deficient Ames dwarf mice. Endocrine. 2003;20:149–154. doi: 10.1385/ENDO:20:1-2:149. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hynes MA, van Wyk JJ, Brooks PJ, D’Ercole AJ, Jansen M, Lund PK. Growth hormone dependence of somatomedin-c/insulin-like growth factor I and insulin-like growth factor II messenger ribonucleic acids. Mol. Endocrinol. 1987;1:233–242. doi: 10.1210/mend-1-3-233. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: correlation to extended longevity. J. Gerontol. 2003;58A:291–296. doi: 10.1093/gerona/58.4.b291. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM. Longevity, genes and aging. Science. 1996;273:54. doi: 10.1126/science.273.5271.54. [DOI] [PubMed] [Google Scholar]
- Kajiura LJ, Rollo CD. A mass budget for transgenic ‘supermice’ engineered with extra rat growth hormone genes: evidence for energetic limitation. Can. J. Zool. 1994;72:1010–1017. [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Khansari DN, Gustad T. Effects of long-term, low-dose growth hormone therapy on immune function and life expectancy of mice. Mech. Ageing Dev. 1991;57:87–100. doi: 10.1016/0047-6374(91)90026-v. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm. Behav. 2001a;39:277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Coschigano KT, Kopchick JJ, Bartke A. Evidence that age-induced decline in memory retention is delayed in growth hormone resistant GH-R-KO (Laron) mice. Physiol. Behav. 2001b;72:653–660. doi: 10.1016/s0031-9384(01)00423-1. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Crenshaw III BE, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor 1 (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Liu JL, LeRoith D. Insulin-like growth factor 1 is essential for postnatal growth in response to growth hormone. Endocrinology. 2001;140:5178–5184. doi: 10.1210/endo.140.11.7151. [DOI] [PubMed] [Google Scholar]
- Madsen MA, Hsieh CC, Boylston WH, Flurkey K, Harrison D, Papaconstantinou J. Altered oxidative stress response of the long-lived Snell dwarf mouse. Biochem. Biophys. Res. Commun. 2004;318:998–1005. doi: 10.1016/j.bbrc.2004.04.126. [DOI] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat. Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Burke PA, Bartke A, Jensen RA. Inhibitory avoidance learning in transgenic mice overexpressing the growth hormone gene. Neurobiol. Learn. Mem. 1997;68:1–12. doi: 10.1006/nlme.1997.3772. [DOI] [PubMed] [Google Scholar]
- Meyer MM, Swinscoe JC, Brown-Borg HM, Carlson EC. Increased glomerular metallothionein accompanies reduced glomerular basement membrane thickening in the Ames dwarf mouse model of delayed aging. Exp. Biol. 2003 (Abstract #217.1, San Diego, CA) [Google Scholar]
- Miller DB, Bartke A, O’Callaghan JP. Increased glial fibrillary acidic protein (GFAP) levels in the brains of transgenic mice expressing the bovine growth hormone (bGH) gene. Exp Gerontol. 1995;30:383–400. doi: 10.1016/0531-5565(94)00064-a. [DOI] [PubMed] [Google Scholar]
- Mulinos MG, Pomerantz L. Psuedo-hypophysectomy, a condition resembling hypophysectomy produced by malnutrition. J. Nutr. 1940;19:493–500. [Google Scholar]
- Murakami S, Salmon A, Miller RA. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003;17:1565–1566. doi: 10.1096/fj.02-1092fje. [DOI] [PubMed] [Google Scholar]
- Naar EM, Bartke A, Majumdar SS, Buonomo FC, Yun JS, Wagner TE. Fertility of transgenic female mice expressing bovine growth hormone or human growth hormone variant genes. Biol. Reprod. 1991;45:178–187. doi: 10.1095/biolreprod45.1.178. [DOI] [PubMed] [Google Scholar]
- Olsson B, Bohlooly YM, Fitzgerald SM, Frick F, Ljungberg A, Ahren B, Tornell J, Bergstrom G, Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146:920–930. doi: 10.1210/en.2004-1232. [DOI] [PubMed] [Google Scholar]
- Ooka H, Fujita S, Yoshimoto E. Pituitary–thyroid activity and longevity in neonatally thyroxine-treated rats. Mech. Ageing Dev. 1983;22:113–120. doi: 10.1016/0047-6374(83)90104-5. [DOI] [PubMed] [Google Scholar]
- Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J. Gerontol. 1997;52A:B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Pendergrass WR, Li Y, Jiang D, Wolf NS. Decrease in cellular replicative potential in giant mice transfected with the bovine growth hormone gene correlates to shortened life span. J. Cell. Physiol. 1993;156:96–103. doi: 10.1002/jcp.1041560114. [DOI] [PubMed] [Google Scholar]
- Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett H, Stewart TA. IGF-1 is required for normal embryonic growth in mice. Genes Dev. 1996;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- Powers RW, III, Harrison DE, Flurkey K. Pituitary removal in adult mice increases life span. Mech. Ageing Dev. 2006;127:658–659. doi: 10.1016/j.mad.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Quaife CJ, Mathews LS, Pinkert CA, Hammer RE, Brinster RL, Palmiter RD. Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology. 1989;124:40–48. doi: 10.1210/endo-124-1-40. [DOI] [PubMed] [Google Scholar]
- Ramsey MM, Ingram RL, Cashion AB, Ng AH, Cline JM, Parlow AF, Sonntag WE. Growth hormone-deficient dwarf animals are resistant to dimethyl-benzanthracine (DMBA)-induced mammary carcinogenesis. Endocrinology. 2002;143:4139–4142. doi: 10.1210/en.2002-220717. [DOI] [PubMed] [Google Scholar]
- Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc. Soc. Exp. Biol. Med. 1998;218:23–37. doi: 10.3181/00379727-218-44264. [DOI] [PubMed] [Google Scholar]
- Rennels EG, Anigstein DM, Anigstein L. A cumulative study of the growth of sarcoma 180 in anterior pituitary dwarf mice. Tex. Rep. Biol. Med. 1965;23:776–781. [PubMed] [Google Scholar]
- Roberts RC. The lifetime growth and reproduction of selected strains of mice. Heredity. 1961;16:369–381. [Google Scholar]
- Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000;28:505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts life span of mammals. Evol. Dev. 2002;55:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Rollo CD, Carlson J, Sawada M. Accelerated aging of giant transgenic mice is associated with elevated free radical processes. Can. J. Zool. 1996;74:606–620. [Google Scholar]
- Rollo CD, Kajiura LJ, Wylie B, D’Souza S. The growth hormone axis, feeding, and central allocative regulation: lessons from giant transgenic growth hormone mice. Can. J. Zool. 1999;77:1861–1873. [Google Scholar]
- Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech. Ageing Dev. 2004;125:269–281. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Physiol. Endocrinol. Metab. 2005;289:E23–E29. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- Samaras T, Elrick H, Storms L. Is height related to longevity? Life Sci. 2003;72:1781–1802. doi: 10.1016/s0024-3205(02)02503-1. [DOI] [PubMed] [Google Scholar]
- Sanz A, Bartke A, Barja G. Long-lived Ames dwarf mice: oxidative damage to mitochondrial DNA in heart and brain. J. Am. Aging Assoc. 2002;25:119–122. doi: 10.1007/s11357-002-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible R, Gowen JW. A new dwarf mouse. Genetics. 1961;46:896. [Google Scholar]
- Shea BT, Hammer RE, Brinster RL. Growth allometry of the organs in giant transgenic mice. Endocrinology. 1987;121:1924–1930. doi: 10.1210/endo-121-6-1924. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am. J. Pathol. 2002;160:2259–2265. doi: 10.1016/S0002-9440(10)61173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg R. Articular aging and osteoarthrosis in dwarf mice. Pathol. Microbiol. 1972;38:417–430. doi: 10.1159/000162458. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J. Anat. 2000;197:575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriére C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Anderson B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the prophet of pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Steger RW, Bartke A, Cecim M. Premature aging in transgenic mice expressing growth hormone genes. J. Reprod. Fertil. Suppl. 1993;46:61–75. [PubMed] [Google Scholar]
- Sun LY, Evans MS, Hsieh J, Panici J, Bartke A. Increased neurogenesis in dentate gyrus of long-lived Ames dwarf mice. Endrocrinology. 2005;146:1138–1144. doi: 10.1210/en.2004-1115. [DOI] [PubMed] [Google Scholar]
- Swinscoe JC, Brown-Borg HM, Bartke A, Carlson EC. Metallothionein levels and multimeric forms in delayed and premature mouse models. Experimental Biology Annual Meeting.2006. [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant drosophila insulin receptor homolog that extends life-span and impairs neuroen-mutant that lives twicdocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tierney T, Robinson IC. Increased lactotrophs despite decreased somatotrophs in the dwarf (dw/dw) rat: a defect in the regulation of lactotroph/somatotroph cell fate? J. Endocrinol. 2002;175:435–446. doi: 10.1677/joe.0.1750435. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Dhahbi JM, Cui X, Mote PL, Bartke A, Spindler SR. Additive regulation of the hepatic gene expression by dwarfism and caloric restriction. Physiol. Genom. 2004;17:307–315. doi: 10.1152/physiolgenomics.00039.2004. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Altered methionine metabolism in long-living Ames dwarf mice. Exp. Gerontol. 2003;38:491–498. doi: 10.1016/s0531-5565(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Uthus EO, Brown-Borg HM. Methionine flux to transulfuration is enhanced in the long living Ames dwarf mouse. Mech. Ageing Dev. 2006;127:444–450. doi: 10.1016/j.mad.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur. J. Pharmacol. 2004;490:87–95. doi: 10.1016/j.ejphar.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated Snell dwarf mice regain fertility but remain long-lived and disease resistant. J. Gerontol. Biol. Sci. 2004;59A:1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH, Casey PJ, Champion ZJ, Gravance CG, Breier BH. IGF-I treatment increases motility and improves morphology of immature spermatozoa in the GH deficient dwarf (dw/dw) rat. Growth Horm. IGF Res. 1999;9:236–240. doi: 10.1054/ghir.1999.0114. [DOI] [PubMed] [Google Scholar]
- Wang GM, O’Shaughnessy PJ, Chubb C, Robaire B, Hardy MP. Effects of insulin-like growth factor I on steroidogenic enzyme expression levels in mouse Leydig cells. Endocrinology. 2003;144:4058–5064. doi: 10.1210/en.2003-0563. [DOI] [PubMed] [Google Scholar]
- Wanke R, Wolf E, Hermanns W, Folger S, Buchmuller T, Brem G. The GH-transgenic mouse as an experimental model for growth research: clinical and pathological studies. Horm. Res. 1992;3:74–87. doi: 10.1159/000182406. [DOI] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar S, Liu JL, Fernandez AM, Wu Y, Schally AV, Frystyk J, Chernausek SD, Mejia W, LeRoith D. Liver-specific IGF-1 gene deletion leads to muscle insulin insensitivity. Diabetes. 2001;50:1110–1118. doi: 10.2337/diabetes.50.5.1110. [DOI] [PubMed] [Google Scholar]
- Yakar S, Setser J, Zhao H, Stannard B, Haluzik M, Glatt V, Bouxsein ML, Kopchick JJ, LeRoith D. Inhibition of growth hormone action improves insulin sensitivity in liver IGF-1-deficient mice. J. Clin. Invest. 2004;113:96–105. doi: 10.1172/JCI200417763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Beamer W, Huynh HT, Pollak M. Reduced growth of human breast cancer xenografts in host homozygous for the ‘lit’ mutation. Cancer Res. 1996;56:1509–1511. [PubMed] [Google Scholar]
- Yu R, Yakar S, Liu YL, Lu Y, LeRoith D, Miao D, Liu JL. Liver-specific IGF-I gene deficient mice exhibit accelerated diabetes in response to streptozotocin, associated with early onset of insulin resistance. Mol. Cell. Endocrinol. 2003;204:31–42. doi: 10.1016/s0303-7207(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Zaina S, Pettersson L, Thomsen AB, Chai C-M, Qi Z, Thyberg J, Nilsson J. Shortened life span, bradycardia, and hypotension in mice with targeted expression of an Igf2 transgene in smooth muscle cells. Endocrinology. 2003;144:2695–2703. doi: 10.1210/en.2002-220944. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Wagner TE, Cataldo LA, Coschigano K, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (The Laron mouse) Proc. Natl. Acad. Sci. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]


