Abstract
Objectives
The study objective was to determine the prognostic significance of serum CA-125 levels in patients with grade 1 serous ovarian carcinoma (SOC) enrolled in a Phase III study.
Methods
An ancillary analysis of a phase III study of women with advanced epithelial ovarian cancer treated with carboplatin/paclitaxel versus triplet or sequential doublet regimens. Grade 1 SOC was used as a surrogate for low-grade serous carcinoma.
Results
Among 3686 enrolled patients, 184 (5%) had grade 1 disease and CA-125 levels available. For those with grade 1 SOC, the median patient age was 56.5; 87.3% had Stage III disease. Median follow-up was 102 months and there was no difference in pre-chemotherapy CA-125 by treatment arm (P = 0.91). Median pretreatment CA-125 for those with grade 1 SOC was lower (119.1) than for patients with grade 2–3 SOC (246.7; P < 0.001). In those with grade 1, pretreatment CA-125 was not prognostic of outcome. However, patients with CA-125 levels that normalized after cycle 1, 2 or 3 were 60–64% less likely to experience disease progression as compared to those who never normalized or normalized after 4 cycles (P ≤ 0.024). Normalization of CA-125 levels before the second cycle was negatively associated with death, with a HR of 0.45 (P = 0.025).
Conclusions
Pretreatment CA-125 level was significantly lower in women with grade 1 SOC compared to those with high-grade SOC. While pretreatment CA-125 was not associated with survival, serial CA-125 measurements during chemotherapy treatment were prognostic, with normalization before the second chemotherapy cycle asso ciated with a decreased risk of death.
Keywords: Ovarian cancer, Low grade serous carcinoma, CA-125
Introduction
CA-125 (cancer antigen 125) is a protein encoded by the MUC16 gene in humans and is the serum tumor marker most closely associated with epithelial ovarian cancer (EOC) [1]. Originally described by Bast et al. in 1981, it is an antigenic determinant on a high molecular weight glycoprotein found on the epithelial surface of reproductive tract organs and the periotoneum and is recognized by the murine monoclonal antibody OC-125 [1,2]. Elevations in serum CA-125 values >35 U/mL have been documented in greater than 85% of women diagnosed with ovarian cancer [3,4], especially in those with advanced stage disease.
In an ancillary analysis of seven Phase III Gynecologic Oncology Group (GOG) trials, pretreatment CA-125 level was found to be an independent predictor of progression-free survival (PFS) in patients with advanced EOC treated with a standard chemotherapy regimen, particularly in the serous tumor subtype [5–12]. While the utility and prognostic value of CA-125 are well known in those with high-grade serous disease, it has not been well documented for women with lower-grade serous tumors [15]. Recent studies suggest a two-tiered classification for serous tumors into low-grade (which account for 10% of all EOCs) and high-grade serous carcinoma, based upon molecular and pathologic differences [13,15]. Historically, women with grade 1 serous tumors have been included in Phase III GOG studies along with those diagnosed with higher grade tumors. However, the grade 1 tumor cohort has constituted a small proportion of the total study subjects enrolled in these trials. In order to develop a better understanding of the regression rates and prognostic value of CA-125 in those with grade 1 serous carcinoma treated with platinum/taxane therapy, an ancillary analysis of a cooperative group, Phase III trial was conducted. Specifically, the study purpose was to determine the prognostic significance of pre- and post-treatment serum CA-125 levels, with a secondary aim of compare CA-125 levels between those with grade 1 to higher grade serous ovarian carcinoma.
Methods
This was an ancillary data analysis of GOG-182, a multi-center, phase III study of EOC patients with optimal (maximal diameter of residual disease <1.0 cm) and suboptimal (>1.0 cm) residual disease treated with carboplatin/paclitaxel alone or in combination with triplet or sequential doublet regimens [16]. All women received the backbone of intravenous carboplatin/paclitaxel with the addition of pegylated liposomal doxorubicin, topotecan or gemcitabine. Each arm included 8 cycles of triplet or sequential-doublet chemotherapy, which provided a minimum of 4 cycles that incorporated experimental treatments while maintaining at least 4 cycles with carboplatin and paclitaxel. For the current investigation, women diagnosed with grade 1 serous carcinoma (e.g., low-grade serous carcinoma, or LGSC) were the primary focus. However, those with grade 2/3 serous tumors (utilized as a surrogate for high-grade serous carcinoma (HGSC)) were also studied in select comparative demographic and survival analyses. Central pathology review of all tumors studied in the current analysis had been performed by the GOG Pathology Committee (of note, this committee reviewed the pathology of all study subjects enrolled from the U.S., which represented approximately 85% of all trial subjects). Prechemotherapy cycle serum CA-125 levels (units/mL), demographic, clinical and surgical factors were evaluated for their effect on PFS and overall survival (OS) outcomes. Specifically, in GOG-182, serum CA-125 values were drawn from patients within two weeks of initiating chemotherapy, prior to every three-week treatment cycle and post-treatment. For purposes of this analysis, the commonly accepted definition of normal CA-125 ≤35 U/mL was employed.
Residual disease status after primary cytoreductive surgery was defined as optimal microscopic residual (no gross residual disease), optimal (0.1–1.0 cm maximal diameter residual disease), or suboptimal (>1.0 cm maximal diameter residual disease). Because of small counts in some of its categories, race was collapsed into the categories White (N = 172), Black (N = 9), and other (N = 8). Categorical variables were evaluated by the Pearson chi-square test [17] and continuous variables by the Wilcoxon–Mann–Whitney test [18] or the Kruskal–Wallis test [19]. Kaplan–Meier survival curves [20] stratified by residual-disease status, tumor grade, and other clinicopathologic factors were calculated, then compared using the log-rank test [21]. The Cox proportional hazards model [22] was used to evaluate independent prognostic factors (identified from previous GOG studies) and to estimate their covariate-adjusted effects on PFS and OS. All statistical tests were two-tailed with the significance level set at α = 0.05. Statistical analyses were performed using the R programming language and environment [23].
Landmark analysis is a kind of survival analysis that classifies patients according to some intermediate, non-outcome event that is nevertheless a response to treatment. In a landmark analysis, the starting point for measuring survival is moved from a patient's study entry to some later time when the event of interest has been observed in most patients. Patients that have experienced the outcome (i.e. have not survived) before the new landmark point are excluded from analysis, and the event of interest is evaluated at the landmark point; the result is the establishment of a new baseline time before which the event most likely occurs and after which the patient must survive. This procedure reduces the bias inherent in comparing the survival of patient subgroups defined by a treatment response that happens after study entry. To reduce bias resulting from subgroups based on CA-125 normalization throughout treatment, our model to evaluate the effect of that normalization on survival only considered patients who had completed all 8 cycles of chemotherapy (N = 156).
Results
Among the original 3686 eligible patients enrolled in GOG-182, 189 had grade 1 serous carcinoma; 184 patients had baseline CA-125 levels available for analysis. The median patient age of the low-grade cohort was 56.5; 87.3% had Stage III disease and 12.7% had Stage IV disease (Table 1). With respect to residual disease status after primary cytoreductive surgery, 24.9% had microscopic residual, 51.3% had 0.1–1.0 cm and 23.8% had >1 cm residual disease remaining. When compared to the higher-grade cohort, those with grade 1 disease were younger, had a higher BMI and were less likely to have ascites at diagnosis (Table 1; all P < 0.001).
Table 1.
Low grade (LGSC: grade 1) versus high grade serous carcinoma (HGSC: grade 2/3) patient characteristics (GOG-182).
| Variable | N | LGSC N = 189 | HGSC N =1763 | Test statistic |
|---|---|---|---|---|
| Ageyears (IQR) | 1952 | 56.5 (46.6-64.3) | 59.3 (51.6-67.3) | P < 0.0011 |
| Race/ethnicity | 1952 | P = 0.7312 | ||
| White | 91.0% (172) | 90.5% (1596) | ||
| Black | 4.8% (9) | 4.1% (72) | ||
| Other | 4.2% (8) | 5.4% (95) | ||
| BMIkg/m2 (IQR) | 1871 | 26.6 (23.4-30.3) | 25.2 (22.2-29.6) | P = 0.0071 |
| Performance status | 1952 | P = 0.4442 | ||
| Normal, asymptomatic | 49.2% (93) | 48.8% (860) | ||
| Symptomatic, ambulatory | 46.0% (87) | 44.0% (776) | ||
| Symptomatic, in bed | 4.8% (9) | 7.2% (127) | ||
| FIGO stage | 1952 | P = 0.2842 | ||
| III | 87.3% (165) | 84.3% (1487) | ||
| IV | 12.7% (24) | 15.7% (276) | ||
| Cytoreductive status | 1952 | P = 0.1062 | ||
| Microscopic | 24.9% (47) | 20.3% (358) | ||
| Optimal (0.1-1 cm) | 51.3% (97) | 49.1% (866) | ||
| Suboptimal (> 1 cm) | 23.8% (45) | 30.6% (539) | ||
| Baseline CA-125 U/mL (IQR) | 1882 | 119.1 (51.8-323.9) | 246.7 (101.8-719.8) | P < 0.0011 |
| Baseline CA-125 U/mL | 1882 | P < 0.0012 | ||
| <35.1 | 15.2% (28) | 6.8% (116) | ||
| 35.1-70.0 | 20.1% (37) | 8.9% (151) | ||
| ≥70.0 | 64.7% (119) | 84.3% (1431) | ||
| Ascites | 1905 | P < 0.0012 | ||
| No | 36.9% (69) | 24.3% (418) | ||
| Yes | 63.1% (118) | 75.7% (1300) |
N is the number of non-missing values. Median values are given for continuous variables, followed by their interquartile range (IQR). Numbers after percents are frequencies.
Tests used
Wilcoxon test
Pearson test.
Although CA-125 levels were initially elevated in the majority of patients with grade 1 SOC, overall those with grade 1 disease had a significantly lower median pretreatment CA-125 value (119.1) than those with high-grade disease (246.7; P < 0.001). Significantly fewer patients with grade 1 SOC (84.8%) had pretreatment CA-125 values above normal (>35 U/mL) than those with higher grade SOC (93.2%) (Table 1; P < 0.001). Some variation in the interval between surgery and chemo (median 25 days, IQR 18–32 days) existed; however, this did not impact survival for those with grade 1 SOC. Median follow up was 102 months. There was no difference in pretreatment CA-125 levels or outcomes among grade 1 cohorts treated with platinum/taxane doublet versus triplet combinations. When comparing grade 1 to the higher grade cohort, there were no differences in overall best response to chemotherapy or in recurrence outcomes (Table 2). However, significantly more women with grade 1 SOC were alive at the end of the study period than those with higher-grade serous disease (30.7% vs. 22.5%, P = 0.01).
Table 2.
Low grade (LGSC) versus high grade serous carcinoma (HGSC) patient outcomes (GOG-0182).
| N | LGSC N = 189 | HGSC N =1763 | Test statistic | |
|---|---|---|---|---|
| Best response to therapy | 1947 | P = 0.191 | ||
| Stable disease | 7.4% (14) | 7.3% (128) | ||
| Partial response | 6.3% (12) | 9.0% (159) | ||
| Complete response | 2.1% (4) | 4.4% (77) | ||
| Increased disease | 0.0% (0) | 0.5% (9) | ||
| Non-measurable, N/A | 84.1% (159) | 77.7%(1366) | ||
| Not evaluable | 0.0% (0) | 1.1% (19) | ||
| Recurrence | 1952 | P = 0.216 | ||
| No | 13.2% (25) | 16.7% (295) | ||
| Yes | 86.8% (164) | 83.3% (1468) | ||
| Survival | 1952 | P = 0.012 | ||
| Censored | 30.7% (58) | 22.5% (397) | ||
| Death | 69.3% (131) | 77.5% (1366) |
N is the number of non-missing values.
Numbers after percents are frequencies.
Test used: Pearson test.
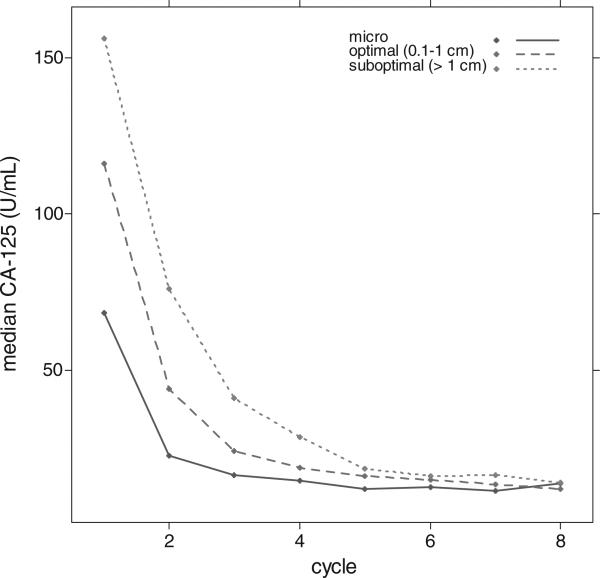
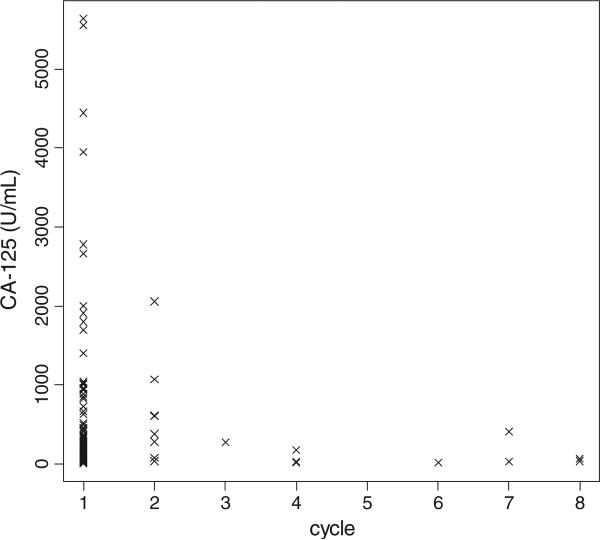
When stratified by residual disease status after primary surgery, the median pre-chemotherapy-treatment CA-125 levels for patients with grade 1 serous and microscopic residual, optimal residual (0.1–1.0 cm), and suboptimal residual (>1.0 cm) disease were 68.3, 116.0, and 156.0, respectively (Fig. 1). In most patients with grade 1 SOC, a maximum CA-125 value was noted immediately prior to the first cycle of chemotherapy (90.7%; Fig. 2).
Fig. 1.
Median CA-125 (U/mL) by chemotherapy cycle and residual disease status.
Fig. 2.
Patients’ (N = 184) peak CA-125 values by the cycle when they occurred.
On multivariate analysis, only residual disease status was associated with PFS and OS (P ≤ 0.006; Table 3). Pretreatment CA-125 was not associated with outcome. However, patients with CA-125 levels that normalized after cycle 1, 2 or 3 were 60–64% less likely to experience disease progression as compared to those who never normalized or normalized after 4 cycles (P ≤ 0.024; Table 4). The adjusted hazard ratios for death from disease in patients that normalized after 1, 2 or 3 cycles were 0.45 (P = 0.025), 0.65 (P = 0.24), and 0.42 (P = 0.06), respectively (Table 3 and Fig. 2). Compared to the other disease-residual cohorts, a higher percentage of patients with suboptimal residual disease never achieved CA-125 normalization (27% versus 19% for 0.1–1.0 cm residual and 2% for microscopic disease, P = .003).
Table 3.
PFS and OS of LGSC population by prognostic factors (multivariate analysis).
| N | Adj. HRa (PFS) | P † | Adj. HRa (OS) | P † | |
|---|---|---|---|---|---|
| Age years | 189 | 1.00 (0.99–1.01) | 0.769 | 1.00 (0.99–1.02) | 0.838 |
| Race/ethnicity | |||||
| White | 172 | Referent | – | Referent | – |
| Black | 9 | 0.42 (0.19–0.92) | 0.031 | 0.59 (0.21–1.66) | 0.317 |
| Other | 8 | 0.65 (0.28–1.48) | 0.304 | 1.32 (0.54–3.24) | 0.538 |
| Performance status | |||||
| 0 | 93 | Referent | – | Referent | – |
| 1–2 | 87 | 1.26 (0.90–1.78) | 0.181 | 1.47 (1.00–2.17) | 0.048 |
| 3 | 9 | 0.84(0.40–1.78) | 0.648 | 1.16 (0.53–2.53) | 0.719 |
| FIGO stage | |||||
| III | 165 | Referent | – | Referent | – |
| IV | 24 | 1.30 (0.81–2.08) | 0.282 | 1.04 (0.61–1.76) | 0.890 |
| Baseline CA-125U/mL | 184 | 1.11 (0.97–1.27) | 0.137 | 1.13 (0.98–1.30) | 0.094 |
| Ascites | |||||
| No | 69 | Referent | – | Referent | – |
| Yes | 118 | 1.21 (0.81–1.80) | 0.344 | 1.18 (0.76–1.82) | 0.465 |
| Residual disease status | |||||
| Microscopic | 47 | Referent | – | Referent | – |
| 0.1–1.0 cm | 97 | 3.13 (1.96–4.98) | <0.001 | 2.31 (1.37–3.90) | 0.002 |
| >1.0 cm | 45 | 3.31 (1.87–5.85) | <0.001 | 2.45 (1.30–4.64) | 0.006 |
Tests used
Wald test.
Hazard ratios (HR) are unitless. HRs are followed by their 95% confidence intervals.
Table 4.
CA-125 normalization and survival in LGSC cohort of GOG-182.
| CA-125 normalization | N | Adj. HRa (PFS) | P † | Adj. HRa (OS) | P † |
|---|---|---|---|---|---|
| No normalization | 18 | Referent | – | Referent | – |
| Before cycle 2 | 67 | 0.38 (0.20–0.73) | 0.004 | 0.45 (0.22–0.91) | 0.025 |
| Between cycles 2 and 3 | 35 | 0.36 (0.18–0.73) | 0.004 | 0.65 (0.32–1.33) | 0.242 |
| Between cycles 3 and 4 | 18 | 0.40 (0.18–0.89) | 0.024 | 0.42 (0.17–1.03) | 0.059 |
| After cycle 4 | 18 | 0.70 (0.32–1.52) | 0.365 | 0.91 (0.41–2.02) | 0.826 |
PFS is progression-free survival; OS is overall survival.
Tests used
Wald test.
Hazard ratios (HR) are unitless. HRs are followed by their 95% confidence intervals.
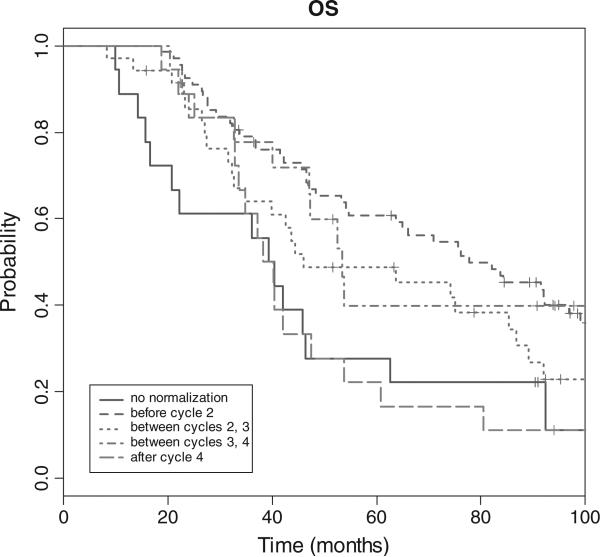
Lastly, the Kaplan Meier OS curve stratified by CA-125 level is illustrated in Fig. 3. Median OS for those who did not experience normalization of CA-125 was 23.0 months, compared with 77.7, 46.8, 53.4 and 38.1 months for those who normalized before cycles 2, 3, 4, and 5, respectively.
Fig. 3.
Overall survival based on CA-125 normalization during chemotherapy treatment.
Discussion
In recent years, serum CA-125 levels have been established as a valuable tool for the diagnosis of EOC, in defining disease prognosis and monitoring of treatment [24,25]. Serum CA-125 values before and after treatment with first-line platinum/taxane-based chemo-therapy are well characterized in those with high grade SOC. A study conducted by the Medical Research Council in the United Kingdom concluded that the CA-125 at the third course of chemo-therapy carried the greatest prognostic significance of any measurement during treatment of primary disease [24]. Additionally, in an ancillary analysis of seven GOG Phase III trials, pretreatment CA-125 level was an independent predictor of PFS in women with advanced serous EOC treated with a standard chemotherapy regimen [5]. Greater than 90% of the patients treated on these trials had grade 2 or 3 disease, and the survival improvements observed based on CA-125 levels were particularly notable in the setting of disease cytoreduction to microscopic residual.
The prognostic value of CA-125 measurements in those with lower grade SOC is not as well studied. In the current report, median pretreatment CA-125 levels were elevated in most women with grade 1 SOC (84.8%; Table 1) but were significantly lower (median 119.1 U/mL vs. 246.7 U/mL, P < 0.001) than those with higher-grade serous disease. It is not clear why CA-125 levels are generally lower in those with grade 1 disease. It is possible that the higher-grade serous tumors produce more CA-125 antigen on the surface of the ovarian cancer tumor cells, or that the lower mitotic index of the low-grade tumors may result in less antigen shed into the blood stream. In the current study, significantly more study subjects with high-grade serous disease were diagnosed with ascites at presentation (75.7%) than their stage-matched counterparts with low-grade disease (63.1%, P < 0.001; Table 1); this may also account for the differences observed in baseline serum CA-125 levels between the cohorts.
Pretreatment CA-125 level was not associated with survival in the grade 1 cohort; this may be partly explained by the fact that approximately one-third of the cohort had a pretreatment CA-125 of less than 70 U/mL. However, serial CA-125 measurements during chemotherapy treatment were prognostic of disease progression and survival, with normalization before the fourth chemotherapy cycle associated with improved PFS and normalization before the second cycle associated with OS. These results are fairly concordant with those observed in the Medical Research Council study, although normalization of CA-125 prior to the second instead of the third cycle of chemotherapy was associated with improved OS in the low-grade GOG-182 cohort. Overall, these data suggest that CA-125 may be a sensitive biomarker of response to treatment and is predictive of outcome in this low-grade serous carcinoma subgroup.
A recent ancillary study of GOG 182 demonstrated that in women with grade 1 serous carcinoma, surgical cytoreduction to microscopic residual after primary surgery was the most compelling prognostic factor associated with PFS and OS [26]. In fact, the survival differences in those who underwent surgery to microscopic residual compared with those who were left with macroscopic disease were more striking in the low grade than in the high-grade serous cohort. This may be due to the relative chemoresistance of the low-grade versus the high-grade serous tumors [27,28], and therefore, the potential benefit associated with maximal cytoreductive surgery may be more pronounced in this cohort than in those with high grade disease. In the current ancillary study, patients with suboptimal residual disease were less likely to ever achieve CA-125 normalization. Overall, CA-125 correlated well with cytoreductive status after primary surgery and is a serum bio-marker predictive of disease progression and survival outcome after chemotherapy treatment. CA-125 may be particularly useful to the clinician in the surveillance of women with grade 1 serous carcinoma, as it appears to be a somewhat sensitive indicator of residual disease status and progression. Given that grade 1 tumors are slow growing and that the mainstay of treatment is surgery, early detection of recurrences potentially amenable to resection via measurement of CA-125 levels is of interest.
In the last decade, low-grade serous carcinoma has been recognized as a distinct entity, with clear biologic and pathologic evidence indicating that these tumors develop via different pathways than their high grade counterparts [14–20,28,29]. While the high grade tumors exhibit an abundance of p53 mutations and grow rapidly, the low grade tumors are distinguishable by mutations in the RAS– RAF–MAPK pathways and for their indolent nature [26]. These clinicopathologic factors may account for the fact that conventional cytotoxic chemotherapy agents have not exhibited exceptional activity against low-grade serous carcinoma tumors. A recent Phase II GOG study of selumetinib, a MEK 1/2 inhibitor, demonstrated tolerability and excellent activity in recurrent low-grade serous carcinoma [30]. The development of specific targeted therapies with benefit in this cohort of patients further underscores the importance of identifying those with low-grade disease by the two-tiered criteria. Additionally, identification of novel biomarkers that may predict response to targeted therapies and may be used in conjunction with serum CA-125 is needed to better tailor the therapies for women with advanced, low-grade disease.
Study limitations include the retrospective data collection and that precise timing of the CA-125 collection was not specified by the protocols, resulting in specimens collected at various times before chemo-therapy was initiated. However, while there was some variation in the interval between surgery and chemo, the length of the interval was not associated with survival in the grade 1 serous patients. Because of the lack of paired preoperative and postoperative CA-125 values, the immediate impact of cytoreductive surgery on baseline CA-125 values could also not be assessed. Further, previous studies suggest that surgical trauma may temporarily elevate the CA-125 level in patients with benign disease and a previously normal level, while cytoreductive surgery for ovarian cancer commonly leads to a decrease in an elevated CA-125 level [5,31]. Finally, in evaluating the association between CA-125 normalization cycles and survival, we excluded patients that did not complete 8 cycles from the analysis, in order to minimize bias. Study strengths include the centralized pathology and data review and the analysis of a large cohort of women with advanced, grade 1 SOC treated in a randomized, cooperative group trial with a consistent chemotherapy regimen.
This is one of the first reports addressing CA-125 response rates and their prognostic significance in women with grade 1 SOC, a surrogate for low-grade serous disease, treated with first-line platinum/taxane-based chemotherapy. Pretreatment CA-125 level was significantly lower in women with grade 1 compared to those with grade 2/3 disease. Although pretreatment CA-125 was not associated with survival, serial CA-125 measurements during chemotherapy treatment were prognostic of outcome, with normalization prior to the fourth chemotherapy cycle associated with survival decreased risk of progression and normalization before the second cycles associated with a decreased risk of death. While CA-125 appears to be a prognostic tool and biomarker of chemotherapy response in this cohort, further studies are needed to identify more refined biomarkers and genes with potential prognostic significance.
HIGHLIGHTS.
In this ancillary GOG study, pre-treatment CA-125 was not associated with survival in women with grade 1 serous ovarian carcinoma.
Serial CA-125 measurements during platinum/taxane-based chemotherapy were associated with disease progression and overall survival.
CA-125 is a biomarker for residual disease status and response to treatment in this patient cohort.
Footnotes
This paper was presented at the Society of Gynecologic Oncology Annual Meeting on Women's Cancer, March 24, 2012, Austin, TX. The authors have no conflicts of interest to report.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: University of Alabama at Birmingham, Oregon Health Sciences University, Duke University Medical Center, Abington Memorial Hospital, University of Rochester Medical Center, Walter Reed Army Medical Center, Wayne State University, University of Minnesota Medical School, University of Southern California at Los Angeles, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, University of Miami School of Medicine, Milton S. Hershey Medical Center, Georgetown University Hospital, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, Albany Medical College, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, University of Kentucky, Eastern Virginia Medical School, The Cleveland Clinic Foundation, Johns Hopkins Oncology Center, State University of New York at Stony Brook, Eastern Pennsylvania GYN/ONC Center, P.C., Southwestern Oncology Group, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, University of Massachusetts Medical School, Fox Chase Cancer Center, Medical University of South Carolina, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, University of Arizona Health Science Center, Tacoma General Hospital, Eastern Collaborative Oncology Group, Thomas Jefferson University Hospital, Case Western Reserve University, and Tampa Bay Cancer Consortium.
Conflict of interest
Dr. Michael Bookman serves as Chair, Ovarian Committee, GOG (with limited financial compensation), and he also served as PI for the primary study GOG-182. All other coauthors have no conflicts of interest to declare.
References
- 1.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–7. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;20:27371–5. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Klug TL, St. John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmuno-assay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–7. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs I, Bast RC., Jr The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4:1–12. doi: 10.1093/oxfordjournals.humrep.a136832. [DOI] [PubMed] [Google Scholar]
- 5.Zorn KK, Tian C, McGuire WP, Hoskins WJ, Markman M, Muggia FM, et al. Prognostic value of pretreatment CA-125 in advanced ovarian carcinoma: a Gynecologic Oncology Group study. Cancer. 2009;115:1028–35. doi: 10.1002/cncr.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 7.Markman M, Bundy BN, Alberts DS, Fowler JM, Clarke-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 8.Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2000;18:106–15. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 9.Rose PG, Nerenstone S, Brady MF, Clarke-Pearson D, Olt G, Rubin SC, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. N Engl J Med. 2004;351:2489–97. doi: 10.1056/NEJMoa041125. [DOI] [PubMed] [Google Scholar]
- 10.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected Stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 11.Spriggs D, Brady MF, Rubin SC, Hanley M, Copeland LJ, Clarke-Pearson D, et al. A phase III randomized trial of cisplatin and paclitaxel administered by either 24 hour or 96 hour infusion in patients with selected stage III or stage IV epithelial ovarian cancer. Proc ASCO. 2004;449(23) (abstr #5004) [Google Scholar]
- 12.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 13.Malpica A, Deavers MT, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Bodurka DC, Deavers MT, Tian C, Sun CC, Malpica A, Coleman RL. Reclassification of serous ovarian carcinoma by a 2-tier system: a Gynecologic Oncology Group Study. Cancer. 2012;118:3087–94. doi: 10.1002/cncr.26618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer InterGroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Phil Mag. 1900;(Series 5):157–75. [Google Scholar]
- 17.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 18.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 20.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Stat Methodol) 1972;34:187–220. [Google Scholar]
- 22.R Development Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. ( http://www.R-project.org/, 3-900051-07-0) [Google Scholar]
- 23.Fayers PM, Rustin G, Wood M, Nelstrop A, Leonard RC, Wilkinson P, et al. The prognostic value of serum CA125 in patients with advanced ovarian cancer: an analysis of 573 patients by the Medical Research Council Working Party on Gynaecological Cancer. Int J Gynaecol Oncol. 1993;3:286–92. doi: 10.1046/j.1525-1438.1993.03050285.x. [DOI] [PubMed] [Google Scholar]
- 24.Meyer T, Rustin GJ. Role of tumor markers in monitoring epithelial ovarian cancer. Br J Cancer. 2000;82:1535–8. doi: 10.1054/bjoc.2000.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fader AN, Java J, Ueda SM, Bookman M, Bristow RE, Armstrong D, et al. Survival outcomes in women with low grade (grade 1) serous ovarian carcinoma: a Gynecologic Oncology Group study. Obstet Gynecol. 2013;122(2, PART 1):225–32. doi: 10.1097/AOG.0b013e31829ce7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmeler KM, Gershenson DM. Low-grade serous ovarian cancer: a unique disease. Curr Oncol Rep. 2008;10:519–23. doi: 10.1007/s11912-008-0078-8. [DOI] [PubMed] [Google Scholar]
- 27.Gershenson DM, Sun CC, Lu KH, Coleman RL, Ak Sood, Malpica A, et al. Clinical behavior of stage II–IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–8. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 28.Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations and restricted to low-grade serous tumours. J Pathol. 2004;202:336–40. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 29.Singer G, Oldt R, III, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 30.Farley J, Brady WE, Vathipadiekal V, Lankes HA, Coleman R, Morgan MA, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–40. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talbot RW, Jacobsen DJ, Nagorney DM, Malkasian GD, Ritts RE., Jr Temporary elevation after abdominal surgical treatment for benign disease and cancer. Surg Gynecol Obstet. 1989;168:407–12. [PubMed] [Google Scholar]