Abstract
Significance: Mitochondria play a vital role in cellular homeostasis and are susceptible to damage from inflammatory mediators released by the host defense. Cellular recovery depends, in part, on mitochondrial quality control programs, including mitochondrial biogenesis. Recent Advances: Early-phase inflammatory mediator proteins interact with PRRs to activate NF-κB-, MAPK-, and PKB/Akt-dependent pathways, resulting in increased expression or activity of coactivators and transcription factors (e.g., PGC-1α, NRF-1, NRF-2, and Nfe2l2) that regulate mitochondrial biogenesis. Inflammatory upregulation of NOS2-induced NO causes mitochondrial dysfunction, but NO is also a signaling molecule upregulating mitochondrial biogenesis via PGC-1α, participating in Nfe2l2-mediated antioxidant gene expression and modulating inflammation. NO and reactive oxygen species generated by the host inflammatory response induce the redox-sensitive HO-1/CO system, causing simultaneous induction of mitochondrial biogenesis and antioxidant gene expression. Critical Issues: Recent evidence suggests that mitochondrial biogenesis and mitophagy are coupled through redox pathways; for instance, parkin, which regulates mitophagy in chronic inflammation, may also modulate mitochondrial biogenesis and is upregulated through NF-κB. Further research on parkin in acute inflammation is ongoing. This highlights certain common features of the host response to acute and chronic inflammation, but caution is warranted in extrapolating findings across inflammatory conditions. Future Directions: Inflammatory mitochondrial dysfunction and oxidative stress initiate further inflammatory responses through DAMP/PRR interactions and by inflammasome activation, stimulating mitophagy. A deeper understanding of mitochondrial quality control programs' impact on intracellular inflammatory signaling will improve our approach to the restoration of mitochondrial homeostasis in the resolution of acute inflammation. Antioxid. Redox Signal. 22, 965–976.
Introduction and Brief Overview of Mitochondrial Biogenesis
Mitochondria play a vital cellular role not only in maintaining normal energy homeostasis but also in the response to pathological conditions that cause stress to the energy metabolism. Although their most well-known function is cellular energy conservation, they are integral to heme and steroid biosynthesis, cell cycle regulation, programed cell death, calcium signaling, and redox homeostasis and signaling. The operation of such centrally important organelles must, therefore, be actively regulated in the physiological state and also protected from stressors by a comprehensive set of adaptive quality control mechanisms. These quality control mechanisms optimize the overall mitochondrial number, distribution, and function through a group of interrelated inducible processes, including mitophagy, mitochondrial fission and fusion, and mitochondrial biogenesis (76, 137).
As the replacement of any cellular component is metabolically expensive, mitochondrial biogenesis is particularly costly, because it may involve the synthesis of hundreds or, in some cases, thousands of new proteins. As a result, mitochondrial biogenesis is strictly controlled by intra- or extracellular signals communicating energy imbalance from increased energy demand, decreased energy production, or both. Mitochondrial biogenesis can be induced by exercise, fasting, cold exposure (thermogenesis), oxidative stress, and inflammatory cell stress. Depending on the stimulus, the program is executed through a variety of signaling pathways that converge on a handful of coactivators and nuclear transcription factors (including the peroxisome proliferator-activated receptor gamma-1 coactivator family [PGC-1α, PGC-1β, and PRC] and nuclear respiratory factors [NRF-1 and NRF-2]) (94). PGC-1α, in particular, has been identified as an important coordinator of the biogenesis response and has been found to orchestrate a wide variety of anti-inflammatory and metabolic nuclear genes; those most directly related to mitochondrial biogenesis in inflammation are covered here, including the NRF-1, NRF-2 (also called GA-binding protein A [GABPA]), and nuclear factor erythroid 2-related factor 2 (Nfe2l2 or Nrf2) transcription factors (16, 120, 129).
These transcription factors and coactivators coordinate the complex bigenomic programs of biogenesis by participating in feedback loops for the precise regulation of mitochondrial quality control (such as reactive oxygen species [ROS], Ca2+, and anti-inflammatory signaling [reviewed in Dominy and Puigserver (24)]) and by modulating specific gene expressions at various regulatory levels within the process. For instance, NRF-1 and NRF-2 upregulate the transcription of many nuclear-encoded mitochondrial proteins, which are transported across the mitochondrial membranes by processes that are themselves modulated by external stress stimuli and some of the same central transcription factors (45). These imported proteins serve as the building blocks for mitochondrial proliferation, while those same central coactivators and transcription factors upregulate expression of mitochondrial DNA (mtDNA)-binding proteins (mitochondrial transcription factor A [mtTFA], B1 [mtTFB1], B2 [mtTFB2], and DNA polymerase [Pol] γ). The mtDNA-binding proteins also undergo mitochondrial importation, where they directly activate mitochondrial transcription and replication (93, 94).
The induction of host inflammatory processes has a direct impact on mitochondrial function and quality control; mitochondrial biogenesis, in particular, is upregulated in response to both mitochondrial damage and the concomitant increases in energetic demand associated with severe inflammation. However, inflammatory processes have a multitude of different effects on various immune and somatic cell types that depend on the nature, severity, and timing of the inflammatory stimulus. The host responses to these stimuli (i.e., the production of pro-inflammatory cytokines, chemokines, and/or anti-inflammatory factors) also impact mitochondrial function and energy balance and so, in turn, have been found to participate both directly and indirectly in the regulation of mitochondrial biogenesis programming over the inflammatory cycle. Finally, the consequences of inflammation on mitochondria themselves signal for adaptive modulation of the inflammatory response. In this study, we briefly review the intersection of innate inflammation and mitochondrial biogenesis by examining proinflammatory effects on mitochondrial function and biogenesis and, conversely, mitochondrial feedback on counter-inflammation, with an emphasis on redox signals that influence cellular and tissue homeostasis during the course of the inflammatory cycle.
Impacts of Inflammation on Mitochondrial Function
Oxidative phosphorylation and impairment of mitochondrial respiration
The impact of inflammation on mitochondrial function has often been modeled by exposure to bacterial endotoxin; as early as the late 1960s, it was known that gram-negative lipopolysaccharide (LPS) exposure decreased respiration in both the isolated mitochondria (46, 97) and in various tissues in vivo (68, 69, 115). Animal models of bacterial sepsis have confirmed that mitochondrial function and/or metabolic profiles are also significantly impacted by septic inflammation, with the most profound changes typically seen early in the host response (4, 9, 52, 62, 63). Finally, in the most relevant model of all, in human patients with sepsis from various pathogens and sources, there is also clear evidence of proinflammatory mitochondrial dysfunction (Fig. 1). Importantly, the degree of mitochondrial dysfunction appears to be roughly associated with clinical outcomes, with nonsurvivors of severe sepsis demonstrating an early decrease in the ATP/ADP ratio in skeletal muscle (8), impaired fatty acid utilization (58), and later a failed induction of upregulation of mitochondrial biogenesis markers when compared with survivors (13).
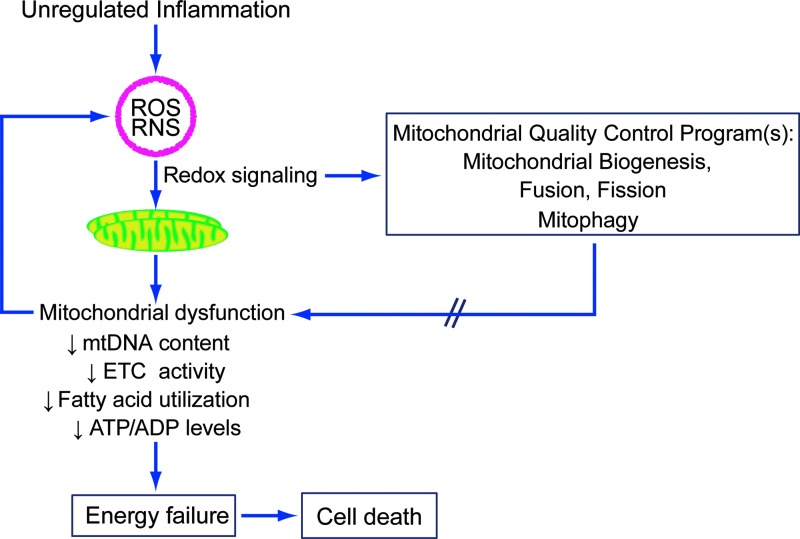
FIG. 1.
Inflammation-induced reactive oxygen and nitrogen species modulate mitochondrial quality control programs through redox signaling. However, higher levels of oxidative stress may also contribute to inflammatory mitochondrial dysfunction, resulting in energetic failure and cell death. ETC, electron transport chain; mtDNA, mitochondrial DNA; ROS, reactive oxygen species; RNS, reactive nitrogen species. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
There are clear advantages and limitations associated with various experimental models of acute inflammation (22); actual pathogenic inflammation within an intact organism involves far more complex stimuli and responses than a time-limited exposure to one or a mixture of inflammatory mediators. Although LPS administration models are simple and reliably produce profound biological effects that lend themselves to mechanistic investigations of the nature of proinflammatory mitochondrial dysfunction and recovery during the resolution phase, the doses of LPS required for mitochondrial damage are supraphysiological and confounded by serious hypotension and tissue ischemia. Animal and human models of bacterial infection have fewer controlled variables, but allow examination of the effects of systemic-level inflammation on mitochondrial function, as well as on survival and other outcomes. Nonetheless, these various models show that the reduction of mitochondrial oxygen consumption in early inflammation is accompanied by decreased ATP or ATP/ADP levels (4, 11, 27) corresponding to nitric oxide synthase 2 (NOS2) induction (6, 115), which may result in, for example, complex I inhibition (9). There is also evidence of mitochondrial dysfunction through mtDNA depletion (109) due to cytochrome c oxidase (COX) inhibition (6, 31, 62, 63) and resulting in decreased State 3 respiration in several tissues (11, 111). Also, while decreased electron transport chain (ETC) protein activities certainly contribute to impaired respiration, it has also been suggested that ETC protein levels are also decreased early after endotoxin/tumor necrosis factor-α (TNF-α) exposure (11, 111). These relatively rapid changes in the mitochondrial protein content may reflect protein disposal mechanisms and/or the activation of selective mitochondrial autophagy (mitophagy) along with perhaps altered antibody peptide recognition due to ROS- or NO-induced post-translational protein modifications (62).
In any event, inflammation-induced mitochondrial dysfunction is a common, but sometimes subtle feature of inflammatory tissue damage in a variety of cell types and inflammatory models. Although profound and permanent respiratory impairment is incompatible with cellular survival, conservation of this response across models and organisms suggests that some type of temporary energetic conservation mechanism in early inflammation may be activated for its adaptive advantages (103). On the tissue level, decreased ATP availability in areas of localized inflammation may prevent pathogenic hijacking of host cell energetic infrastructure and help contain infection, preventing systemic spread (62). More globally, the example of a hibernating myocardium in response to ischemic threat may be analogous to the temporary downregulation of mitochondrial respiration in the systemic inflammation of sepsis, but just as the hibernating myocardium is not sustainable indefinitely, decreased mitochondrial respiration in sepsis eventually results in irreversible energetic deficits that lead to cell death (104).
Reactive oxygen and nitrogen species
In the setting of such metabolic stasis, the temporary decrease in oxidative phosphorylation not only potentially conserves limited mitochondrial substrate for respiration but it may also mitigate pathologic mitochondrial ROS production. This aspect may be of particular importance in the setting of inflammation, where systemic oxygen (or carbon substrate) supply is not thought to be a limiting factor (32) (notwithstanding limitations in local areas of hypoperfusion [reviewed in Trzeciak and Rivers (119)]). Small amounts of ROS are generated in the course of normal oxidative phosphorylation (primarily by complexes I and III of the ETC) (82); however, the production of both ROS and reactive nitrogen species (RNS) is increased in the setting of mitochondrial damage, such as that encountered in severe inflammation. Inflammatory ROS/RNS production may eventually overwhelm the cellular antioxidant capacity and lead to damage to proteins, lipids, and DNA by oxidation or nitration. Oxidative damage to cellular and mitochondrial components, particularly to the ETC complexes (10, 18, 71), leads to further dysfunction and ROS/RNS production.
Inflammation-induced changes in mitochondrial function and ROS/RNS production vary depending on the intensity and duration of the inflammatory stimulus. Low-level ROS production is important in redox signaling and in prosurvival cellular adaptations (95), particularly evident is H2O2 leakage from mitochondria as a retrograde signal to nuclear-targeted cytosolic pathways that warn the cell of internal oxidative stress. On the other hand, more profound energetic deficits and ROS production not only lead to damage of cellular components but also to loss of ROS signal localization (1). If repair mechanisms become overwhelmed, the intrinsic apoptotic cascade may be triggered in the setting of only moderate inflammation (132). In severe inflammation, mitochondrial dysfunction and ROS/RNS-induced damage may lead to extensive cellular necrosis and release of cellular contents, including mitochondria and mitochondrial fragments, which act as further inflammatory stimuli (91, 122).
Inflammatory Regulation of Mitochondrial Biogenesis
Since the level of mitochondrial dysfunction, ROS/RNS production, and mitochondrial damage induced by the host response depends on the magnitude and timing of that stimulus, it follows that successful restoration of a fully functional mitochondrial population is dependent on the timely upregulation of mitochondrial quality control programs (Fig. 2). Following acute inflammation and an initial decrease of mitochondrial function, there may be a restoration of respiratory capacity (42, 111). Data from animal studies and septic patients show that evidence of mitochondrial recovery is predictive of (9, 33) and/or significantly associated with clinical recovery (8, 13, 55). In this study, we have focused on the induction of mitochondrial biogenesis pathways in inflammation, but all of the quality control elements are important for sepsis resolution (104).
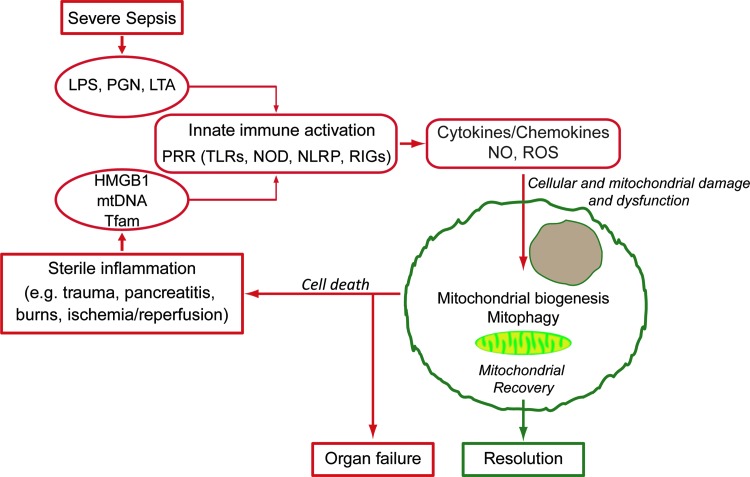
FIG. 2.
An overview of the impact of inflammation on mitochondrial function and quality control. Acute infectious or sterile inflammation leads to innate immune activation through interaction with PRRs, resulting in inflammatory mediator production. These inflammatory mediators affect mitochondrial damage and dysfunction; successful resolution requires activation of mitochondrial quality control pathways, including mitochondrial biogenesis. Inadequate mitochondrial quality control results in cell death, organ dysfunction, and propagation of sterile inflammation through the release of DAMPs. DAMPs, danger-associated molecular patterns; HMGB1, high-mobility group box 1; LAN, lipoteichoic acid; LPS, lipopolysaccharide; NLRP, NACHT, LRR, and PYD domains-containing protein; NOD, nucleotide-binding oligomerization domain; PGN, peptidoglycan; PRR, pattern recognition receptor; RIG, retinoic acid-inducible gene; Tfam, mitochondrial transcription factor A; TLR, toll-like receptor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
For nonimmune cells (immune cells are discussed later), mitochondrial dysfunction in the early phase of septic inflammation is stimulated, in part, by circulating proinflammatory cytokines like TNF-α (96) and a subset of the interleukins. A plethora of pathways participate in regulating the mitochondrial response (Fig. 3), and many of the intermediate cellular transcription factors and coactivators can also participate in regulating adaptive responses and/or amplifying the inflammatory signal. The inhibition of respiration and initiation of mitochondrial damage also initiate retrograde signaling to the nucleus for the genetic activation of mitochondrial quality control programs, pro- and anti-inflammatory elements, and antioxidant enzymes. Each is discussed briefly below.
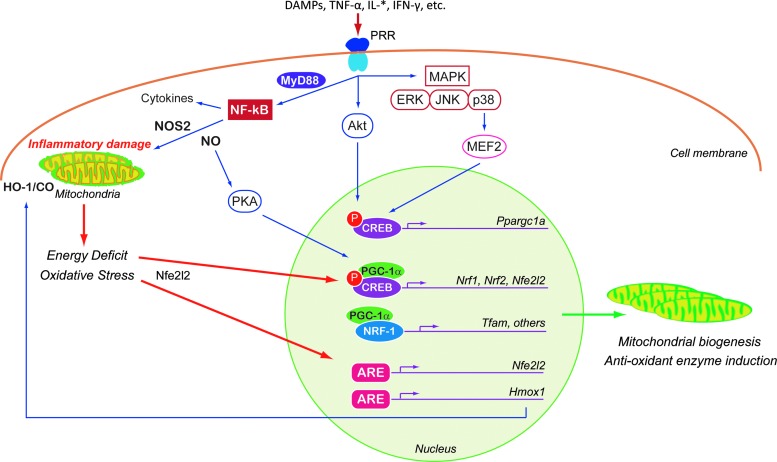
FIG. 3.
An overview of the pathways regulating mitochondrial biogenesis in response to inflammatory stimuli in nonimmune cells. Early-phase inflammatory protein mediators interact with PRRs to activate the NF-κB, PKB/Akt, or MAPK pathways. A shared component of these pathways is increased expression or activity of the core coactivators and/or transcription factors controlling mitochondrial biogenesis (PGC-1α, NRF-1, NRF-2, and Nfe2l2) by both CREB-dependent and CREB-independent mechanisms. Inflammatory upregulation of NOS2-induced NO not only contributes to oxidative stress but also upregulates mitochondrial biogenesis via PGC-1α. Similarly, oxidative stress activates the HO-1/CO system, which stimulates both mitochondrial biogenesis and antioxidant enzyme genes. More generic energy-sensing and redox-sensitive pathways (such as AMPK, SIRT1, CREB, and HO-1/CO) also modulate mitochondrial biogenesis in the setting of inflammatory stress. Akt, protein kinase B; ARE, antioxidant response element; CO, carbon monoxide; CREB, cAMP response element-binding protein; ERK, extracellular signal-related kinases; HO-1 or Hmox1, heme oxygenase-1; JNK, c-Jun NH2-terminal kinases; IL, interleukin; IFN-γ, interferon gamma; MAPK, mitogen-activated protein kinase; MEF2, myocyte enhancer factor-2; MyD88, myeloid differentiation primary response protein; Nfe2l2, nuclear factor erythroid 2-related factor 2; PKA, protein kinase A; NOS2 or iNOS, nitric oxide synthase 2; NRF or Nrf, nuclear respiratory factors; PGC-1 or Ppargc1, peroxisome proliferator-activated receptor gamma-1 coactivator; TNF-α, tumor necrosis factor-α.
Pathways and mediators of inflammatory mitochondrial biogenesis
Toll-like receptors, inflammatory mediators, and biogenesis gene regulation
The innate immune response is activated through the recognition of microbial antigens (pathogen-associated molecular patterns [PAMPs]) or intrinsic factors released into the circulation (alarmins) (74). Together these factors, called danger-associated molecular patterns (DAMPs), are sensed by cellular pattern recognition receptors (PRRs) such as toll-like receptors (TLRs). In general, PRR activation upregulates the release of early-phase inflammatory protein mediators (TNF-α, interleukins [IL-*], interferon gamma [IFN-γ], etc.), growth factors, hormones (cortisol and adrenaline), and ROS/RNS. These are far from being the only mediators of immune signaling [reviewed in Castellheim et al. (14)], but the pathways by which these modulate inflammatory responses in nonimmune cells are relatively well studied; these are encapsulated here. The adrenergic and hormonal responses to inflammation certainly also lead to adaptations in mitochondrial function and quality control (37, 117, 125, 127), but are not covered here in detail.
The circulating inflammatory proteins released by cellular components of the innate immune system provide important information to nonimmune cells through cell-type-specific arrays of inflammatory protein receptors and PRRs (including TLRs) for the recognition of relevant DAMPs (17). Many of these receptors (TLRs as well as PRRs for TNF-α, IFN-γ, and interleukins [specifically IL-6]) activate one or several well-defined inflammatory signaling pathways. The first of these is via the NF-κB family of transcription factors, which activates pathways that contribute to inflammatory, apoptotic, proliferative, and tumorigenic processes. NF-κB is normally present in the cell in an inhibited state until myeloid differentiation primary response protein (MyD88)-dependent or -independent phosphorylation of the NF-κB inhibitor (IκB); NF-κB subunits are then free to translocate to the nucleus to regulate transcription in combination with other transcription factors (39). Many targets of TLR signaling through NF-κB are proinflammatory and induce further oxidative stress (114). The p65 subunit may even suppress the PGC-1α activity and related metabolic activity in some tissues such as the heart (3). On the other hand, LPS-stimulated TLR4 signaling, operating through NF-κB, can promote (cAMP response element-binding protein [CREB]) upregulation of PGC-1α, NRF-1, NRF-2, and other components of the mitochondrial biogenesis framework in both CREB-dependent (110, 112) and CREB-independent pathways (113). Evidence also suggests that parkin, best known for its role in mitophagy regulation in the setting of Parkinson's disease and other chronic inflammatory states, may also regulate mitochondrial biogenesis (35, 92). There is recent evidence that parkin binds to mtDNA via mtTFA, enhancing transcription (56, 88). Parkin has also been found to regulate degradation of PARkin-interacting substrate (PARIS, ZNF746) by ubiquitination, which then leads to decreased repression of Ppargcla transcription (102). Intriguingly, in an LPS model, parkin was increased by a MyD88/NF-κB-dependent pathway, suggesting a potential for parkin-modulated mitochondrial biogenesis in acute inflammation (118).
Another group of pathways commonly activated by TLRs or other inflammatory receptors are those involving the mitogen-activated protein kinases (MAPKs) and protein kinase B (PKB/Akt). Core MAPK signaling pathways include extracellular signal-related kinases (ERK), p38, and c-Jun NH2-terminal kinases (JNK). The ERKs, p38s, and JNKs phosphorylate a vast array of other kinases and transcription factors (57); activities specifically relevant to the mitochondrial function include activation of activating protein-1 (AP-1) by all three core pathways (100) and p38 activation of myocyte enhancer factor-2 (MEF2) (43). AP-1 participates in apoptosis regulation, cell differentiation, and proliferation, and MEF2 participates in a positive feedback loop (which is enhanced by Ca2+ signaling), increasing PGC-1α expression (44). Akt/PKB is a prosurvival kinase that also has a role in controlling many inflammatory response processes, including protein synthesis, chemotaxis regulation, apoptosis, and cell metabolism (14). In the context of mitochondrial biogenesis, Akt phosphorylates and activates the NRF-1 transcription factor, resulting in induction of mtTFA (80), and it stimulates the expression of several biogenesis genes via the CREB/CBP nuclear transduction pathway (26). However, Akt can also be proinflammatory through the regulation of NF-κB expression (21) and, when chronically overexpressed, has been shown to lead to decreased PGC-1α levels in the heart (19).
Nitric oxide
One of the most important NF-κB-dependent responses in sepsis is the upregulation of NOS2 (iNOS) in both immune and nonimmune cells. TNF-α, IL-1β, IFN-γ, and platelet-activating factor (PAF) can also activate NOS2 expression individually or in concert in many cell types (53, 116), acting through a variety of other transcription factors, including interferon regulatory factor-1 (IRF-1), signal transducer and activator of transcription-1a (STAT-1a), CREB, and AP-1 (77). Because NOS2 is regulated mainly at the transcriptional level and not by calcium, it can in some cells produce very high levels of NO, which is important to antimicrobial defense, but also cytotoxic to the host cell (34). In the setting of oxidative stress, NO will react with superoxide to produce peroxynitrite (ONOO−), a highly RNS that can lead to damage of mitochondrial ETC components by chemical nitration and hydroxylation (10). At high levels, NO also inhibits complex I, cytochrome c, and COX (6, 10, 59).
Apart from those relatively direct effects on mitochondrial function, NO also functions as an intracellular signal. The effect most relevant here is that NO stimulates mitochondrial biogenesis via the induction of the PGC-1α coactivator (41, 73, 84). There is also evidence that S-nitros(yl)ation of certain heat shock chaperone proteins by endogenous NO production is associated with induction of mitochondrial biogenesis in murine sepsis (108). In addition, Nfe2l2-mediated antioxidant gene expression is dependent on NO production in cardiomyoblasts (54), neuroblastoma cells (23), endothelial cells (7), and others. NO also modulates inflammatory signals—NO leads to suppression of NF-κB [via the inhibitory protein IκB (78)] and upregulates endothelin-1 (ET-1), a proinflammatory mediator, for instance, in the liver in sepsis (30).
Heme oxygenase-1 and carbon monoxide
The induction of heme catabolism during a variety of cell stresses ranging from hypoxia to inflammation has been recognized for many years. This is the function of the two isoforms of the heme oxygenases (HO) (90). HO-1 upregulation is seen consistently with LPS exposure in most tissues (12, 27, 36, 85). In addition, NO plays a role in HO-1 expression in the liver during sepsis (30). Heme metabolism by HO-1 produces carbon monoxide (CO) as a product, which through its interactions with reduced transition metals, is itself a source of oxidative stress and ROS signaling. Otherwise, HO-1 and CO exert anti-inflammatory effects (decreased TNF-α, IL-1β, and macrophage inflammatory protein-1 β; and increased IL-10) (75, 81, 126), thus providing negative feedback control on the inflammatory response and facilitating the proresolution state. Of mention, in the setting of chronic metabolic inflammation, the loss of HO-1 in hepatic tissue has been reported to reduce steatosis and toxicity—the mechanism underlying these findings is unexplained as yet (51). In addition to these adaptive processes, HO-1/CO increases mitochondrial biogenesis and antioxidant gene expression via upregulated Nfe2l2 activation (65, 80, 124). Nfe2l2, itself a redox-sensitive transcription factor, is capable of promoting further expression of HO-1 (2).
Energy deficit and oxidative stress
Apart from the mechanisms of induction of mitochondrial biogenesis that are relatively specific to inflammatory states, the energetic deficits and oxidative stress induced by inflammation activate more general pathways for mitochondrial quality control recruited by the cell stress response. These will be mentioned only briefly as more complete detailed reviews of signaling and mitochondrial biogenesis in response to cellular energetic sensing can be found elsewhere (94, 131).
Cellular energy deficits and mitochondrial dysfunction may become manifest by an increase in the AMP/ATP or the NAD+/NADH ratios. Increased AMP/ATP induces PGC-1α phosphorylation via AMPK signaling (50); a rise in NAD+increases deacetylation of PGC-1α via sirtuin 1 (SIRT1) (86). As mentioned, PGC-1α is a key regulator of mitochondrial biogenesis, and it also modulates anti-inflammatory (28) and antioxidant pathways (16, 64, 106). Phosphorylation of PGC-1α increases its translocation into the nucleus and coactivation of the mitochondrial biogenesis transcriptional process (5); SIRT1 deacetylation increases the activity of the PGC-1α protein and its regulation of nuclear and mitochondrial gene transcription (38). Finally, CREB1, an intermediate transcription factor that also has a number of roles in regulating gene transcription (often in partnership with PGC-1α) in response to metabolic needs (128), also helps mediate ROS signaling (106).
This overview leads us to a brief discussion of mitochondrial biogenesis in response to oxidative stress. Mitochondrial mass has been shown to increase in cells under external sublethal oxidative stress (61). The generation of ROS, primarily in the form of relatively diffusible H2O2 and through oxidation of various proteins, can provide signal transduction communicating the mitochondrial oxidative state, as feedback for control of nuclear programs for antioxidant defense or mitochondrial quality control. Even in the absence of a clear inflammatory stimulus, oxidative stress can upregulate many of the transcription factors already discussed and induce some of the mitochondrial biogenesis signaling pathways mentioned. These include increased CREB promoter binding (106), AMPK activation of PGC-1α and-1β (49), and expression of NRF-1 (80) and NRF-2. Additionally, oxidative stress induces the HO-1/CO system via Akt/PKB, activating Nfe2l2 and ultimately enhancing NRF-1 expression resulting in mitochondrial biogenesis and an increase in mitochondrial mass in the mouse heart (79).
Inflammation and mitochondrial biogenesis in immune cells
A perhaps more neglected component of the mitochondrial biogenesis response to inflammatory stimuli is the role of mitochondrial biogenesis in immune effector cells for the support of increased cellular energy requirements, as well as for cell proliferation and differentiation. This is addressed briefly below and leads naturally to novel concepts of how inflammatory damage to mitochondria modulates subsequent pro- or anti-inflammatory processes. Sepsis induces both proliferation and apoptosis in immune cells (87). Increased respiration and mitochondrial proliferation have been reported in circulating peripheral immune cells of septic patients, although different results have been reported (105). In vitro and animal models support this finding: AMPK stimulation in macrophages induces autophagy and improved clearance of mycobacterium, and also increased PGC-1α and mtDNA as markers for accompanying mitochondrial biogenesis (130). During activation of murine T cells, T-cell receptor activation increases mitochondrial mass and mtDNA content also via AMPK (20). Moreover, IL-15 influences mitochondrial biogenesis to provide for spare respiratory capacity for maintenance of the memory phase of immunity (CD8+ T memory cells) following clonal expansion in response to infection (121).
Mitochondrial dysfunction and damage as modulators of inflammation
The mitochondrial dysfunction induced by inflammatory stress is also known to have a signaling role in modulating further inflammation. Severe stress induces cell death and the extracellular release of varying amounts and types of intracellular products depending on the specific cell death pathway (91). These extracellular DAMPs, including mitochondrial DAMPs [e.g., mtDNA (134), cytochrome c (29), mtTFA (15), high-mobility group box 1 (HMGB1) (83, 123)], ATP (40), and other mitochondria-derived peptides [e.g., humanin, which has a cDNA sequence virtually identical to mitochondrial 16S rRNA (47)], interact with PRRs to trigger pro- or anti-inflammatory responses as reviewed above (67, 98). Additionally, the signaling activities of many cytokines and cell death-associated proteins are redox regulated through cysteine modifications and are therefore sensitive to inflammatory oxidative stress (89).
Inflammatory mitochondrial dysfunction also induces intracellular inflammatory signaling (Fig. 4); mitochondrial dysfunction increases oxidative stress and also leads to membrane permeability transition (MPT) and mtDNA translocation to the cytoplasm (72). The presence of cytoplasmic mtDNA upregulates NF-κB through TLR9 signaling (133). Cytosolic mtDNA and increased ROS—via thioredoxin-interacting protein (135)—initiate NACHT, LRR, and PYD domains-containing protein 3 (NLRP3) inflammasome assembly (25, 101); this allows caspase-1 activation with subsequent proinflammatory IL-1β and IL-18 release (66). Macroautophagy, specifically mitophagy, is a negative regulator of inflammasome activation (72, 136). Impaired autophagy reduces clearance of damaged mitochondria, resulting in increased ROS production and mtDNA accumulation in the cytoplasm, leading to inflammasome activation and inflammatory mediator release. The translocation of mtDNA into the cytoplasm itself seems to be partially dependent on inflammasome activation (72). More recent evidence also supports roles for both mitochondrial antiviral signaling protein [MAVS; previously known to induce NF-κB (99) and IRFs (60)] (107) and mitochondrial Ca2+ signaling in NLRP3 inflammasome activation (48, 70).
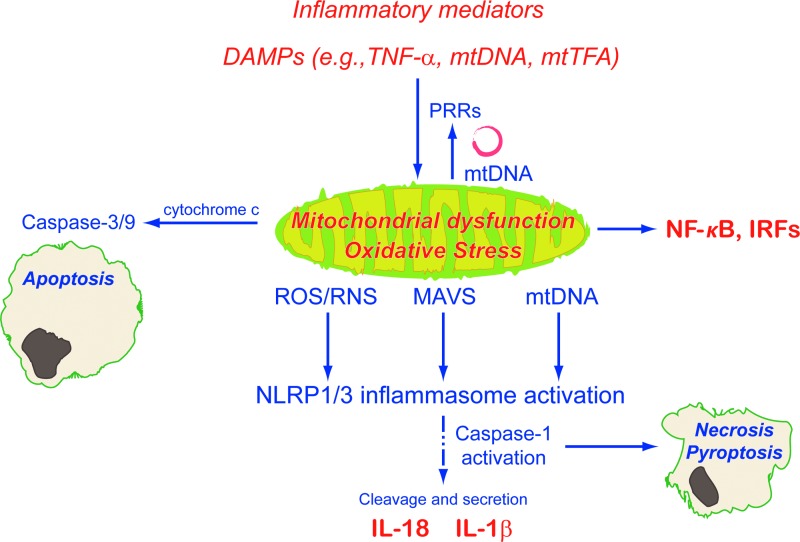
FIG. 4.
Inflammatory mitochondrial dysfunction and oxidative stress initiate further inflammatory responses and apoptosis through inflammasome activation. Cytosolic mtDNA, mitochondrial antiviral signaling (MAVS) protein, and other by-products of mitochondrial dysfunction also induce NF-κB and interferon upregulation. IRF, interferon regulatory factor; MAVS, mitochondrial antiviral signaling protein; mtTFA, mitochondrial transcription factor A; NLRP, NACHT, LRR, and PYD domains-containing protein.
Summary and Conclusions
In summary, mitochondria not only play a vital role in cellular homeostasis but also in the response to environmental stimuli. Initially, inflammation-induced mitochondrial dysfunction results in decreased oxidative phosphorylation and increased oxidative stress. Host recovery is dependent, in part, on the upregulation of mitochondrial quality control programs. Mitochondrial biogenesis, one component of these programs, is tightly controlled by specific coactivators and transcription factors that regulate the expression of components of the nuclear and mitochondrial genome. These are found to be stimulated by various elements of the inflammatory response; in nonimmune cells, early-phase inflammatory protein mediators interact with PRRs to activate the NF-κB, MAPK, or PKB/Akt pathways. A shared component of these pathways is increased expression or activity of the core coactivators and/or transcription factors controlling mitochondrial biogenesis (PGC-1α, NRF-1, NRF-2, and Nfe2l2). Inflammatory upregulation of NOS2-induced NO, although directly impacting negatively on mitochondrial function as a reactive species, also functions as a signaling molecule; NO upregulates mitochondrial biogenesis via PGC-1α, participates in Nfe2l2-mediated antioxidant gene expression, and itself has a role in inflammatory mediation. Similarly, NO and other components of the host inflammatory response induce the HO-1/CO system, which stimulates both mitochondrial biogenesis and antioxidant genes. More generic energy-sensing and redox-sensitive pathways (such as AMPK, SIRT1, CREB, and HO-1/CO) also modulate mitochondrial biogenesis in the setting of inflammatory stress. Finally, inflammatory mitochondrial dysfunction and oxidative stress can initiate further inflammatory responses through DAMP interaction with PRRs and by inflammasome activation. In short, the host response depends profoundly on a complex network of pro- and anti-inflammatory pathways that impact, and are impacted by, mitochondrial dysfunction. Recovery of mitochondrial function and cell redox status is vital to cellular survival and organ function in unregulated acute inflammatory states such as severe sepsis.
Abbreviations Used
- AP-1
activating protein-1
- ARE
antioxidant response element
- CO
carbon monoxide
- COX
cytochrome c oxidase
- CREB
cAMP response element-binding protein
- DAMP
danger-associated molecular pattern
- ERK
extracellular signal-related kinases
- ET-1
endothelin-1
- ETC
electron transport chain
- GABPA
GA-binding protein A (also NRF-2)
- HMGB1
high-mobility group box 1
- HO-1 or Hmox1
heme oxygenase-1
- IFN-γ
interferon gamma
- IL
interleukin
- IRF-1
interferon regulatory factor-1
- IκB
NF-κB inhibitor
- JNK
c-Jun NH2-terminal kinases
- LAN
lipoteichoic acid
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MAVS
mitochondrial antiviral signaling protein
- MEF2
myocyte enhancer factor-2
- MPT
membrane permeability transition
- mtDNA
mitochondrial DNA
- mtTFA or Tfam
mitochondrial transcription factor A
- MyD88
myeloid differentiation primary response protein
- Nfe2l2 or Nrf2
nuclear factor erythroid 2-related factor 2
- NLRP
NACHT, LRR, and PYD domains-containing protein
- NOD
nucleotide-binding oligomerization domain
- NOS2 or iNOS
nitric oxide synthase 2
- NRF
nuclear respiratory factor
- PAF
platelet-activating factor
- PAMP
pathogen-associated molecular pattern
- PARIS
PARkin-interacting substrate, ZNF746
- PGC-1 or Ppargc1
peroxisome proliferator-activated receptor gamma-1 coactivator
- PGN
peptidoglycan
- PKA
protein kinase A
- PKB or Akt
protein kinase B
- Pol γ
DNA polymerase γ
- PRR
pattern recognition receptor
- RIG
retinoic acid-inducible gene
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- STAT-1a
signal transducer and activator of transcription-1a
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
Acknowledgment
This work was supported, in part, by the NIH P01 HL108801-04, Project 2 (CAP).
References
- 1.Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, and Gillespie MN. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal 5: ra47, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, and Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Guardia D, Palomer X, Coll T, Davidson MM, Chan TO, Feldman AM, Laguna JC, and Vazquez-Carrera M. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc Res 87: 449–458, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Astiz M, Rackow EC, Weil MH, and Schumer W. Early impairment of oxidative metabolism and energy production in severe sepsis. Circ Shock 26: 311–320, 1988 [PubMed] [Google Scholar]
- 5.Austin S. and St-Pierre J. PGC1alpha and mitochondrial metabolism—emerging concepts and relevance in ageing and neurodegenerative disorders. J Cell Sci 125: 4963–4971, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bolanos JP, Peuchen S, Heales SJ, Land JM, and Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem 63: 910–916, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Borniquel S, Valle I, Cadenas S, Lamas S, and Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. FASEB J 20: 1889–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, and Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 360: 219–223, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Brealey D, Karyampudi S, Jacques TS, Novelli M, Stidwill R, Taylor V, Smolenski RT, and Singer M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am J Physiol Regul Integr Comp Physiol 286: R491–R497, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Brown GC. and Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta 1658: 44–49, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Callahan LA. and Supinski GS. Downregulation of diaphragm electron transport chain and glycolytic enzyme gene expression in sepsis. J Appl Physiol (1985) 99: 1120–1126, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Cantoni L, Rossi C, Rizzardini M, Gadina M, and Ghezzi P. Interleukin-1 and tumour necrosis factor induce hepatic haem oxygenase. Feedback regulation by glucocorticoids. Biochem J 279 (Pt 3): 891–894, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carre JE, Orban JC, Re L, Felsmann K, Iffert W, Bauer M, Suliman HB, Piantadosi CA, Mayhew TM, Breen P, Stotz M, and Singer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 182: 745–751, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellheim A, Brekke OL, Espevik T, Harboe M, and Mollnes TE. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand J Immunol 69: 479–491, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Chaung WW, Wu R, Ji Y, Dong W, and Wang P. Mitochondrial transcription factor A is a proinflammatory mediator in hemorrhagic shock. Int J Mol Med 30: 199–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherry AD, Suliman HB, Bartz RR, and Piantadosi CA. Peroxisome proliferator-activated receptor gamma co-activator 1-alpha as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J Biol Chem 289: 41–52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chtarbanova S. and Imler JL. Microbial sensing by Toll receptors: a historical perspective. Arterioscler Thromb Vasc Biol 31: 1734–1738, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, and Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett 345: 50–54, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Cook SA, Matsui T, Li L, and Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem 277: 22528–22533, 2002 [DOI] [PubMed] [Google Scholar]
- 20.D'Souza AD, Parikh N, Kaech SM, and Shadel GS. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion 7: 374–385, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, and Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev 22: 1490–1500, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock 9: 1–11, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Dhakshinamoorthy S. and Porter AG. Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J Biol Chem 279: 20096–20107, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Dominy JE. and Puigserver P. Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harb Perspect Biol 5: pii:, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, and Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du K. and Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273: 32377–32379, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Duvigneau JC, Piskernik C, Haindl S, Kloesch B, Hartl RT, Huttemann M, Lee I, Ebel T, Moldzio R, Gemeiner M, Redl H, and Kozlov AV. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab Invest 88: 70–77, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Eisele PS, Salatino S, Sobek J, Hottiger MO, and Handschin C. The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J Biol Chem 288: 2246–2260, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V, and Stefanidis I. Damage-associated molecular patterns derived from mitochondria may contribute to the hemodialysis-associated inflammation. Int Urol Nephrol 46: 107–112, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Eum HA, Park SW, and Lee SM. Role of nitric oxide in the expression of hepatic vascular stress genes in response to sepsis. Nitric Oxide 17: 126–133, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Eyenga P, Roussel D, Morel J, Rey B, Romestaing C, Teulier L, Sheu SS, Goudable J, Negrier C, and Viale JP. Early septic shock induces loss of oxidative phosphorylation yield plasticity in liver mitochondria. J Physiol Biochem 70: 285–296, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Fink MP. Bench-to-bedside review: cytopathic hypoxia. Crit Care 6: 491–499, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried RC, Bailey PM, Mullen JL, Stein TP, Crosby LO, and Buzby GP. Alterations in exogenous substrate metabolism in sepsis. Arch Surg 121: 173–178, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Galea E. and Feinstein DL. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. FASEB J 13: 2125–2137, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Gaweda-Walerych K. and Zekanowski C. Integrated pathways of parkin control over mitochondrial maintenance—relevance to Parkinson's disease pathogenesis. Acta Neurobiol Exp (Wars) 73: 199–224, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Gemsa D, Woo CH, Fudenberg HH, and Schmid R. Stimulation of heme oxygenase in macrophages and liver by endotoxin. J Clin Invest 53: 647–651, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerhart-Hines Z, Dominy JE, Jr., Blattler SM, Jedrychowski MP, Banks AS, Lim JH, Chim H, Gygi SP, and Puigserver P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol Cell 44: 851–863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, and Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass CK. and Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol 6: 44–55, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Gordon JL. Extracellular ATP: effects, sources and fate. Biochem J 233: 309–319, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutsaeva DR, Carraway MS, Suliman HB, Demchenko IT, Shitara H, Yonekawa H, and Piantadosi CA. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J Neurosci 28: 2015–2024, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haden DW, Suliman HB, Carraway MS, Welty-Wolf KE, Ali AS, Shitara H, Yonekawa H, and Piantadosi CA. Mitochondrial biogenesis restores oxidative metabolism during Staphylococcus aureus sepsis. Am J Respir Crit Care Med 176: 768–777, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han J, Jiang Y, Li Z, Kravchenko VV, and Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386: 296–299, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Handschin C, Rhee J, Lin J, Tarr PT, and Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci U S A 100: 7111–7116, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, and Meisinger C. The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell Metab 19: 357–372, 2014 [DOI] [PubMed] [Google Scholar]
- 46.Harris RA, Harris DL, and Green DE. Effect of Bordetella endotoxin upon mitochondrial respiration and energized processes. Arch Biochem Biophys 128: 219–230, 1968 [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, and Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta. Proc Natl Acad Sci U S A 98: 6336–6341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol 35: 253–261, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irrcher I, Ljubicic V, and Hood DA. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am J Physiol Cell Physiol 296: C116–C123, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Jager S, Handschin C, St-Pierre J, and Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104: 12017–12022, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jais A, Einwallner E, Sharif O, Gossens K, Lu TT, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, Duvigneau JC, Lindroos-Christensen J, Zapf TC, Amann S, Saluzzo S, Jantscher F, Stiedl P, Todoric J, Martins R, Oberkofler H, Muller S, Hauser-Kronberger C, Kenner L, Casanova E, Sutterluty-Fall H, Bilban M, Miller K, Kozlov AV, Krempler F, Knapp S, Lumeng CN, Patsch W, Wagner O, Pospisilik JA, and Esterbauer H. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell 158: 25–40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kantrow SP, Taylor DE, Carraway MS, and Piantadosi CA. Oxidative metabolism in rat hepatocytes and mitochondria during sepsis. Arch Biochem Biophys 345: 278–288, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Kleinert H, Schwarz PM, and Forstermann U. Regulation of the expression of inducible nitric oxide synthase. Biol Chem 384: 1343–1364, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Kolamunne RT, Dias IH, Vernallis AB, Grant MM, and Griffiths HR. Nrf2 activation supports cell survival during hypoxia and hypoxia/reoxygenation in cardiomyoblasts; the roles of reactive oxygen and nitrogen species. Redox Biol 1: 418–426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kreymann G, Grosser S, Buggisch P, Gottschall C, Matthaei S, and Greten H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit Care Med 21: 1012–1019, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Kuroda Y, Mitsui T, Kunishige M, Shono M, Akaike M, Azuma H, and Matsumoto T. Parkin enhances mitochondrial biogenesis in proliferating cells. Hum Mol Genet 15: 883–895, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Kyriakis JM. and Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 92: 689–737, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, Chen B, Carin L, Suarez A, Mohney RP, Freeman DH, Wang M, You J, Wulff J, Thompson JW, Moseley MA, Reisinger S, Edmonds BT, Grinnell B, Nelson DR, Dinwiddie DL, Miller NA, Saunders CJ, Soden SS, Rogers AJ, Gazourian L, Fredenburgh LE, Massaro AF, Baron RM, Choi AM, Corey GR, Ginsburg GS, Cairns CB, Otero RM, Fowler VG, Jr., Rivers EP, Woods CW, and Kingsmore SF. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci Transl Med 5: 195ra95, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen FJ, Schiffer TA, Weitzberg E, and Lundberg JO. Regulation of mitochondrial function and energetics by reactive nitrogen oxides. Free Radic Biol Med 53: 1919–1928, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, Thackray L, Brassil MM, Virgin HW, Nikolich-Zugich J, Moses AV, Gale M, Jr., Fruh K, and Diamond MS. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog 9: e1003118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HC, Yin PH, Lu CY, Chi CW, and Wei YH. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J 348Pt 2: 425–432, 2000 [PMC free article] [PubMed] [Google Scholar]
- 62.Lee I. and Huttemann M. Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta 1842: 1579–1586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levy RJ, Vijayasarathy C, Raj NR, Avadhani NG, and Deutschman CS. Competitive and noncompetitive inhibition of myocardial cytochrome C oxidase in sepsis. Shock 21: 110–114, 2004 [DOI] [PubMed] [Google Scholar]
- 64.Lu Z, Xu X, Hu X, Fassett J, Zhu G, Tao Y, Li J, Huang Y, Zhang P, Zhao B, and Chen Y. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal 13: 1011–1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacGarvey NC, Suliman HB, Bartz RR, Fu P, Withers CM, Welty-Wolf KE, and Piantadosi CA. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am J Respir Crit Care Med 185: 851–861, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martinon F, Burns K, and Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10: 417–426, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Mela L, Bacalzo LV, Jr., and Miller LD. Defective oxidative metabolism of rat liver mitochondria in hemorrhagic and endotoxin shock. Am J Physiol 220: 571–577, 1971 [DOI] [PubMed] [Google Scholar]
- 69.Mela L. and Miller LD. Efficacy of glucocorticoids in preventing mitochondrial metabolic failure in endotoxemia. Circ Shock 10: 371–381, 1983 [PubMed] [Google Scholar]
- 70.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, and Horng T. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A 109: 11282–11287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musatov A. and Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res 46: 1313–1326, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, Fitzgerald KA, Ryter SW, and Choi AM. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12: 222–230, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, and Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299: 896–899, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Oppenheim JJ. and Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 17: 359–365, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, and Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Palikaras K. and Tavernarakis N. Mitochondrial homeostasis: the interplay between mitophagy and mitochondrial biogenesis. Exp Gerontol 56: 182–188, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Pautz A, Art J, Hahn S, Nowag S, Voss C, and Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 23: 75–93, 2010 [DOI] [PubMed] [Google Scholar]
- 78.Peng HB, Libby P, and Liao JK. Induction and stabilization of I kappa B alpha by nitric oxide mediates inhibition of NF-kappa B. J Biol Chem 270: 14214–14219, 1995 [DOI] [PubMed] [Google Scholar]
- 79.Piantadosi CA, Carraway MS, Babiker A, and Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103: 1232–1240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piantadosi CA. and Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem 281: 324–333, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE, and Suliman HB. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem 286: 16374–16385, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poyton RO, Ball KA, and Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab 20: 332–340, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Pusterla T, de Marchis F, Palumbo R, and Bianchi ME. High mobility group B2 is secreted by myeloid cells and has mitogenic and chemoattractant activities similar to high mobility group B1. Autoimmunity 42: 308–310, 2009 [DOI] [PubMed] [Google Scholar]
- 84.Reynolds CM, Suliman HB, Hollingsworth JW, Welty-Wolf KE, Carraway MS, and Piantadosi CA. Nitric oxide synthase-2 induction optimizes cardiac mitochondrial biogenesis after endotoxemia. Free Radic Biol Med 46: 564–572, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rizzardini M, Carelli M, Cabello Porras MR, and Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: synergism by glutathione depletion and protection by N-acetylcysteine. Biochem J 304 (Pt 2): 477–483, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, and Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Roger PM, Hyvernat H, Ticchioni M, Kumar G, Dellamonica J, and Bernardin G. The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J Crit Care 27: 384–393, 2012 [DOI] [PubMed] [Google Scholar]
- 88.Rothfuss O, Fischer H, Hasegawa T, Maisel M, Leitner P, Miesel F, Sharma M, Bornemann A, Berg D, Gasser T, and Patenge N. Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum Mol Genet 18: 3832–3850, 2009 [DOI] [PubMed] [Google Scholar]
- 89.Rubartelli A. and Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 28: 429–436, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Ryter SW. and Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol 41: 251–260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sangiuliano B, Perez NM, Moreira DF, and Belizario JE. Cell death-associated molecular-pattern molecules: inflammatory signaling and control. Mediators Inflamm 2014: 821043, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scarffe LA, Stevens DA, Dawson VL, and Dawson TM. Parkin and PINK1: much more than mitophagy. Trends Neurosci 37: 315–324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev 88: 611–638, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Scarpulla RC, Vega RB, and Kelly DP. Transcriptional integration of mitochondrial biogenesis. Trends Endocrinol Metab 23: 459–466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schieber M. and Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schulze-Osthoff K, Bakker AC, Vanhaesebroeck B, Beyaert R, Jacob WA, and Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem 267: 5317–5323, 1992 [PubMed] [Google Scholar]
- 97.Schumer W, Erve P, Kapica SK, and Moss GS. Endotoxin effect on respiration of rat liver mitochondria. J Surg Res 10: 609–612, 1970 [DOI] [PubMed] [Google Scholar]
- 98.Seong SY. and Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4: 469–478, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Seth RB, Sun L, Ea CK, and Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122: 669–682, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Shaulian E. and Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136, 2002 [DOI] [PubMed] [Google Scholar]
- 101.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, and Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, and Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson's disease. Cell 144: 689–702, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singer M. Mitochondrial function in sepsis: acute phase versus multiple organ failure. Crit Care Med 35: S441–S448, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5: 66–72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sjovall F, Morota S, Persson J, Hansson MJ, and Elmer E. Patients with sepsis exhibit increased mitochondrial respiratory capacity in peripheral blood immune cells. Crit Care 17: R152, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, and Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Subramanian N, Natarajan K, Clatworthy MR, Wang Z, and Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153: 348–361, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Suliman HB, Babiker A, Withers CM, Sweeney TE, Carraway MS, Tatro LG, Bartz RR, Welty-Wolf KE, and Piantadosi CA. Nitric oxide synthase-2 regulates mitochondrial HSP60 chaperone function during bacterial peritonitis in mice. Free Radic Biol Med 48: 736–746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suliman HB, Carraway MS, and Piantadosi CA. Postlipopolysaccharide oxidative damage of mitochondrial DNA. Am J Respir Crit Care Med 167: 570–579, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Suliman HB, Sweeney TE, Withers CM, and Piantadosi CA. Co-regulation of nuclear respiratory factor-1 by NFkappaB and CREB links LPS-induced inflammation to mitochondrial biogenesis. J Cell Sci 123: 2565–2575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, and Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 64: 279–288, 2004 [DOI] [PubMed] [Google Scholar]
- 112.Sweeney TE, Suliman HB, Hollingsworth JW, and Piantadosi CA. Differential regulation of the PGC family of genes in a mouse model of Staphylococcus aureus sepsis. PLoS One 5: e11606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sweeney TE, Suliman HB, Hollingsworth JW, Welty-Wolf KE, and Piantadosi CA. A toll-like receptor 2 pathway regulates the Ppargc1a/b metabolic co-activators in mice with Staphylococcal aureus sepsis. PLoS One 6: e25249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, and Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11: 443–451, 1999 [DOI] [PubMed] [Google Scholar]
- 115.Tatsumi T, Akashi K, Keira N, Matoba S, Mano A, Shiraishi J, Yamanaka S, Kobara M, Hibino N, Hosokawa S, Asayama J, Fushiki S, Fliss H, Nakagawa M, and Matsubara H. Cytokine-induced nitric oxide inhibits mitochondrial energy production and induces myocardial dysfunction in endotoxin-treated rat hearts. J Mol Cell Cardiol 37: 775–784, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Thiemermann C. Nitric oxide and septic shock. Gen Pharmacol 29: 159–166, 1997 [DOI] [PubMed] [Google Scholar]
- 117.ThyagaRajan SPD. and Priyanka HP. Bidirectional communication between the neuroendocrine system and the immune system: relevance to health and diseases. Ann Neurosci 19: 40–46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tran TA, Nguyen AD, Chang J, Goldberg MS, Lee JK, and Tansey MG. Lipopolysaccharide and tumor necrosis factor regulate Parkin expression via nuclear factor-kappa B. PLoS One 6: e23660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trzeciak S. and Rivers EP. Clinical manifestations of disordered microcirculatory perfusion in severe sepsis. Crit Care 9Suppl 4: S20–S26, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, and Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res 66: 562–573, 2005 [DOI] [PubMed] [Google Scholar]
- 121.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, and Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vanlangenakker N, Vanden Berghe T, and Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ 19: 75–86, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, and Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251, 1999 [DOI] [PubMed] [Google Scholar]
- 124.Wang X, Qin W, Qiu X, Cao J, Liu D, and Sun B. A novel role of exogenous carbon monoxide on protecting cardiac function and improving survival against sepsis via mitochondrial energetic metabolism pathway. Int J Biol Sci 10: 777–788, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weitzel JM, Iwen KA, and Seitz HJ. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol 88: 121–128, 2003 [DOI] [PubMed] [Google Scholar]
- 126.Willis D, Moore AR, Frederick R, and Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med 2: 87–90, 1996 [DOI] [PubMed] [Google Scholar]
- 127.Wills LP, Trager RE, Beeson GC, Lindsey CC, Peterson YK, Beeson CC, and Schnellmann RG. The beta2-adrenoceptor agonist formoterol stimulates mitochondrial biogenesis. J Pharmacol Exp Ther 342: 106–118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu Z, Huang X, Feng Y, Handschin C, Feng Y, Gullicksen PS, Bare O, Labow M, Spiegelman B, and Stevenson SC. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A 103: 14379–14384, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, and Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999 [DOI] [PubMed] [Google Scholar]
- 130.Yang CS, Kim JJ, Lee HM, Jin HS, Lee SH, Park JH, Kim SJ, Kim JM, Han YM, Lee MS, Kweon GR, Shong M, and Jo EK. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy 10: 785–802, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoboue ED. and Devin A. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int J Cell Biol 2012: 403870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Youle RJ. and Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59, 2008 [DOI] [PubMed] [Google Scholar]
- 133.Zhang JZ, Liu Z, Liu J, Ren JX, and Sun TS. Mitochondrial DNA induces inflammation and increases TLR9/NF-kappaB expression in lung tissue. Int J Mol Med 33: 817–824, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 134.Zhang Q, Itagaki K, and Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock 34: 55–59, 2010 [DOI] [PubMed] [Google Scholar]
- 135.Zhou R, Tardivel A, Thorens B, Choi I, and Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11: 136–140, 2010 [DOI] [PubMed] [Google Scholar]
- 136.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]
- 137.Zhu J, Wang KZ, and Chu CT. After the banquet: mitochondrial biogenesis, mitophagy, and cell survival. Autophagy 9: 1663–1676, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]