Abstract
Using a transgenic mouse (mus musculus) that labels nestin-expressing progenitors with eGFP, we previously characterized the expression of GltI and Glast on early neural progenitors in vivo. To address their functional role in this cell population, we manipulated their expression in P7 neurospheres isolated from the dentate gyrus. We observed that knockdown of GltI or Glast was associated with decreased BrdU incorporation and neurosphere formation. Moreover, we determined that both glutamate transporters regulate progenitor proliferation in a calcium- and metabotropic glutamate receptor-dependent manner. To address the relevance of this in vivo, we utilized models of acquired brain injury, which are known to induce hippocampal neurogenesis. We observed that GltI and Glast are specifically upregulated in progenitors following brain injury and that this increased expression is maintained for many weeks. Additionally, we found that recurrently injured animals with increased expression of glutamate transporters within the progenitor population were resistant to subsequent injury-induced proliferation. These findings demonstrate that GltI and Glast negatively regulate calcium-dependent proliferation in vitro and their upregulation after injury is associated with decreased proliferation after brain trauma.
Keywords: neural stem cells, hippocampus, glutamate, neuroregeneration, mus musculus
Introduction
During adult neurogenesis glial fibrillary acidic protein (GFAP)-expressing quiescent type I cells self-renew, divide and give rise to transient amplifying type IIa cells which will differentiate into neuronal nuclei (NeuN)-expressing mature granular cell neurons (Mullen et al., 1992; Gage et al., 1995; Gage et al., 1998; Kempermann & Gage, 2000; Blaiss et al., 2011). We previously developed and characterized a transgenic animal that labels type I and IIa progenitor cells with green fluorescent protein (GFP) under the control of the nestin promoter and its second intron enhancer (Miles & Kernie, 2006; Shi et al., 2007; Koch et al., 2008; Miles & Kernie, 2008; Yu et al., 2008; Gilley et al., 2011). Using this transgenic line, we created a developmental profile of the postnatal dentate gyrus that documented cell-autonomous proliferative phenotypes and identified several candidate genes that may regulate this neurogenic niche. Excitatory amino acid transporter 2 (GltI), a glutamate transporter, was identified in this screen and found to be upregulated about ten-fold in postnatal day 28 (P28) progenitors compared to those isolated at P7. In addition we confirmed that GltI and its closely related partner Glast (Eaat1) were expressed on type I and IIa neural progenitors in vivo (Gilley et al., 2011).
Although the function of glutamate transporters has been well-documented in astrocytes, their role in early neural progenitors has not been established. In the synaptic cleft, glutamate transporters are neuroprotective and function on astrocytes to regulate extracellular concentrations of glutamate, the main neurotransmitter in the brain (Danbolt, 2001; Fonnum & Lock, 2004; Maragakis et al., 2004). In addition to its role as a neurotransmitter, glutamate has also been shown to positively regulate neural progenitor proliferation (Luk et al., 2003; Brazel et al., 2005; Suzuki et al., 2006). We therefore chose to examine how glutamate transporters regulate progenitor proliferation in vitro.
We used a combination of neurosphere cultures and models of acquired brain injury to elucidate the function of GltI and Glast in early neural progenitors. Using neurospheres derived exclusively from the hippocampal dentate gyrus, we manipulated glutamate transporter expression with small interfering RNAs (siRNAs) and overexpression plasmids and analyzed the effect on proliferation. Our results indicate that glutamate transporters function on neural progenitors to negatively regulate calcium-dependent proliferation. By using models of brain injury to induce hippocampal neurogenesis we were also able to demonstrate prolonged upregulation of GltI and Glast after injury. Moreover, increased transporter expression is associated with decreased proliferation after subsequent brain injury. We have therefore identified a novel functional role for GltI and Glast in regulating neural progenitor proliferation in vitro, which is closely correlated with in vivo models of induced neurogenesis.
Methods
Cloning
GltI cDNA was purchased in plasmid form (pFLCI-GltI) from Geneservice Ltd. (Cambridge, CB4 OFE, UK, catalog #6330500I22) while Glast mouse cDNA (pCMV6-Glast-Myc) was purchased from Origene (Rockville, MD, US, catalog #MR208665). GltI cDNA was subcloned into pCMV-IRES-DsRed2 (Clontech, Mountain View, CA, US, catalog #632420) in order to create the GltI overexpression plasmid pCMV-GltI-DsRed2. The Glast cDNA construct (pCMV6-Glast-Myc) was directly used to overexpress Glast.
Transient Transfections
P7 dentate gyrus-derived neurospheres were dissociated into single cells and transfected with either siRNA compounds or overexpression plasmids driven by the CMV promoter. Lipofectamine RNAi Max (Invitrogen, Eugene, OR, US, catalog #56531) was used to reverse transfect 10 nM GltI siRNA (Sigma-Aldrich, St. Louis, MO, US, catalog #SASI_Rn01_00062046), 10 nM Glast siRNA (Sigma-Aldrich, St. Louis, MO, US, catalog #SASI_Rn02_00262083) or 10 nM scrambled siRNA (Applied Biosystems, Carlsbad, CA, US, catalog #AM4611). Lipofectamine 2000 (Invitrogen, Eugene, OR, US, catalog #52887) was used to transiently transfect 300 ng pCMV-GltI-DsRed2 (final concentration: 150 ng/ml), 300 ng pCMV-Glast-Myc (final concentration: 150 ng/ml) or 300 ng of pCMV-DsRed2 (Clontech, Mountain View, CA, US, catalog #632420) (final concentration: 150 ng/ml). Seven days after cells were transfected, some neurospheres were taken from each experiment and misexpression of GltI and Glast was confirmed using quantitative PCR (QPCR) analysis. Only transfections with confirmed knockdown or overexpression were used for further experimentation. The siRNA sequences are as follows: GltI sense: 5’-GAU AGU GAC UGU AAG CCU U-3’; GltI antisense: 5’-AAG GCU UAC AGU CAC UAU C-3’; Glast sense: 5’-CUG UCA UUG UGG GUA CAA U-3’; Glast antisense: 5’AUU GUA CCC ACA AUG ACA G-3’.
Quantitative PCR (QPCR)
Knockdown and overexpression of GltI and Glast were confirmed using methods adapted from previous publications (Gilley et al., 2011). Seven days after transfection, RNA was isolated from neurospheres, reversed transcribed into cDNA and relative mRNA levels were measured. mRNA levels were normalized to either GltI or Glast gene expression. The primer information is as follows: GAPDH forward: 5’-CTC AAC TAC ATG GTC TAC ATG TTC CA-3’; GAPDH reverse: 5’-CCA TTC TCG GCC TTG ACT GT-3’; GltI forward: 5’-GGA AGA TGG GTG AAC AGG C-3’; GltI reverse: 5’-TTC CCA CAA ATC AAG CAG G-3’; Glast forward: 5’-ACG GTC ACT GCT GTC ATT G-3’; Glast reverse: 5’-TGT GAC GAG ACT GGA GAT GA-3’.
Neurosphere growth and assays
All media used in the preparation and growth of neurospheres was done so in Neurobasal A media (Invitrogen, Eugene, OR, US, catalog #10888-022) lacking glutamate and aspartate. Preparation of activated papain, neural stem cell media and semi-solid media was adapted from previously described protocols. All neurospheres were derived from P7 dentate gyrus and grown in neural stem cell media for seven days before they used for further experimentation (see (Gilley et al., 2011) for prior protocols).
Misexpression neurosphere assays were performed in glutamate free media on neurospheres transfected with either siRNA or overexpression plasmids (see transient transfections). Briefly, P7 neurospheres were dissociated into single cells, transiently transfected using Lipofectamine and were grown in neural stem cell media for seven days. Transfected neurospheres were dissociated again, counted and plated in semi-solid media before they were quantified seven days later. This experiment was repeated four times and analyzed for significance using One Way ANOVA and Bonferonni post hoc analysis.
For the glutamate neurosphere assays, neurospheres were grown in glutamate-free media for seven days, mechanically dissociated into a single-cell solution and plated in semi-solid media supplemented with either 0 µM or 5 µM glutamate. Additionally, we treated some of our cells with 10 µM (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) which serves as a nonselective metabotropic glutamate receptor (mGluR) antagonist at this concentration (Kingston et al., 1998). Another group of cells were treated with 1 µM 1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N’,N’-tetra acetic acid tetrakis ester (BAPTA-AM) which serves as an intracellular calcium chelator (Santa Cruz, Santa Cruz, CA, catalog #sc-202488). After seven days in culture, neurospheres were quantified and the diameters of at least twenty neurospheres from each group were measured (Gilley et al., 2011). An Unpaired t test and Bonferonni post hoc analysis were used to calculate significance. The experiment was repeated at least four times for each condition.
Bromodeoxyuridine (BrdU) Incorporation
Transiently transfected neurospheres were pulsed with 10 µM BrdU for fifteen minutes, dissociated with activated papain and triturated into a single-cell solution before they were fixed overnight with 100% ethanol. 2N HCl/0.5% TritonX in PBS was used to denature DNA for thirty minutes at room temperature. Afterwards the reaction was neutralized with 0.1 M NaB407 before being incubated in staining solution (1.3 µl BrdU-APC (BD Pharmingen, San Diego, CA, US, catalog #552598), 5 µl RNase and 50 µl 0.5% Tween/1% BSA in PBS) overnight at four degrees. Cells were then washed and resuspended in PBS with propidium iodide (PI) and analyzed for BrdU incorporation via flow cytometry. Results are displayed as the percentage of cells that were BrdU-positive and PI-negative. The experiment was performed in quadruplicate. Statistical significance was measured using a One Way ANOVA followed by Bonferonni post hoc analysis.
Intracellular Calcium Measurement
After misexpression was confirmed seven days after transfection, all neurospheres were dissociated and stained with Fluo4-AM calcium indicator (Invitrogen, Eugene, OR, US, catalog #F14201) after cells had been exposed to a variety of compounds including an intracellular calcium chelator and a mGluR antagonist. Cells were incubated with 1 µM BAPTA-AM according to the manufacturer’s protocols. Additionally, 10 µM LY341495 was added to a portion of dissociated progenitors that acts as a nonselective mGluR antagonist at high concentrations (Kingston et al., 1998). Cells were then analyzed using flow cytometry methods. Prior to supplementation with glutamate, baseline intracellular calcium levels were measured for thirty seconds. Cells were then spiked with 5 µM glutamate and calcium measurements were recorded for an additional minute and a half. The percentage of cells containing increased intracellular calcium levels (compared to baseline) is presented. The final percentage of cells expressing Fluo4-AM was normalized to the percentage of positive cells at baseline levels (% induced cells/% baseline cells). Intracellular calcium was measured in quadruplicate for each condition and significance was calculated using One Way ANOVA and Bonferonni post hoc analysis.
Mice
All animal experiments were approved by the Institutional Animal Use and Care Committee at UT Southwestern Medical Center. The Animal Resource Center within UT Southwestern, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care humanely housed and cared for all animals.
All experiments were performed using nestin-rtTA-eGFP transgenic mice in a CD1 background which have been well characterized (Miles & Kernie, 2006; Shi et al., 2007; Koch et al., 2008; Miles & Kernie, 2008; Yu et al., 2008; Gilley et al., 2011). P28 transgenic mice were exposed to either HI injury or sham injury before being treated with traumatic brain injury (TBI). Both groups of animals (Sham + TBI or HI + TBI) were anesthetized, placed in a supine position, had their right carotid artery ligated and were allowed to recover 30–60 minutes (see below). Mice in the HI+TBI group were then exposed to 8% oxygen for one hour in a hypoxic chamber before they were allowed to recover in room air. Animals in the Sham+TBI group were not treated with hypoxia. At P60 animals from both groups were injured using controlled cortical impact (CCI) in order to induce TBI. From P64-P67 mice were injected with a single daily dose of 100 mg/kg BrdU and were perfused and sacrificed two hours after injection on P67.
The Rice-Vanucci model was used to emulate HI injury and has been described in previously published protocols (Koch et al., 2008; Miles & Kernie, 2008). Briefly, mice were anesthetized and maintained on isoflurane mixed with oxygen and nitrogen. A midline incision was made and the right carotid artery was isolated and ligated with suture. In sham injuries, the artery was exposed but not ligated. After the incision was closed animals were allowed to recover up to one hour. Only animals that fully recovered were used for further experimentation. After recovery mice were placed in a hypoxic chamber and treated with 8% oxygen for one hour. Mice were then allowed to recover in their cage.
A CCI devise was used to induce TBI and the methodology has been described elsewhere (Kernie et al., 2001). 32 days after HI or sham injuries (P60), mice were anesthetized, placed in a stereotactic frame and part of the skull was removed before the brain was impacted with a steel tipped device in order to generate the injury. The incision was closed and the mice were allowed to recover. Daily injections of BrdU (100 mg/kg) were started four days after TBI at P64 and mice were sacrificed and perfused at P67.
Immunostaining
One day, three days, seven days and 45 days after hypoxic-ischemic (HI) injury, 50 µm vibratome sections were obtained and stained for the presence of GltI and Glast. After being blocked with normal donkey serum, sections were then stained with chicken anti-GFP 1:500 (Aves Labs, Tigard, OR, US, catalog #GFP-1020) mouse anti-GFAP 1:400 (BD Biosciences, Rockville, MD, US, catalog #556330) and guinea pig anti-GltI (catalog #AB1783) or anti-Glast (catalog #AB1782) (both 1:200) (Millipore, Temecula, CA, US). Fluorescent secondary antibodies were used at a concentration of 1:200 (Santa Cruz, Santa Cruz, CA, US). See (Gilley et al., 2011) for details.
Vibratome sections from HI+TBI and Sham+TBI animals were blocked in normal donkey serum, stained with rat anti-BrdU 1:400 (Abcam, Cambridge, MA, US, catalog #AB6326) and chicken anti-GFP 1:500 (Aves Labs, Tigard, OR, US, catalog #GFP-1020) according to previously described protocols (Gilley et al., 2011).
Only sections in which antibody and signal penetration were even were used for confocal imaging. Percentages of BrdU- and GFP-double positive cells were quantified (nonblinded) by colocalizing BrdU-positive nuclei with GFP which was entirely included in the section throughout the z axis. The top and bottom of the sections were identified by the presence of the first or last nuclei that could be entirely visualized by the z plane. Colocalization was determined by scanning serially through the z-axis of each cell using a depth of focus optical slice of 2.72 µm.
Imaging
Confocal images were acquired on a Zeiss LSM 510 (Zeiss, Jena, Gottingen, Germany) scanning confocal multicolor microscopy using Argon 488, He 543 and He 633 lasers (version 3.2 SP2, Aim interface) as previously described (Gilley et al., 2011). A Zeiss Neofluar 40x/1.3 and 63x/1.3 oil DIC lens were used. A pinhole aperture of 3.46 Airy units was used.
Fluorescent Activated Cell Sorting (FACS)
To determine the extent of GltI and Glast upregulation after injury, cells from the ipsilateral and contralateral dentate gyrus of injured transgenic animals were stained with mouse anti-polysialic acid neural cell adhesion molecule (PSA-NCAM) 1:200 (Millipore, Billerica, MA, US, catalog #MAB5324) for twenty minutes on ice. After washing away unbound primary antibody, cells were stained with donkey anti-mouse Alexa Fluor 647 1:200 (Molecular Probes, catalog #A31571) secondary antibody for an additional twenty minutes on ice. Cells were then washed and four different populations were sorted on a MoFlo (Beckman Coulter, Brea, CA, US, catalog #ML99030): ipsilateral GFP+/PSA-NCAM+ and GFP+/PSA-NCAM- and contralateral GFP+/PSA-NCAM+ and GFP+/PSA-NCAM-. Afterwards RNA was extracted and normalized across samples before being analyzed by QPCR.
Results
Glutamate transporter misexpression influences neurosphere formation and progenitor proliferation in vitro
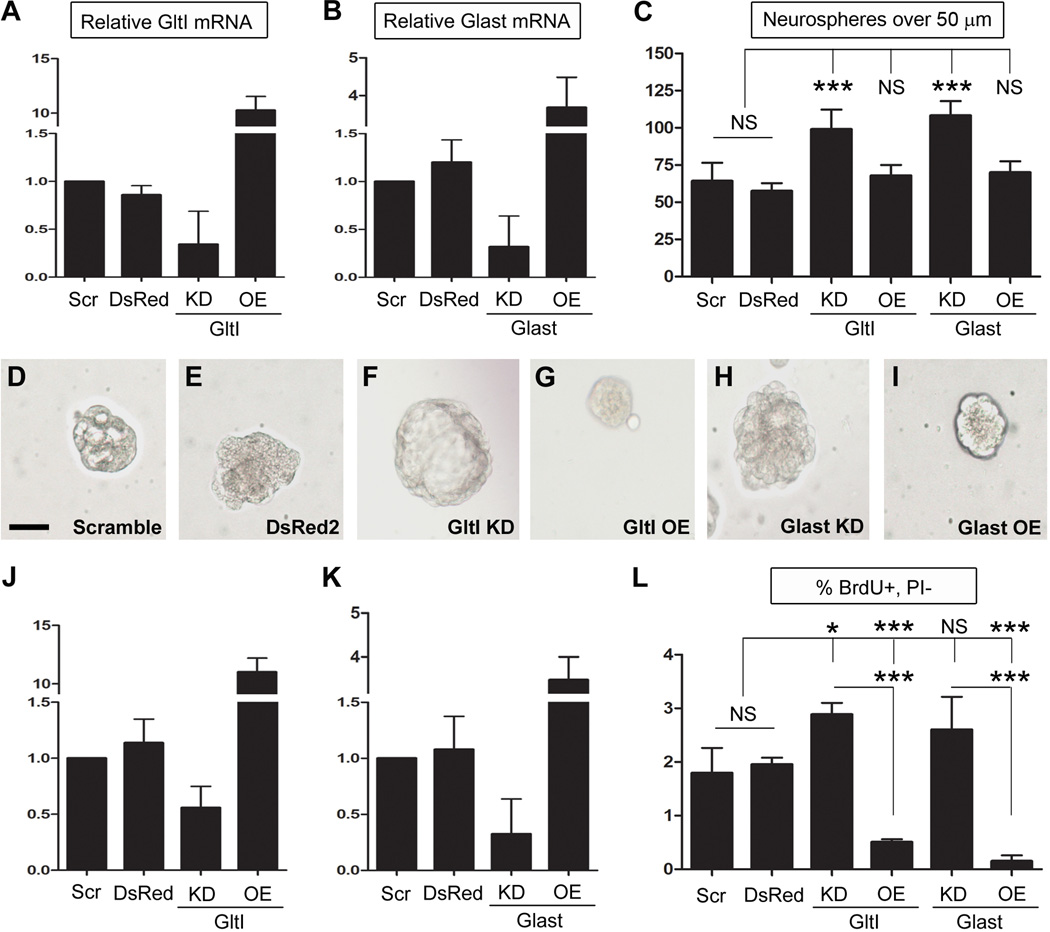
To determine if GltI and Glast contribute to regulating hippocampal neurogenesis, we performed gain- and loss-of-function experiments on neurosphere cultures grown in the absence of glutamate. First, we performed neurosphere assays on cells previously transfected with GltI and Glast siRNA or overexpression plasmids. Seven days after transfection, neurospheres were assayed for misexpression using quantitative PCR (QPCR) techniques (n=4). Relative mRNA levels were normalized to GltI and Glast expression in cells transfected with scrambled siRNA, a negative control (Figure 1, A–B). Relative levels of GltI expression were 0.9 in cells transfected with DsRed2, 0.3 for cells lacking GltI and 10.3 in cells overexpressing GltI (Figure 1, A). Glast expression levels after transfection were 1.2 for DsRed2, 0.3 for the knockdown and 3.7 for the overexpression experiment (Figure 1, B). These data suggest that both glutamate transporters are being misexpressed appropriately in culture compared to controls (cells transfected with Scramble or DsRed2).
Figure 1. Knockdown of glutamate transporters results in increased proliferation in vitro.
Seven days after neurospheres were transfected with DNA constructs or gene-specific siRNA, they were assayed for misexpression using QPCR (A–B and D–E). GltI and Glast relative mRNA expression was normalized to their respective expression levels in cells transfected with the Scramble negative control. (A, J) and (B, K) show that both GltI and Glast are knocked down and overexpressed appropriately. After misexpression was confirmed, neurospheres were dissociated and plated in semi-solid media for seven days before those over 50 µm were quantified (C). Knockdown of either GltI or Glast, resulted in an increase in the number of neurospheres formed compared to controls, while their overexpression did not have a significant effect on neurosphere formation. (D–I) shows representative neurospheres from each experiment and suggest that GltI and Glast expression levels affect neurosphere size as well. Progenitors were also pulsed with BrdU and stained with an anti-BrdU-APC antibody before being analyzed for incorporation via flow cytometry. Knockdown of GltI and Glast resulted in increased BrdU incorporation while their overexpression drastically decreased the percentage of BrdU- and PI- double-labeled cells (L). These results suggest that glutamate transporters regulate neurosphere proliferation in vitro. Statistical analysis in (C and F) represents Bonferonni post hoc analysis (*P<0.05; ***P<0.0001). Scale bar in (D) represents 50 µm. Scr, Scrambled siRNA; DsRed, DsRed2 plasmid; KD, knockdown; OE, overexpression; NS, not significant; PI, propidium iodide.
After overexpression and knockdown levels were confirmed, the remaining neurospheres were dissociated and replated in semisolid media containing neural stem cell media (lacking glutamate) and 1.6% methylcellulose at a density of 20 cells/µl. After seven days in culture, neurospheres over 50 µm were quantified (Figure 1, C). The average number of spheres per well was 64 for DsRed2, 57 in Scramble, 99 for GltI KD, 68 for GltI OE, 108 for Glast KD, and 69 in Glast OE. Analysis by One Way ANOVA suggest that these differences are significant (***P<0.0001, F=13.4, n=4). Representative neurospheres are shown for each condition and suggest that sphere size is dependent on the expression level of each glutamate transporter (Figure 1, D–I). Moreover, these results show that knockdown of glutamate transporters results in increased neurosphere formation in vitro.
To confirm this phenotype, we pulsed transfected neurospheres with bromodeoxyuridine (BrdU) and analyzed its incorporation using flow cytometry. In addition, the fidelity of misexpression of GltI and Glast was analyzed (Figure 1, J–K) (n=4). The average relative expression of GltI and Glast in cells transfected with DsRed2 were 1.1 for each. Knockdown with either Glast or GltI siRNA resulted in relative mRNA levels of 0.3 and 0.6, respectively, suggesting that both genes are knocked down by at least 44%. Cells transfected with overexpression plasmids demonstrated increased GltI (11.0) and Glast (3.5) expression. From this we can conclude that glutamate transporter knockdown and overexpression results in altered gene expression of GltI and Glast. These results are similar to those previously observed and suggest that we are able to consistently manipulate GltI and Glast expression levels in vitro.
Once misexpression was confirmed, the remaining neurospheres were pulsed with BrdU and stained with an anti-BrdU-APC antibody. We used flow cytometry to assess the effect of glutamate transporter misexpression on proliferation and the results are reported as a percentage of BrdU-positive, propidium iodide (PI)-negative cells (Figure 1, L). Baseline BrdU incorporation was 1.8% for DsRed2 and 2.0% for Scramble, both negative controls, which were not significantly different from one another. Knockdown of GltI resulted in 2.9% BrdU incorporation while its overexpression decreased the percentage of BrdU-positive, PI-negative cells to 0.5% when compared to negative controls. Similarly, Glast siRNA increased the percentage of BrdU-expressing cells to 2.6% however, its overexpression decreased BrdU incorporation to 0.2%. Statistical analysis with One Way ANOVA indicate that these results are statistically different (****P<0.0001, F=51.2, n=4). These experiments taken together indicate that GltI and Glast negatively regulate neural progenitor proliferation in vitro.
Glutamate-induced neurosphere proliferation is dependent on calcium and metabotropic glutamate receptors (mGluRs)
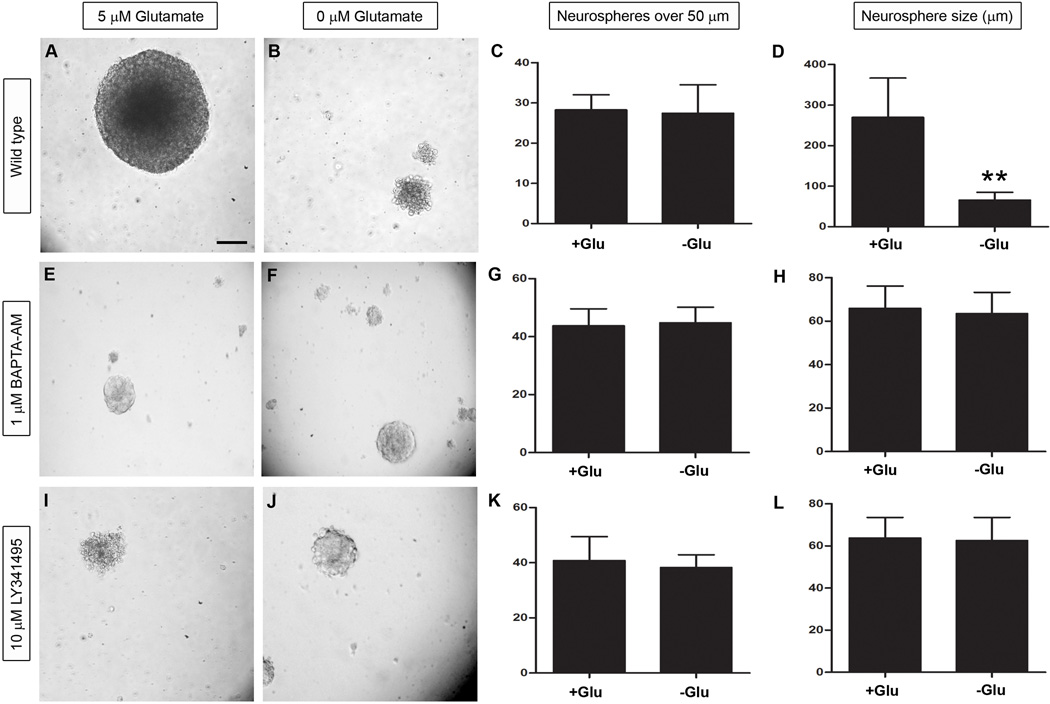
Although previous studies have shown that glutamate stimulates neurosphere proliferation in a number of different cell types (Luk et al., 2003; Brazel et al., 2005; Suzuki et al., 2006), there are no studies that specifically demonstrate glutamate’s effect on neurospheres derived exclusively from the dentate gyrus. To control for the presence of glutamate in most media, we prepared our neurospheres in media lacking glutamate (Neurobasal A) before supplementing them with either 0 µM or 5 µM glutamate prior to final plating. In addition to plating neurospheres under wild type conditions (Figure 2, A–D), we also repeated the assay in the presence of 1 µM 1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N’,N’-tetra acetic acid tetrakis ester (BAPTA-AM), a cell permeable calcium chelator which neutralizes intracellular calcium (Tsien, 1980; Dieter et al., 1993)(Figure 2, E–H). Finally, to determine the role of mGluRs in proliferation, we also supplemented neurospheres with 10 µM LY341495 which nonselectively inhibits all mGluRs at this concentration (Figure 2, I–L) (Kingston et al., 1998). After seven days in vitro, we quantified the number of neurospheres over 50 µm and their diameter as shown in Figure 2. Under wild type conditions, the number of spheres present was 27 and 28 in the presence and absence of exogenous glutamate, respectively (Figure 2, C). However, in media supplemented with glutamate, the average neurosphere size was 270 µm compared to 66 µm in media lacking glutamate (Figure 2, D). Although there was no difference in sphere number (Unpaired t test, P=0.96, F=9.0, n=7), the size of the neurospheres was statistically significant when analyzed by an Unpaired t test (****P<0.0001, F=24.9, n=20). When we supplemented the media with BAPTA-AM or LY341495 in the presence and absence of glutamate, we saw a similar number of neurospheres. In cells exposed to 1 µM BAPTA-AM, those treated with glutamate formed 44 neurospheres per well which averaged 66 µm in diameter (Figure 2, E and G). In the absence of glutamate, there were 45 spheres per well and their average diameter was 63 µm (Figure 2, F and H). Similarly, cells treated with LY341495 and 5 µM glutamate, exhibited 41 neurospheres with an average diameter of 64 µm (Figure 2, I and K) while those grown in media lacking glutamate formed 38 spheres with an average diameter of 63 µm (Figure2, J and L). According to statistical analysis with an Unpaired t tests, cells treated with BAPTA-AM were not significantly different in neurosphere formation (P=0.81, F=1.2, n=4) or neurosphere size (P=0.49, F=1.1, n=20). Similarly, neither neurosphere number (Unpaired t test, P=0.57, F=3.4, n=5) nor diameter (Unpaired t test, P=0.72, F=1.3, n=20) was significantly different in wells treated with LY341495. These results suggest that intracellular calcium and mGluRs are both required for glutamate-induced neurosphere proliferation in vitro.
Figure 2. Glutamate enhances mGluR-dependent neurosphere proliferation.
Neurospheres derived from P7 transgenic animals were grown in the presence (B, F and J) and absence (A, E and I) of exogenous glutamate and were quantified and measured as shown in (C, G and K) and (D, H and L), respectively. Although the number of neurospheres in each group were similar under wild type conditions (C), the overall size of the spheres was statistically significant as shown in (D), signifying that exogenous glutamate positively regulates neurosphere proliferation. In cells treated with either 1 µM BAPTA-AM or 10 µM LY341495 both the number and size of neurospheres were similar to one another in the presence and absence of glutamate suggesting that intracellular calcium and mGluRs are required for glutamate-induced neurosphere proliferation. Statistical analysis with a Bonferonni post hoc test is shown (**P<0.001). The scale bar in (A) represents 50 µm.
Glutamate transporter misexpression affects intracellular calcium levels
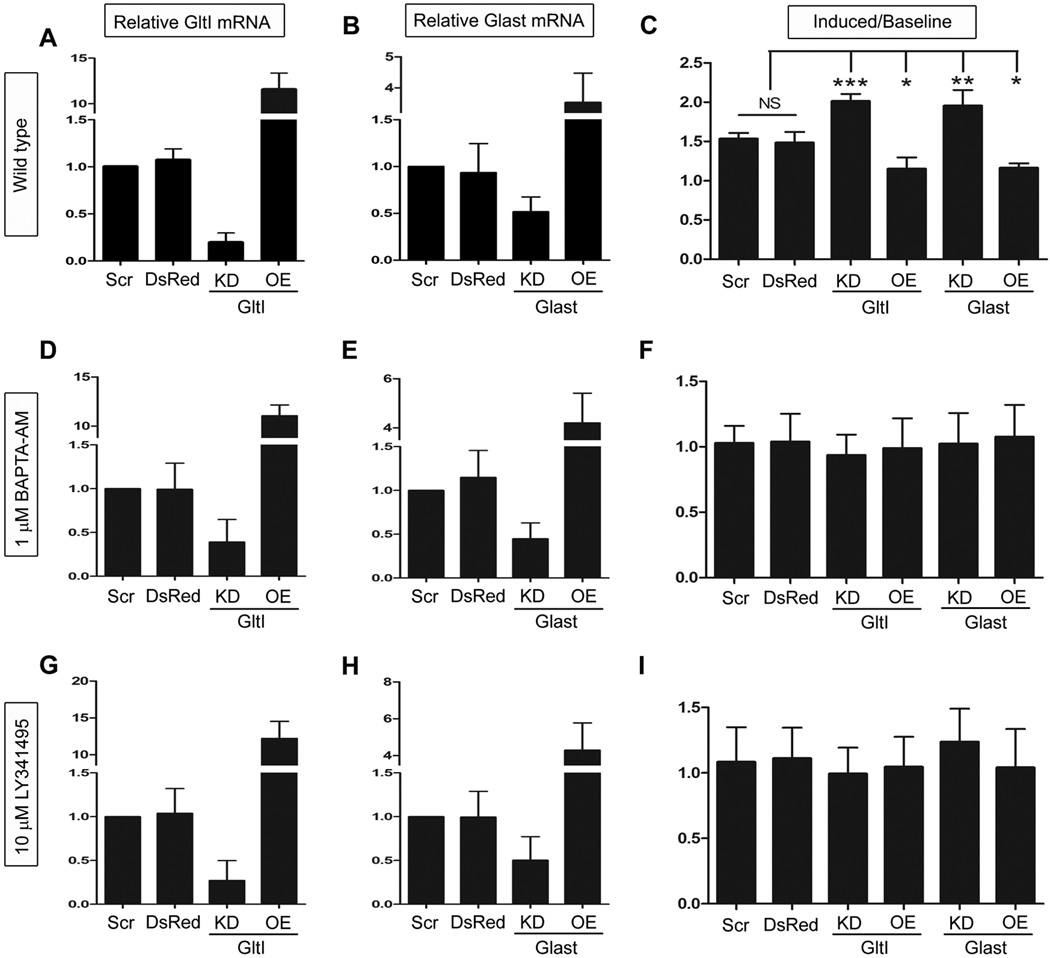
Although the exact mechanisms underlying neurogenesis remain unclear, several studies have suggested that the activation of certain ionotropic (ligand-gated ion channels) and/or mGluRs (G protein coupled) might be required (Choi et al., 1988; Luk et al., 2003; Deisseroth et al., 2004; Brazel et al., 2005; Nacher & McEwen, 2006; Nacher et al., 2007). Evidence suggests that activation of these receptors can also be associated with increases in intracellular calcium (Deisseroth et al., 2004; Brazel et al., 2005). We therefore chose to use calcium flux to assay for pro-proliferative pathways. To determine the effect of GltI and Glast misexpression on glutamate-mediated calcium flux, we incubated transfected neurospheres with a Fluo4-AM calcium indicator and assayed for changes in intracellular calcium using flow cytometry. Prior to stimulation with exogenous glutamate, baseline levels of intracellular calcium were recorded for thirty seconds. Afterwards, cells were stimulated with 5µM glutamate and we immediately began recording increased fluorescence indicating a proportional increase in intracellular calcium. The results are presented as a percentage of cells that are Fluo4-AM-positive and are normalized to the percentage of positive cells at baseline levels prior to stimulation with glutamate (Induced/Baseline) (Figure 3, C).
Figure 3. Glutamate transporters regulate intracellular calcium stores.
Once again, misexpression of GltI and Glast mRNA were confirmed after transfection (A–B, D–E and G–H) and progenitors were assayed for changes in glutamate-mediated intracellular calcium (C, F and I). Under wild type conditions knockdown of either GltI or Glast resulted in significant increases in intracellular calcium as a result of glutamate transporter knockdown. In cells treated with BAPTA-AM, an intracellular calcium chelator, the changes in calcium flux disappeared (F). Moreover, progenitors treated with 10 µM LY341495 which serves as a nonselective mGluR inhibitor at this concentration (Kingston et al., 1998) also demonstrated no change in intracellular calcium flux (I). These results suggest that GltI and Glast regulate calcium- and mGluR-dependent proliferation of neural progenitors in vitro. In (C, F and I) a Bonferonni post hoc analysis was used to determine statistical significance (*P<0.05; **P<0.001; ***P<0.0001). Scr, Scrambled siRNA; DsRed, DsRed2 plasmid; KD, knockdown; OE, overexpression; NS, not significant.
Before we conducted the experiment, we once again confirmed misexpression in vitro (Figure 3, A–B) (n=4). GltI and Glast relative expression was 1.1 and 0.9, respectively, in cells transfected with DsRed2. Cells transfected with either GltI or Glast siRNA exhibited expression levels of 0.2 and 0.5, respectively. Overexpression experiments demonstrated relative mRNA levels of 11.5 for GltI and 3.5 for Glast. Once again these results show that GltI and Glast mRNA can be reliably manipulated in vitro. About 4% of cells above baseline levels were Fluo4-AM-positive in cells transfected with either DsRed2 or scrambled siRNA prior to their stimulation with glutamate. After glutamate was added to the cells, the ratio of induced/baseline cells was 1.5 for both DsRed2 and Scramble. While knockdown of GltI resulted in a ratio of 2.0, its overexpression decreased the percentage of positive cells to 1.2. Glast overexpression also had a percentage at 1.2 while its knockdown resulted in a ratio of 2.0. Analysis with One Way ANOVA indicates that these results are different from one another (****P<0.0001, F=24.3, n=4). These results suggest that glutamate transporter knockdown leads to increased intracellular calcium, presumably through activation of glutamate receptors. This therefore allows the cells to proliferate more readily than when glutamate transporters are present at baseline or increased levels.
We next wanted to determine if this proliferative phenotype was calcium-dependent. To test this we repeated the experiment in the presence of 1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N’,N’-tetra acetic acid tetrakis ester (BAPTA-AM), a cell permeable calcium chelator which neutralizes intracellular calcium (Tsien, 1980; Dieter et al., 1993)(Figure 3, F). Prior to incubation with BAPTA-AM, misexpression was confirmed in transfected cells (n=4). GltI relative mRNA levels were 1.0 for Scramble, 1.0 for DsRed2, 0.4 for knockdown and 11.1 for overexpression. Glast expression was 1.0 in Scramble, 1.2 in DsRed2, 0.5 in knockdown and 4.2 in cells overexpressing Glast (Figure 3, D–E). After misexpression was confirmed we incubated transfected cells with BAPTA-AM prior to staining with Fluo4-AM. This allowed us to determine if intracellular calcium is required for the induction observed in Figure 3, C. Once again we recorded baseline levels of calcium before we stimulated the cells with glutamate at which point we calculated the ratio of Induced/Baseline cells. Results are shown in Figure 3, F. In cells transfected with Scramble, the ratio was 1.0 while cells transfected with DsRed2 also exhibited a ratio of 1.0. Cells knocked down with either GltI or Glast siRNA had ratios of 0.9 and 1.0, respectively. Lastly, cells overexpressing GltI and Glast both demonstrated ratios of 1.0. One Way ANOVA suggests that none of these results were statistically significant from one another (P=0.95, F=0.21, n=4). This suggests that intracellular calcium is required for the phenotype observed in Figure 3, C.
To confirm that mGluRs are required for the calcium flux observed in Figure 3, C, we repeated the experiment in the presence of 10 µM (2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) to inhibit all mGluR subtypes. Prior to experimentation, cells to be treated with LY341495 were analyzed for misexpression (n=4). Relative GltI expression levels for Scramble, DsRed2, knockdown and overexpression were 1.0, 1.0, 0.3 and 12.2, respectively while mRNA levels for Glast were 1.0, 1.0, 0.5 and 4.3, respectively (Figure 3, G–H). After misexpression was confirmed, cells previously treated with LY341495 were analyzed for glutamate-induced calcium flux. Results obtained were similar to those observed in Figure 3, F. The ratios of Induced/Baseline calcium flow for cells transfected with Scramble, DsRed2, GltI siRNA, GltI overexpression, Glast siRNA or Glast overexpression were 1.1, 1.1, 1.0, 1.0, 1.2 and 1.4, respectively (Figure 3, I). Analysis by One Way ANOVA were not statistically significant (P=0.80, F=0.46, n=4). Therefore, inhibiting mGluRs stifles the phenotype first seen in Figure 3, C. These results suggest that glutamate transporters regulate intracellular calcium-dependent proliferation of neural progenitors in a mGluR-dependent fashion.
Glutamate transporters are persistently upregulated after hypoxic-ischemic (HI) injury
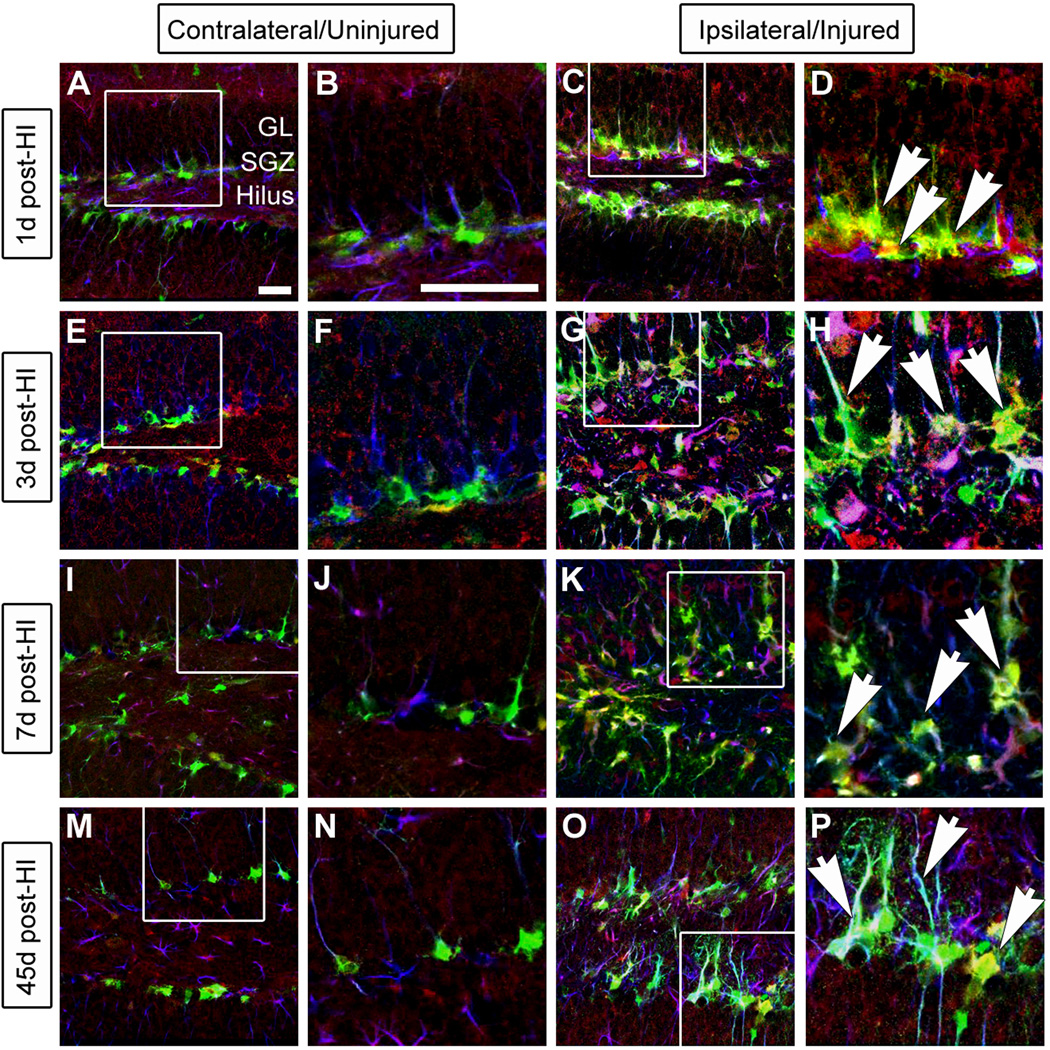
We previously demonstrated that GltI and Glast are expressed on type I cells in vivo (Gilley et al., 2011). Moreover, injury-induced activation of type I cells and subsequent neurogenesis has been well-documented (Kernie et al., 2001; Miles & Kernie, 2006; Shi et al., 2007; Koch et al., 2008; Miles & Kernie, 2008; Yu et al., 2008; Blaiss et al., 2011). We therefore hypothesized that GltI and Glast would be expressed on activated progenitors after injury. Following unilateral HI injury in nestin-GFP-expressing transgenic mice, we performed immunostaining for GFP, GFAP and either GltI (Figure 4) or Glast (Figure 5) to typify their expression at various time points after injury (n=6). GFP in this transgenic line is a well established marker of slowly dividing type I cells as well as the more rapidly dividing type IIa population (Miles & Kernie, 2006; 2008; Yu et al., 2008). GFAP in conjunction with GFP marks the type I cells only so expression of both can be used to distinguish type I from type IIa progenitors (Shi et al., 2007; Miles & Kernie, 2008; Yu et al., 2008). On the uninjured (contralateral) side of the brain, glutamate transporter expression remained at baseline levels at all indicated time points (Figure 4 and Figure 5). However, GltI and Glast were both upregulated on type I cells on the ipsilateral (injured) side of the brain up to 45 days after injury (Figure 4 and Figure 5).
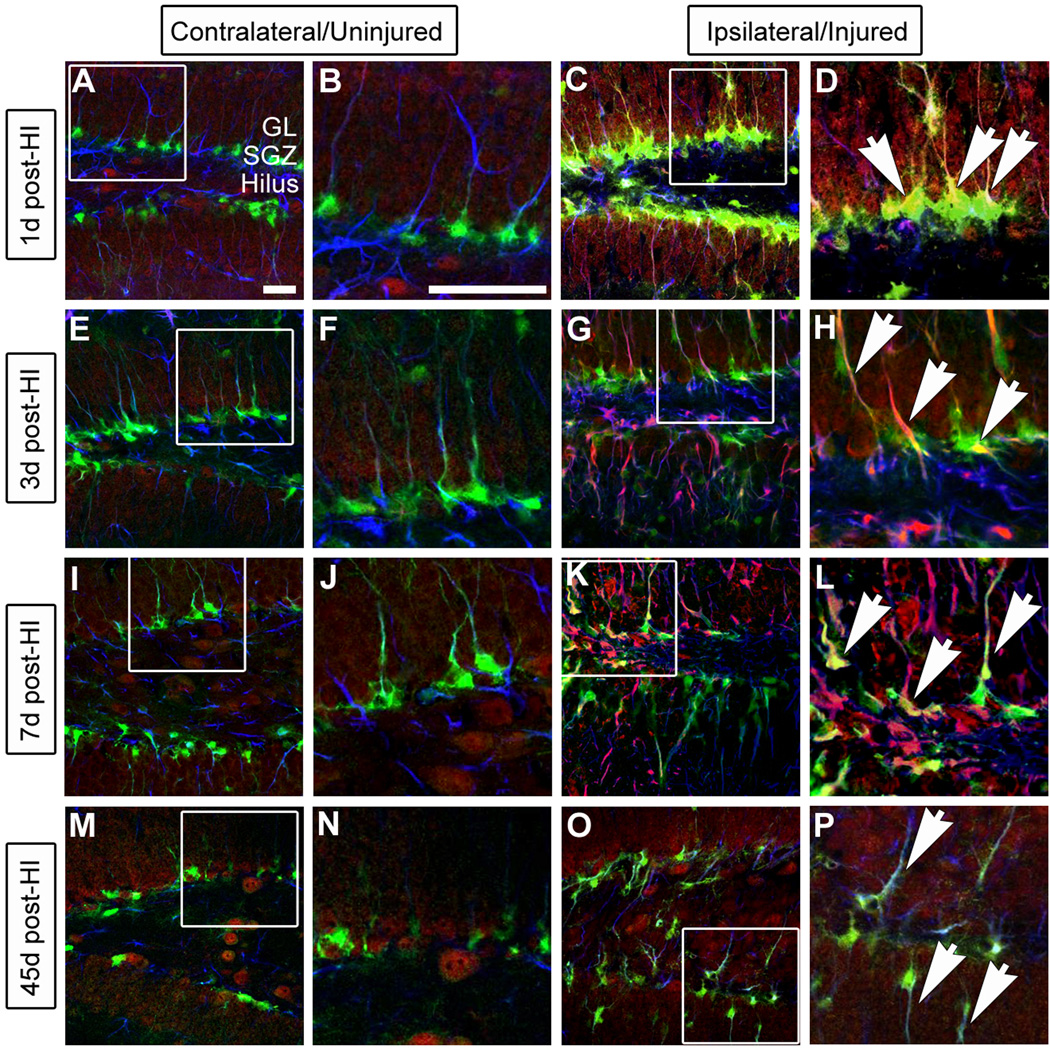
Figure 4. GltI is indefinitely upregulated after HI injury.
Animals were sacrificed one day (A–D), 3 days (E–H), 7 days (I–L) and 45 days (M–P) post-HI and stained for the presence of GFP (green), GltI (red) and GFAP (blue). The contralateral or uninjured side of the brain is shown in the left panel (A–B, E–F, I–J and M–N) while the ipsilateral (injured) side is on the right (C–D, G–H, K–L, O–P). White boxes represent the magnified areas shown. 1 day (D), 3 days (H), 7 days (L) and 45 days (P) post-HI, magnified images show activation of GltI-expressing type I cells (arrows) on the injured side of the brain. These findings indicate that GltI expression is permanently increased subsequent to HI injury. Scale bars in (A) and (B) are 25 µm and 100 µm, respectively. SGZ, subgranular zone; GL, granular layer; post-HI, post- hypoxic-ischemia.
Figure 5. Glast is upregulated after HI injury.
Animals were sacrificed and stained for GFP (green), Glast (red) and GFAP (blue) 1 day (A–D), 3 days (E–H), 7 days (I–L) and 45 days (M–P) after injury. Similar to GltI expression, Glast expression is increased on the ipsilateral side (D, H, L and P) of the brain compared to the contralateral side (B, F, J and N). White boxes represent the magnified areas shown. Scale bars in (A) and (B) are 25 µm and 100 µm, respectively. SGZ, subgranular zone; GL, granular layer; post-HI, post-hypoxic-ischemia.
Cell-specific elevation of glutamate transporter expression in injured progenitor cells
There are two possible explanations to corroborate the results observed in Figure 4 and Figure 5. Either increased numbers of GFP-positive cells on the injured side of the brain result in higher glutamate transporter expression, or GltI or Glast’s expression is elevated at the cellular level. To distinguish between these two possibilities, we FAC-sorted early and late progenitors from transgenic mice that were injured three days prior. We used GFP and poly-sialated neural cell adhesion molecule (PSA-NCAM) expression to distinguish early (type I and IIa: GFP+/PSANCAM-) and late (type III: GFP-/PSANCAM+) progenitors (Chumley et al., 2007). We collected both populations from the ipsilateral (injured) and contralateral (uninjured) sides. Afterwards we isolated the RNA and normalized the concentrations across all samples before synthesizing cDNA and analyzing relative expression levels using QPCR. Expression for a particular gene was normalized to its own expression on the uninjured side of the brain. The results are shown in Figure 6.
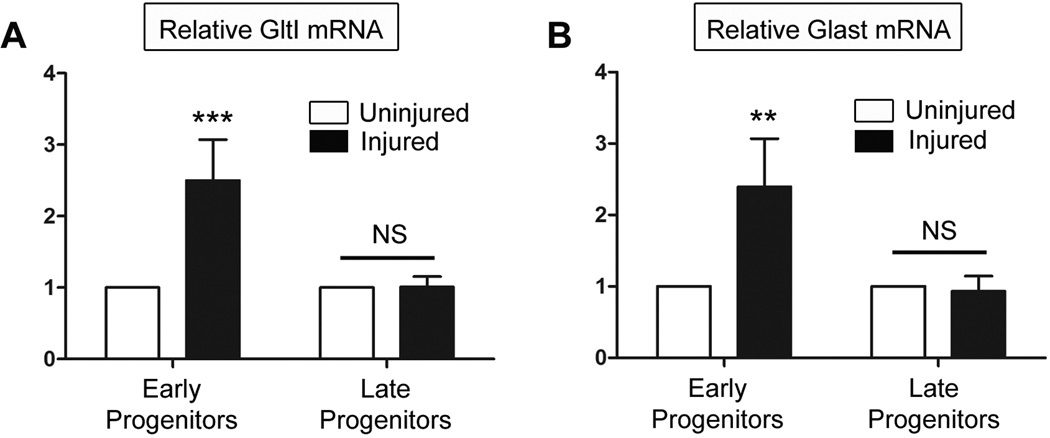
Figure 6. Upregulation of glutamate transporters is cell-specific following HI injury.
Three days following HI injury, cells were sorted for their expression of GFP and PSA-NCAM signifying either early (GFP+/PSA-NCAM-) or late (GFP+/PSA-NCAM+) neural progenitors. Afterwards relative expression levels of GltI (A) and Glast (B) were measured in each population obtained from the injured and uninjured sides of the brain. Both glutamate transporters were upregulated in early progenitors from the injured side of the brain. However their expression was not elevated in late progenitors from the injured or uninjured brain. This suggests that GltI and Glast are specifically induced in early progenitor cells isolated from the injured dentate gyrus. A Bonferonni post hoc analysis was used to calculate significance (**P<0.001; ***P<0.0001). NS, not significant.
We observed that GltI mRNA is 2.5 fold higher in early progenitor cells from the injured side of the brain when compared to those from the uninjured side (Figure 6, A) (Two Way ANOVA, **P=0.002, F=25.2, n=4). However, relative expression of GltI is unchanged in late progenitors from injured and uninjured dentate gyrus. The latter result is not surprising considering that neither GltI nor Glast colocalize with markers indicative of late type III progenitors (Gilley et al., 2011). This suggests that GltI is upregulated in early progenitors at the cellular level as a result of HI injury. Similar to results for GltI, Glast is also upregulated on early progenitors isolated from the injured side of the brain but its expression is not significantly different in later progenitors (Figure 6, B) (Two Way ANOVA, **P=0.01, F=14.01, n=4). Together, these results indicate that elevated expression of glutamate transporters on the injured side of the brain (Figure 4 and Figure 5) are due to changes in gene expression at the single cell level.
Recurrent injury impairs progenitor proliferation
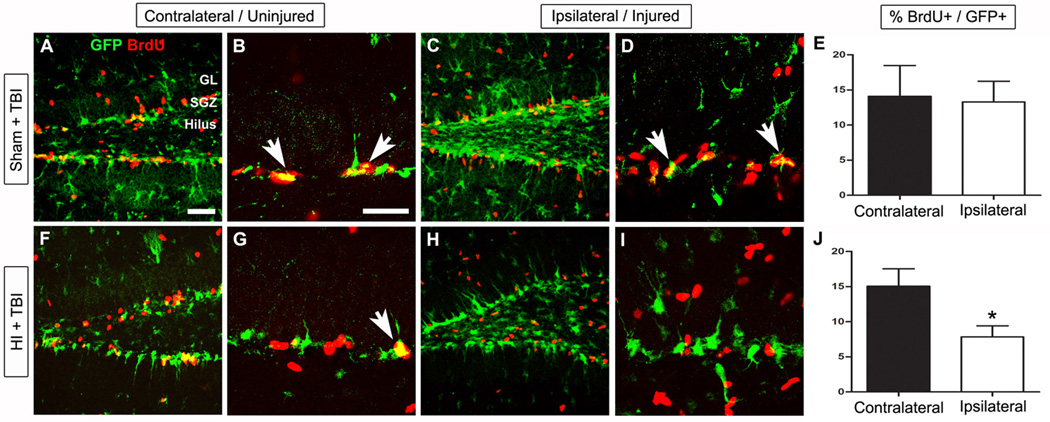
Since we observed long-lasting changes in expression of GltI and Glast at the cellular level following injury, we next wanted to determine whether injured progenitors maintained their proliferative potential in the context of gene expression changes. One month after the HI injury, a controlled cortical impact (CCI) model was used to induce traumatic brain injury (TBI) in a well established model of increasing progenitor proliferation (Kernie et al., 2001; Shi et al., 2007; Yu et al., 2008; Blaiss et al., 2011). To analyze the subsequent effects on progenitor proliferation, we injected both groups with BrdU, performed immunostaining, and quantified the number of GFP- and BrdU- double-labeled cells. In animals exposed to the sham HI injuries (ligation with no hypoxia), there were no observed differences in the percentage of proliferative cells following TBI (Figure 7, E) (Unpaired t test, P=0.74, F=2.2, n=5). However Figure 7, J shows there was a significant difference between co-localized cells within the contralateral (15.1%) and ipsilateral (7.8%) side in animals injured with both HI and CCI (Unpaired t test, **P=0.003, F=2.5, n=4). These results suggest that repetitive brain injuries lead to decreased capacity for injury-induced neurogenesis that may in part reflect changes in glutamate transporter expression.
Figure 7. Upregulation of GltI and Glast after HI is associated with decreased proliferation following TBI.
32 days prior to inducing injury with TBI, P28 transgenic mice were exposed to either sham injury (A–E) or HI (F–J). To assess changes in proliferation, the percentage of GFP- and BrdU- double-labeled cells was quantified on each side of the brain for both groups of animals (E and J). In the Sham + TBI group, there was no observed difference (E) in proliferative cells on the uninjured (A–B) and injured (C–D) sides of the brain. However, in animals exposed to HI and TBI, the percentage of double-positive cells is significantly decreased (J) on the ipsilateral side (F–G) compared to the contralateral side (H–I). The scale bars in (A) and (B) are 1 mm and 35 µm, respectively. Bonferonni post hoc analysis was used to calculate significance (*P<0.05). HI, hypoxic-ischemia; TBI, traumatic brain injury.
Discussion
Appropriate concentrations of extracellular glutamate are thought to regulate neurogenesis by activating glutamate receptors on the surface of the cell (Choi et al., 1988; Luk et al., 2003; Melchiorri et al., 2007). Although the exact mechanism remains unclear, there is increasing evidence suggesting that activation of ionotropic and metabotropic glutamate receptors results in elevated calcium levels which is thought to contribute to both cellular proliferation and cell toxicity (Choi et al., 1988; Luk et al., 2003). Results from the current study suggest that glutamate transporters indirectly regulate calcium-dependent proliferation of neural progenitors via mGluRs. It appears that GltI and Glast function to regulate the availability of extracellular glutamate to various receptors expressed on the cell surface thereby indirectly affecting calcium-induced proliferation. Moreover, when glutamate concentrations rise after injury (Andine et al., 1991; Globus et al., 1995; Bullock et al., 1998), glutamate transporters are able to transport excess glutamate into the cell thus preventing overstimulation of glutamate receptors (Yi & Hazell, 2006);(Danbolt, 2001; Fonnum & Lock, 2004). This may explain why in vitro knockdown of GltI or Glast consistently resulted in increased proliferation (Figure 1 and Figure 2).
Although some extracellular glutamate may be beneficial to the cell, excessive amounts can overstimulate glutamate receptors leading to excitotoxicity that may ultimately exacerbate neuronal damage as a result of brain injury (Choi et al., 1988; Jensen, 2002; Yi & Hazell, 2006). Glutamate transporters are therefore essential in regulating extracellular concentrations of glutamate in order to prevent excitotoxicity (Tanaka et al., 1997; Watase et al., 1998; Matsugami et al., 2006). During synaptic transmission astrocytic GltI and Glast are neuroprotective because they transport excess glutamate, a key neurotransmitter, into the cell to prevent cytotoxic neuronal damage (see (Danbolt, 2001) for review). Once glutamate is inside the astrocyte, it is converted to glutamine and then transported outside the cell. Presynaptic neurons are then able to uptake glutamine and convert it back to glutamate so that it may be reused as a neurotransmitter (Meldrum, 2000; Maragakis et al., 2004). Mice with traditional knockouts of GltI or Glast (or both) are not able to recycle excess glutamate in this manner, which can ultimately lead to excitotoxicity, neuronal damage and premature death (Tanaka et al., 1997; Watase et al., 1998; Matsugami et al., 2006).
In the current study we show that manipulation of GltI and Glast mRNA in early postnatal dentate gyrus progenitors affects neurosphere formation, BrdU incorporation and intracellular calcium signaling. Although the results from the neurosphere assay are not as striking as those from the BrdU incorporation assay, we believe the variable sensitivities of these assays may explain this discrepancy. In the BrdU experiment, we utilized flow cytometry to assess the percentage of live cells expressing BrdU, which is extremely sensitive. In the neurosphere assay however, our quantification is based only on the presence of absence of neurospheres. Moreover, we used P7 neurospheres which highly express Glast but not GltI (Gilley et al., 2011). This may explain why overexpression of Glast in these neurospheres did not produce a significant decrease in neurosphere formation as expected. These findings suggest that glutamate transporters function on neural progenitors to indirectly regulate calcium-dependent proliferation.
We also demonstrated that GltI and Glast overexpression correlated with decreased proliferation of neural progenitors after TBI (Figure 7). Acquired brain injury has been shown to induce adult neurogenesis within the dentate gyrus and has recently been shown to underlie recovery (Blaiss et al., 2011). We have previously demonstrated that this activation of progenitors is required to replace dying neuroblasts and for long-term neuronal remodeling in the hippocampus (Miles & Kernie, 2008). In addition, we have recently shown that injury-induced neurogenesis is required for cognitive recovery following TBI (Blaiss et al., 2011). Other studies have demonstrated a relationship between the severity and frequency of brain trauma and decreased capacity to recover from such injuries (Spettell et al., 1991; Slemmer & Weber, 2005). The decreased proliferation of neural progenitors associated with HI and TBI (Figure 7) might therefore explain these phenotypes and provide insight into the mechanisms underlying recovery from multiple brain injuries. Here we provide evidence that glutamate transporters may play a critical role in injury-induced proliferation.
Several studies have begun to characterize the role glutamate transporters might play in neuroprotection. For instance, preconditioning with normobaric hyperoxia or middle cerebral artery occlusion increased expression of GltI and Glast in astrocytes and was associated with marked brain protection (Bigdeli et al., 2009). Furthermore, less neuronal damage was observed in animals pretreated with 8% oxygen one day prior to unilateral HI injury. GltI was also shown to be upregulated in these animals (Cimarosti et al., 2005). When rat cortical cultures were preconditioned via glucose-oxygen deprivation, similar results were obtained. This study also concluded that GltI is a target of peroxisome proliferator-activated receptor gamma, because injection of an agonist immediately after middle cerebral artery occlusion resulted in neuroprotection and increased levels of GltI (Romera et al., 2007). Moreover, several groups have induced glutamate transporter expression with β-lactam antibiotics, transgenic mice and ceftriaxone and have also demonstrated neuroprotection in vivo (Rothstein et al., 2005; Chu et al., 2007; Weller et al., 2008). Results from the current study are consistent with these and suggest that GltI and Glast are upregulated after injury and might be involved in hypoxic preconditioning and neuroprotection. However, this present study further suggests a mechanism underlying the reduction of injury-induced neurogenesis upon subsequent brain injuries.
The results presented here are consistent with findings from experimental models of recurrent brain injury and clinical evidence associated with multiple brain insults. Several studies have shown increased expression of markers of central nervous system damage including S-100 beta and neuron-specific enolase in animals affected with multiple brain maladies, especially TBI (Slemmer & Weber, 2005). More relevant are findings that demonstrate a relationship between the severity and frequency of brain trauma and decreased capacity to recover from such an insult (Spettell et al., 1991; Salcido & Costich, 1992; Slemmer & Weber, 2005). The decreased proliferation of neural progenitors associated with HI and TBI (Figure 6, J) might therefore explain these phenotypes and provide insight into the mechanisms underlying recovery from multiple brain injuries. However, to elucidate the exact mechanisms involved, more focused studies on glutamate transporters and their role in injury-induced proliferation are necessary.
In summary, we provide a novel functional role for glutamate transporters in regulating neural progenitor proliferation in a calcium- and mGluR-dependent manner. In addition, we demonstrate decreased proliferation associated with multiple brain injuries, which correlate with increased glutamate transporter expression. Taken together the current study suggests that glutamate transporters contribute to regulating progenitor proliferation during development and may also affect induced proliferation after injury.
Acknowledgments
The authors would like to thank Gui Zhang, Kyle Lieppman and Darryl Miles for their technical support and assistance with this publication.
Support: NIH grant R01 NS048192 (SGK).
Glossary
Abbreviations
- BAPTA-AM
1,2-bis(2-amino-5-fluorophenoxy)ethane-N,N,N’,N’-tetra acetic acid tetrakis ester
- BrdU
bromodeoxyuridine
- CCI
controlled cortical impact
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- Glast
excitatory amino acid transporter 1
- GltI
excitatory amino acid transporter 2
- HI
hypoxic-ischemic or hypoxia-ischemia
- LY341495
(2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid
- PI
propidium iodide
- QPCR
quantitative PCR
- siRNA
small interfering RNA
- TBI
traumatic brain injury
Footnotes
The authors have no other financial interest to disclose.
References
- Andine P, Sandberg M, Bagenholm R, Lehmann A, Hagberg H. Intra- and extracellular changes of amino acids in the cerebral cortex of the neonatal rat during hypoxic-ischemia. Brain Res Dev Brain Res. 1991;64:115–120. doi: 10.1016/0165-3806(91)90214-4. [DOI] [PubMed] [Google Scholar]
- Bigdeli MR, Rahnema M, Khoshbaten A. Preconditioning with subreconditioning with sublethal ischemia or intermittent normobaric hyperoxia up-regulates glutamate transporters and tumor necrosis factor-alpha converting enzyme in the rat brain. J Stroke Cerebrovasc Dis. 2009;18:336–342. doi: 10.1016/j.jstrokecerebrovasdis.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazel CY, Nunez JL, Yang Z, Levison SW. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131:55–65. doi: 10.1016/j.neuroscience.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, Young HF. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Lee ST, Sinn DI, Ko SY, Kim EH, Kim JM, Kim SJ, Park DK, Jung KH, Song EC, Lee SK, Kim M, Roh JK. Pharmacological Induction of Ischemic Tolerance by Glutamate Transporter-1 (EAAT2) Upregulation. Stroke; a journal of cerebral circulation. 2007;38:177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- Chumley MJ, Catchpole T, Silvany RE, Kernie SG, Henkemeyer M. EphB receptors regulate stem/progenitor cell proliferation, migration, and polarity during hippocampal neurogenesis. J Neurosci. 2007;27:13481–13490. doi: 10.1523/JNEUROSCI.4158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimarosti H, Jones NM, O’Shea RD, Pow DV, Salbego C, Beart PM. Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett. 2005;385:52–57. doi: 10.1016/j.neulet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Dieter P, Fitzke E, Duyster J. BAPTA induces a decrease of intracellular free calcium and a translocation and inactivation of protein kinase C in macrophages. Biol Chem Hoppe Seyler. 1993;374:171–174. doi: 10.1515/bchm3.1993.374.1-6.171. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Lock EA. The contributions of excitotoxicity, glutathione depletion and DNA repair in chemically induced injury to neurones: exemplified with toxic effects on cerebellar granule cells. Journal of neurochemistry. 2004;88:513–531. doi: 10.1046/j.1471-4159.2003.02211.x. [DOI] [PubMed] [Google Scholar]
- Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, Peterson DA, Suhr ST, Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gilley JA, Yang CP, Kernie SG. Developmental profiling of postnatal dentate gyrus progenitors provides evidence for dynamic cell-autonomous regulation. Hippocampus. 2011;21:33–47. doi: 10.1002/hipo.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus MY, Alonso O, Dietrich WD, Busto R, Ginsberg MD. Glutamate release and free radical production following brain injury: effects of posttraumatic hypothermia. J Neurochem. 1995;65:1704–1711. doi: 10.1046/j.1471-4159.1995.65041704.x. [DOI] [PubMed] [Google Scholar]
- Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. Int J Dev Neurosci. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–235. discussion 235–241, 302–226. [PubMed] [Google Scholar]
- Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001;66:317–326. doi: 10.1002/jnr.10013. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Koch JD, Miles DK, Gilley JA, Yang CP, Kernie SG. Brief exposure to hyperoxia depletes the glial progenitor pool and impairs functional recovery after hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2008;28:1294–1306. doi: 10.1038/jcbfm.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kennedy TE, Sadikot AF. Glutamate promotes proliferation of striatal neuronal progenitors by an NMDA receptor-mediated mechanism. J Neurosci. 2003;23:2239–2250. doi: 10.1523/JNEUROSCI.23-06-02239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Dietrich J, Wong V, Xue H, Mayer-Proschel M, Rao MS, Rothstein JD. Glutamate transporter expression and function in human glial progenitors. Glia. 2004;45:133–143. doi: 10.1002/glia.10310. [DOI] [PubMed] [Google Scholar]
- Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, Ogawa M, Obata K, Watanabe M, Hashikawa T, Tanaka K. From the Cover: Indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci U S A. 2006;103:12161–12166. doi: 10.1073/pnas.0509144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiorri D, Cappuccio I, Ciceroni C, Spinsanti P, Mosillo P, Sarichelou I, Sale P, Nicoletti F. Metabotropic glutamate receptors in stem/progenitor cells. Neuropharmacology. 2007;53:473–480. doi: 10.1016/j.neuropharm.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130 doi: 10.1093/jn/130.4.1007S. 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- Miles DK, Kernie SG. Activation of neural stem and progenitor cells after brain injury. Prog Brain Res. 2006;157:187–197. doi: 10.1016/s0079-6123(06)57012-8. [DOI] [PubMed] [Google Scholar]
- Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nacher J, McEwen BS. The role of N-methyl-D-asparate receptors in neurogenesis. Hippocampus. 2006;16:267–270. doi: 10.1002/hipo.20160. [DOI] [PubMed] [Google Scholar]
- Nacher J, Varea E, Miguel Blasco-Ibanez J, Gomez-Climent MA, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS. N-methyl-d-aspartate receptor expression during adult neurogenesis in the rat dentate gyrus. Neuroscience. 2007;144:855–864. doi: 10.1016/j.neuroscience.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, Serena J, Vivancos J, Nombela F, Lorenzo P, Lizasoain I, Moro MA. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007;27:1327–1338. doi: 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Salcido R, Costich JF. Recurrent traumatic brain injury. Brain injury : [BI] 1992;6:293–298. doi: 10.3109/02699059209029671. [DOI] [PubMed] [Google Scholar]
- Shi J, Miles DK, Orr BA, Massa SM, Kernie SG. Injury-induced neurogenesis in Bax-deficient mice: evidence for regulation by voltage-gated potassium channels. Eur J Neurosci. 2007;25:3499–3512. doi: 10.1111/j.1460-9568.2007.05624.x. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Weber JT. The extent of damage following repeated injury to cultured hippocampal cells is dependent on the severity of insult and inter-injury interval. Neurobiol Dis. 2005;18:421–431. doi: 10.1016/j.nbd.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Spettell CM, Ellis DW, Ross SE, Sandel ME, O'Malley KF, Stein SC, Spivack G, Hurley KE. Time of rehabilitation admission and severity of trauma: effect on brain injury outcome. Arch Phys Med Rehabil. 1991;72:320–325. [PubMed] [Google Scholar]
- Suzuki M, Nelson AD, Eickstaedt JB, Wallace K, Wright LS, Svendsen CN. Glutamate enhances proliferation and neurogenesis in human neural progenitor cell cultures derived from the fetal cortex. Eur J Neurosci. 2006;24:645–653. doi: 10.1111/j.1460-9568.2006.04957.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tsien RY. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980;19:2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- Weller ML, Stone IM, Goss A, Rau T, Rova C, Poulsen DJ. Selective overexpression of excitatory amino acid transporter 2 (EAAT2) in astrocytes enhances neuroprotection from moderate but not severe hypoxia-ischemia. Neuroscience. 2008;155:1204–1211. doi: 10.1016/j.neuroscience.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]