Asthma is a chronic inflammatory disease of the airways manifested by reversible airflow obstruction and airway hyperresponsiveness.1 One of the challenges of treating patients with asthma is the known heterogeneity of the disease and differential responses to standard treatments.2–4 For example, in a large clinical trial of patients with moderate asthma, one of three patients had asthma that was not well controlled despite the regular use for 1 year of fluticasone, an inhaled glucocorticoid, and salmeterol, a long-acting beta-agonist.3 In another study involving patients with more severe asthma, 40% were unable to gain control of their asthma with the addition of omalizumab, a biologic agent that binds to IgE.4 An improved understanding of the mechanisms underlying the heterogeneity of the treatment response in patients with asthma represents an unmet need.
An asthma subphenotype associated with an “interleukin-13 signature surrogate” or a high type 2 helper T-cell (Th2) phenotype has been described recently.5 This high-Th2 phenotype has been defined as an IgE level greater than 100 ng per milliliter and more than 0.14×109 eosinophils per liter in the peripheral blood.5 In patients with asthma, the high-Th2 phenotype has been associated with an increase in circulating periostin, a matricellular protein induced by interleukin-13 and expressed by airway structural cells. The presence of interleukin-13, which shares a receptor with interleukin-4, is critical to the expression of the Th2 phenotype, which has been shown in studies in animals to lead to the formation of IgE antibody.6,7 Interleukin-13 is found in the airways of patients with asthma and is thought to mediate several features of asthma, including airway hyperreponsiveness, inflammation, mucous metaplasia, and activation and proliferation of airway fibroblasts, which contribute to adverse airway remodeling8,9 (Fig. 1). Thus, interleukin-13 is a relevant target for asthma therapy, but it is only one of the pathways that can lead to the expression of an asthma phenotype.
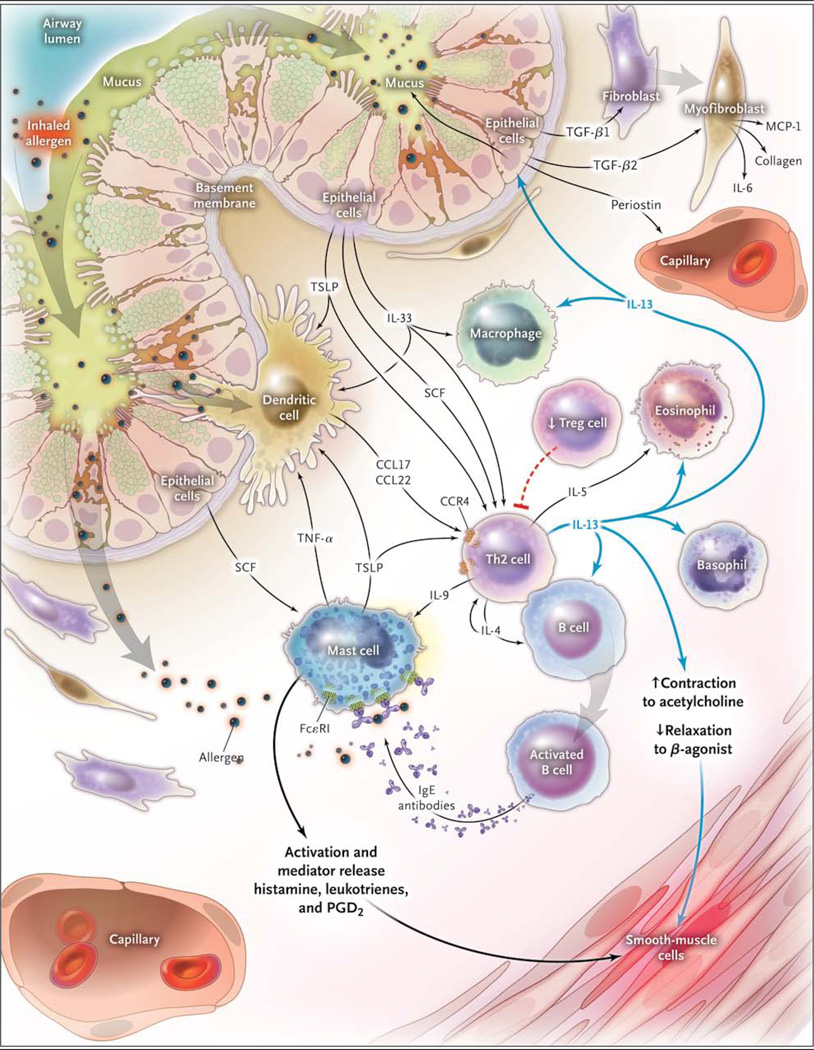
Figure 1. Interleukin-13 and Non–Interleukin-13 Inflammatory Pathways in Asthma.
Inhaled allergen activates mast cells, which are maintained by stem-cell factor (SCF) produced by epithelial cells and by dendritic cells, through cross-linking with IgE on their cell surfaces via FcεR1 to release mediators that induce bronchoconstriction, such as histamine, cysteinyl leukotrienes, and prostaglandin D2 (PGD2). Allergens are processed by dendritic cells, which are induced to secrete the CC chemokine ligand (CCL) 17 and CCL22 by thymic stromal lymphopoietin (TSLP). Dendritic cells then attract and activate type 2 helper T-cells (Th2) by the binding of CCL17 and CCL22 with CC chemokine receptor 4 (CCR4) on the Th2 cell surface. Another driver of the allergen sensitization process is interleukin-33 (IL-33), produced by airway epithelial cells, which activates dendritic cells and Th2 through mast-cell–derived tumor necrosis factor alpha (TNF-α). Th2 secrete IL-4 and IL-13, which induce B cells to produce IgE; IL-5, which is necessary for the development and survival of eosinophils; and IL-9, which activates mast cells. T regulatory (Treg) cells inhibit this inflammatory cascade, and there are data that suggest that they may be reduced in asthma, thus promoting ongoing Th2 inflammation. Once IL-13 is produced, it can promote the survival and migration of eosinophils and promotes activation of macrophages to create an M2, or an allergic cell phenotype. Through modulation of the barrier function of airway epithelial cells and subsequent production of transforming growth factor β1 (TGF-β1), the permeability of airway epithelial cells and the production of mucous are increased, and airway fibroblasts transform to myofibroblasts, with subsequent production of monocyte chemoattractant protein 1 (MCP-1), IL-6, and collagen. IL-13 also has direct effects on airway smooth muscle, leading to increased contraction to agonists such as acetylcholine and decreased relaxation with beta-agonists.
In this issue of the Journal, Corren and colleagues report the effects of an interleukin-13 inhibitor, lebrikizumab, in a cohort of patients with moderate asthma who were symptomatic despite taking inhaled glucocorticoids and, in most cases, an additional long-acting beta-agonist.10 Although there was an effect on airflow obstruction in all the patients who were treated with lebrikizumab, the effect was greater in patients who had circulating levels of periostin above the median and exhibited the high-Th2 phenotype than in those without this phenotype. These data provide proof of the concept that anti–interleukin-13 therapy can be targeted to susceptible patients.
As we look toward the goal of treating patients with this heterogeneous disease in a more personalized fashion, it is refreshing to see a trial in which there is acknowledgement that not all patients will respond similarly to an intervention. Although larger studies are needed to verify this observation, future trials should strive to include stratification of patients according to the expected phenotype to help us personalize the response to asthma treatment.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Szefler SJ, Martin RJ, King TS, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Beasley R, Ayres J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–316. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 5.Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [Erratum, Am J Respir Crit Care Med 2009;180:796.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grünig G, Warnock M, Wakil AE, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, sub-epithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saha SK, Berry MA, Parker D, et al. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J Allergy Clin Immunol. 2008;121:685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingram JL, Huggins MJ, Church TD, et al. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med. 2011;183:1625–1632. doi: 10.1164/rccm.201009-1452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corren J, Lemanske RF, Jr, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]