Abstract
Implementation of an ecosystem approach to fisheries requires advice on trade-offs among fished species and between fisheries yields and biodiversity or food web properties. However, the lack of explicit representation, analysis and consideration of uncertainty in most multispecies models has limited their application in analyses that could support management advice.
We assessed the consequences of parameter uncertainty by developing 78 125 multispecies size-structured fish community models, with all combinations of parameters drawn from ranges that spanned parameter values estimated from data and literature. This unfiltered ensemble was reduced to 188 plausible models, the filtered ensemble (FE), by screening outputs against fish abundance data and ecological principles such as requiring species' persistence.
Effects of parameter uncertainty on estimates of single-species management reference points for fishing mortality (FMSY, fishing mortality rate providing MSY, the maximum sustainable yield) and biomass (BMSY, biomass at MSY) were evaluated by calculating probability distributions of estimated reference points with the FE. There was a 50% probability that multispecies FMSY could be estimated to within ±25% of its actual value, and a 50% probability that BMSY could be estimated to within ±40% of its actual value.
Signal-to-noise ratio was assessed for four community indicators when mortality rates were reduced from current rates to FMSY. The slope of the community size spectrum showed the greatest signal-to-noise ratio, indicating that it would be the most responsive indicator to the change in fishing mortality F. Further, the power of an ongoing international monitoring survey to detect predicted responses of size spectrum slope was higher than for other size-based metrics.
Synthesis and applications: Application of the ensemble model approach allows explicit representation of parameter uncertainty and supports advice and management by (i) providing uncertainty intervals for management reference points, (ii) estimating working values of reference points that achieve a defined reduction in risk of not breaching the true reference point, (iii) estimating the responsiveness of population, community, food web and biodiversity indicators to changes in F, (iv) assessing the performance of indicators and monitoring programmes and (v) identifying priorities for data collection and changes to model structure to reduce uncertainty.
Keywords: biodiversity, ecosystem-based management, fish community, fisheries, food web, large fish indicator, power analysis, risk, size-based, trade-off
Introduction
One facet of the adoption of an ecosystem approach to fisheries is the need to specify and meet objectives for ecosystem components and attributes as well as target species (Garcia & Cochrane 2005; Rice 2011). This requires that objectives can be defined and advice provided on management actions to meet them. In practice, both agreeing on objectives and advising how to meet them have been challenging, in part because advice on consequences of adopting alternate objectives and the probability of meeting them is uncertain and this uncertainty is not quantified.
Several models have been developed to assess fishing effects on community or ecosystem properties (e.g. Plagányi 2007). They have improved understanding of fisheries interactions with ecosystems and are often used to underpin state assessments, but relatively few are used operationally; the main exceptions being those that consider interactions among small groups of species (e.g. Gjosaeter, Bogstad & Tjelmeland 2002). One reason for limited uptake is that model outputs are assumed to be highly uncertain, and yet there have been few attempts to express uncertainty and its implications (e.g. Hill et al. 2007; Espinoza-Tenorio et al. 2012; Gaichas et al. 2012; Link et al. 2012). This contrasts with analysis and advice on single-species exploitation, for which advisers and managers have longstanding experience presenting and addressing uncertainty (e.g. Francis & Shotton 1997).
Effects of parameter uncertainty on complex ecosystem models are poorly understood and may be challenging to address owing to practical constraints such as long run-times and few data for parameterization or validation. However, the complexity of these models is not a prerequisite for addressing many operational management questions (e.g. Fulton, Smith & Johnson 2003; Butterworth & Plaganyi 2004), and a range of less complex models that further simplify ecosystems and/or deal with subsets of ecosystems can be adopted. One class of these models includes size-based multispecies models. These focus on groups of interacting fish species in an otherwise generalized food web and seek to predict changes in species' abundance at size as a function of fishing mortality F (e.g. Hall et al. 2006; Rochet et al. 2011; Blanchard et al. 2014). These models are practical to apply as they are based on measurable properties of species' life histories and dynamics, are relatively straightforward to parameterize with empirical data and have short run-times. They are also suitable for supporting management advice as they predict the effects of changes in F on both size and abundance of species in the modelled community and can therefore be used to estimate single-species fisheries and conservation reference points in a multispecies context as well as a range of community metrics proposed or specified as indicators for evaluating the fishing effects (e.g. Shin et al. 2005; EU 2008, 2010; Greenstreet et al. 2011; Shephard, Reid & Greenstreet 2011).
To support development of operational management advice on the effects of fishing, we develop and apply a method to assess and describe parameter uncertainty and its consequences in a size-based multispecies model. Parameter uncertainty is explicitly incorporated into the analysis by developing multiple models based on the full range of possible parameter values, rather than applying a single model based on a best estimate of each individual parameter. Emergent population dynamics predicted by each model are then screened against data to generate a reduced set of models displaying credible dynamics. The approach is used to describe uncertainty in single species and community reference points and single species and community responses to alternate management actions. Predicted uncertainties are used to assess the power of monitoring surveys to detect responses in community metrics and to identify where investment in future data collection should be targeted to reduce uncertainty.
Materials and methods
Analyses were performed using a modified version of the length-based multispecies model initially developed by Hall et al. (2006) to represent the Georges Bank fish community and subsequently applied to the North Sea community (Rochet et al. 2011). The model represents 21 fish species in 32 equal length classes (each c. 5 cm), spanning the full size range of species represented in the model (Table 1). Progression of individuals through length classes is represented using the deterministic von Bertalanffy growth equation (VBGE). Individuals mature when they reach a threshold size defined by a logistic model, with 50% of individuals mature at the length of maturity (Lmat) defined in Table 1. Reproduction is described with a spawner–recruit relationship, which determines the numbers of recruits entering the smallest size class from the biomass of mature individuals. Species' dynamics are linked via predation mortality (M2), which varies with predator abundance, and size and species preference. Size preference is described with a preference function based on a log-normal distribution and species preference with a diet matrix indicating who eats whom (Hall et al. 2006). In each length class, individuals are also susceptible to residual natural mortality (M1) and fishing mortality (F). Other details of model structure and implementation are provided in Hall et al. (2006) and Rochet et al. (2011).
Table 1.
Default parameter values and species' abundances for modelled species. Spawning stock biomass (SSB) was obtained from survey data or ICES stock assessments, minimum and maximum estimates of SSB from ICES stock assessments were used to filter model variants. FMSY and FHIST were used in simulations. Lifetype indicates if the species is pelagic (P), semi-pelagic and demersal (D1) or strictly demersal (D2), and a and b are the species-specific ‘shape’ parameters, enabling conversion from length (L, cm) to weight (W, g) via the relation W = aLb, L∞ is the asymptotic length, Lmat is the length at 50% maturity and ĸ is the von Bertalanffy growth parameter. Maximum SSB was estimated with ICES stock assessment data if these were available; otherwise, estimates were based on ICES North Sea International Bottom Trawl Survey data
| Common name | Species | Lifetype | L∞ (cm) | K (year−1) | Lmat (cm) | a (×1000) | b | Maximum SSB (t) | Minimum SSB (t) | Maximum SSB (t) | FMSY (year−1) | FHIST (year−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sprat | Sprattus sprattus | P | 17 | 0·681 | 12 | 5·9 | 3·109 | 380 236 | 78 083 | 380 236 | 1·30 | 1·06 |
| Norway Pout | Trisopterus esmarkii | D1 | 24 | 0·849 | 14 | 7·5 | 3·024 | 462 487 | 63 034 | 462 487 | 0·35 | 0·48 |
| Sandeel | Ammodytes spp. | P | 24 | 1·000 | 12 | 4·9 | 2·783 | 1 243 757 | 148 723 | 1 243 757 | 0·35 | 0·57 |
| Poor Cod | Trisopterus minutus | D1 | 23 | 0·520 | 15 | 8·2 | 3·087 | 786 976 | – | – | 0·72 | 0·55 |
| Long Rough Dab | Hippoglossoides platessoides | D2 | 25 | 0·340 | 15 | 5·3 | 3·143 | 46 140 | – | – | 0·60 | 0·55 |
| Dab | Limanda limanda | D2 | 32 | 0·536 | 13 | 15·9 | 2·864 | 276 779 | – | – | 0·41 | 0·55 |
| Herring | Clupea harengus | P | 33 | 0·606 | 23 | 4·8 | 3·198 | 2 857 134 | 691 752 | 2 857 134 | 0·25 | 0·25 |
| Horse Mackerel | Trachurus trachurus | P | 28 | 0·380 | 19 | 31·6 | 2·652 | 193 771 | – | – | 0·50 | 0·63 |
| Lemon Sole | Microstomus kitt | D2 | 37 | 0·420 | 27 | 12·3 | 2·971 | 24 915 | – | – | 0·33 | 0·55 |
| Sole | Solea solea | D2 | 47 | 0·284 | 21 | 8·9 | 3·017 | 90 300 | 17 600 | 90 300 | 0·22 | 0·53 |
| Mackerel | Scomber scombrus | P | 38 | 0·510 | 26 | 5·2 | 3·167 | 248 438 | – | – | 0·32 | 0·63 |
| Whiting | Merlangius merlangus | D2 | 52 | 0·323 | 21 | 9·9 | 2·943 | 527 871 | 181 713 | 527 871 | 0·21 | 0·37 |
| Witch | Glyptocephalus cynoglossus | D2 | 44 | 0·200 | 29 | 1·7 | 3·389 | 38 129 | – | – | 0·27 | 0·55 |
| Gurnard | Eutriglia gurnardus | D2 | 43 | 0·266 | 18 | 16·0 | 2·815 | 166 957 | – | – | 0·27 | 0·55 |
| Plaice | Pleuronectes platessa | D2 | 65 | 0·122 | 22 | 12·5 | 2·943 | 500 800 | 182 000 | 500 800 | 0·25 | 0·55 |
| Starry Ray | Amblyraja radiata | D2 | 66 | 0·230 | 46 | 10·7 | 2·940 | 74 341 | – | – | 0·15 | 0·55 |
| Haddock | Melanogrammus aeglefinus | D2 | 71 | 0·271 | 27 | 9·2 | 3·013 | 524 367 | 63 074 | 524 367 | 0·30 | 0·58 |
| Cuckoo Ray | Leucoraja naevus | D2 | 92 | 0·110 | 59 | 2·6 | 3·217 | 7809 | – | – | 0·11 | 0·55 |
| Monkfish | Lophius piscatorius | D2 | 106 | 0·180 | 61 | 29·7 | 2·841 | 15 000 | – | – | 0·10 | 0·55 |
| Cod | Gadus morhua | D2 | 150 | 0·216 | 54 | 8·1 | 3·050 | 86 562 | 24 534 | 86 562 | 0·19 | 0·88 |
| Saithe | Pollachius virens | D1 | 119 | 0·175 | 49 | 8·5 | 3·024 | 327 700 | 105 400 | 327 700 | 0·30 | 0·38 |
Expected ranges of parameter uncertainty were expressed for seven key parameters with a default estimate and four alternate values that spanned the range of uncertainty reported in published literature or estimated from data (Table S1). Model variants were established for all combinations of parameter values, generating an initial set of 57 (78 125) variants. Each variant was run under ‘no-fishing’ and ‘historic fishing’ scenarios, and outputs were screened to establish whether all species persisted (with no-fishing) and species' biomass was consistent with independent estimates (with ‘historic fishing’, details below). Model variants that produced outputs that did not meet these criteria were excluded. The remaining model variants were treated as equally plausible and dubbed the filtered ensemble (FE). Evaluations of fishing scenarios were conducted using the FE and thus generated probability distributions of outputs.
Eleven species in the model are assessed by ICES with age-based single-species models; Norway pout Trisopterus esmarkii (Nilsson), sandeel Ammodytes spp., herring Clupea harengus Linnaeus, horse mackerel Trachurus trachurus (Linnaeus), sole Solea solea (Linnaeus), mackerel Scomber scombrus Linnaeus, whiting Merlangius merlangus (Linnaeus), plaice Pleuronectes platessa Linnaeus, haddock Melanogrammus aeglefinus (Linnaeus), cod Gadus morhua Linnaeus and saithe Pollachius virens (Linnaeus). For these species, default VBGE parameters were calculated by fitting the VBGE to ICES weight-at-age data. For remaining species, VBGE parameter estimates were taken from Rochet et al. (2011) and Blanchard et al. (2014). Prey species that were not individually modelled were represented as a constant resource of 1010 g of ‘other food’ available to all predator length classes at each time step. This equates to a total of 2·5 × 106 tonnes of other food available to the whole community at each time step. Preliminary sensitivity tests involving 10-fold changes in other food, and varying its size profile, confirmed that while some outputs from the FE were sensitive to the quantity and representation of other food, these choices led to unreasonable M2 values (Figs S1–S3, Sparholt 1990). The modelling approach assumes that the modelled community is closed (dynamics determined solely by internal processes), an assumption that is necessarily violated to some extent in a continuous marine environment.
Recruitment was assumed to be deterministic, with individuals recruiting at the mid-point of the smallest length class. The Ricker spawner–recruit relationship used by Hall et al. (2006) was replaced with a hockey-stick spawner–recruit relationship (HS-SRR, Barrowman & Myers 2000). This was changed because the Ricker relationship led to a steeper size spectrum slope (SSS) when unfished than at modest fishing pressures, in contrast with theory and observation (e.g. Rice & Gislason 1996; Bianchi et al. 2000; Andersen & Beyer 2006) and because the HS-SRR provided a better fit to ICES data which included few recruitment estimates at relatively high spawner biomass. For the default model parameterization, α (the initial slope of the HS-SRR) was estimated as a function of L∞ from data (Denney, Jennings & Reynolds 2002), and an upper limit for β, the fraction of maximum spawning stock biomass (SSBmax) above which recruitment is assumed constant, was estimated by fitting the HS-SRR to ICES spawner and recruit abundance data from single-species stock assessments (ICES 2013). Lower values of β were selected from a linear series of successive 20% reductions in β (Table S1). For each species, we then estimated α from L∞ and the inflection point in the segmented regression as a fraction of maximum spawner biomass (SSBmax). SSBmax for assessed species was defined as the highest species' SSB reported in ICES stock assessments from 1990 to 2010. For non-assessed species, SSBmax was each species' highest SSB as estimated from swept area in the North Sea Quarter 1 International Bottom Trawl Survey (IBTS) from 1990 to 2010 (ICES 2012a). Swept area abundance estimates were corrected for selectivity and availability by species, following the method of Fraser, Greenstreet & Piet (2009).
Natural mortality was divided into non-predation (M1) and predation (M2) mortality, where M2 varied with predator abundance. Total predation mortality imposed by each predator was calculated based on the food requirements needed to support growth, taking account of growth efficiency. Predators consumed prey species defined as suitable in a diet interaction matrix that specifies possible interactions among all species. A predator of a given size could consume individuals of an available prey species within a defined predator: prey size ratio (Hall et al. 2006; Table S1). All modelled species also consume individuals of a suitable size in ‘other food’. Mortality imposed by predators was partitioned among available prey according to relative prey abundance at size. The default diet interaction matrix from Rochet et al. (2011) was supplemented with two variants based on empirical diet data, where predators were allowed to consume prey species if the frequency of occurrence of the prey in diet data was greater than a specified threshold (Tables S1–S4). For two remaining variants of the diet matrix, we assumed that all species ate all other species or that pelagic species only ate pelagic species, and demersal species only ate demersal species.
Fishing mortality was applied assuming species-specific relative size selectivity established from fits to ICES (2012b) F at length data. Model scenarios were fishing at MSY (FMSY, the fishing mortality that produced the maximum sustainable yield, Table 1, Fig. S4) and fishing with ‘historic’ fishing patterns (FHIST, Table 1, Fig. S4). For the FHIST scenario, the F applied to each of the 11 modelled and assessed species was mean F for 1990–2010 (ICES 2012b). Non-assessed species were grouped with assessed species of similar morphology and behaviour and were assumed to be fished at the same F (Pope et al. 2000). Poor cod, gurnard Eutriglia gurnardus (Linnaeus) and monkfish Lophius piscatorius Linnaeus were grouped with haddock, cod and whiting. Long rough dab Hippoglossoides platessoides (Fabricius), dab Limanda limanda (Linnaeus), lemon sole Microstomus kitt (Walbaum), witch Glyptocephalus cynoglossus (Linnaeus), starry ray Amblyraja radiata (Donovan) and cuckoo ray Leucoraja naevus (Müller & Henle) were grouped with plaice. For the FMSY scenario, FMSY values for assessed species were obtained from ICES (2012b). For the non-assessed species, the F leading to 40% spawner-per-recruit (F40) was assumed to be a proxy for FMSY, and F40 values were estimated following Le Quesne & Jennings (2012).
Output from each of the 78 125 model variants was tested against two test criteria to filter out variants with parameter sets leading to unrealistic community properties. Each model variant was run for 45 years with F = 0 to predict an unfished equilibrium community state, followed by 30 years fishing at FHIST. The first test criterion was that all species should persist in the unfished model system, with biomass exceeding 1% of current biomass estimates from ICES assessments or survey data analyses. The second test criterion was applied after 30 years fishing at FHIST. It specified that mean predicted biomass of assessed species during the last 15 years of the FHIST simulation should be more than half the lowest, but less than twice the highest, biomass estimate from 1990 to 2010 (assessment data from ICES 2012b). We used a factor of two because biomass estimates from the single-species assessments are uncertain, and because we adopted a deterministic spawner–recruit relationship when environmental trends and stochastic processes influence recruitment dynamics in the real world. Using the screening criteria, each of the 78 125 model variants was classified as plausible or rejected. Consequences of modifying the factor, dubbed the tolerance factor, from 1·5 to 3·0 were examined (Figs S5, S6 and S7). Selected parameter options in the FE were examined to identify which parameters introduced most uncertainty in model outputs, and to assess whether model dynamics were independent of any given parameter choice.
Accepted model variants, referred to as the FE, were run for 30 years into the future under given fishing scenarios to generate ensemble predictions of the potential response of individual species and community metrics to F. For the FMSY scenario, all species were fished at their predicted FMSY, and at F = 0 to F = 5FMSY, in 0·1 steps. This set of FMSY scenarios did not allow for examination of yield trade-offs among species, as all F were scaled equally (same proportional change in F applied to each modelled species), but was used to provide a measure of the relative magnitude of uncertainty in predictions of the single-species reference points FMSY, BMSY (the SSB at FMSY) and YMSY (yield at FMSY) derived in a multispecies context.
During simulations, biomass and yield of each species and the value of each community metric were calculated annually. Community metrics calculated were as follows: mean length (ML), biomass fraction >40 cm (dubbed the large fish indicator, LFI), slope of the size spectrum (slope of relationship between log numbers in each log size class and log size, SSS) and mean maximum weight by biomass (biomass-weighted mean of species' W∞; a measure of the life-history composition of the community (mean maximum weight, MMW, Shin et al. 2005). Metrics were calculated using abundance estimates for individuals >15 cm, approximating sizes adequately selected in trawl surveys in the North Sea (Fraser, Greenstreet & Piet 2009). When assessing fishing effects on individual species, we referred to species as collapsed when SSB fell below 10% of unexploited SSB. Outputs of simulations are presented as frequency distributions, treating all variants in the FE as equally plausible. Uncertainty was expressed as 50th and 90th percentile intervals within the FE. The 50% and 90% intervals spanned the 25th to 75th and 5th to 95th percentiles of the frequency distribution of output values generated by the FE, respectively.
We calculated the statistical power of the North Sea IBTS Quarter 1 survey to detect predicted changes in community metrics during a transition from fishing at FHIST to FMSY. Statistical power was defined as the probability that the predicted change in metric value achieves a statistical significance (here set at 0·10) over a 5- or 15-year period. As well as the statistical significance and sampling period, power depends on the magnitude of the residual variance, which was estimated by taking residuals from a loess smoother (Fryer & Nicholson 1993, span = 1) fitted to a time series (1990–2011) of metrics calculated from North Sea IBTS Quarter 1 survey data. Metrics were calculated from survey data using only the species and size classes included in the multispecies model. Power to detect the predicted change in each metric during the transition from fishing at FHIST to FMSY was estimated for each model variant in the FE based on 10 000 random draws from a normal distribution fitted to residuals of the empirical time series.
Results
Individual species' biomass predictions from the models in the unfiltered ensemble (UE), when fishing according to the FMSY scenario, tended to span 2 or more orders of magnitude (Fig. 1), indicating that initial spread of parameter choices provided little constraint on predictions. Uncertainty in predicted biomass is greatly reduced for the FE where uncertainty is reduced to between one-half and one order of magnitude (Fig. 1). Only 188 of 78 125 possible model variants passed the screening criteria. The default model did not pass the screening criteria. Changing the screening criteria for biomass at FHIST to allow a tolerance factor of 1·5× to 3× for the relationship between ICES single-species biomass estimates and model outputs increased the size of the FE (Fig. S5), but had a small effect on outputs and uncertainty and did not change qualitative behaviour and relative performance of species and community metrics (Figs S6 and S7).
Fig 1.
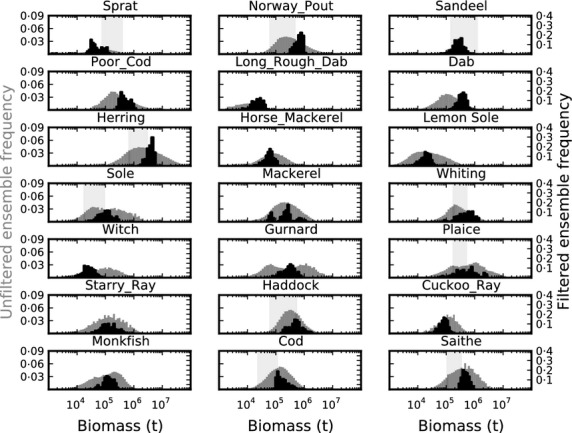
Frequency distribution of estimated biomass of modelled species at FMSY. Grey histograms denote the frequency distribution of biomass estimates from models in the unfiltered ensemble (UE), black histograms the biomass estimates from models in the filtered ensemble (FE). The vertical shaded band represents the biomass range reported by ICES for assessed species for years 1990–2010.
To evaluate impacts of parameter uncertainty on estimates of management reference points for each individual species in the multispecies model, the spread of FMSY and BMSY estimates for all models in the FE was compared with mean FMSY calculated across all models in the FE. For FMSY, there was 50% probability that a given value was within ±25% of the mean and 90% probability that it was within ±50% of the mean (Table 2), for all species except plaice. For BMSY, there was a 50% probability of predicting single-species values to within ±40%; 90% intervals were much wider, typically spanning a factor of 5–10. Probability distributions of outputs from the FE provided information on uncertainty that could inform decisions such as how to set FMSY to achieve a defined reduction in the risk that true F exceeds FMSY. For example, if FMSY for cod was 0·23, there would be 50% confidence that the actual FMSY limit was not breached. However, if the FMSY limit was set to increase confidence to 75%, or 95%, FMSY would need to be reduced to 0·21 or 0·17, respectively (Table 2).
Table 2.
Estimates of single-species fisheries reference points with the filtered ensemble (FE). Mean estimates for FMSY and BMSY are presented as uncertainty intervals (UI) spanning 50% and 90% of the members of the FE. FMSY ratio is the ratio of the FMSY predicted by the multispecies model to estimates of FMSY from ICES single-species stock assessment models
| Common name | FMSY (year−1) | 50% UI FMSY (year−1) | 90% UI FMSY (year−1) | FMSY ratio | BMSY (×105 t) | 50% UI BMSY (proportion) | 90% UI BMSY (proportion) |
|---|---|---|---|---|---|---|---|
| Sprat | 0·78 | 0·65–0·91 | 0·52–1·04 | 0·60 | 1·26 | 0·64–1·32 | 0·46–1·95 |
| Norway Pout | 0·82 | 0·67–0·98 | 0·52–1·16 | 2·35 | 5·07 | 0·76–1·24 | 0·50–1·45 |
| Sandeel | 1·11 | 0·84–1·36 | 0·67–1·57 | 3·18 | 2·13 | 0·69–1·24 | 0·46–1·69 |
| Poor Cod | 0·77 | 0·65–0·86 | 0·50–1·08 | 1·07 | 6·88 | 0·69–1·25 | 0·54–1·66 |
| Long Rough Dab | 0·84 | 0·72–1·02 | 0·54–1·14 | 1·39 | 0·32 | 0·67–1·25 | 0·41–1·69 |
| Dab | 0·85 | 0·70–0·98 | 0·57–1·15 | 2·07 | 3·19 | 0·79–1·19 | 0·53–1·55 |
| Herring | 0·54 | 0·45–0·62 | 0·35–0·73 | 2·17 | 28·3 | 0·82–1·20 | 0·52–1·48 |
| Horse Mackerel | 0·54 | 0·45–0·60 | 0·35–0·80 | 1·07 | 0·80 | 0·64–1·24 | 0·33–1·96 |
| Lemon Sole | 0·48 | 0·36–0·56 | 0·33–0·69 | 1·45 | 0·20 | 0·68–1·29 | 0·40–1·80 |
| Sole | 0·68 | 0·55–0·77 | 0·44–0·99 | 3·08 | 0·90 | 0·62–1·23 | 0·41–2·16 |
| Mackerel | 0·49 | 0·42–0·58 | 0·32–0·67 | 1·55 | 2·59 | 0·47–1·04 | 0·17–3·20 |
| Whiting | 0·75 | 0·59–0·90 | 0·46–1·03 | 3·55 | 4·26 | 0·62–1·23 | 0·39–2·15 |
| Witch | 0·34 | 0·27–0·41 | 0·24–0·49 | 1·27 | 0·28 | 0·57–1·27 | 0·33–2·04 |
| Gurnard | 0·84 | 0·70–0·97 | 0·54–1·19 | 3·12 | 2·81 | 0·65–1·20 | 0·41–1·96 |
| Plaice | 0·31 | 0·23–0·38 | 0·15–0·50 | 1·24 | 8·64 | 0·26–1·06 | 0·13–3·89 |
| Starry Ray | 0·21 | 0·17–0·24 | 0·14–0·26 | 1·38 | 1·49 | 0·42–1·19 | 0·23–2·49 |
| Haddock | 0·48 | 0·36–0·57 | 0·30–0·72 | 1·61 | 4·70 | 0·52–1·19 | 0·26–2·61 |
| Cuckoo Ray | 0·11 | 0·10–0·12 | 0·09–0·13 | 1·02 | 0·86 | 0·70–1·18 | 0·49–1·82 |
| Monkfish | 0·20 | 0·17–0·23 | 0·15–0·25 | 2·01 | 0·90 | 0·65–1·31 | 0·32–1·88 |
| Cod | 0·23 | 0·21–0·27 | 0·17–0·27 | 1·21 | 1·72 | 0·55–1·25 | 0·45–2·44 |
| Saithe | 0·34 | 0·24–0·42 | 0·21–0·45 | 1·13 | 5·54 | 0·69–1·21 | 0·51–1·99 |
Model FMSY predictions were consistent with single-species estimates for 12 species, higher for eight and lower for sprat (Fig. 2). FMSY estimates were higher for mid-sized species, the likely explanation being predation release as large fish were depleted at high F. Relationships between BMSY and B0 followed theoretical expectations of BMSY ≈ B0/2 (e.g. Punt et al. 2014).
Fig 2.
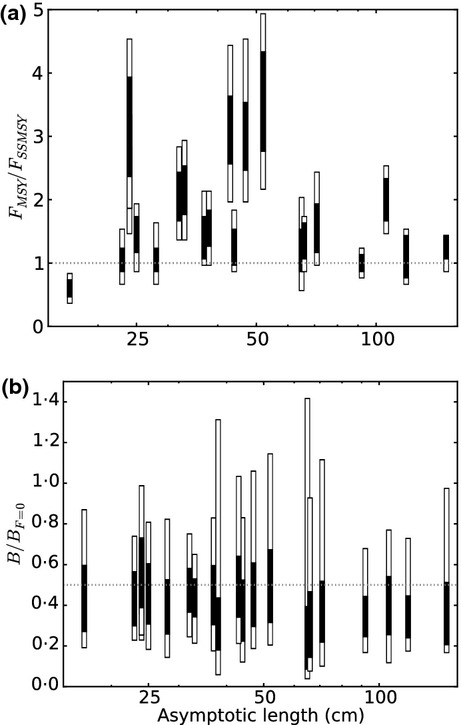
Relationship (a) between FMSY predicted with model variants in the filtered ensemble (FE) as a proportion of FMSY from ICES single-species stock assessment models (FSSMSY) and asymptotic length, and (b) between BMSY as a proportion of B0 predicted with model variants in the FE and asymptotic length. Black bars and open bars denote 50% and 90% uncertainty intervals, respectively.
Community metrics showed qualitatively similar responses to changes in F (Fig. 3), although the SSS showed a near linear decline in response to increasing F, while the LFI, ML and MMW displayed a nonlinear relationship with F. There was considerable uncertainty in predicted values of the community metrics at FMSY and across the full range of F (Fig. 3).
Fig 3.
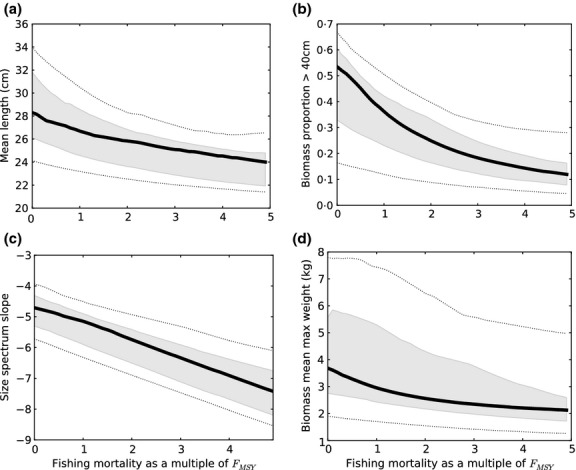
Relationship between the community metrics and fishing mortality predicted by the model variants in the filtered ensemble (FE). Black line is the mean value, and the grey and broken lines denote uncertainty intervals including 50% and 90% of FE members, respectively. Metrics are (a) mean length, (b) proportion of large fish, (c) size spectrum slope and (d) mean maximum weight.
If a community metric is to provide feedback on effects of management action, then it should show a response to these effects and this should be detectable in monitoring data. This requires that the change in metric value following a defined management action should be greater than uncertainty in prediction, and the predicted change in the metric value should be detectable given interannual variation in data. To compare theoretically expected change in community metrics with uncertainty in prediction, the ratio of the metric at FHIST:FMSY, for each FE variant, was compared with the 50% and 90% uncertainty intervals for the prediction at FHIST, where the 50% and 90% uncertainty intervals were expressed as a proportion of the mean metric value (Fig. 4). The mean change in the values of the metrics fell outside the 50% uncertainty interval for MMW, LFI and SSS, but not for ML. For MMW, LFI and SSS, the predicted change in indicator values for the entire FE lay outside the 50% uncertainty interval, and for SSS, predicted change also lay outside the 90% interval. Thus, SSS is expected to be the most responsive community indicator.
Fig 4.
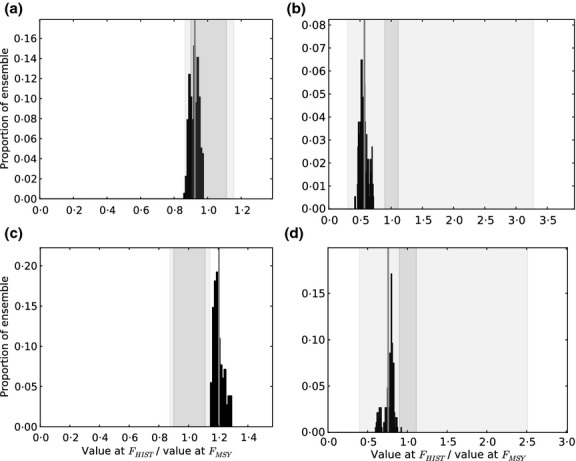
Frequency distribution of community metrics predicted with models in the filtered ensemble (FE) and expressed as the ratio of values of each metric at FHIST and FMSY (black histogram). The vertical grey line is the mean ratio of values of each metric at FHIST and FMSY. Vertical shaded bands indicate 50% (dark) and 90% (light) uncertainty intervals for the prediction at FHIST, with intervals presented as a proportion of the mean metric value. Metrics are (a) mean length, (b) proportion of large fish, (c) size spectrum slope and (d) mean maximum weight.
Power of the North Sea IBTS to detect changes in community metrics was assessed by comparing the magnitude of change in mean metric values when model F transitioned from FHIST to FMSY with the magnitude of interannual variation in metric values from IBTS data. For community metrics applied to all demersal species (Table 1, see Lifetype D1), there was a 0·6 to 0·8 mean power of detecting a change in ML, MMW or LFI that occurred over 5 years and a mean power of >0·9 of detecting a change in SSS (Fig. 5). After 15 years simulated monitoring, mean power was still <0·8 for ML, ≈0·9 for MMW, ≈0·95 for the LFI and 1·0 for SSS. For all community metrics except SSS, there was significant variation in power among FE variants after 15 years. For example, for LFI, power ranged from 0·7 to 1·0. Power to predict changes in community metrics for all demersal species was generally a little lower than for strictly demersal species (Table 1, see Lifetype D2), but SSS still provided greatest power after 5 and 15 years (Fig. S8).
Fig 5.
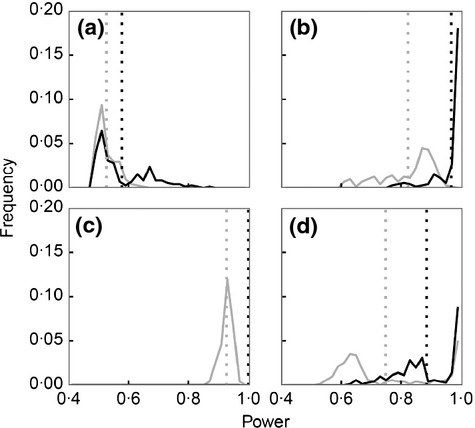
Frequency distributions for the predicted power of the North Sea International Bottom Trawl Survey (IBTS) to detect modelled changes in the values of community metrics for all demersal fishes (Lifetype D1, Table 1) when fishing mortality is reduced from FHIST to FMSY over 5-year (grey) and 15-year (black) periods. Metrics are (a) mean length, (b) proportion of large fish, (c) size spectrum slope and (d) mean maximum weight. Vertical lines denote mean power for all model variants in the filtered ensemble (FE).
Total community yield was maximized when F ≈ 1·6 × FMSY (Fig. 6). Fishing mortality had to be reduced to FMSY or below to avoid all possibility of species' collapse. At 3 × FMSY, an approximation of maximum ‘historic’ F for North Sea target species, mean total yield was predicted to be similar to yield at FMSY, or around 85% of the maximum, but two species were expected to collapse, and probability of collapse was >0·1 for another five species. In these fishing simulations, F across all species was increased and decreased evenly, with F ratios among species defined from single-species estimates of FMSY. This fishing strategy tends to predict relatively high yields for all species in the community at FMSY (Fig. S9) and few trade-offs that result from more uneven distributions of F in relation to FMSY. The sensitivity of sprat to collapse may be an indication of the differing age at selection applied in our model and the ICES assessment models and/or may reflect relatively high estimates of FMSY in the ICES assessment.
Fig 6.
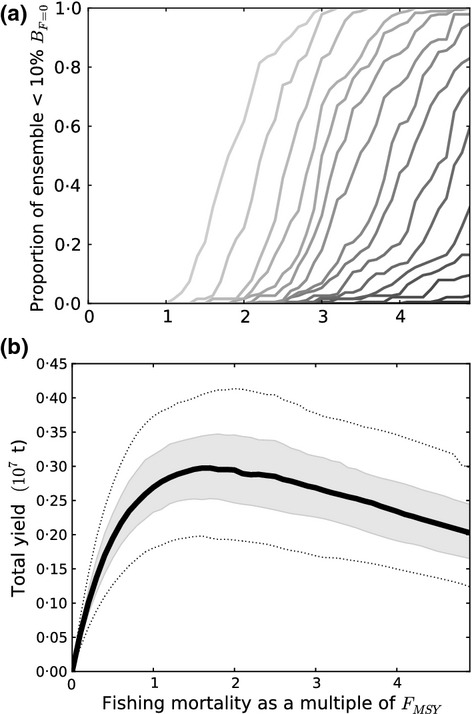
(a) Probability of species' collapse as a function of fishing mortality, with probability expressed as the proportion of variants in the filtered ensemble (FE) which result in collapses (B < 10% B0). The lightest grey tone indicates probability for the most sensitive species and successively darker tones the probabilities for two or more (up to 14) species. (b) Relationship between mean total yield from the community and fishing mortality. Grey shading and broken lines denote 50% and 90% uncertainty intervals, respectively.
We assessed contributions of each parameter to uncertainty in model outputs (Table 3). Parameters contributing most to overall uncertainty in the UE were L∞ followed by the diet matrix, where only one option gave successful outcomes. The choices of M1, predator–prey size ratio, growth efficiency and HS-SRR parameters all made similar contributions to overall variance in model outputs; in each case, these parameter choices only accounted for about 60% of the variance generated by choice of L∞. Only the diet matrix of Rochet et al. (2011) was incorporated in FE model variants. A single parameter choice was responsible for >50% of successful outcomes for predator–prey size ratio and growth efficiency, while the distribution was more even for M1, HS-SRR α and L∞.
Table 3.
Proportion of accepted model variants in the filtered ensemble (FE) that incorporated each parameter option, and the relative variance in biomass projections related to each parameter in the unfiltered ensemble (UE). The relative variance describes the variance in biomass projections related to each parameter as a proportion of the variance in biomass projections related to asymptotic length. For parameter choices, see Table S1
| Parameter choice (FE) |
Relative variance (UE) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Diet matrix | 1·00 | 0·00 | 0·00 | 0·00 | 0·00 | 0·83 |
| Predation size selection | 0·60 | 0·20 | 0·06 | 0·06 | 0·08 | 0·58 |
| Natural mortality (M1) | 0·25 | 0·17 | 0·36 | 0·19 | 0·03 | 0·60 |
| Spawner–recruit (β) | 0·04 | 0·09 | 0·22 | 0·45 | 0·20 | 0·59 |
| Growth efficiency (Ge) | 0·07 | 0·62 | 0·10 | 0·03 | 0·18 | 0·61 |
| Asymptotic length (L∞) | 0·15 | 0·08 | 0·16 | 0·23 | 0·38 | 1·00 |
| Spawner–recruit (α) | 0·21 | 0·09 | 0·31 | 0·19 | 0·20 | 0·65 |
Discussion
Incorporating plausible parameter uncertainty in model inputs led to many implausible outputs, and screening against data removed >99% of variants. The resulting FE was used to assess uncertainty in single species and community reference points and single species and community responses to alternate management actions. Probability distributions of outputs from the FE provided information on uncertainty. This can inform decisions on the values of reference points to achieve a defined reduction in risk that reference points are not exceeded. Some management frameworks are already designed to take account of information on uncertainty. For example, in the United States, the ‘Overfishing Limit’ (OL) corresponds to our central estimate of BMSY while the ‘Acceptable Biological Catch’ (ABC) is set lower than the OL, with the difference between OL and ABC determined by the uncertainty in the OL prediction and the risk that the manager accepts (National Marine Fisheries Service 2009). Outputs from our FE can also be used to assess power of monitoring surveys to detect responses in different community metrics and to identify parameters where investment in future data collection or changes to model structure may be targeted to reduce uncertainty.
Results are clearly contingent on assumptions. In particular, assumed selectivity patterns and relative F among species have a significant influence on estimates of species' abundance, size composition and yield. For simplicity, we scaled F to all species uniformly in relation to FMSY. This does not provide an accurate representation of the way F is applied to North Sea stocks, nor did it attempt to reflect a realistic alteration in F in response to management measures, although both issues could be addressed in future. Our preferred emphasis was on evaluating the extent and implications of uncertainty associated with multispecies and community predictions, rather than re-evaluating reference points for North Sea stocks.
Our main measure of indicator performance was sensitivity; the capacity to differentiate responses to changes in F. Another relevant measure is the specificity of response to fishing, the extent to which changes in the indicator are driven by fishing as opposed to other causes (e.g. Houle et al. 2012). To evaluate specificity would require a model that sought to describe environmental and other influences on the fish community. Our evaluation of the capacity of four community indicators to describe change in state when transiting from historic North Sea F to the current management target of fishing at, or below, FMSY (EU 2013) demonstrated that SSS tracked change with lowest uncertainty. If model predictions of change were realistic then power analysis demonstrated that the NS-IBTS was more likely to detect trends in SSS, as defined here, than any other indicator. Power to detect changes in community indicators for strictly demersal species was better than for all demersal species, likely because the former are more effectively sampled by survey gear (Fraser, Greenstreet & Piet 2009) resulting in lower interannual variation. The more consistent and detectable response of SSS to changes in F compared with other community indicators, including the LFI, was consistent with results in Blanchard et al. (2014) based on a single parameterization of an alternate multispecies size-structured model that accounted for food-dependent growth and incorporated stochastic recruitment variation. Our power calculations assume that community changes associated with moving from FHIST to FMSY take 5 or 15 years. This allowed relative performance of indicators to be compared. We did not attempt to predict absolute rates of change in community indicators because they are highly dependent on stochastic processes, especially recruitment.
The frequency with which different parameter choices appear in the FE, coupled with relative variance in outputs associated with different parameters, helps identify when investment in refining parameter values may be desirable. Results suggested that the diet matrix was especially influential with neither empirically based diet matrix leading to accepted models. This may be due to limitations of diet studies that may not provide evidence of consumption when some prey species are small, relatively delicate and/or highly digestible, because they quickly become unidentifiable in stomach contents. This could apply to sprat consumption for example. Developing diet matrices likely requires consideration of other evidence for feeding interactions, as attempted by Rochet et al. (2011). Further, in models with no explicit representation of spatial structure, the diet matrix also identifies feeding interactions that are not feasible owing to species' spatial separation. The simple assumption that everything eats everything was not supported in our size- and species-based model. Some species require a refuge from predators in the modelled community for their dynamics to mimic those observed in data.
Our conclusions about the effects of parameter uncertainty may not hold for other models with more tightly coupled species dynamics or including food-dependent growth (e.g. Blanchard et al. 2014). Further, much density dependence in our model is generated by the HS-SRR which fits quite poorly to data that are already expensive to generate. It is unlikely that more data would reduce our uncertainty about a relationship that has to be used in population models to capture a number of unspecified density-dependent processes that may be impossible to characterize given other sources of variation (e.g. Szuwalski et al. 2014). Consequently, the advantages of a model structure that is robust to parameter uncertainty are balanced by disadvantages when model structure omits potentially important processes.
In summary, there have been few attempts to explore effects of uncertainty in multispecies models, which limits interpretation of outputs and their use in management advice. This study has demonstrated one practical method for explicitly introducing parameter uncertainty in model outputs and describing uncertainty in single species and community reference points and responses to fishing mortality. The research also demonstrates the level of caution that needs to be applied in interpretation of outputs from community models based on single parameter sets.
Acknowledgments
This work was funded by the UK Department of the Environment Food and Rural Affairs (Project MF1225) and additionally by the EU FP7 project “Ocean-Certain” in its later stages. We thank two anonymous referees and Matthew Spencer for detailed and well-considered comments that helped us to improve the analyses and presentation.
Data accessibility
The data used in this article are publicly available via the International Council for the Exploration of the Sea (ICES) Data Portal and from the Centre for Environment, Fisheries and Aquaculture Science (CEFAS) Integrated Database and Portal for Fish Stomach Records (DAPSTOM). More detailed descriptions of data sources are provided in the ‘Materials and methods’ section of this article.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Parameter values in the unfiltered ensemble.
Table S2. Diet matrix option 1 (Rochet et al. 2011).
Table S3. Diet matrix option 3 with a cut-off diet fraction by numbers of 0·001 (Pinnegar & Platts, 2011).
Table S4. Diet matrix option 4 with a cut-off diet fraction by numbers of 0·01 (Pinnegar & Platts, 2011).
Table S5. Diet matrix option 5.
Fig. S1. Estimated predation mortality rates (M2) in the unfished system based on outputs from model variants in the filtered ensemble (FE) with 1010 g ‘other food’.
Fig. S2. Estimated predation mortality rates (M2) in the unfished system based on outputs from model variants in the filtered ensemble (FE) with 109 g ‘other food’.
Fig. S3. Estimated predation mortality rates (M2) in the unfished system based on model variants in the filtered ensemble (FE) with 1011 g ‘other food’.
Fig. S4. Fishing mortality as a function of length for the FHIST (historic average 1990–2010, red line) and FMSY (single species MSY estimates, blue line) scenarios.
Fig. S5. The number of models in the filtered ensemble (FE) as a function of the tolerance factor accepted when screening model outputs against ICES stock assessment data.
Fig. S6. The effects of changes to the tolerance factor on mean FMSY (red) and associated uncertainty (50% uncertainty intervals: black bars; 90% uncertainty intervals: open bars).
Fig. S7. The effects of changes to the tolerance factor on values of four indicators and the associated uncertainty (50% uncertainty intervals: black bars; 90% uncertainty intervals: open bars).
Fig. S8. Frequency distributions for the predicted power of the North Sea International Bottom Trawl Survey (IBTS) to detect modelled changes in the values of community metrics for strictly demersal fishes (Lifetype D2, Table 1, accompanying paper) when fishing mortality is reduced from FHIST to FMSY over 5 year (grey) and 15 year (black) periods.
Fig. S9. Species' yields as a function of fishing mortality, as predicted with model variants in the filtered ensemble.
References
- Andersen KH, Beyer JE. Asymptotic size determines species abundance in the marine size spectrum. American Naturalist. 2006;168:54–61. doi: 10.1086/504849. [DOI] [PubMed] [Google Scholar]
- Barrowman NJ, Myers RA. Still more spawner-recruit curves: the hockey stick and its generalisations. Canadian Journal of Fisheries and Aquatic Science. 2000;57:665–676. [Google Scholar]
- Bianchi G, Gislason H, Graham K, Hill L, Jin X, Koranteng K, et al. Impact of fishing on size composition and diversity of demersal fish communities. ICES Journal of Marine Science. 2000;57:558–571. [Google Scholar]
- Blanchard JL, Andersen KH, Scott F, Hintzen NT, Piet GJ, Jennings S. Evaluating targets and trade-offs among fisheries and conservation objectives using a multispecies size spectrum model. Journal of Applied Ecology. 2014;51:612–622. [Google Scholar]
- Butterworth D, Plaganyi EE. A brief introduction to some approaches to multispecies/ecosystem modelling in the context of their possible application in the management of South African fisheries. African Journal of Marine Science. 2004;26:53–61. [Google Scholar]
- Denney NH, Jennings S, Reynolds JD. Life-history correlates of maximum population growth rates in marine fishes. Proceedings of the Royal Society B-Biological Sciences. 2002;269:2229–2237. doi: 10.1098/rspb.2002.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza-Tenorio A, Wolff M, Taylor MH, Espejel I. What model suits ecosystem-based fisheries management? a plea for a structured modelling process. Reviews in Fish Biology and Fisheries. 2012;22:81–94. [Google Scholar]
- EU. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy (Marine Strategy Framework Directive) Official Journal of the European Union. 2008;L164:19–40. [Google Scholar]
- EU. Commission decision of 1 September 2010 on criteria and methodological standards on good environmental status of marine waters. Official Journal of the European Union. 2010;L232:14–24. [Google Scholar]
- EU. Regulation (EU) No 1380/2013 of the European Parliament and of the Council of 11 December 2013 on the Common Fisheries Policy, amending Council Regulations (EC) No 1954/2003 and (EC) No 1224/2009 and repealing Council Regulations (EC) No 2371/2002 and (EC) No 639/2004 and Council Decision 2004/585/EC. Official Journal of the European Union. 2013;L354:22–61. [Google Scholar]
- Francis RICC, Shotton R. “Risk” in fisheries management: a review. Canadian Journal of Fisheries and Aquatic Sciences. 1997;54:1699–1715. [Google Scholar]
- Fraser HM, Greenstreet SPR, Piet GJ. Selecting MPAs to conserve ground fish biodiversity: the consequences of failing to account for catchability in survey trawls. ICES Journal of Marine Science. 2009;66:82–89. [Google Scholar]
- Fryer RJ, Nicholson MD. The power of a temporal trend monitoring programme to detect linear trends and incidents. ICES Journal of Marine Science. 1993;50:161–168. [Google Scholar]
- Fulton EA, Smith ADM, Johnson CR. Effect of complexity on marine ecosystem models. Marine Ecology Progress Series. 2003;253:1–16. [Google Scholar]
- Gaichas SK, Odell G, Aydin KY, Francis RICC. Beyond the defaults: functional response parameter space and ecosystem-level fishing thresholds in dynamic food web model simulations. Canadian Journal of Fisheries and Aquatic Sciences. 2012;69:2077–2094. [Google Scholar]
- Garcia SM, Cochrane KL. Ecosystem approach to fisheries: a review of implementation guidelines. ICES Journal of Marine Science. 2005;62:311–318. [Google Scholar]
- Gjosaeter H, Bogstad B, Tjelmeland S. Assessment methodology for Barents Sea capelin, Mallotus villosus (Muller) ICES Journal of Marine Science. 2002;59:1086–1095. [Google Scholar]
- Greenstreet SPR, Rogers SI, Rice JC, Piet GJ, Guirey EJ, Fraser HM, Fryer RJ. Development of the EcoQO for the North Sea fish community. ICES Journal of Marine Science. 2011;68:1–11. [Google Scholar]
- Hall SJ, Collie JS, Duplisea DE, Jennings S, Bravington M, Link J. A length-based multispecies model for evaluating community responses to fishing. Canadian Journal of Fisheries and Aquatic Sciences. 2006;1359:1344–1359. [Google Scholar]
- Hill SL, Watters GM, Punt AE, McAllister MK, Quéré CL, Turner J. Model uncertainty in the ecosystem approach to fisheries. Fish and Fisheries. 2007;8:315–336. [Google Scholar]
- Houle JE, Farnsworth KD, Rossberg AG, Reid DG. Assessing the sensitivity and specificity of fish community indicators to management action. Canadian Journal of Fisheries and Aquatic Sciences. 2012;69:1065–1079. [Google Scholar]
- ICES. 2012a. p. 443. Copenhagen ICES Report of the ICES Advisory Committee 2012, Book 6: North Sea pp.
- ICES. 2012b. p. 68. Copenhagen ICES Manual for the International Bottom Trawl Surveys. Series of ICES Survey Protocols. SiSP 1-IBTS VIII, pp.
- ICES. 2013. Copenhagen ICES Stock Assessment Summary Database http://www.ices.dk/marine-data/dataset-collections/Pages/Fish-catch-and-stock-assessment.aspx (accessed 18/06/2013)
- Le Quesne WJF, Jennings S. Predicting species vulnerability with minimal data to support rapid risk assessment of fishing impacts on biodiversity. Journal of Applied Ecology. 2012;49:20–28. [Google Scholar]
- Link JS, Ihde TF, Harvey CJ, Gaichas SK, Field JC, Brodziak JKT, Townsend HM, Peterman RM. Dealing with uncertainty in ecosystem models: the paradox of use for living marine resource management. Progress in Oceanography. 2012;102:102–114. [Google Scholar]
- National Marine Fisheries Service (NMFS) 2009. pp. 3178–3212. Final rule national standard guidelines. 74 Federal Register (January 16, 2009)
- Plagányi ÉE. 2007. p. 108. Rome FAO Models for an Ecosystem Approach to Fisheries. FAO Fisheries Technical Paper No. 477, pp.
- Pope JG, MacDonald DS, Daan N, Reynolds JD, Jennings S. Gauging the vulnerability of non-target species to fishing. ICES Journal of Marine Science. 2000;57:689–696. [Google Scholar]
- Punt AE, Smith ADM, Smith DC, Tuck GN, Klaer NL. Selecting relative abundance proxies for BMSY and BMEY. ICES Journal of Marine Science. 2014;71:469–483. [Google Scholar]
- Rice JC. Managing fisheries well: delivering the promises of an ecosystem approach. Fish and Fisheries. 2011;12:209–231. [Google Scholar]
- Rice JC, Gislason H. Patterns of change in the size spectra of numbers and diversity of the North Sea fish assemblage, as reflected in surveys and models. ICES Journal of Marine Science. 1996;53:1214–1225. [Google Scholar]
- Rochet MJ, Collie JS, Jennings S, Hall SJ. Does selective fishing conserve community biodiversity? Predictions from a length-based multispecies model. Canadian Journal of Fisheries and Aquatic Sciences. 2011;68:469–486. [Google Scholar]
- Shephard S, Reid DG, Greenstreet SPR. Interpreting the large fish indicator for the Celtic Sea. ICES Journal of Marine Science. 2011;68:1963–1972. [Google Scholar]
- Shin Y-J, Rochet M-J, Jennings S, Field J, Gislason H. Using size-based indicators to evaluate the ecosystem effects of fishing. ICES Journal of Marine Science. 2005;62:384–396. [Google Scholar]
- Sparholt H. Improved estimates of the natural mortality rates of nine commercially important fish species included in the North Sea multispecies VPA model. Journal du Conseil, Conseil International pour L'Exploration de la Mer. 1990;46:211–223. [Google Scholar]
- Szuwalski CS, Vert-Pre KA, Punt AE, Branch TA, Hilborn R. Examining common assumptions about recruitment: a meta-analysis of recruitment dynamics for worldwide marine fisheries. Fish and Fisheries. 2014 DOI: 10.1111/faf.12083. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameter values in the unfiltered ensemble.
Table S2. Diet matrix option 1 (Rochet et al. 2011).
Table S3. Diet matrix option 3 with a cut-off diet fraction by numbers of 0·001 (Pinnegar & Platts, 2011).
Table S4. Diet matrix option 4 with a cut-off diet fraction by numbers of 0·01 (Pinnegar & Platts, 2011).
Table S5. Diet matrix option 5.
Fig. S1. Estimated predation mortality rates (M2) in the unfished system based on outputs from model variants in the filtered ensemble (FE) with 1010 g ‘other food’.
Fig. S2. Estimated predation mortality rates (M2) in the unfished system based on outputs from model variants in the filtered ensemble (FE) with 109 g ‘other food’.
Fig. S3. Estimated predation mortality rates (M2) in the unfished system based on model variants in the filtered ensemble (FE) with 1011 g ‘other food’.
Fig. S4. Fishing mortality as a function of length for the FHIST (historic average 1990–2010, red line) and FMSY (single species MSY estimates, blue line) scenarios.
Fig. S5. The number of models in the filtered ensemble (FE) as a function of the tolerance factor accepted when screening model outputs against ICES stock assessment data.
Fig. S6. The effects of changes to the tolerance factor on mean FMSY (red) and associated uncertainty (50% uncertainty intervals: black bars; 90% uncertainty intervals: open bars).
Fig. S7. The effects of changes to the tolerance factor on values of four indicators and the associated uncertainty (50% uncertainty intervals: black bars; 90% uncertainty intervals: open bars).
Fig. S8. Frequency distributions for the predicted power of the North Sea International Bottom Trawl Survey (IBTS) to detect modelled changes in the values of community metrics for strictly demersal fishes (Lifetype D2, Table 1, accompanying paper) when fishing mortality is reduced from FHIST to FMSY over 5 year (grey) and 15 year (black) periods.
Fig. S9. Species' yields as a function of fishing mortality, as predicted with model variants in the filtered ensemble.
Data Availability Statement
The data used in this article are publicly available via the International Council for the Exploration of the Sea (ICES) Data Portal and from the Centre for Environment, Fisheries and Aquaculture Science (CEFAS) Integrated Database and Portal for Fish Stomach Records (DAPSTOM). More detailed descriptions of data sources are provided in the ‘Materials and methods’ section of this article.


