Abstract
The combination of clarithromycin, lenalidomide and dexamethasone (BiRd) has led to highly durable responses in newly diagnosed myeloma. However, the ability of clarithromycin to overcome resistance to lenalidomide and dexamethasone (Rd) is not known. To study this, we performed a retrospective analysis of 24 patients with myeloma for which clarithromycin was added to Rd at the time of progression on Rd. The median number of prior therapies was 3 (range 1–8). The best response was complete response (CR) in one (4.2%), very good partial response (VGPR) in one (4.2%) and partial response in eight (33.3%) patients. Ten patients, 41.7% (95% CI: 22.1, 63.4), achieved ≥PR. The median time to response was 4.4 months (range 1–13.6 months) and the median duration of response was 6.9 months (range 3–52.2 months). The clinical benefit rate (CR + VGPR + PR + MR) was 45.8% (95% CI 25.6, 67.2). The median progression-free survival was 4 months. Median overall survival was 25 months with a median follow-up of 27.5 months. The regimen was well tolerated and only 2 patients needed a clarithromycin dose reduction. Addition of clarithromycin to Rd can overcome resistance to Rd in a subset of patients and lead to durable clinical responses.
Introduction
Multiple myeloma (MM) is a neoplastic plasma cell disorder which accounts for approximately 1% of neoplastic diseases and 10% of hemato-logic malignancies [1]. In Western countries, the annual age-adjusted incidence is 5.6 cases per 100,000 persons [2]. The use of immunomodula-tory agents (thalidomide, lenalidomide) and a protesome inhibitor (bortezomib) have contributed to an improvement in the overall survival (OS) in MM [3,4]. Despite these advances, patients eventually develop disease refractory to all available agents including thalidomide, lenalidomide, bortezomib, and alkylating agents posing a major challenge for the treatment of refractory MM.
The addition of clarithromycin to thalidomide and dexamethasone has yielded responses in patients who are refractory to thalidomide and dexamethasone [5]. Clarithromycin appears to optimize the pharmacologic effect of glucocorticoids by increasing the area under the curve and the maximum concentration levels of certain corticosteroids [6–9]. Clarithromycin, lenalidomide and dexamethasone (BiRd) in newly diagnosed MM has yielded an overall response rates (ORR) of 93% and a progression-free survival (PFS) of 43 months [10,11]. In a case-matched study, the ORR, time to progression (TTP) and PFS, were superior with BiRd compared to lenalidomide and dexamethasone (Rd) in newly diagnosed MM [12]. Recently, Kato et al. [13] described the case of a 54-year-old patient with MM refractory to Rd, where addition of clarithromycin to Rd led to decrease in IgG levels. In our study, we report the clinical activity of BiRd in MM refractory to Rd.
Methods
Patients
After obtaining approval from the Johns Hopkins University Institutional Review Board (IRB), we retrospectively analyzed 24 consecutive patients with MM in whom clarithromycin was added to Rd at the time of progression on Rd between January 1, 2007 and March 31, 2013. An electronic database search was used to capture all patients in whom clarithromycin was added to Rd. From that group, patients who had evidence of progressive disease at the time of addition of clarithromycin were included in the study. Clinical notes, laboratory reports, pathology reports and radiology reports up to July 2013 were reviewed. High risk MM was defined as having any one of the following: del(13q) by cytogenetics or t(4;14), t(14;16), t(14;20), −17p,+1q21 on FISH/cytogenetics. Cytogenetics and MM FISH were available for 23 (96%) patients at diagnosis. International Staging System (ISS) stage could not be assessed for seven (29%) patients due to missing data for beta2 microglobulin and albumin at diagnosis of symptomatic MM.
Treatment
Following baseline assessment and confirmation of progressive disease (PD) on Rd, clarithromycin was added to Rd without making any adjustment to the dose of Rd. In 22 (91.7%) patients, the clarithromycin dose was 500 mg twice daily. In two patients the starting dose of clarithromycin was 250 mg twice daily. In one patient, a lower dose of clarithromycin 250 mg twice daily was used due to interactions with other medications the patient was taking. A second patient also received clarithromycin at a dose of 250 mg twice daily because of pre-existing gastrointestinal symptoms. 13 (54.2%) patients were on lenalidomide 25mg daily for 21 days followed by 1 week off at the time of adding clarithromycin. Of the remaining 11 patients, 3 were on lenalidomide 15 mg, 7 were on lenalidomide 10 mg and 1 patient was on lenalidomide 5 mg at the time of adding clarithromycin. The lower doses of lenalidomide in these patients were due to standard indications for dose reduction. In 18 (75%) patients, the dexamethasone dose was 40 mg weekly. In six patients (25%), the dexamethasone dose was 20 mg weekly. The lower doses of dexamethasone in these patients were due to standard indications for dose reduction. After starting BiRd, clarithromycin, lenalidomide, and dexamethasone doses were not escalated for any patient. Dose reductions or stopping of the individual drugs were based on standard guidelines and at the discretion of the providing physician.
Responses and survival
Assessment of disease response were based on International Myeloma Working Group (IMWG) criteria [14]. PFS is defined as the interval between start of BiRd and evidence of progressive disease (PD) as defined by the IMWG criteria. OS is defined as interval between start of BiRd and the time of death.
Statistics
Probabilities of OS and PFS were estimated with the method of Kaplan and Meier [15], and compared using the log-rank statistic [16] or the Cox proportional hazards regression model [17]. Factors associated with BiRd response were selected based on cross tabulations and logistic regression modeling [18]. Factors evaluated for an association with response, OS, and PFS included age, race, gender, high risk MM status, stem cell transplant, prior number of therapies and prior Rd response. All P values are two-sided. Computations were performed using the Statistical Analysis System [19], or R [20].
Results
Patient characteristics and prior therapy
Table I shows the baseline patient characteristics. Median age was 61 (range 41–80 years), 11 patients (47.8%) had high risk features by either cytogenetics or FISH. Of the 17 patients in whom albumin and beta 2 microglobulin were available at the time of diagnosis of symptomatic MM, 7 (41.2%) had ISS I, 5 (29.4%) has ISS II, and 5 (29.4%) had ISS III disease. This was a heavily pre-treated group of patients. Median prior therapy was 3 (range 1–8) and six (25%) patients had received a prior stem cell transplant. Of the 18 patients who had not undergone an autologous transplant in the past, the primary reason was poor response to immediate prior therapy. Other reasons included, advanced age, social factors and patient preference. 13 (54.2%) patients had received either thalidomide or bortezomib or both in the past. The immediate prior therapy for all 24 patients was Rd. 10 (41.7%) patients had a partial response (PR) to Rd, 3 (12.5%) had a minimal response (MR), 7 (29.2%) had stable disease (SD) and 4 (16.7%) patients were primary refractory to Rd. All patients had developed progressive disease (PD) on Rd at the time of addition of clarithromycin.
TABLE I.
Patient's Clinical Characteristics (Total N = 24)
| Characteristic | Result |
|---|---|
| Age, year, median (range) | 61 (41-80) |
| Male sex, n (%) | 14 (58.3) |
| Race, n (%) | |
| White | 16 (66.7) |
| Black | 6 (25) |
| Other | 2 (8.3) |
| International staging system at diagnosis, n (% of 17) | |
| I | 7 (41.2) |
| II | 5 (29.4) |
| III | 5 (29.4) |
| Cytogenetics/FISH, n (% of 23) | |
| Standard risk | 12 (52.2) |
| High risk | 11 (47.8) |
| Time from diagnosis to BiRd, median (range) months | 36.7 (6.6-59) |
| Median prior therapy, n (range) | 3 (1-8) |
| Prior stem cell transplant, n (%) | 6 (25) |
| Prior treatment with bortezomib, n (%) | 13 (54.2) |
| Prior treatment with thalidomide, n (%) | 13 (54.2) |
| Prior treatment with Rd, n (%) | 24 (100%) |
| Best response to Rd, n (%) | |
| PR | 10 (41.7) |
| MR | 3 (12.5) |
| SD | 7 (29.2) |
| PD | 4 (16.7) |
| Median duration on Rd, mo, range | 5.2 (1.6-37.8) |
Responses
Ten patients, 41.7% (95% CI: 22.1, 63.4), achieved ≥PR (Table II). One patient achieved a complete response (CR) and an additional patient achieved a very good partial response (VGPR). The clinical benefit rate (CR + VGPR + PR + MR) was 45.8%. (95% CI: 25.6, 67.2) The median time to response was 4.4 months (range: 1–13.6 months) and the median duration of response was 6.9 months (range: 3–52.2 months). Patients initially responding to Rd (n = 10) were more likely to respond to BiRd, ORR 60% (95% CI: 26.2, 87.8) compared to patients that did not have an initial response to Rd (n = 14), ORR 28.6% (95% CI: 8.4, 58.1), OR = 3.75, P = 0.13. Of the 14 patients who initially achieved less than a PR to Rd, four patients responded after addition of clarithromycin at the time of disease progression, there were two PRs, one VGPR, and one CR. High risk genetics and prior stem cell transplant did not impact the response to BiRd.
TABLE II.
Responses to BiRd (total N = 24)
| Responses | Number (%) | 95% CI |
|---|---|---|
| Complete response | 1 (4.2) | (0.1%, 21.1%) |
| Very good partial response | 1 (4.2) | (0.1%, 21.1%) |
| Partial response | 8 (33.3) | (15.6%, 55.3%) |
| Stable disease | 5 (20.8) | (7.1%, 42.2%) |
| Overall response | 10 (41.7) | (22.1%, 63.4%) |
| Clinical benefit | 11 (45.8) | (25.6%, 67.2%) |
| Progressive disease | 8 (33.3) | (15.6%, 55.3%) |
Survival
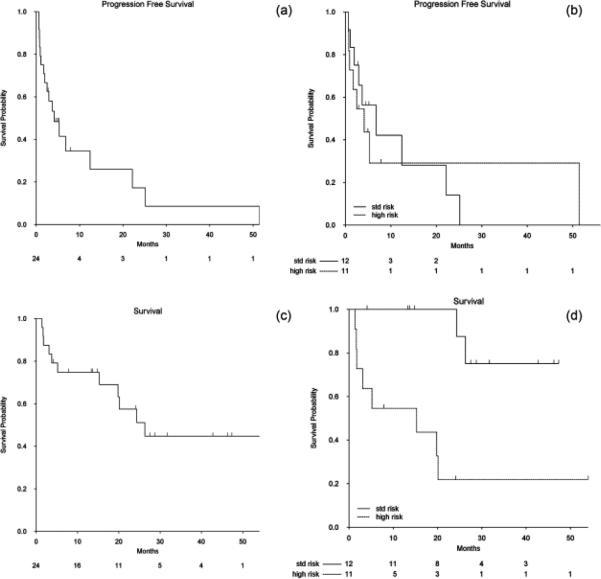
The median PFS was 4 months (Fig. 1a). In univariate Cox regression analyses, increasing age and more prior therapies significantly increased the risk of this outcome (Table III). Patients over the age of 60 had a higher hazard of progression or death than patients under the age of 60, HR 3.48 (95% CI 1.09–11.09), P = 0.04. The hazard of progression or death was increased by a factor of 1.59 for each additional prior therapy, HR= 1.59 (95% CI: 1.19, 2.11), P = 0.002. When adjusted for age in a multivariate regression analysis, the estimated effect of prior therapy on PFS remained relatively unchanged, HR = 1.47 (95% CI: 1.07–2.01), P = 0.02. Age greater than 60 was not a significant factor in this model, HR = 2.03 (95% CI: 0.56, 7.35), P = 0.28. High risk genetics (Fig. 1b), prior stem cell transplant, and prior response to Rd did not correlate with PFS.
Figure 1.
Kaplan–Meier survival estimates. (a) PFS for all patients (n = 24). (b) PFS stratified by risk. Patients with standard risk cytogenetics/FISH (n = 12, solid line) compared with patients with high risk cytogenetics/FISH (n = 11, dashed line), p = 0.85. (c) OS for all patients (n = 24). (d) OS stratified by risk. Patients with standard risk cytogenetics/FISH (n = 12, solid line) compared with patients with high risk cytogenetics/FISH (n = 11, dashed line), p = 0.001.
TABLE III.
Univariate Cox Regression Models for PFS
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Number of prior therapies | 1.59 | 1.19-2.11 | 0.002 |
| Age ≥60 | 3.48 | 1.09-11.09 | 0.04 |
| Prior stem cell transplant | 1.66 | 0.51-5.44 | 0.4 |
| Prior response to Rd | 0.76 | 0.27-2.1 | 0.59 |
| High risk MM | 1.07 | 0.39-2.9 | 0.89 |
Median OS was 25 months with a median follow-up of 27.5 months (Fig. 1c). The strongest factor associated with OS was high risk MM, HR = 9.73 (95% CI: 1.91, 49.49), P = 0.01 (Fig. 1d). Increasing number of prior therapies increased the risk of death by a factor of 1.31 for each additional prior therapy, HR=1.31 (95% CI: 1.00, 1.72), P = 0.05 (Table IV). High risk genetics remained a significant factor when adjusted for the number of prior therapies.
TABLE IV.
Univariate Cox Regression Models for OS
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Number of prior therapies | 1.31 | 1-1.72 | 0.05 |
| Age ≥60 | 2.94 | 0.81-10.64 | 0.1 |
| Prior stem cell transplant | 2.56 | 0.77-8.54 | 0.13 |
| Prior response to Rd | 0.5 | 0.13-1.9 | 0.31 |
| High risk MM | 9.73 | 1.91-49.49 | 0.01 |
Tolerability
Treatment with BiRd was well tolerated. Treatment was discontinued in two patients due to toxicity attributed to the regimen. In one case, the patient developed GI side effects including dyspepsia, dysgeusia, nausea and diarrhea that necessitated treatment discontinuation. One patient developed grade 2 anemia, hoarseness and fatigue leading to treatment discontinuation. Transient grade 3 transaminitis was seen in one patient and this resolved without dose reductions in clarithromycin. Lenalidomide dose reductions were required due to pancytopenia in two patients. Dexamethasone was dose reduced in one patient due to emotional lability. No venous thromboembolic events (VTE) were seen. All patients were maintained on standard VTE prophylaxis as recommended with the use of lenalidomide.
Discussion
Our retrospective study demonstrates that the addition of clarithromycin to patients relapsing on Rd was able to overcome resistance to Rd in a subset of patients. This can be attributed to the addition of clarithromycin as the doses of Rd were not escalated or changed at the time when clarithromycin was added. Interestingly, patients who had initially achieved a >PR to Rd appeared to have benefitted the most by adding clarithromycin at time of progression. Even in those patients with less than a PR to Rd, BiRd showed a benefit, including CR in one patient. The combination was well tolerated with only two patients discontinuing treatment early due to toxicity. Responses were durable and the median duration of response was 6.9 months.
Lenalidomide has been shown to inhibit production of pro-inflammatory cytokines, TNF-α, IL-1, IL-6, and IL-12 [21]. IL-6 inhibits the apoptosis of MM cells and helps in their proliferation [22]. Lenalidomide down-regulates the production of IL-6 directly and by inhibiting the interaction between MM cells and bone marrow stromal cells (BMSC) [23,24], thereby augmenting apoptosis of MM cells [25–27]. T cell co-stimulation by lenalidomide [28] leads to an increased Th1 type cytokine response resulting in increased secretion of IFN-γ and IL-2 that in turn stimulate clonal T cell proliferation and NK cell activity [21,27].
How does the addition of clarithromycin overcome resistance to Rd? One possibility is by optimizing pharmacologic effect of dexamethasone by increasing the area under the curve and maximum concentration levels of corticosteroids. This could be mediated through CYP3A inhibition by clarithromycin [29]. Second, clarithromycin can decrease IL-6 levels, and increase IL-10 and IFN γ levels [30] and can down regulate Th1/Th17 regulatory T cell responses [31]. Therefore, clarithromycin has immunomodulatory properties and may be synergistic with lenalidomide. Third, clarithromycin can attenuate autophagy in MM cells and have a direct anti-myeloma effect [32].
Treatment of relapsed and refractory MM has benefitted from the development of new targeted agents. However, refractory disease develops in almost all patients [33]. Recently, pomalidomide and carfilzomib have been approved by the US FDA for the use in relapsed and refractory MM after prior exposure to lenalidomide and bortezomib. In patients refractory to leanlidomide, pomalidomide and dexamethasone has an ORR of 47%, 4.8 months PFS and an OS of 13.9 months [34]. Other studies using pomalidomide which have included both bortezomib and lenalidomide refractory patients have shown response rates from 21 to 43% and PFS ranging 3.2 to 6.5 months [35–37]. In patients refractory or intolerant to bortezomib and lenalidomide, carfilzomib, has an ORR of 22.9% and the duration of response is 7.8 months [38]. In a study where thalidomide was used for patients who relapsed after lenalidomide, an ORR of 40%, PFS of 5.5 months and OS of 18 months was reported [39]. The combination of clarithromycin, lenalidomide and dexamethasone (BiRd) in our retrospective study for patients with MM refractory to Rd showed a 41.7% ORR, PFS of 4 months and a median duration of response lasting 6.9 months. Although limited by small numbers and the retrospective nature of our study, the results indicate a viable therapeutic option for patients who are refractory to lenalidomide and dexamethasone.
Despite the availability of newer agents in relapsed and refractory MM, the duration of response to post-relapse therapies becomes shorter with subsequent lines of therapy. This is confirmed in our study where the hazard of progression or death was increased for each additional prior therapy. This poses a significant challenge and one approach has been to maximize the efficacy of available treatment through use of the most appropriate combinations and sequences of agents [40]. For disease refractory to Rd, it has been feasible to re-administer lenalidomide in combination with other agents in order to reestablish a response. In a small retrospective study in 14 patients who were refractory to Rd, a clinical response rate of 64.3% was achieved using lenalidomide plus cyclophosphamide plus prednisone [41]. In a phase I study of 38 patients who had relapsed or refractory MM following treatment with thalidomide, lenalidomide, and/or bortezomib, the combination of lenalidomide + bortezomib showed promising activity, with 61% of patients achieving a minimal response or better and median PFS was 6.9 months [42]. Our study clearly shows that addition of clarithromycin to lenalidomide and dexamethasone can lead to durable responses in patients who were previously refractory Rd. The added toxicity from clarithromycin is low and has an economic advantage due to a low average monthly cost considering that clarithromycin is a generic antibiotic.
As new drugs become available for MM, it is critical to evaluate survival outcomes in the context of how heavily pretreated and refractory to treatment the patient population is. We observed that PFS is longest in patients who had the fewest number of prior regimens. By univariate analysis, age ≥60 years inversely correlated with PFS but this effect was not seen on multivariate analysis likely because older patients had also been exposed to more prior therapies. Importantly, high risk cytogenetics/FISH did not have a negative impact on responses and PFS. This is consistent with previously published results of BiRd for the treatment of newly diagnosed MM [10,11]. Even though high risk cytogenetics/FISH did not have a significant negative impact on PFS, it adversely affected OS, indicating that patients in this group had a poor response to subsequent therapy. Median survival for the standard risk group of patients was not reached during the period of the study.
In conclusion, addition of clarithromycin to lenalidomide and dexamethasone (BiRd) can overcome resistance to lenalidomide and dexamethasone in a subset of patients and lead to clinical durable responses. Although, this does not substitute for the incorporation of new anti-MM drugs, salvaging response to existing therapies increases the therapeutic treatment options and is likely to improve the overall duration of effective therapy which may impact survival. This retrospective study supports the prospective evaluation of BiRd in MM refractory to lenalidomide and dexamethasone.
Acknowledgments
We are grateful to the hospital staff who are committed to providing high-quality care for all our patients. We thank Jason Miller and Kymberlee Olson from Clinical Resource Management and Department of Utilization Management at Johns Hopkins Hospital, Baltimore for data acquisition.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Author Contributions
NG designed the study, collected and analyzed the data and wrote the manuscript. NT performed data collection and generated the database. MZ performed biostatistical analysis. JW helped with data collection. CAH contributed in the idea generation, treatment program concept, data collection and provided expert patient care. IB contributed in the treatment program concept, data collection and provided expert patient care. All authors reviewed and approved the manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111:2521–2526. doi: 10.1182/blood-2007-08-104984. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman M, Leonard J, Lyons L, Pekle K, Nahum K, Pearse R, Niesvizky R, Michaeli J. BLT-D (clarithromycin [Biaxin], low-dose thalidomide, and dexamethasone) for the treatment of myeloma and Waldenstrom’s macroglobulinemia. Leuk Lymphoma. 2002;43:1777–1782. doi: 10.1080/1042819021000006303. [DOI] [PubMed] [Google Scholar]
- 6.Durie B, Villarete L, Farvard A, Ornopia M, Urnovitz HB. Clarithromycin (Biaxin) as primary treatment for myeloma. Blood. 1997;90:579a. [Google Scholar]
- 7.Spahn JD, Fost DA, Covar R, Martin RJ, Brown EE, Szefler SJ, Leung DY. Clarithromycin potentiates glucocorticoid responsiveness in patients with asthma: results of a pilot study. Ann Allergy Asthma Immunol. 2001;87:501–505. doi: 10.1016/S1081-1206(10)62264-8. [DOI] [PubMed] [Google Scholar]
- 8.Niesvizky R, Pekle K, Lyons L. Dexamethasone alone, or in combination with low-dose thalidomide as induction therapy for advanced multiple myeloma, and the effect of the addition of clarithromycin (Biaxin) on response rate. Interim results of a prospective, sequential, randomized trial [abstract]. Blood. 2003:102. Abstract 832. [Google Scholar]
- 9.Vescio R, Sjak-Shie NN, Manyak SJ, Yang H, Berenson JR. Clarithromycin (Biaxin) adds to the efficacy and toxicity of staroid therapy in multiple myeloma [abstract]. Blood. 2001:98. Abstract 5007. [Google Scholar]
- 10.Niesvizky R, Jayabalan DS, Christos PJ, Furst JR, Naib T, Ely S, Jalbrzikowski J, Pearse RN, Zafar F, Pekle K, Larow A, Lent R, Mark T, Cho HJ, Shore T, Tepler J, Harpel J, Schuster MW, Mathew S, Leonard JP, Mazumdar M, Chen-Kiang S, Coleman M. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1119. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]
- 11.Rossi A, Mark T, Jayabalan D, Christos P, Zafar F, Pekle K, Pearse R, Chen-Kiang S, Coleman M, Niesvizky R. BiRd (clarithromycin, lenalidomide, dexamethasone): An update on long-term lenalidomide therapy in previously untreated patients with multiple myeloma. Blood. 2013;121:1982–1985. doi: 10.1182/blood-2012-08-448563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay F, Rajkumar SV, Coleman M, Kumar S, Mark T, Dispenzieri A, Pearse R, Gertz MA, Leonard J, Lacy MQ, Chen-Kiang S, Roy V, Jayabalan DS, Lust JA, Witzig TE, Fonseca R, Kyle RA, Greipp PR, Stewart AK, Niesvizky R. Clarithromycin (Biaxin)-lenalidomide-low-dose dexamethasone (BiRd) versus lenalidomide-low-dose dexamethasone (Rd) for newly diagnosed myeloma. Am J Hematol. 2010;85:664–669. doi: 10.1002/ajh.21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato H, Onishi Y, Okitsu Y, Katsuoka Y, Fujiwara T, Fukuhara N, Ishizawa K, Takagawa M, Harigae H. Addition of clarithromycin to lenalidomide/low-dose dexamethasone was effective in a case of relapsed myeloma after long-term use of lenalidomide. Ann Hematol. 2013;92:1711–1712. doi: 10.1007/s00277-013-1761-x. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Harousseau JL, Miguel JS, Blad e J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–480. [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 17.Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 18.Cox DR. The Analysis of Binary Data. Methuen; London: 1970. [Google Scholar]
- 19.SAS Institute Inc. SAS User’s Guide: Statistics, Version 5 Edition. SAS Institute Inc.; Cary, NC: 1985. [Google Scholar]
- 20.R Development Core Team. R . A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. ISBN 3-900051-07-0. http://www.R-project.org. [Google Scholar]
- 21.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163:380–386. [PubMed] [Google Scholar]
- 22.Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 23.Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, Chauhan D, Okawa Y, Munshi NC, Richardson PG, Anderson KC. Lena-lidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–1932. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 24.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31:213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 25.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. [PubMed] [Google Scholar]
- 26.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, Schlossman RL, Richardson P, Ralph P, Wu L, Payvandi F, Muller G, Stirling DI, Anderson KC. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: Therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 27.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, Treon SP, Richardson PG, Schlossman RL, Morgan GJ, Muller GW, Stirling DI, Anderson KC. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 28.LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, Mitsiades C, Cheema P, Chauhan D, Richardson PG, Anderson KC, Munshi NC. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–1790. doi: 10.1182/blood-2003-02-0361. [DOI] [PubMed] [Google Scholar]
- 29.Pinto AG, Wang YH, Chalasani N, Skaar T, Kolwankar D, Gorski JC, Liangpunsakul S, Hamman MA, Arefayene M, Hall SD. Inhibition of human intestinal wall metabolism by macro-lide antibiotics: Effect of clarithromycin on cyto-chrome P450 3A4/5 activity and expression. Clin Pharmacol Ther. 2005;77:178–188. doi: 10.1016/j.clpt.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Demartini G, Esposti D, Marthyn P, Lapidari A, Fraschini F, Scaglione F. Effect of multiple doses of clarithromycin and amoxicillin on IL-6, IFN-gamma and IL-10 plasma levels in patients with community acquired pneumonia. J Chemother. 2004;16:82–85. doi: 10.1179/joc.2004.16.1.82. [DOI] [PubMed] [Google Scholar]
- 31.Ding FM, Zhu SL, Shen C, Jiang YQ. Low-dose clarithromycin therapy modulates CD4(1) T-cell responses in a mouse model of chronic Pseudomonas aeruginosa lung infection. Respirology. 2012;17:727–734. doi: 10.1111/j.1440-1843.2012.02166.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura M, Kikukawa Y, Takeya M, Mitsuya H, Hata H. Clarithromycin attenuates autophagy in myeloma cells. Int J Oncol. 2010;37:815–820. [PubMed] [Google Scholar]
- 33.van de Donk NW, Lokhorst HM, Dimopoulos M, Cavo M, Morgan G, Einsele H, Kropff M, Schey S, Avet-Loiseau H, Ludwig H, Goldschmidt H, Sonneveld P, Johnsen HE, Blad e J, San-Miguel JF, Palumbo A. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37:266–283. doi: 10.1016/j.ctrv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Lacy MQ, Hayman SR, Gertz MA, Short KD, Dispenzieri A, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Zeldenrust S, Fonseca R, Bergsagel PL, Roy V, Mikhael JR, Stewart AK, Laumann K, Allred JB, Mandrekar SJ, Rajkumar SV, Buadi F. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multi ple myeloma (MM). Leukemia. 2010;24:1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacy MQ, Allred JB, Gertz MA, Hayman SR, Short KD, Buadi F, Dispenzieri A, Kumar S, Greipp PR, Lust JA, Russell SJ, Dingli D, Zeldenrust S, Fonseca R, Bergsagel PL, Roy V, Stewart AK, Laumann K, Mandrekar SJ, Reeder C, Rajkumar SV, Mikhael JR. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: Comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118:2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leleu X, Attal M, Arnulf B, Moreau P, Traulle C, Marit G, Mathiot C, Petillon MO, Macro M, Roussel M, Pegourie B, Kolb B, Stoppa AM, Hennache B, Br echignac S, Meuleman N, Thielemans B, Garderet L, Royer B, Hulin C, Benboubker L, Decaux O, Escoffre-Barbe M, Michallet M, Caillot D, Fermand JP, Avet-Loiseau H, Facon T. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myelome 2009-02. Blood. 2013;121:1968–1975. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 37.Richardson PG, Siegel D, Baz R, Kelley SL, Munshi NC, Laubach J, Sullivan D, Alsina M, Schlossman R, Ghobrial IM, Doss D, Loughney N, McBride L, Bilotti E, Anand P, Nardelli L, Wear S, Larkins G, Chen M, Zaki MH, Jacques C, Anderson KC. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121:1961–1967. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, Trudel S, Kukreti V, Bahlis N, Alsina M, Chanan-Khan A, Buadi F, Reu FJ, Somlo G, Zonder J, Song K, Stewart AK, Stadtmauer E, Kunkel L, Wear S, Wong AF, Orlowski RZ, Jagannath S. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120:2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guglielmelli T, Petrucci MT, Saglio G, Palumbo A. Thalidomide after lenalidomide: A possible treatment regimen in relapse refractory multiple myeloma patients. Br J Haematol. 2011;152:108–110. doi: 10.1111/j.1365-2141.2010.08416.x. [DOI] [PubMed] [Google Scholar]
- 40.San Miguel JF. Relapse/refractory myeloma patient: Potential treatment guidelines. J Clin Oncol. 2009;27:5676–5677. doi: 10.1200/JCO.2009.24.3683. [DOI] [PubMed] [Google Scholar]
- 41.van de Donk NW, Wittebol S, Minnema MC, Lokhorst HM. Lenalidomide (Revlimid) combined with continuous oral cyclophosphamide (endoxan) and prednisone (REP) is effective in lenalidomide/dexamethasone-refractory myeloma. Br J Haematol. 2010;148:335–337. doi: 10.1111/j.1365-2141.2009.07931.x. [DOI] [PubMed] [Google Scholar]
- 42.Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, Mazumder A, Munshi NC, Ghobrial IM, Doss D, Warren DL, Lunde LE, McKenney M, Delaney C, Mitsiades CS, Hideshima T, Dalton W, Knight R, Esseltine DL, Anderson KC. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009;27:5713–5719. doi: 10.1200/JCO.2009.22.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]