Abstract
Scope
Increased body mass index (BMI) and decreased serum vitamin D are both known to be associated with increased mortality from breast cancer. However, vitamin D levels are lower in obese individuals in general. Recent studies have sought to determine whether serum vitamin D levels can account for some of the association between higher BMI and increased risk for breast cancer and found that low vitamin D levels in the overweight and obese account for up to 40% of the BMI-attributable risk of developing breast cancer.
Methods and results
Here we reviewed the literature to determine if a similar relationship exists between vitamin D, BMI, and breast cancer mortality. Utilizing previously reported independent associations of low vitamin D and high BMI to increases in breast cancer mortality, as well as the known decrement in vitamin D per unit increase in BMI, we estimated that low vitamin D levels may be responsible for roughly 16% of the increased mortality from breast cancer in overweight and obese patients.
Conclusion
Although this is a relatively small proportion of the effect of obesity, supplements to increase serum vitamin D levels may represent a way to reduce obesity-associated disparities in breast cancer mortality.
Keywords: Breast cancer, BMI, Cancer mortality, Obesity, Vitamin D
1 Introduction
In the past decade, a large body of work has focused on identifying the contributions of various risk factors to the development and prognosis of breast cancer. In the United States, breast cancer accounts for nearly one third of cancer diagnoses in women and is the second leading cause of death among women [1]. Factors known to be associated with survival include stage at diagnosis, age at diagnosis, race/ethnicity, socioeconomic factors, tumor characteristics (such as grade and HER2 status), as well as obesity [2–6], physical activity, and diet [7–9]. Recently, vitamin D levels have also been shown to correlate with survival after breast cancer diagnosis [10].
Breast cancer patients who are obese at diagnosis have an approximately 30% higher risk of death from breast cancer when compared to patients with a healthy body weight [8,11]. In 2010, a large meta-analysis by Protani et al. [11] reported poorer overall (hazard ratio (HR) = 1.33; 95% confidence interval (95% CI): 1.21, 1.47) and breast cancer-specific (HR = 1.33; 95% CI: 1.19, 1.50) survival among obese compared with non-obese women with breast cancer. Two of the studies included in the meta-analysis reported linear associations between increasing body mass index (BMI) and overall mortality from breast cancer [12, 13]. Several mechanisms explaining the effect of obesity on the prognosis of breast cancer have been suggested, including circulating or tissue levels of sex and metabolic hormones, levels of hormone binding proteins, cytokine levels and inflammation, and chemotherapy underdosing in obese patients [8].
Although vitamin D status has been investigated numerous times as a possible risk factor for the development of breast cancer [14–23], contributions of vitamin D status, specifically the inactive form, 25-hydroxy-vitamin D (25(OH)D; referred to as vitamin D from here on), to breast cancer mortality have been less thoroughly studied. Studies in Norway have shown that the prognosis for breast cancer varies significantly with season of diagnosis, with the greatest survival for summer and fall [24], a 15–25% decrease in mortality for diagnoses in summer versus winter [25], and variation in survival by latitude, which the authors contributed to measured variations in annual UV exposure [25]. These and other studies suggested a role for vitamin D3 generated from UV exposure in the progression of breast cancer and led some to question the value of circulating levels of vitamin D in determining and augmenting prognosis. While these questions resulted in identification of significant categorical associations between vitamin D status and both breast cancer recurrence [26] and overall mortality [26,27], continuous associations were either insignificant [27] or not reported [26] and a later study was unable to confirm these associations [28]. However, more recently a large prospective cohort study in Germany [10] reported a linear association between decreasing vitamin D levels and overall mortality from breast cancer (HR = 1.08 per 10 nmol/L decrement in 25(OH)D; 95% CI: 1.00, 1.17) in postmenopausal women that was independent of other factors including BMI and physical activity. These findings were consistent with other studies [29], wherein disease stage independently predicted serum vitamin D levels after controlling for age, BMI, race/ethnicity, geography, season, physical activity, and cancer treatment. The mechanisms underlying the effect of vitamin D on breast cancer mortality are still unclear. Though the active form of vitamin D and its derivatives are known to exert anti-tumor effects through down regulation of growth-factor signaling [30] and their effects on proliferation, differentiation, apoptosis, angiogenesis, and metastatic potential [31], studies have shown that malignant transformation of mammary tissue is associated with a decreased ability of tumor cells to synthesize the active form of vitamin D, reduced responses to vita-min D-receptor (VDR)-mediated signaling, and an increased ability of these cells to degrade vitamin D [32]. In light of these findings, it has been suggested that supplementation with vitamin D and calcium may represent an alternative method of reducing both the incidence and mortality of breast cancer [33].
Complicating the studies of the individual associations of obesity and vitamin D status to both the increased risk of developing breast cancer and the prognosis of breast cancer is the relationship between BMI and vitamin D levels, in which vitamin D levels have been shown to decrease with increasing BMI not only in healthy patients [34], but also in those with a variety of cancers [35]. The results from a number of studies [34, 36–39] suggest that for each increase in BMI of 1 kg/m2 there is an approximately 1 nmol/L decrement in vitamin D. The association between increasing BMI and decreasing vitamin D may be explained by a single factor such as volumetric dilution [40] or by a number of factors, including sequestration of vitamin D in adipose tissue [41], reduced consumption of vitamin D [42] and decreased sun exposure [43] in the overweight and obese, and the effects of low vitamin D levels on lipogenesis and adipogenesis that may potentially favor obesity; however, further studies are necessary to entirely elucidate this complex relationship [41].
In a recent publication, Lagunova et al. [39] examined the effects of increasing BMI and decreasing vitamin D levels on the risk of developing a variety of cancers, including those of the breast, and demonstrated that up to 40% of the BMI-attributable risk of developing breast cancer may be contributed by decreasing vitamin D levels. Here we use available data from the literature on obesity, vitamin D, and breast cancer mortality to estimate the proportion of the increase in mortality from breast cancer seen with increasing BMI that can be attributed to decreasing vitamin D levels.
2 Materials and methods
2.1 Literature search
We performed a literature search of PubMed using the following three separate search terms: “Breast Neoplasms/mortality”[MeSH] AND “Vitamin D”[Majr], “Breast Neoplasms/mortality”[MeSH] AND “Body Mass Index”[Majr], and “Body Mass Index”[Majr] AND “Vitamin D”[Majr]. These were used to identify previous studies regarding associations between vitamin D levels and breast cancer mortality, BMI and breast cancer mortality, and BMI and vitamin D levels, respectively. After identification of a number of studies reporting associations of vitamin D and BMI to breast cancer mortality, we further limited our search to focus specifically on those reporting associations wherein either vitamin D or BMI were modeled as continuous variables.
2.2 Analysis
From our literature review, we extrapolated the relationships between BMI and overall risk of death after breast cancer, between vitamin D levels and BMI, and, lastly, between vitamin D levels and overall risk of death after breast cancer. The meta-analysis by Protani et al. [11] from 2010 reported a categorical association of obesity with an increase in breast cancer mortality when compared to non-obese participants (HR = 1.33; 95% CI: 1.21, 1.47), but did not report a linear association per unit increase in BMI. A review of the studies included in the meta-analysis revealed two that reported linear associations of BMI to breast cancer mortality: one from Barnett et al. [13] in 2008 reporting the association for their combined study population of pre- and postmenopausal women (HR = 1.03 per 1 kg/m2 increase in BMI; 95% CI: 1.01, 1.04) and one from Cleveland et al. [12] in 2007 that was reported only for premenopausal participants (HR = 1.07 per 1 kg/m2 increase in BMI; 95% CI: 1.02, 1.13). The study by Barnett et al. reported no significant difference between their pre- and postmenopausal participants with respect to the effect of BMI on breast cancer mortality. Additionally, although the study by Cleveland et al. only reported continuous associations for premenopausal women, they did report significant categorical associations between BMI and breast cancer mortality for both pre- and postmenopausal women. We used the average of these reported continuous associations in our analysis (HR = 1.05 per 1 kg/m2 increase in BMI) to estimate the effect of BMI on breast cancer mortality.
We estimated the relationship between vitamin D levels and BMI based on that reported in the meta-analysis by Lagunova et al. [39] in 2010 (~1 nmol/L decrease in 25(OH)D per 1 kg/m2 increase in BMI). We then identified the only article that estimated the association of vitamin D levels to breast cancer mortality in a continuous manner (HR = 1.08 per 10 nmol/L decrease in 25(OH)D; 95% CI: 1.00, 1.17) [10] and used this as our estimate of the relationship of vitamin D with breast cancer mortality. Utilizing the estimated relationship between vitamin D levels and BMI, we expressed the association between vitamin D levels and breast cancer mortality as a function of BMI (HR = 1.08 per 10 kg/m2 increase in BMI). Next, we plotted this relationship, along with the effect of BMI on breast cancer mortality, in order to estimate the contribution of vitamin D levels to the effect of increasing BMI on breast cancer mortality. We limited the range of BMI values to 20–40 kg/m2 because of the loss of significance of the linear association between breast cancer mortality and BMIs outside of this range, especially those < 20 kg/m2. Lastly, we compared the area under the two curves as a measure to quantify the contribution of vitamin D levels to the effect of BMI on mortality.
3 Results
Our literature search of breast cancer mortality and vita-min D initially provided 13 results. Of these, only three were relevant original research articles and only a single one provided data on vitamin D as a continuous measure [10]. Our second literature search of breast cancer mortality and BMI yielded 30 hits, of which eight were relevant original research and two included BMI as a continuous measure [12, 13]. Our final literature search, on BMI and vitamin D, originally identified 27 articles, including six original research articles reporting on this association, only one of which used both vitamin D and BMI continuously.
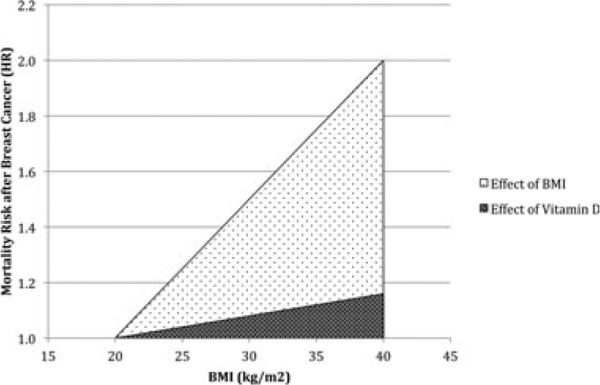
A summary of the studies utilized for our analysis is provided in Table 1. The results of our estimation of the effect of vitamin D in explaining the association of obesity with breast cancer mortality are presented in Fig. 1. The area encompassed by the line depicting the effect of decreasing vitamin D status with increasing BMI on breast cancer mortality represents 16% of the area encompassed by the area depicting the overall effect of BMI. This suggests that approximately 16% of the increase in breast cancer mortality attributed to increasing BMI levels in obese and overweight patients may be the result of decreased circulating levels of vitamin D.
Table 1.
Summary of studies reporting linear relationships of serum vitamin D or BMI to breast cancer mortality
| Study | Sample size (number of deaths) | Age at diagnosis (years) | Follow-up (years) | Body mass index (kg/m2) | Vitamin D (nmol/L) | Measurement, time | Menopause status | Factors included in multivariate analyses |
|---|---|---|---|---|---|---|---|---|
| Studies of BMI and breast cancer mortality | ||||||||
| Cleveland, 2007 [12] | 1491 (196) | 58.8 (mean) | 5.56 (mean) | Not reported | Not applicable | BMI, 1 year prior to diagnosis | Pre- and post-menopausal | Age at diagnosis, demographic factors (race, income, education, marital status), reproductive history (parity, age at 1st live birth, breast feeding), menstruation (age at menarche and menopause), exogenous hormone use, medical history, lifestyle factors, history of comorbidities, tumor characteristics (tumor stage, tumor size, nodal status), and breast cancer treatment |
| Barnett, 2008 [13] | 4560 (620) | 51.5 (median) | 6.82 (median) | Not reported | Not applicable | BMI, calculated from self-reported height and weight at enrollment | Pre- and post-menopausal | Age at diagnosis, tumor characteristics (tumor stage, tumor grade, ER status), reproductive history (parity, age at first full-term pregnancy, total full-term pregnancies, years since last pregnancy), menstruation (age at menarche and menopause, menopausal status at diagnosis), lifestyle factors |
| Studies of vitamin D and breast cancer mortality | ||||||||
| Vrieling, 2011 [10] | 1295 (137) | 63.4 (mean) | 5.8 (median) | 26.6 (mean) | 44.9 (median) | Serum 250HD, median 83 days post-diagnosis | Post-menopausal | Age at diagnosis, season of serum collection, tumor characteristics (tumor size, tumor grade, nodal status, metastasis, ER/PR status), prevalent diabetes, mode of tumor detection |
Abbreviations: ER: estrogen receptor; PR: progesterone receptor.
Figure 1.
Individual contributions of increasing BMI and decreasing vitamin D to mortality from breast cancer. Vitamin D was plotted as a function of BMI by utilizing the known relationship between the two (~1 nmol/L decrease in 25(OH)D per 1 kg/m2 increase in BMI).
4 Discussion
By utilizing estimates from previous studies of associations between BMI and breast cancer mortality [12, 13], vitamin D levels and breast cancer mortality [10], and BMI and vitamin D levels [34, 35, 39, 41], we attempted to estimate the proportion of the BMI-attributable increase in mortality after breast cancer that can be accounted for by decreasing vitamin D levels. We found that over the range of BMIs from 20 to 40 kg/m2, approximately 16% of the BMI-related increase in mortality after breast cancer is likely to be accounted for by decreasing levels of vitamin D.
There were several limitations to our analysis that are related to the studies from which we derived our estimations. The first of these limitations arises from previous studies of increasing breast cancer mortality associated with increasing BMI. Only a handful of these studies reported linear associations for increasing BMI and breast cancer mortality [12,13]. Those studies that either did not explore linear associations or did not report them chose to do so based on studies showing that underweight individuals with BMIs below 20 kg/m2 had increased mortality following a diagnosis of breast cancer [11]. This suggests that the relationship between BMI and breast cancer mortality assumes more of a ‘J’ shape than a linear form, with increasing mortality after breast cancer at both ends of the BMI scale. For this reason, we chose to limit the range of BMIs used in our analysis to 20–40 kg/m2. Only one of the studies we used for the association of BMI to breast cancer mortality had a relevant limitation, which was the use of self-reported weight for BMI calculations. However, self-reported weight has been well-correlated with measured weight in women [12].
The studies that previously reported associations of vitamin D to breast cancer mortality also included several limitations. First, many of these studies have utilized various cut-points for vitamin D in their categorical analyses, such as defining patients either as deficient, insufficient, or sufficient, or, in another study, by separating them into quartiles or by the median value for the study population [26,27]. These variations limited the ability to reliably compare the categorical associations reported by these studies, however, because we sought out reported associations wherein vitamin D was modeled as a continuous variable, cut-point variability was not a limiting factor in our analysis. Additionally, only one of the studies that we examined reported a linear association of vitamin D levels to breast cancer mortality [10], thereby eliminating the problem of vitamin D cut-point variability from our analysis. This study by Vrieling et al. in 2011 contained several limitations that should be considered when interpreting our analysis. First, the study population consisted primarily of postmenopausal women. This is particularly important given the previously discussed disparity in breast cancer mortality between pre- and post-menopausal women [1] and suggests that our analysis is more relevant in establishing guidelines for post-menopausal rather than pre-menopausal patients. Another limitation of the study is that the significance of the relationship of vitamin D levels to breast cancer mortality was limited to women who had not received chemotherapy prior to blood collection, though this design was similar to other studies [26]. One possible limitation to our analysis is the assumption of a linear relationship between vitamin D and breast cancer mortality. Previous studies of the effect of vitamin D on risk of cancer showed a nonlinear relationship between vitamin D and odds ratios for a variety of cancers that asymptotically approaches a lower odds ratio as vitamin D increases [21]. Goodwin et al. [26] noted that the maximum benefit of vitamin D levels with respect to all cause mortality might be in the 80–110 nmol/L range. However, this was not statistically significant. Furthermore, to our knowledge there is no evidence in the literature that the effect of vitamin D on breast cancer specific mortality is nonlinear. It should also be noted that follow-up time has been shown to affect the relationship of pre-diagnostic serum vitamin D to all-cause mortality in prospective cohort studies [44], with shorter follow-up time being associated with stronger effects, and may represent a limitation to the studies on which we base our analyses.
Limitations to our analysis also arose from the studies we utilized to estimate the association of BMI to vitamin D levels. In one of these, the cross-sectional study design limited the conclusions that could be drawn, specifically those regarding causality in the relationship between vitamin D and BMI. Factors that may selectively affect overweight and obese individuals, but were not examined, included lifestyle, cultural reasons for limiting skin exposure, and UV light levels sufficient for vitamin D3 synthesis [36]. In another study, the conclusions were limited to young, healthy, and moderately overweight white women [37]. In the third study that we examined, the lack of non-obese controls, variations in vitamin D intake, the moderate sample size, and the self-reporting methods used to assess dietary intake of calcium and vitamin D, as well as sunlight exposure, were considered limitations [38].
Our analysis was also somewhat limited by publication bias. This is a result of both the limited number of studies from which the estimates we used were extracted and the tendency for more significant results to be submitted and accepted for publication.
This work illustrates the impact of decreasing vitamin D levels with increasing BMI on breast cancer mortality and suggests that vitamin D supplementation could potentially serve to significantly decrease the risk of death after breast cancer, especially for overweight and obese patients. Additionally, for many patients vitamin D supplementation may represent an easier approach to reducing breast cancer mortality than lifestyle modifications intended to decrease BMI. Though dietary vitamin D intake has been shown to have little effect on serum vitamin D levels [37], the effect of supplements on serum concentrations in the obese is still somewhat unclear, with several studies reporting varying effects [37, 38, 45]. Because studies of vitamin D and cancer mortality are frequently performed using vitamin D concentrations taken around the time of diagnosis, the effect of supplementation after diagnosis on mortality has yet to be clarified. Further studies are necessary to elucidate the benefits of correcting vitamin D status after a breast cancer diagnosis and to determine appropriate recommendations for duration and dosing of supplements, as well as optimal targets for serum vitamin D [46].
Acknowledgments
This work was supported by the National Cancer Institute (grant number K07 CA136758).
Abbreviations
- 25(OH)D
25-hydroxy-vitamin D
- VDR
vitamin D receptor
Footnotes
The authors have declared no conflict of interest.
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J. Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Dignam JJ, Wieand K, Johnson KA, Raich P, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2006;97:245–254. doi: 10.1007/s10549-005-9118-3. [DOI] [PubMed] [Google Scholar]
- 3.Caan BJ, Kwan ML, Hartzell G, Castillo A, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–1328. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, Lu W, Zheng W, Gu K, et al. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res. Treat. 2010;122:823–833. doi: 10.1007/s10549-009-0708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emaus A, Veierod MB, Tretli S, Finstad SE, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res. Treat. 2010;121:651–660. doi: 10.1007/s10549-009-0603-y. [DOI] [PubMed] [Google Scholar]
- 6.Hauner D, Janni W, Rack B, Hauner H. The effect of over-weight and nutrition on prognosis in breast cancer. Dtsch. Arztebl. Int. 2011;108:795–801. doi: 10.3238/arztebl.2011.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmichael AR. Obesity and prognosis of breast cancer. Obes. Rev. 2006;7:333–340. doi: 10.1111/j.1467-789X.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J. Clin. Oncol. 2010;28:4074–4080. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Society AC. Breast Cancer Facts & Figures 2011–2012. American Cancer Society, Inc.; Atlanta: 2011. [Google Scholar]
- 10.Vrieling A, Hein R, Abbas S, Schneeweiss A, et al. Serum 25-hydroxyvitamin D and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res. 2011;13:R74. doi: 10.1186/bcr2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res. Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 12.Cleveland RJ, Eng SM, Abrahamson PE, Britton JA, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol. Biomarkers Prev. 2007;16:1803–1811. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- 13.Barnett GC, Shah M, Redman K, Easton DF, et al. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J. Clin. Oncol. 2008;26:3310–3316. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- 14.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women's Health Initiative (WHI) limited-access data set. Am. J. Clin. Nutr. 2011;94:1144–1149. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan QJ, Kimler BF, Fabian CJ. The relationship between vitamin D and breast cancer incidence and natural history. Curr. Oncol. Rep. 2010;12:136–142. doi: 10.1007/s11912-010-0081-8. [DOI] [PubMed] [Google Scholar]
- 16.Mohr SB, Gorham ED, Alcaraz JE, Kane CJ, et al. Serum 25-hydroxyvitamin D and prevention of breast cancer: pooled analysis. Anticancer Res. 2011;31:2939–2948. [PubMed] [Google Scholar]
- 17.Shin MH, Holmes MD, Hankinson SE, Wu K, et al. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J. Natl. Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 18.Welsh J. Vitamin D and prevention of breast cancer. Acta Pharmacol. Sin. 2007;28:1373–1382. doi: 10.1111/j.1745-7254.2007.00700.x. [DOI] [PubMed] [Google Scholar]
- 19.Yin L, Grandi N, Raum E, Haug U, et al. Meta-analysis: serum vitamin D and breast cancer risk. Eur. J. Cancer. 2010;46:2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 20.Gandini S, Boniol M, Haukka J, Byrnes G, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int. J. Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- 21.Grant WB. Relation between prediagnostic serum 25-hydroxyvitamin D level and incidence of breast, colorectal, and other cancers. J. Photochem. Photobiol. B. 2010;101:130–136. doi: 10.1016/j.jphotobiol.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk-a prospective nested case-control study. Int. J. Cancer. 2010;127:2159–2168. doi: 10.1002/ijc.25215. [DOI] [PubMed] [Google Scholar]
- 23.Amir E, Cecchini RS, Ganz PA, Costantino JP, et al. 25-Hydroxy vitamin-D, obesity, and associated variables as predictors of breast cancer risk and tamoxifen benefit in NSABP-P1. Breast Cancer Res. Treat. 2012;133:1077–1088. doi: 10.1007/s10549-012-2012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon-and prostate cancer (Norway). Cancer Causes Control. 2004;15:149–158. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 25.Porojnicu AC, Lagunova Z, Robsahm TE, Berg JP, et al. Changes in risk of death from breast cancer with season and latitude: sun exposure and breast cancer survival in Norway. Breast Cancer Res. Treat. 2007;102:323–328. doi: 10.1007/s10549-006-9331-8. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J. Clin. Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 27.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J. Natl. Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs ET, Thomson CA, Flatt SW, Al-Delaimy WK, et al. Vitamin D and breast cancer recurrence in the Women's Healthy Eating and Living (WHEL) Study. Am. J. Clin. Nutr. 2011;93:108–117. doi: 10.3945/ajcn.2010.30009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuhouser ML, Sorensen B, Hollis BW, Ambs A, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am. J. Clin. Nutr. 2008;88:133–139. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga M, Eisman JA, Sutherland RL. Regulation of epidermal growth factor receptor levels by 1,25-dihydroxyvitamin D3 in human breast cancer cells. Cancer Res. 1988;48:2734–2739. [PubMed] [Google Scholar]
- 31.Ingraham BA, Bragdon B, Nohe A. Molecular basis of the potential of vitamin D to prevent cancer. Curr. Med. Res. Opin. 2008;24:139–149. doi: 10.1185/030079908x253519. [DOI] [PubMed] [Google Scholar]
- 32.Lopes N, Sousa B, Martins D, Gomes M, et al. Alterations in vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garland CF, Garland FC, Gorham ED. Calcium and vitamin D. Their potential roles in colon and breast cancer prevention. Ann. N. Y. Acad. Sci. 1999;889:107–119. doi: 10.1111/j.1749-6632.1999.tb08728.x. [DOI] [PubMed] [Google Scholar]
- 34.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–3720. [PubMed] [Google Scholar]
- 35.Vashi PG, Lammersfeld CA, Braun DP, Gupta D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr. J. 2011;10:51. doi: 10.1186/1475-2891-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008;7:4. doi: 10.1186/1475-2891-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Rodriguez E, Navia B, Lopez-Sobaler AM, Ortega RM. Vitamin D in overweight/obese women and its relationship with dietetic and anthropometric variables. Obesity (Silver Spring) 2009;17:778–782. doi: 10.1038/oby.2008.649. [DOI] [PubMed] [Google Scholar]
- 38.Stein EM, Strain G, Sinha N, Ortiz D, et al. Vitamin D insufficiency prior to bariatric surgery: risk factors and a pilot treatment study. Clin. Endocrinol. (Ox.) 2009;71:176–183. doi: 10.1111/j.1365-2265.2008.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagunova Z, Porojnicu AC, Grant WB, Bruland O, Moan JE. Obesity and increased risk of cancer: does decrease of serum 25-hydroxyvitamin D level with increasing body mass index explain some of the association? Mol. Nutr. Food Res. 2010;54:1127–1133. doi: 10.1002/mnfr.200900512. [DOI] [PubMed] [Google Scholar]
- 40.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin d status of obesity. Obesity (Silver Spring) 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 41.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int. J. Obes. (Lond) 2012;36:387–396. doi: 10.1038/ijo.2011.119. [DOI] [PubMed] [Google Scholar]
- 42.Kamycheva E, Joakimsen RM, Jorde R. Intakes of calcium and vitamin d predict body mass index in the population of Northern Norway. J. Nutr. 2003;133:102–106. doi: 10.1093/jn/133.1.102. [DOI] [PubMed] [Google Scholar]
- 43.Florez H, Martinez R, Chacra W, Strickman-Stein N, Levis S. Outdoor exercise reduces the risk of hypovitaminosis D in the obese. J. Steroid. Biochem. Mol. Biol. 2007;103:679–681. doi: 10.1016/j.jsbmb.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 44.Grant WB. Effect of follow-up time on the relation between prediagnostic serum 25-hydroxyvitamin D and all-cause mortality rate. Dermatoendocrinol. 2012;4:1–5. doi: 10.4161/derm.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garland CF, French CB, Baggerly LL, Heaney RP. Vitamin D supplement doses and serum 25-hydroxyvitamin D in the range associated with cancer prevention. Anticancer Res. 2011;31:607–611. [PubMed] [Google Scholar]
- 46.Goodwin PJ. Vitamin D in cancer patients: above all, do no harm. J. Clin. Oncol. 2009;27:2117–2119. doi: 10.1200/JCO.2008.20.8629. [DOI] [PubMed] [Google Scholar]