Abstract
Objective
To determine whether automated mechanical stimulation of the whisker pad improves whisking recovery after facial nerve transection and repair in a rat model.
Methods
Sixty-one rats underwent unilateral facial nerve transection and suture repair, and were randomized into 8 groups. Six groups received daily automated whisker or whisker pad mechanical stimulation, including 0.5, 1.5, and 8 Hz patterns. Two control groups received restraint without stimulation. Treatment started on postoperative day 8, occurred 5 days per week, and lasted throughout 15 weeks of recovery. Whisking amplitude, velocity, and acceleration were quantified weekly for 15 weeks.
Results
Rats receiving the low frequencies of stimulation of the whiskers or whisker pad did not demonstrate enhanced whisking recovery, and rats receiving stimulation at 8 Hz showed significantly worse whisking recovery than controls and previously published groups receiving lower dose manual stimulation.
Conclusions
Although daily manual whisker pad stimulation has been shown to enhance whisking recovery, rats in this study did not demonstrate improved whisking recovery after automated mechanical stimulation across a wide range of driving frequencies. Moreover, faster stimulation (8 Hz) was actually detrimental to recovery. Future work is needed to understand the relationship between stimulation patterns and the physiological mechanisms underlying improved or worsened functional outcomes after facial nerve transaction and repair.
Introduction
Facial paralysis is a disorder with profound consequences, both functional and psychosocial. Causes of facial nerve paralysis are myriad, stemming from surgical, infectious, traumatic, congenital, and idiopathic causes. Amongst the various consequences, incomplete eye closure (leading to exposure keratopathy), external nasal valve obstruction, oral incompetence, speech and articulation problems, esthetic impairments, and the inability to express emotions through facial musculature are most clinically important. Treatment options comprise physical therapy, nerve transfers, muscle transfers and static surgical techniques.1 The results, even following aggressive treatment, remain variable and often disappointing. Following nerve repair, the slow rate of nerve regeneration can lead to degeneration of the motor end organ and permanent loss of function. In addition, axonal misrouting can develop, leading to synkinesis.2
One driving question for facial nerve regeneration research groups is how to both accelerate and improve facial nerve regeneration. The rat model is widely employed to study interventions that affect speed and completeness of facial nerve recovery. The rat facial nerve is anatomically comparable to the human facial nerve3, and recovery is highly quantifiable by measurement of whisking kinematics.4 Research has focused upon pharmacological5-8, electrical9-10, and mechanical11-14 interventions to accelerate and improve facial nerve regeneration; the latter intervention potentially demonstrating the most promise to date. The application of mechanical stimulation to the facial muscles during regeneration of the facial nerve could be relatively easy to administer, and would be of a great value in clinical settings where recovery is expected. However, more thorough exploration of the therapeutic potential of this treatment option is required and the underlying physiologic mechanisms must be better understood, before it can become part of routine clinical care of patients recovering from facial paralysis.
In previous studies from our and other laboratories10-14, mechanical whisker and whisker pad stimulation has been delivered manually. Our laboratory recently developed a “Whisk Assist” (WA) system for delivering controlled and quantifiable patterns of mechanically driven whisking after rat facial nerve injury.15 This WA apparatus drives or assists whisker movement on the horizontal (dominant) plane of natural whisking, and is well tolerated by head-fixed (restrained) animals. In the current study, we examined the effects of several pre-programmed WA patterns during recovery from facial nerve transection and suture repair. We studied larger groups of rats of previously promising conditions15 and piloted additional new conditions, under the hypothesis that such WA treatment would enhance the speed and/or completeness of whisking recovery compared with control animals.
Methods
Conditioning and head fixation
Sixty-one female Wistar-Hannover rats (Charles River Laboratorium, Wilmington, MA), weighing 200-250 g were handled on a daily basis, 5 days per week for 1 week, to acclimate to human handling. All rats then underwent implantation of a titanium head fixation device, as previously described.16 The device has four lateral extensions that provide points of attachment with an external framework for head fixation. Two weeks after head fixation device implantation, the rats were progressively conditioned to a body and head restraint apparatus 5 days per week.16 When animals were sufficiently conditioned to undergo head and body restraint without struggling or signs of stress (typically after 2 weeks), unilateral facial nerve cut and suture repair surgery was performed. All experimentation was conducted under protocols approved by the Massachusetts Eye and Ear Infirmary Animal Care and Use Committee.
Facial nerve cut and suture repair surgery
Rats were anesthetized with an intramuscular injection of ketamine (50mg/kg) (Fort Dodge Animal Health, Fort Dodge, IA) and dexmedetomidine hydrochloride (0.5 mg/kg) (Orion Corporation, Espoo, Finland). The left facial nerve was exposed via a preauricular incision, and ipsilateral parotidectomy was performed. The main trunk and dominant branches of the facial nerve were identified. The main trunk of the nerve was sharply transected and microsurgically reconnected with two or three 10-0 epineural nylon sutures (Ethicon Inc, Somerville, NJ). The wound was closed in a single layer with absorbable suture. All surgical procedures were performed by a single microsurgeon with substantial neurorrhaphy experience.
The anesthetic was reversed with a subcutaneous injection of atipamezole hydrochloride (0.05 mg/kg) (Orion Corporation, Espoo, Finland). Postoperatively the rats were monitored for signs of discomfort, weight maintenance, cage behavior and wound issues.
Mechanical Stimulation with the Whisk Assist System
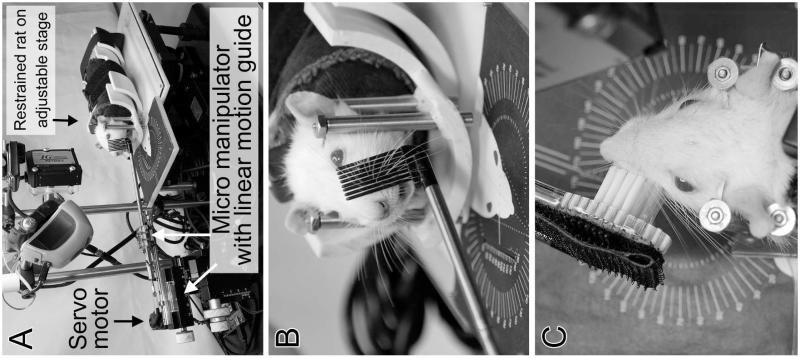
The WA system has been previously described.15 Briefly, rats are placed in a body and head restraint half-pipe, which is then positioned in the apparatus. The WA system is designed to move the whiskers on one side of the face in the horizontal plane. The automated mechanical stimulation is delivered via a servomotor-controlled rod holding either a comb with 8 vertical tines that contact all of the prominent whiskers when the rod is moved horizontally, or a brush that contacts the whisker pad for direct pad surface stimulation (Figure 1). Animals were randomized into 8 groups (Table 1). The most promising treatment patterns of Heaton et al15 were chosen to test in larger groups of rats; for three experimental groups, the mechanical stimulation treatment patterns moved the whiskers 60-70 degrees at a rate of 8 Hz, with these three groups differing in how many treatment sessions were delivered per day, or whether the stimulation was continuous or intermittent during the treatment sessions. Three additional treatment patterns of the same amplitude were also tested; one was whisker movement at 1.5 Hz, and for two groups the WA apparatus was altered to provide both whisker and whisker pad stimulation at 0.5 Hz via the head of a soft-bristled tooth brush (in place of the comb; see Figure 1). These latter three low-frequency conditions were chosen to better emulate the manual mechanical stimulation studied in prior reports.10-14 All treatment patterns started at postoperative day 8. Control animals were restrained for 20 minutes per day in a similar apparatus as the WA system, with the WA comb against the whisker pad, but without comb movement.
Figure 1.
Rat in the Whisk Assist (WA) apparatus, showing a system overview (A), the comb in position to drive whisker movement (B), and the bristle brush in position to mechanically stimulate the whisker pad (C).
Table 1.
Whisk Assist Treatment Programs
| Group | n | WA Pattern | Sessions per Day |
Total Minutes in Apparatus per Day |
|---|---|---|---|---|
| CNTR-A | 8 | no stimulation | 1 | 20 |
| CNTR-B | 8 | no stimulation | 3 | 20 |
| 8HZ-A | 16 | 8 Hz constant | 1 | 20 |
| 8HZ-B | 8 | 8 Hz constant | 3 | 20 |
| 8HZ-C | 8 | 8 Hz for 5 sec per 30 sec | 3 | 20 |
| 1.5Hz | 3 | 1.5 Hz constant | 1 | 5 |
| 0.5HZ-A* | 5 | 0.5 Hz constant | 1 | 5 |
| 0.5HZ-B* | 5 | 0.5 Hz constant | 3 | 15 |
These conditions included direct stimulation of the whisker pad surface; WA = whisk assist; CNTR = control group
Functional Recovery Testing
Whisking function was assessed weekly throughout the 15-week recovery period using our previously validated testing apparatus.17, 18 Briefly, the rats were placed in head and body restraint and positioned in the testing apparatus for five minutes of continuous recording per recording session. Movement of light-weight markers threaded onto a representative, prominent whisker (C1) on the right and left was tracked by laser micrometers (MetraLight, San Mateo, CA) positioned adjacent to each whisker pad, and whisker movement was saved by custom data acquisition software.4, 19, 20
Data Analysis
For each recording session, the three largest amplitude whisks on each side of the face were automatically identified and measured for amplitude, velocity and acceleration using software adapted from Bermejo et al.4, 19, 20 Whisking function on the recovering side was analyzed in relation to whisking on the healthy side in order to account for daily variation in whisking effort, because whisking is typically symmetrical across the two sides of the face.21 The recovery parameters of whisking amplitude, acceleration, and velocity were each averaged by week across weeks 3-15 of recovery, and one-way ANOVAs were performed to test for overall treatment effects among the 8 Hz experimental and control groups for each parameter. Multiple post-hoc Tukey’s tests were performed (as appropriate) after establishing main effects to determine which mechanical stimulation treatment groups differed from each other and from control rats. These data were compared with our previously published data11, with a one-way ANOVA and Tukey’s post hoc tests. The small group sizes of the three low-frequency stimulation groups (see Table 1) precluded meaningful statistical comparison, but data from these groups were presented descriptively with the other groups for comparison. Statistical testing was performed with SPSS software (Ver. 16.0, SPSS Inc., Chicago, IL).
Results
During the 15 week post-operative recovery period, 8 animals (13.1%) were excluded from the study due to head fixation device failure. This attrition rate was consistent with our prior findings using the head fixation device,10, 11 and we have found this to be equal to or lower than attrition when simple head-mount screws are reinforced with adhesives over extended survival periods (e.g. 15 week). The excluded animals were relatively equally spread across the groups (n=1 CNTR-A, n=2 CNTR-B, n=2 8Hz-A, n=1 8Hz-B, n=1 8Hz-C, n=0 1.5Hz, n=0 0.5Hz-A, n=1 0.5Hz-B). There were no post-surgical wound infections after either the head fixation device implantation or facial nerve transection and repair surgery, and all rats demonstrated normal feeding and social behavior.
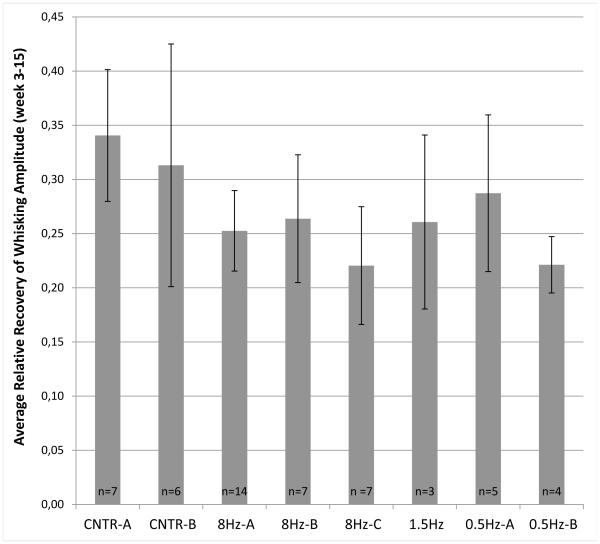
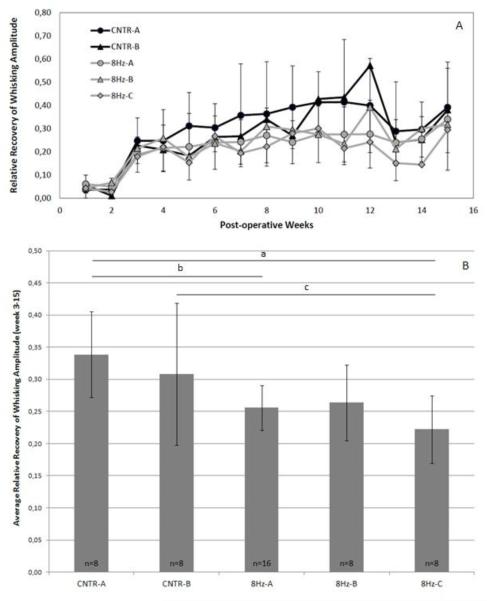
Figure 2 shows the average recovery of whisking amplitude across week 3 to 15 as a ratio of whisking amplitude for the nerve-repaired side divided by the healthy side. No trend of improvement was observed in animals undergoing these pilot whisk assist conditions. Statistical analyses were performed with the 8Hz experimental groups. Post-operative whisking amplitude data for the 8 Hz experimental and control groups are shown in Figure 3. A one-way ANOVA demonstrated an overall statistically significant difference among these five experimental groups in relative whisking amplitude across weeks 3-15 (P<.001). The asterisks in Figure 3B indicate where Tukey’s post hoc analysis found statistically significant differences between CNTR-A and 8Hz-A (P=.018), between CNTR-A and 8Hz-C (P<.001), and between CNTR-B and 8Hz-C (P=.009), with the whisk assisted groups performing more poorly. Results of whisking velocity and acceleration recovery were qualitatively and quantitatively similar to whisking amplitude recovery (data not shown).
Figure 2.
Average recovery of whisking amplitude across week 3 to 15. The different groups are described in Table 1. Standard error bars are shown.
Figure 3.
A. Recovery of amplitude for the 8Hz experimental and control groups during 15 post-operative weeks. Standard error bars are shown. B. Columns represent the average recovery of amplitude across week 3 to 15 for the 8Hz experimental groups. Standard error bars are shown. Horizontal bars with asterisks indicate statistically significant differences (P < 0.05).
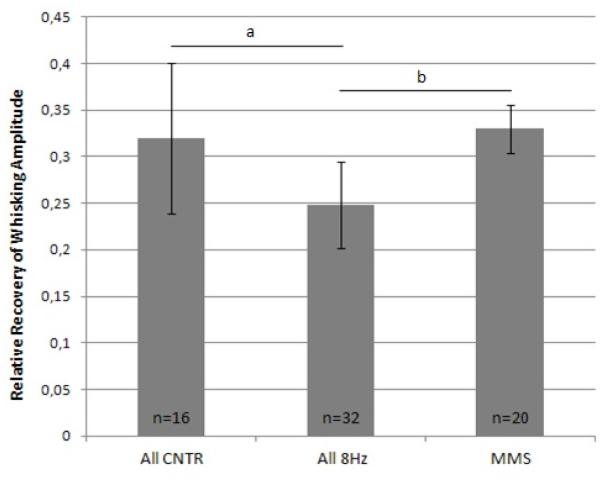
Since there were no statistically significant differences between the control groups and among the 8 Hz treatment conditions in the present report, CNTR-A and CNTR-B were combined (All CNTR), as were 8Hz-A, -B, and -C (All 8 Hz) for further comparison with manual mechanical stimulation (MMS) data from our prior report.11 The average recovery of whisking amplitude across week 3 to 15 in the MMS group was used for comparison. A statistically significant difference was found among these three groups (P<.001; one way ANOVA) (Figure 4), and Tukey’s post hoc analysis showed statistically significant differences between All 8Hz and MMS (P=.004), and between All CNTR and All 8Hz (P=.001), with control animals and manual mechanical stimulated animals performing better than whisk assisted animals. The average recovery of whisking amplitude across week 3 to 15 for the MMS group from our prior report11 did not significantly differ from the All CNTR group of the present report (P>.05).
Figure 4.
Columns represent average recovery of whisking amplitude across weeks 3 to 15 of the current study (combined groups) and manual mechanical stimulation (MMS).11 All CNTR = combination of CNTR-A and CNTR-B, All 8Hz = combination of the three 8Hz conditions (groups 8Hz-A, -B, and -C), MMS = manual mechanical stimulation.11 Standard error bars are shown. Horizontal bars with asterisks indicate statistically significant differences (P < 0.05).
Discussion
Functional recovery from facial nerve transection and surgical repair is typically poor in both rats and humans, providing an opportunity to test interventions intended to enhance facial nerve regeneration in rats, which might ultimately translate to humans. Rat whisking movement begins to re-appear by approximately 3 weeks after unilateral facial nerve transection and repair, improves steadily for several weeks, and generally plateaus by 2-4 months at only about 25% of the whisking amplitude relative to the contralateral, healthy side of the face.6, 10-12, 14 Previous studies have found enhanced functional recovery from brief, daily manual mechanical stimulation of the whiskers and/or whisker pad delivered during recovery from unilateral facial nerve transection and repair.10-14 Such enhancement has ranged from a modest 10% improvement in relative whisking amplitude reported by our laboratory10, 11, to complete (symmetrical) whisking recovery observed by others.12-14
We sought to deliver multiple patterns of whisker and whisker pad mechanical stimulation under greater experimental control than previous studies, with the goal of identifying optimal treatment patterns and potentially resolve discrepancies among prior outcomes. Based upon pilot data15, we anticipated that the 8 Hz stimulation patterns delivered in the present report would show greater enhancement of functional outcome compared to our prior, moderate levels of stimulation.10, 11, 15 To the contrary, functional outcome after high-dose WA treatment was impaired relative to controls, suggesting that high-dose WA treatment can cause deleterious overstimulation of the whisker pad. Moreover, lower-dose WA movement of the whiskers (1.5 Hz via comb interface) and/or whisker pad surface (0.5 Hz via brush interface) likewise failed to improve whisking recovery in the present study, drawing into question the cause of the complete recovery achieved after hand-delivered mechanical stimulation performed in a different laboratory.12-14
One possible mechanism underlying the apparent deleterious effects of high-dose (8 Hz) stimulation is fatigue of the facial mechanoreceptors during prolonged activation.22 The work of Pavlov et al13 indicates that intact sensory input is required for mechanical stimulation to provide a benefit, and that mechanical stimulation delivered in the absence of normal whisker pad sensation (after infraorbital nerve cut) not only fails to enhance functional outcome, but also worsens whisking recovery. Whisker pad sensation may have been diminished or interrupted during intensive mechanical stimulation in our study due to mechanoreceptor fatigue,22 thereby resembling the sensory nerve lesion effect reported by Pavlov et al13, and leading to reduced functional outcome. However, this does not explain why lower-frequency stimulation (0.5 – 1.5 Hz) failed to enhance whisking recovery, and indicates that further experimentation is required to shed light on the interaction of sensory feedback and motor axon regeneration in the whisker pad.
An additional explanation for the lack of benefit from the 8 Hz WA stimulation is that direct mechanical contact with the whisker pad may be required to evoke a beneficial regenerative effect. A denervated muscle undergoes various changes depending on the delay before reinnervation, including loss of muscle mass, diminished blood circulation, shrinking of connective tissue, and adhesion (fibrosis).23 It is possible that mechanical stimulation of the whisker pad itself (instead of just whisker movement) may help minimize these sequelae of denervation by maintaining whisker pad health while the facial nerve is regenerating, and perhaps provide an optimal interaction between trigeminal and facial brainstem nuclei (see paragraph above). In the initial version of our WA system,13 the oscillating comb contacted whiskers close to their exit from the pad (Figure 1A), but the comb had little contact with the pad itself. Given that the prior studies which have found the greatest enhancement of facial nerve regeneration involved stimulation of both the whiskers and the pad through finger tip stroking of the pad,10-14 we modified the WA hardware for groups 0.5Hz-A and 0.5Hz-B in the present study to provide direct pad contact via a soft bristle brush pressing against the pad surface (to emulate finger tip pressure; see Figure 1C). However, this pad-stimulating condition failed to enhance whisking recovery, leaving us at a loss for why relatively low-frequency stimulation did not enhance regeneration as had been seen with manual stimulation in prior reports.12-14
One potentially important difference between whisker pad stimulation delivered in this study versus hand-held delivery used in prior studies is the heightened stress rats may have experienced under rigid restraint within the WA system. Rats were extensively conditioned to human handling and placement in restraint in the weeks prior nerve injury/repair and WA treatment, and they did not exhibit signs of heightened stress under restraint (vocalizing, struggling, etc.) while the WA system delivered stimulation compared to the stationary comb control condition. However, it is possible that WA treatment produced occult stress that offset potentially beneficial effects of the treatment, explaining why whisking recovery that was similar to, or worse than, that of the restrained control rats, as has been demonstrated in prior studies; Van Meeteren et al demonstrated that chronic intermittent stress impaired nerve regeneration in a sciatic nerve model.24 The nerve regeneration process is controlled by neuroendocrine, immunologic and autonomic nervous system factors. Chronic stress deteriorates the efficiency of these processes; for example, activation of the autonomic nervous system causes epineural vasoconstriction and reduces endoneural nerve blood flow.24 Likewise, Amako et al found suppressed sciatic nerve recovery after water-immersion stress in rats.25
Variability of the whisking amplitude within groups (shown in Figure 3) and the fluctuation in average whisking kinematics across all groups at most recovery time points is consistent with our prior observations, and represents an inherent weakness in employing this particular functional recovery measure. Because rats whisk with variable amplitude based both upon muscle strength and intactness of innervation, and upon mood, state of curiosity and arousal, interest in the surrounding environment, amongst other factors, the assay itself may be relatively insensitive to small but real influences and interventions.
Future investigations of mechanical stimulation effects on nerve regeneration will require exploring ways to mitigate the stress associated with restraint during treatment delivery, such as sedation or delivery of appetitive rewards.
The effects of mechanical stimulation have been studied in other animal models of peripheral nerve regeneration26-31, sometimes with conflicting results. For example, Van Meeteren et al demonstrated that mild daily exercise (4 hours of hindpaw stretching) augmented functional recovery in the early phase (persisting into the late phase) after sciatic nerve crush in the rats27, whereas Herbison et al showed that intense swimming did not enhance the repair of reinnervated muscle, and that treadmill running led to a deleterious effect on muscle function recovery.32, 33
Potential explanations for these inconsistencies include variations in the type of nerve injury, whether or not the nerve contains sensory axons,30 the type of mechanical stimulation delivered, and the duration and intensity of the stimulation.34 With specific regard to the effect of mechanical stimulation on regeneration of the facial nerve, there are likewise conflicting results in the literature as described above. It is possible that, in our hands or apparatus, mechanical stimulation has not led to true enhancement in whisking recovery when you consider the performance of control and experimental groups across our studies. The relative recovery of whisking amplitude for regenerated nerves versus the contralateral (healthy) side within rats has been approximately 10% better (on average, within studies) compared to simultaneous nerve-repaired controls10, 15 or historical controls.11 However, had the present nerve-repaired control group served as the point of comparison in our prior studies, then none of our previous stimulated groups would have shown an enhancement. This is illustrated by comparing the greatest prior enhancement of manual, mechanical stimulation11 with the present combined control groups (see Figure 5), yielding similar whisking amplitude after 15-16 weeks of recovery. This suggests that either daily restraint for 20 min (with a stationary whisker comb) provides the same degree of recovery enhancement as mechanical stimulation in all of the manual and automated forms we have tested to date10, 11, 15, or that the impact of mechanical stimulation by our group has been negligible. Either way, we have repeatedly failed to replicate the complete, symmetrical recovery caused by manual stroking of the whisker pad as previously reported.12-14 Differences in stimulation delivery techniques or whisking quantification methods across laboratories may contribute to these disparate findings,35 but our studies have shown that enhancing nerve regeneration through whisker pad manipulation is difficult to achieve at best, and potentially detrimental at worst. Our failure to demonstrate recovery benefit despite our exhaustive employment of myriad regimens of automated whisk-assisting under systematic, highly controlled circumstances, with and without direct whisker pad stimulation, leads us to conclude that the benefit originally proposed may not actually represent a true, reproducible phenomenon.
Conclusions
Recovery of horizontal whisking was followed for 15 weeks after unilateral facial nerve transection and repair in 61 rats; 45 of which received daily, automated “whisk assist” mechanical therapy, and 16 were sham-stimulated controls. Automated mechanical stimulation failed to enhance whisking recovery for low-frequency stimulation (0.5 Hz or 1.5 Hz) resembling the manually delivered patterns used in prior reports, and higher-frequency stimulation (8 Hz) resulted in worse recovery than controls. Moreover, the current control group performed as well as experimental groups in our prior reports, where recovery was believed to have been enhanced by manually delivered stimulation (via whisker pad stroking with a finger tip10 or paint brush11), drawing into question whether mechanical stimulation actually led to meaningful enhancement of whisking in those reports. The discrepancy between the modest or non-existent whisking recovery enhancement caused by mechanical stimulation in some studies10, 11, 15 versus the complete recovery reported by other groups12-14 requires further clarification given the potential clinical importance of this physical therapy intervention.
Acknowledgment
The authors want to acknowledge Julie S. Weinberg for her contributions in data collection and analysis, and Marc H. Hohman for performing our microsurgeries.
Ingrid J. Kleiss and Tessa A. Hadlock had full access to all data in this study, and take responsibility for the integrity of the data, and the accuracy of the data analysis.
The contents of this manuscript have not been published elsewhere, and likewise the manuscript is not being submitted elsewhere. There is no potential conflict of interest for the individual authors and we have no financial interest to declare in relation to the content of this article.
References
- 1.Hadlock TA, Greenfield LJ, Wernick-Robinson M, Cheney ML. Multimodality approach to management of the paralyzed face. Laryngoscope. 2006;116:1385–1389. doi: 10.1097/01.mlg.0000225980.38147.c6. [DOI] [PubMed] [Google Scholar]
- 2.Husseman J, Mehta RP. Management of synkinesis. Facial Plast Surg. 2008;24:242–249. doi: 10.1055/s-2008-1075840. [DOI] [PubMed] [Google Scholar]
- 3.Mattox DE, Felix H. Surgical anatomy of the rat facial nerve. Am J Otol. 1987;8:43–47. [PubMed] [Google Scholar]
- 4.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking--I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:341–346. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay RW, Heaton JT, Edwards C, Smitson C, Hadlock TA. Nimodipine and acceleration of functional recovery of the facial nerve after crush injury. Arch Facial Plast Surg. 2010;12:49–52. doi: 10.1001/archfacial.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vakharia KT, Lindsay RW, Knox C, et al. The effects of potential neuroprotective agents on rat facial function recovery following facial nerve injury. Otolaryngol Head Neck Surg. 2011;144:53–59. doi: 10.1177/0194599810390892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh C, Bowers D, Hadlock TA. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch Facial Plast Surg. 2007;9:333–339. doi: 10.1001/archfaci.9.5.333. [DOI] [PubMed] [Google Scholar]
- 8.Angelov DN, Neiss WF, Streppel M, Andermahr J, Mader K, Stennert E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996;16:1041–1048. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinis N, Horn F, Genchev B, et al. Electrical stimulation of paralyzed vibrissal muscles reduces endplate reinnervation and does not promote motor recovery after facial nerve repair in rats. Ann Anat. 2009;191:356–370. doi: 10.1016/j.aanat.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Hadlock T, Lindsay R, Edwards C, et al. The effect of electrical and mechanical stimulation on the regenerating rodent facial nerve. Laryngoscope. 2010;120:1094–1102. doi: 10.1002/lary.20903. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay RW, Heaton JT, Edwards C, Smitson C, Vakharia K, Hadlock TA. Daily facial stimulation to improve recovery after facial nerve repair in rats. Arch Facial Plast Surg. 2010;12:180–185. doi: 10.1001/archfacial.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelov DN, Ceynowa M, Guntinas-Lichius O, et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol Dis. 2007;26:229–242. doi: 10.1016/j.nbd.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Pavlov SP, Grosheva M, Streppel M, et al. Manually-stimulated recovery of motor function after facial nerve injury requires intact sensory input. Exp Neurol. 2008;211:292–300. doi: 10.1016/j.expneurol.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 14.Skouras E, Merkel D, Grosheva M, et al. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor Neurol Neurosci. 2009;27:237–251. doi: 10.3233/RNN-2009-0474. [DOI] [PubMed] [Google Scholar]
- 15.Heaton JT, Knox CJ, Malo JS, Kobler JB, Hadlock TA. A system for delivering mechanical stimulation and robot-assisted therapy to the rat whisker pad during facial nerve regeneration. Transactions on Neural Systems & Rehabilitation Engineering. doi: 10.1109/TNSRE.2013.2244911. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadlock T, Kowaleski J, Mackinnon S, Heaton JT. A novel method of head fixation for the study of rodent facial function. Exp Neurol. 2007;205:279–282. doi: 10.1016/j.expneurol.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hadlock T, Kowaleski J, Lo D, et al. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008;118:1744–1749. doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 18.Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171:197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- 20.Bermejo R, Friedman W, Zeigler HP. Topography of whisking II: interaction of whisker and pad. Somatosens Mot Res. 2005;22:213–220. doi: 10.1080/08990220500262505. [DOI] [PubMed] [Google Scholar]
- 21.Kleinfeld D, Berg RW, O'Connor SM. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens Mot Res. 1999;16:69–88. doi: 10.1080/08990229970528. [DOI] [PubMed] [Google Scholar]
- 22.Barker DJ, Shepard PD, McDermott KL. Fatigue in cat facial mechanoreceptors. Neurosci Lett. 1982;30:117–122. doi: 10.1016/0304-3940(82)90282-8. [DOI] [PubMed] [Google Scholar]
- 23.Belal A., Jr. Structure of human muscle in facial paralysis. J Laryngol Otol. 1982;96:325–334. doi: 10.1017/s0022215100092562. [DOI] [PubMed] [Google Scholar]
- 24.van Meeteren NL, Brakkee JH, Helders PJ, Wiegant VM, Gispen WH. Functional recovery from sciatic nerve crush lesion in the rat correlates with individual differences in responses to chronic intermittent stress. J Neurosci Res. 1997;48:524–532. [PubMed] [Google Scholar]
- 25.Amako M, Nemoto K. Influence of water immersion stress on peripheral nerve recovery in the rat. J Orthop Sci. 1998;3:32–41. doi: 10.1007/s007760050019. [DOI] [PubMed] [Google Scholar]
- 26.Marqueste T, Alliez JR, Alluin O, Jammes Y, Decherchi P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol. 2004;96:1988–1995. doi: 10.1152/japplphysiol.00775.2003. [DOI] [PubMed] [Google Scholar]
- 27.van Meeteren NL, Brakkee JH, Hamers FP, Helders PJ, Gispen WH. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch Phys Med Rehabil. 1997;78:70–77. doi: 10.1016/s0003-9993(97)90013-7. [DOI] [PubMed] [Google Scholar]
- 28.Udina E, Puigdemasa A, Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve. 2011;43:500–509. doi: 10.1002/mus.21912. [DOI] [PubMed] [Google Scholar]
- 29.Evgenieva E, Schweigert P, Guntinas-Lichius O, et al. Manual stimulation of the suprahyoid-sublingual region diminishes polynnervation of the motor endplates and improves recovery of function after hypoglossal nerve injury in rats. Neurorehabil Neural Repair. 2008;22:754–768. doi: 10.1177/1545968308316387. [DOI] [PubMed] [Google Scholar]
- 30.Sinis N, Guntinas-Lichius O, Irintchev A, et al. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Exp Brain Res. 2008;185:469–483. doi: 10.1007/s00221-007-1174-y. [DOI] [PubMed] [Google Scholar]
- 31.Herbison GJ, Jaweed MM, Ditunno JF, Scott CM. Effect of overwork during reinnervation of rat muscle. Exp Neurol. 1973;41:1–14. doi: 10.1016/0014-4886(73)90176-3. [DOI] [PubMed] [Google Scholar]
- 32.Herbison GJ, Jaweed MM, Ditunno JF. Effect of swimming on reinnervation of rat skeletal muscle. J Neurol Neurosurg Psychiatry. 1974;37:1247–1251. doi: 10.1136/jnnp.37.11.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbison GJ, Jaweed MM, Ditunno JF. Effect of activity and inactivity on reinnervating rat skeletal muscle contractility. Exp Neurol. 1980;70:498–506. doi: 10.1016/0014-4886(80)90176-4. [DOI] [PubMed] [Google Scholar]
- 34.Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat. 2011;193:347–353. doi: 10.1016/j.aanat.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Knutsen PM, Derdikman D, Ahissar E. Tracking whisker and head movements in unrestrained behaving rodents. J Neurophysiol. 2005;93:2294–2301. doi: 10.1152/jn.00718.2004. [DOI] [PubMed] [Google Scholar]