Abstract
Purpose
This prospective phase II pilot study evaluated safety and efficacy of transarterial chemoembolization (TACE) with drug-eluting beads (DEBs) loaded with doxorubicin in patients with unresectable hepatocellular carcinoma (HCC).
Methods
Twenty patients with unresectable HCC (75% Child's A, 95% Eastern Cooperative Oncology Group performance status 0 to 1, 60% Barcelona Clinic Liver Cancer C, tumor size 6.9 cm) underwent 34 DEBTACE sessions. Primary endpoints were tumor response, assessed by contrast-enhanced magnetic resonance imaging at 1 month after treatment, using size (response evaluation criteria in solid tumors [RECIST]), contrast-enhancement (European Association for the Study of the Liver) and apparent diffusion coefficient values, and safety assessed by National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE). Secondary endpoints included feasibility, progression-free survival, and overall survival.
Results
DEB-TACE was successfully performed in 34 sessions and demonstrated a favorable safety profile. On initial (1 month) postprocedural magnetic resonance imaging, treated lesions had a mean decrease in size of 4% (P = 0.1129). Using RECIST, partial response was achieved in 2 patients (10%), and 18 patients (90%) had stable disease. Treated tumors demonstrated a mean decrease in contrast enhancement of 64% (P < 0.0001). By European Association for the Study of the Liver criteria, 12 patients (60%) had objective tumor response, and 8 (40%) had stable disease. No patients had progression of a treated lesion while undergoing treatment. At 6 months, the disease control rate was 95% using RECIST. Overall survival rates at 1 and 2 years were 65% and 55%, respectively; median overall survival was 26 months.
Discussion
DEB-TACE is safe and effective in achieving local tumor control in patients with unresectable HCC.
Keywords: hepatocellular carcinoma, drug delivery systems, intra-arterial injection, doxorubicin
Hepatocellular carcinoma (HCC) is the third most common cause of cancer death worldwide.1 The majority of patients present with intermediate-advanced disease that is not amenable to curative treatment, and the median survival in this group is 6 to 8 months.2 Well-designed randomized trials have shown the positive impact of transarterial chemoembolization (TACE) on the survival for these patients.3,4 A new type of microspheres with drug-eluting capabilities can be administered intra-arterially in the same manner as the oil/chemotherapy suspension used during conventional TACE. These microspheres or drug-eluting beads (DEBs) allow for controlled and sustained drug delivery and minor blood dispersion of the drug compared with conventional TACE.5,6
The first clinical studies with TACE using DEBs (DEBTACE) in the treatment of HCC showed a high index of tumor necrosis, a low incidence of toxicities, and overall response rates varying from 50% to 75%.7–9 These initial studies included mainly asymptomatic patients, with Child-Pugh A cirrhosis, no vascular invasion, and Barcelona Clinic Liver Cancer (BCLC) stage A or B (early or intermediate stage HCC). Because DEB-TACE seems to have a more favorable safety profile than conventional TACE, there might be a potential benefit for patients with advanced HCC (BCLC C). Llovet et al (Study of Heart and Renal Protection study) showed that sorafenib, a multikinase inhibitor, significantly increased overall survival by 2.6 months in patients with advanced HCC. However, the median survival in these patients is still less than 1 year.10
In this prospective study, we, therefore, assessed the safety and efficacy of DEB-TACE in patients with unresectable advanced HCC using toxicity data and magnetic resonance imaging (MRI) findings as primary endpoints. Secondary endpoints included feasibility, progression free, and overall survival.
METHODS
Patient Selection
The study was approved by our institutional review board and the Food and Drug Administration, with a physician sponsored Investigational Device Exemption to treat 20 patients with surgically unresectable HCC.
The diagnosis of HCC was confirmed by biopsy (15/20), or typical radiologic findings, in addition to an elevated serum α-feto-protein >400 ng/mL.
All patients were reviewed at our weekly multidisciplinary liver conference; those with disease deemed unresectable by the surgeons (due to locally advanced lesions, proximity to a vessel, and associated liver cirrhosis, ect.) and referred for TACE were considered for DEB-TACE. Eligibility criteria included Eastern Cooperative Oncology Group performance status ≤2, Child-Pugh classification A or B; absent or trace ascites, albumin >2.5 g/dL, alanine aminotransferase and aspartate aminotransferase < 5 × upper normal limit, total bilirubin <3.0 mg/dL, creatinine <2.0 mg/dL, platelet count ≥50,000/mm3, international normalized ratio ≤1.5, and a left ventricle ejection fraction of ≥50%.
Exclusion criteria included previous therapy for HCC other than liver resection and complete occlusion of the portal venous system.
Treatment Protocol
Patients received a full clinical examination, laboratory assessment, and MRI at baseline. After DEB-TACE treatment, patients were admitted overnight and given analgesics via a patient-controlled pump. The 24-hour pain medication doses were recorded. Follow-up visits were performed at 2- to 3-month intervals and included clinical examination, laboratory assessment, and MRI. Lesions with residual contrast enhancement on MRI (<90% necrosis) received up to 2 additional DEB-TACE treatments. All patients were followed up until disease progression or death.
DEB-TACE Technique
All DEB-TACE procedures were performed by an experienced interventional radiologist using a consistent approach. LC Beads (2 mL, BioCompatibles Ltd., UK) with a diameter of 100 to 300 μm or 300 to 500 μm were loaded with 100 mg of doxorubicin hydrochloride (25 mg/mL, Pharmacia-UpJohn) and mixed with an equal volume of nonionic contrast media. Access to the common femoral artery was obtained, and a catheter was positioned as closely to the tumor bed as possible before infusions of the DEBs. This means that every injection was performed in a superselective manner preferably through a microcatheter. DEBs (up to 4 mL) were administered by alternating injections of aliquots of the beads and contrast, until complete delivery or when the blood flow of the feeding artery slowed down substantially. Complete occlusion of the main feeding artery was avoided to allow for retreatment if necessary as we have reported before with our conventional TACE technique.11
Safety Assessment
Safety evaluation, at each study visit, included a physical examination, clinical laboratory evaluations, and assessments of adverse events. Start and stop dates for all adverse events, degree of severity (according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.), and causal relationship to DEB-TACE treatment were recorded. Criteria specific for the diagnosis of postembolization syndrome (pain, fever, nausea, and vomiting) were recorded.
Efficacy Assessment
Objective response rates were evaluated by change in targeted tumor size (response evaluation criteria in solid tumors [RECIST], modified to allow for measurement of targeted tumors),12,13 contrast-enhancement (European Association for the Study of the Liver [EASL] and American Association for the Study of Liver Diseases Guidelines),14,15 and apparent diffusion coefficient (ADC) value (motion of water molecules) using MRI scans performed 1 month after initial DEB-TACE treatment and 6 months after completion of the entire DEB-TACE cycle. For each patient, the treated lesion with the largest axial diameter was evaluated. For patients with a baseline α-fetoprotein >200 ng/mL, changes in α-fetoprotein levels were monitored for correlation with imaging response.
Secondary Endpoints
Feasibility, local progression-free survival, general progression-free survival, and overall survival were evaluated as secondary endpoints. Overall survival was calculated from death of any cause. General progression-free survival was defined as disease progression at any site or patient death. Local progression-free survival was defined as local progression of the treated lesion or patient death. Surviving patients (9/20) were censored at the last day of follow-up.
Statistical Analysis
A paired Student t test was used to compare tumor size, contrast enhancement, and ADC values before and after DEBTACE. Correlation between change in tumor size and contrast-enhancement was performed with linear regression analysis. Survival curves were computed by Kaplan-Meier method. Multivariate analysis of the influence of BCLC stage on overall survival was made by the Cox proportional hazards model. For all statistical analyses, SPSS statistical software (V17.0; SPSS Inc, Chicago, IL) was used. A P value <0.05 was considered statistically significant.
RESULTS
Patient Characteristics
Twenty patients with advanced unresectable HCC (median age 64 years) were enrolled between December 2005 and December 2007 in this prospective, single-arm, single-institution study (Table 1). Most patients (80%, 16/20) had cirrhosis, and 60% were classified as Barcelona Clinic Liver Cancer (BCLC) grade C (A/B/C: 6/2/12). The mean size of the treated lesion was 6.9 cm (range, 1.9–16.2 cm). The median number of DEB-TACE procedures per patient was 2 (range, 1–3). Median follow-up time was 14.5 months (mean 14.9 months; range, 1–39 months).
TABLE 1.
Patient Characteristics
| Variable | Value |
|---|---|
| No. patients | 20 |
| Mean age, years (range) | 64 (41-85) |
| Sex (M/F) | 12/8 |
| Hepatitis | |
| HBV | 5 |
| HCV | 8 |
| Child-Pugh class | |
| A | 15 |
| B | 5 |
| C | 0 |
| ECOG PS | |
| 0 | 9 |
| 1 | 10 |
| 2 | 1 |
| BCLC stage | |
| A | 6 |
| B | 2 |
| C* | 12 |
| Okuda | |
| I | 13 |
| II | 6 |
| III | 1 |
| Tumor burden (%) | |
| ≤50 | 18 |
| >50 | 2 |
| Mean tumor size in cm (range) | 6.9 (1.9-16.2) |
| Number of nodules (1/1 + satellites/2/multifocal) | 10/6/2/2 |
| Portal vein thrombosis (Y/N) | 4/16 |
| AFP (ng/mL) | 1215 |
| DEB-TACE treatments received† (range) | 2 (1-3) |
BCLC C: advanced stage: portal invasion, N1, Ml, PST 1-2.
DEB-TACE treatment: a single DEB-TACE procedure. DEB-TACE cycle: the number of DEB-TACE treatments needed to treat a targeted lesion.
HBV indicates hepatitis B virus; HCV, hepatitis C virus; ECOG PS, Eastern Cooperative Oncology Group performance status; and AFP, α-fetoprotein.
Safety
Toxicities were evaluated 1 and 3 months after initial treatment (n = 20, n = 18). Toxicity was generally modest and the frequency of grades 3 to 4 events in this study was low (Table 2). A post-TACE syndrome was observed only in 1 of the 20 patients, requiring supportive care. Liver function parameters were not significantly altered after most of the procedures. Leukocytopenia grade 3 was the most severe hematologic side effect (n = 1). Two patients died within 30 days of the procedure; the first died of multiorgan failure at an outside facility. This patient experienced diarrhea and dehydration after DEB-TACE 2, was treated at an outside facility with diuretics for persistent edema, but remained hypovolemic and hypotensive. At that time, she was placed on comfort measures. The other patient died of rapid progression of liver disease that had taken place between the baseline inclusion assessment and the first treatment. Neither death was attributed to the DEB-TACE procedure.
TABLE 2.
Reported Adverse Events in 19 Patients
| Toxicity Grade |
||||
|---|---|---|---|---|
| 30-d Postinitial DEB-TACE Treatment* (n = 20) |
3-mo (1-3 mo) Post-DEB-TACE Cycle† (n = 18) |
|||
| Description of Toxicities | I-II (%) | III (%) | I-II (%) | III (%) |
| Postembolization syndrome | 1 (6) | |||
| Fatigue | 13 (65) | 1 (5) | 7 (39) | |
| Pain-abdomen | 8 (40) | 7 (39) | ||
| Fever | 3 (15) | |||
| Nausea | 3 (15) | 1 (6) | ||
| Vomiting | 1 (5) | |||
| Anemia | 9 (45) | 5 (28) | ||
| Leukocytopenia | 4 (20) | 1 (5) | 2 (11) | |
| Thrombocytopenia | 3 (17) | |||
| ALT/AST elevation | 3 (15) | 5 (28) | 1 (6) | |
| Liver failure | 1 (5) | |||
| Anorexia | 7 (35) | 4 (22) | ||
| Weight loss | 6 (30) | 3 (17) | ||
| Alopecia | 1 (5) | |||
| Decreased libido | 1 (5) | 1 (6) | ||
| Diarrhea | 1 (5) | 1 (6) | ||
| Gastroenteritis | 1 (6) | |||
| Pancreatitis | 1 (6) | |||
| Cholecystitis | 1 (6) | |||
DEB-TACE treatment: a single DEB-TACE procedure.
DEB-TACE cycle: the number of DEB-TACE treatments needed to treat a targeted lesion.
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase.
Efficacy by MRI Parameters
One month after therapy, treated lesions (n = 20) had a mean decrease in size of 4%. No patients achieved a complete response (CR), and only 2 (10%) achieved partial response (PR) (Fig. 1) using modified RECIST guidelines. The other 18 (90%) lesions did not change significantly in size. On contrast-enhanced MRI, treated tumors demonstrated a mean decrease in contrast enhancement of 64% (P < 0.001). Six (30%) patients achieved CR, 6 (30%) PR, and 8 (40%) stable disease (SD) by EASL and American Association for the Study of Liver Diseases criteria. Using another surrogate marker of tumor necrosis, specifically the ADC value, as measured by functional diffusion-weighted MRI, there was an increase of 18% (P = 0.035) after treatment consistent with a more homogeneous distribution of water molecules throughout the tumor thereby indicative of tumor necrosis (Table 3).
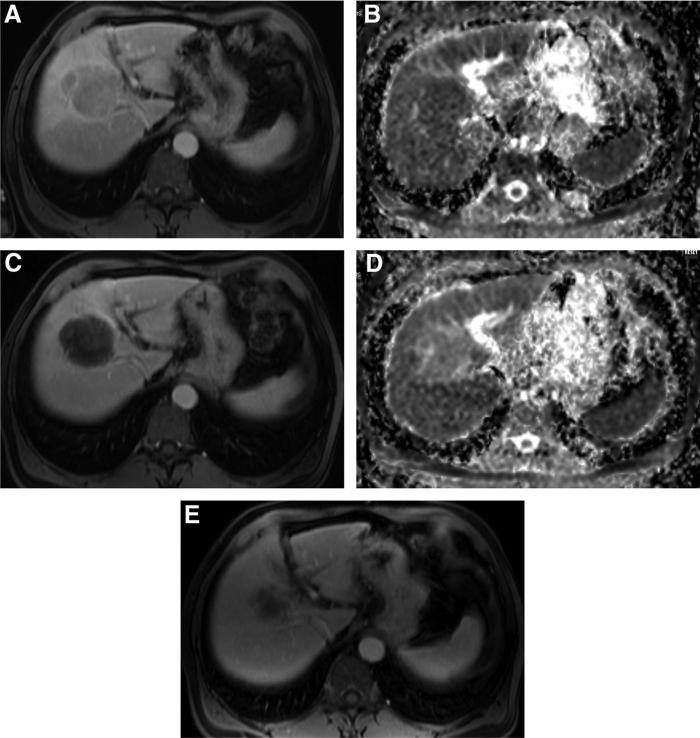
FIGURE 1.
Changes in size, enhancement, and ADC value after DEBTACE. (A) Baseline gadolinium-enhanced MRI of the abdomen of a 55-year-old man with hepatitis C virus and a hypervascular lesion of 6.0 cm in his right liver lobe. (B) Corresponding baseline diffusion-weighted image shows a lesion with an ADC value of 0.996 × 10−3 mm2/s. (C) Gadolinium-enhanced MRI 1 month after DEBTACE shows almost complete necrosis, associated with a small reduction in size (5.7 cm). (D) Corresponding diffusion-weighted after DEB-TACE shows a lesion with an ADC value of 1.785 × 10−3 mm2/s. (E) Six-month posttreatment MRI shows a significant smaller lesion (4.2 cm).
TABLE 3.
Changes in Tumor Size, Enhancement, and ADC Value After DEB-TACE (n = 20)
| Features | Before DEB-TACE | After DEB-TACE | Change (%) | P |
|---|---|---|---|---|
| Size of tumor ± SD (cm) | 6.9 ± 3.9 | 6.7 ± 4.1 | 4 | 0.1129 |
| Enhancement of tumor (%) | ||||
| Arterial | 86 ± 23 | 25 ± 32 | 61 | <0.0001 |
| Venous | 94 ± 13 | 30 ± 34 | 64 | <0.0001 |
| ADC (×10–3 mm2/s) | ||||
| Tumor | 1.48 ± 0.34 | 1.74 ± 0.41 | 18 | 0.035 |
| Liver | 1.28 ± 0.23 | 1.32 ± 0.28 | 3 | 0.218 |
| Spleen | 0.96 ± 0.15 | 0.96 ± .012 | 0 | 0.317 |
Follow-up imaging after 6 months showed a significant mean decrease in tumor size of 24% (P = 0.0024). This correlates with CR in 1 patient (5%), PR in 7 (35%), SD in 11 (55%), and progressive disease (PD) in 1 (5%) when using RECIST. Linear regression analysis demonstrated a significant but moderate correlation between this decrease in size on long-term follow-up imaging (6 months) and early change in contrast enhancement seen 1 month after DEB-TACE (R = 0.6). Conversely, no correlation (R = 0.2) was found between early size change and early change in contrast enhancement 1 month after DEB-TACE.
Histologic and Biochemical Confirmation of Imaging Findings
Four patients (20%), with unresectable liver tumors at enrollment, became surgical candidates after DEB-TACE therapy. Histo-logic examination of the successfully resected liver lesions showed extensive necrosis (80%–95%), which correlated well with the degree of necrosis seen on presurgical MRI scans (Table 4 and Fig. 2).
TABLE 4.
Histologic Findings Associated With Tumor Response
| Type of Surgery | Tumor Size (cm) on MRI Before DEB-TACE | Tumor Size (cm) on MRI After DEB-TACE | Percent Necrosis on MRI Before DEB-TACE* | Percent Necrosis on MRI After DEB-TACE* | Pathology Description |
|---|---|---|---|---|---|
| Right hepatectomy | 13.7 | 9.6 | 0 | >90 | 95% Necrotic |
| Partial hepatectomy segments V/VI | 12.8 | 11.7 | 10 | 85-90 | 80% Necrotic |
| Right hepatectomy | 7.2 | 5.9 | 0 | 85-90 | > 50% Necrotic |
| Hepatic debulking RFA of 3 smaller lesions | 2.5 | 1.7 | 0 | 100 | Not available. Lesion treated by DEB-TACE was ablated |
| Attempted liver transplant (1 d post-DEB-TACE) | 4.8 | 3.8 | 0 | 90 | Well-differentiated fragments of viable (25%) and necrotic (75%) HCC |
RFA indicates radiofrequency ablation.
Percent necrosis in MRI is based on lack of contrast enhancement.
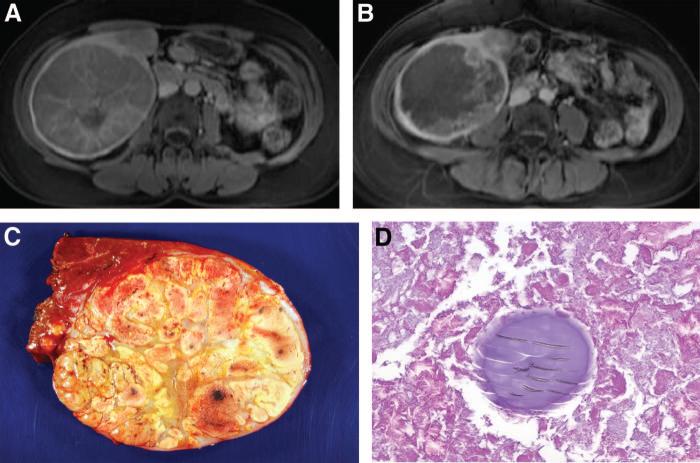
FIGURE 2.
Histologic findings associated with tumor response. (A) Pre-treatment MRI showing a hypervascular lesion in segments 5 to 6. (B) Posttreatment MRI illustrating 85% to 90% tumor necrosis. (C) Gross specimen after resection. (D) Histo-pathology from the tumor documenting extensive necrosis and no viable tumor cells. DEB is noted within the necrotic tumor.
Ten patients with a baseline α-fetoprotein >200 ng/mL, (mean 1873 ng/mL, range, 349–7831 ng/mL) responded with a 72% decrease of α-fetoprotein levels after DEB-TACE (mean 516, range, 7–2289), which correlated well with objective tumor response seen on imaging.
Feasibility
A median dose of 97 mg (range, 50–100 mg) of doxorubicin was successfully administered in 34 procedures according to the endpoint of the embolization procedure. A 3-French microcatheter was used in half of the procedures. Median duration of hospitalization was 1 day (range, 1–10 days), with minimal pain reported (2.7 on a scale of 1–10), and a fentanyl pump was used after 67% of the procedures (median dose of 140 μg of fentanyl over 24 hours). A 4.8-cm subcapsular tumor ruptured within 24 hours after treatment (n = 1). This was discovered at surgery when the patient was getting ready for a possible liver transplantation. The transplantation was aborted, but histopathologic analysis showed complete tumor necrosis. This patient is still alive 16 months after the procedure and more importantly has shown no evidence of tumor recurrence on follow-up imaging. Ischemic damage to the pancreas occurred in 1 patient and likely reflected backflow of microspheres during administration. Both patients were treated conservatively and recovered completely.
Patient Survival
Survival analysis calculated for all 20 patients revealed a median overall survival of 26 months, with overall survival rates at 1 and 2 years of 65% and 55%, respectively (Fig. 3). Patients with BCLC A or B had overall survival rates at 1 and 2 years of 100% and 70%, respectively, whereas patients with BCLC C had overall survival rates at 1 and 2 years of 42% and 33%, respectively. Multivariate analysis identified Okuda stage as the only independent risk factor affecting patient survival (relative risk 4.9, 95% confidence interval 1.8–36.8). All of the following risk factors showed no association: sex, race, age, tumor size, the presence of portal vein thrombosis, α-fetoprotein, Child–Pugh score, Eastern Cooperative Oncology Group performance status, BCLC stage, and number of DEB-TACE treatments.
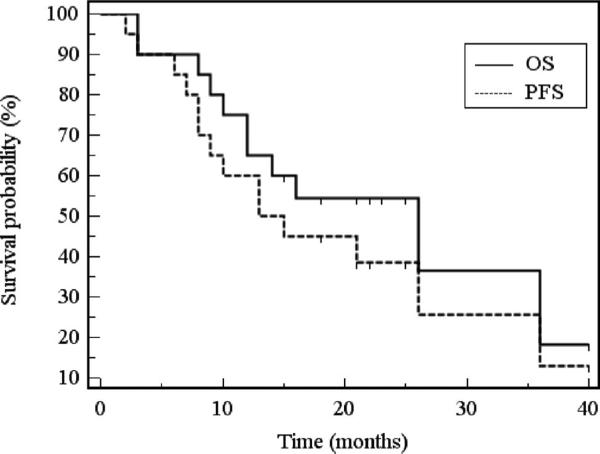
FIGURE 3.
Kaplan-Meier survival curve. Kaplan-Meier estimates of overall survival and progression-free survival (N = 20). OS indicates overall survival; PFS, progression-free survival.
Median progression-free survival was 13 months. General progression-free survival rates at 1 and 2 years were 60% and 39%, respectively (Fig. 3). Local progression-free survival of treated lesions was 21 months, with 1 and 2 year rates of 70% and 47%, respectively.
After completion of DEB-TACE, all patients were followed up with interval imaging. If there was a need for further treatment and protocol eligibility criteria was not met (n = 7), patients were treated with conventional TACE.
DISCUSSION
DEB-TACE is a new approach that enables the delivery of drug-loaded microspheres into the liver tumor in a precise, controlled, and sustained manner. The microspheres delivery principle exploits alterations in hemodynamic arterial flow to deliver the DEBs to the treated tumor via an intra-arterial catheter.16 Once the microspheres are lodged into the tumor, they slowly elute doxorubicin during a course of 14 days, allowing for a concentrated exposure in the tumor and increased cell death.5
Conventional TACE has a limited ability to deliver and maintain chemotherapy selectively in liver lesions and can potentially accelerate underlying liver disease.17 The improved delivery profile of DEBs decreases damage to the surrounding liver parenchyma and systemic toxicity. Therefore, treatment could be repeated more frequently and in a broader patient population. In our study, we were able to administer DEB-TACE treatment to patients with advanced disease (BCLC: C; 60%). These patients are often excluded from standard TACE treatment, according to BCLC schedule. Despite the advanced stage of our cohort, there were no serious adverse events related to the DEB-TACE treatment. The 2 patients who died within 30 days of the DEB-TACE procedure died because of liver failure (due to rapid progression between the screening baseline inclusion assessment and the DEB-TACE treatment) and multiorgan failure as a result of uncompensated hypovolemia, respectively. These 2 deaths were not attributed to the DEB-TACE itself. It is important to note that rates up to 50% for liver failure have been reported after conventional TACE.18 Given the advanced stage of our patients, we could have expected a higher rate of complications.
Postembolization syndrome occurs in up to 90% of patients after TACE and consists of varying degrees of abdominal pain, nausea, vomiting, fever, and elevation of liver enzymes.19–21 Although this syndrome is typically transient and can be managed with supportive care, it is a major complication of TACE, significantly prolongs hospitalization, and increases patient morbidity. Our results showed that DEB-TACE was extremely well tolerated, and postembolization syndrome was observed in only 1 patient. Further- more, in our experience, the majority of the patients undergoing standard TACE require large doses of intravenous narcotics for relief. After DEB-TACE, the mean highest pain score reported in the 24 hours postprocedure was 2.7 on a scale of 1 to 10. These findings support the results from other phase I/II studies with DEB-TACE where a significantly lower rate of postembolization syndrome was found when compared with conventional TACE.7–9
Besides the advantage of reduced toxicity, DEB-TACE also results in high objective tumor response rates. Traditional cross-sectional imaging techniques rely on changes in tumor size to estimate tumor response after treatment (RECIST). Using modified RECIST (measurement of targeted tumors), none of the patients in our study achieved CR, and only 10% achieved PR 1 month after DEB-TACE. Given the fact that extensive tumor necrosis is not typically accompanied by a reduction in tumor size, we also used changes in contrast-enhancement (EASL criteria) and in tumor ADC values (reflecting cellular changes) to estimate tumor response.22 By using these criteria, we showed CR in 30% of the patients and PR in another 30%. There was no correlation (R = 0.2) between size change and change in contrast enhancement on follow-up imaging (1 month after DEB-TACE). Lack of correlation might reflect that the tumor may have experienced extensive necrosis, but no reduction in diameter. More interestingly, a correlation was seen between decrease in tumor size as measured at the 6-month follow-up imaging time point after DEB-TACE and decrease in enhancement on the early MR scans (R = 0.6). Altogether, our data indicate that DEB-TACE seems to have a significant antitumoral effect, best evaluated by early (1 month) decrease in contrast enhancement and followed by a late (6 months) decrease in size.
Histologic examination remains the gold standard to assess tumor response. However, obtaining pathologic confirmation of tumor necrosis in patients with unresectable HCC is challenging, especially in the setting of background hepatic failure and coagulopathy. In our study, 4 patients (20%) with unresectable liver tumors at enrollment became surgical candidates after DEB-TACE therapy and underwent successful liver resection. Histologic examination showed 80% to 95% necrosis, which correlated strongly with early decrease in contrast enhancement (85%–90%), as seen on the MRI. The subset of patients with a baseline α-fetoprotein >200 ng/mL (n = 10), (mean 1873 ng/mL; range, 349–7831) responded with a 72% decrease of α-fetoprotein levels after DEB-TACE (mean 516; range, 7–2289), indicating favorable response.
Treatment was performed successfully in 34 procedures, and a 3-French microcatheter was used in 50% of these procedures to deliver the DEBs superselectively into the tumor bed. LC Beads (100 to 300 μm) were used in most of the procedures to deliver the microspheres more deeply within the tumor bed, allowing for a shorter distance the drug would have to travel to the cancer cells.23 In contrast to lipiodol used during conventional TACE, DEBs cannot be visualized directly, and the flow must be assessed with alternating infusion of contrast. A known risk during TACE is administration of the therapeutic agent to nontarget organs. In our study, 1 patient experienced an episode of pancreatitis, which could be attributed to backflow of microspheres. In 1 patient, a 4.8-cm subcapsular tumor ruptured within 24 hours after treatment. Tumor rupture after TACE has been reported with an incidence of <3% in western countries and up to 14.5% in Hong Kong24 and occurs usually in large subcapsular tumors, as was the case in our study. Therefore, care should be exercised in using DEB-TACE in tumors with exophytic growth or subcapsular location.
In our study, overall survival rates at 1 and 2 years were 65% and 55%, respectively, which is similar to those previously reported for patients treated with TACE (60%–88% at 1 year and 30%–60% at 2 years)25–27 and significantly better than survival probabilities reported for patients receiving only symptomatic control (32%–63% at 1 year and 11%–27% at 2 years).3 Previous studies with DEBTACE, however, did show 1- and 2-year survival rates of 92.5% and 88.9%, respectively.9 The slightly lower survival rates in our study might be related to patient sampling since our study enrolled patients with advanced stage HCC (60% BCLC stage C), when compared with prior studies that included patients with early HCC. The 1- and 2-year survival rate differed between patients with BCLC A or B (100% at 1 year and 70% at 2 years) and patients with BCLC C (42% at 1 year and 33% at 2 years). We also found that overall progression-free survival rates at 1 and 2 years are 60% and 39%, respectively. Progression-free survival for the treated lesions, however, showed 1 and 2 years rates of 70% and 47%, respectively. This demonstrates the strong locoregional effect of DEB-TACE treatment.
This study was designed to test efficacy and safety in a pilot group of patients. A limitation of the study is that it does not offer a sample size calculation designed to test a hypothesis.
In conclusion, this pilot study provides clear evidence of the efficacy and safety of DEB-TACE in obtaining local control in a heterogeneous sample of patients with unresectable HCC. The use of intra-arterially delivered DEB represents a new treatment that has the potential to become the standard of care for unresectable intermediate and advanced stage HCC. Nevertheless, randomized controlled trials comparing DEB-TACE to conventional TACE treatment are warranted to further prove the clinical benefit of this approach, examine adequate patient selection, and validate new imaging techniques to measure response to treatment.
Acknowledgments
Supported by Biocompatibles, Inc., Farnham Surrey, GU9 8QL, United Kingdom
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Ribes J, Díaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(5 suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Real MI, Montaña X, et al. Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 4.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 5.Hong K, Khwaja A, Liapi E, et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 6.Lewis AL, Gonzalez MV, Leppard SW, et al. Doxorubicin eluting beads–1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18:1691–1699. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- 7.Malagari K, Alexopoulou E, Chatzimichail K, et al. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging. 2008;33:512–519. doi: 10.1007/s00261-007-9334-x. [DOI] [PubMed] [Google Scholar]
- 8.Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–1108. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Geschwind JF, Ramsey DE, Cleffken B, et al. Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Intervent Radiol. 2003;26:111–117. doi: 10.1007/s00270-002-2524-6. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchida Y, Therasse P. Response evaluation criteria in solid tumors (RECIST): new guidelines. Med Pediatr Oncol. 2001;37:1–3. doi: 10.1002/mpo.1154. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Bruix J, Sherman M, Llovet JM, et al. EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 16.Brown DB, Geschwind JF, Soulen MC, et al. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2006;17(2 Pt 1):217–223. doi: 10.1097/01.RVI.0000196277.76812.A3. [DOI] [PubMed] [Google Scholar]
- 17.Poon RT, Fan ST, Lo CM, et al. Difference in tumor invasiveness in cirrhotic patients with hepatocellular carcinoma fulfilling the Milan criteria treated by resection and transplantation: impact on long-term survival. Ann Surg. 2007;245:51–58. doi: 10.1097/01.sla.0000225255.01668.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bismuth H, Morino M, Sherlock D, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992;163:387–394. doi: 10.1016/0002-9610(92)90039-t. [DOI] [PubMed] [Google Scholar]
- 19.Chung JW, Park JH, Han JK, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology. 1996;198:33–40. doi: 10.1148/radiology.198.1.8539401. [DOI] [PubMed] [Google Scholar]
- 20.Leung DA, Goin JE, Sickles C, et al. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–326. doi: 10.1016/s1051-0443(07)61911-3. [DOI] [PubMed] [Google Scholar]
- 21.Patel NH, Hahn D, Rapp S, et al. Hepatic artery embolization: factors predisposing to postembolization pain and nausea. J Vasc Interv Radiol. 2000;11:453–460. doi: 10.1016/s1051-0443(07)61377-3. [DOI] [PubMed] [Google Scholar]
- 22.Geschwind JF, Artemov D, Abraham S, et al. Chemoembolization of liver tumor in a rabbit model: assessment of tumor cell death with diffusion-weighted MR imaging and histologic analysis. J Vasc Interv Radiol. 2000;11:1245–1255. doi: 10.1016/s1051-0443(07)61299-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee KH, Liapi E, Vossen JA, et al. Distribution of iron oxide-containing Embosphere particles after transcatheter arterial embolization in an animal model of liver cancer: evaluation with MR imaging and implication for therapy. J Vasc Interv Radiol. 2008;19:1490–1496. doi: 10.1016/j.jvir.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battula N, Srinivasan P, Madanur M, et al. Ruptured hepatocellular carcinoma following chemoembolization: a western experience. Hepatobiliary Pancreat Dis Int. 2007;6:49–51. [PubMed] [Google Scholar]
- 25.Geschwind JF, Ramsey DE, Choti MA, et al. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol. 2003;26:344–349. doi: 10.1097/01.COC.0000020588.20717.BB. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda M, Okada S, Yamamoto S, et al. Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol. 2002;32:455–460. doi: 10.1093/jjco/hyf097. [DOI] [PubMed] [Google Scholar]
- 27.Llado L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]