Abstract
Flavonoids and chalcones are natural plant derived compounds with inherent therapeutic value for a range of human pathologies. In this study, a series of 24 substituted chalcones and flavones were synthesized and subsequently screened for anti-inflammatory effects on lipopolysaccharide (1 µg/ml)-activated BV-2 microglial cells by assessing initial production/release of nitric oxide (NO). The data obtained eliminate the majority of compounds as weak or non-effective, whereas 2′-hydroxy-3,4,5,3′,4′-pentamethoxychalcone (1) and 2′-hydroxy-3,4,5-trimethoxychalcone (2) were potent, having an IC50 of 1.10 and 2.26 µM, respectively; with greater potency than L-N6-(1-iminoethyl)lysine selective iNOS inhibitor (IC50 = 3.1 µM) but less than steroidal dexamethasone (IC50 < 200 nM). The most potent compound (chalcone 1) attenuated NO parallel to reducing iNOS protein expression, events also corresponding to reduction of IL-1α, IL-10 and IL-6 pro-inflammatory cytokines. These findings suggest that the presence of electron donating groups OH and OCH3 on both A and B rings of synthetic compounds correlate to stronger anti-inflammatory potency.
Keywords: Flavonoids, Chalcones, Cytokines, Nitric oxide, Microglial cells
Introduction
Natural flavonoids and chalcones are abundantly distributed throughout fruits, herbs/spices and vegetables, often responsible for therapeutic efficacy of plants in the treatment or prevention of cancer, bacterial infection (Dimmock et al., 1999), asthma (Tanaka and Takahashi, 2013; Toledo et al., 2013), acute (Dou et al., 2013)/chronic low-grade inflammation (Siriwardhana et al., 2013), various inflammatory diseases (Nam et al., 2013; Tao et al., 2013) and infections of diverse pathogenic origin. (Yadav et al., 2011) There are thousands of reported natural food-based polyphenolic compounds within the literature, demonstrated to exert a plethora of anti-inflammatory properties in diverse biological models, consistently known to suppress NF-kappaB, p38MAPK, ERK1/2, JAK/STAT, PI3K-Akt, and subsequently attenuate pro-inflammatory molecules such as NO (Park and Song, 2013; Senggunprai et al., 2014; Wan et al., 2014) oxygen radicals or diverse cytokines (Zhang et al., 2013; Lin et al., 2014) (e.g., tumor necrosis factor-alpha, IL-1beta and IL-6) (Byun et al., 2013). The inherent capacity of natural polyphenolic compounds to down-regulate inflammatory processes could have long-term therapeutic value against neurodegenerative conditions associated with aging or traumatic brain injury. (Stough et al., 2012; Dajas et al., 2013; Raza et al., 2013; Scheff et al., 2013) Central nervous system (CNS) degenerative conditions involve reactive glial cells (astrocytes and microglia) (Spencer et al., 2012) believed to exacerbate pathology of Parkinson’s disease (Phani et al., 2012; Pradhan and Andreasson, 2013), stroke (Monif et al., 2010; Johnson et al., 2013), amyotrophic lateral sclerosis (Corcia et al., 2012) or hypoxic-ischemic incidents (Jellema et al., 2013; Shrivastava et al., 2013).
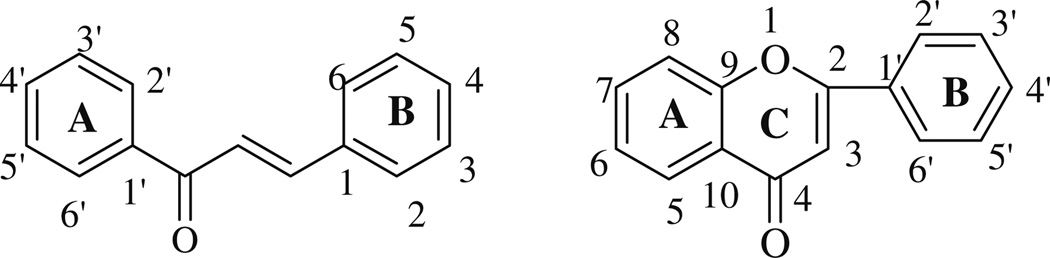
The distinction between structure and function of anti-inflammatory natural molecules may have to do with substitutions on the A and B rings of chalcones and flavones which can result in compounds with different biological activities. The general structure and numbering of flavones and chalcones are shown in Fig. 1.
Fig. 1.
The general structure of chalcones and flavonoids
In this study, we evaluated the efficacy of novel synthetic flavonoids and chalcones to inhibit pro-inflammatory processes in activated microglia cells exposed to bacterial lipopolysaccharide (LPS), a model commonly known to induce NF-kappaB transcriptional activation, iNOS, COX-2, TNF alpha, chemokine (C–C motif) ligand 2 (Hirai et al., 2007; Furusawa et al., 2009) and release of nitric oxide (NO) (Batool et al., 2013; Wong, 2013).
Methods and materials
Hanks balanced salt solution, (4-(2-hydroxyethyl)-1-piper-azineethanesulfonic acid) (HEPES), ethanol, dimethyl sulfoxide (DMSO), 96-well plates, general reagents, solvents, chemicals and supplies were all purchased from Sigma-Aldrich Co. (St. Louis, MO) and VWR International (Radnor, PA). Imaging probes were supplied by Life Technologies (Grand Island, NY). Multi-Analyte sandwich-based ELISAs were purchased from SABiosciences, QIAGEN Inc, (Valencia, CA).
Chemistry
The synthesis of the substituted chalcone analogs was carried out via Claisen–Schmidt condensation, and flavone analogs were synthesized using a three-step Baker-Venk-ataraman rearrangement. Synthesis and characterization of these compounds were reported in our previous papers (Mateeva et al., 2002; Mills and Redda, 2006).
Cell culture
BV-2 microglial cells were cultured in high glucose (4,500 mg/ml) DMEM containing phenol red, 5 % FBS, 4 mM l-glutamine, and penicillin/streptomycin (100 U/0.1 mg/ml) (Blasi et al., 1990). Culture conditions were maintained (37 °C in 5 % CO2/atmosphere) and every 2–5 days, the media was replaced and cells were subcultured. For experiments, plating media consisted of DMEM (minus phenol red), 5 % FBS, penicillin/streptomycin (100 U/0.1 mg/ml), and 3 mM l-glutamine. All experimental compounds were dissolved in DMSO (20 mg/ml) and dilutions were prepared in sterile HBSS + 5 mM HEPES, adjusted to a pH of 7.4 with a final experimental working concentration of DMSO less than 0.5 % v/v. Activation of BV-2 cells was established using 1 µg/ml of LPS from E. coli O111:B4 for 24 h in experimental media, maintaining cell density at 0.5 × 106 cells/ml.
Cell viability
Cell viability was quantified using resazurin (Alamar Blue) indicator dye (Evans et al., 2001). A working solution of resazurin was prepared in sterile PBS—phenol red (0.5 mg/ml) and added (15 % v/v) to each sample. Samples were returned to the incubator for 6–8 h, and reduction of the dye by viable cells (to resorufin, a fluorescent compound) was quantitatively analyzed using a microplate fluorometer, Model 7620, version 5.02 (Cambridge Technologies Inc, Watertown, MA) with settings at (550/580), (excitation/emission) wavelengths. The data were expressed as percent of live untreated controls.
Nitrite (NO2−) determination
Quantification of nitric oxide (NO) was determined using the Griess reagent (Ko et al., 2008). The Griess reagent was prepared by mixing an equal volume of 1.0 % sulfanilamide in 0.5 N HCl and 0.1 % N-(1-naphthyl)-ethylenediamine in deionized water. The Griess reagent was added directly to the cell supernatant suspension and incubated under reduced light at room temperature for 10 min. A standard curve for NO2− was generated from dilutions of sodium nitrite (NaNO2) (1–100 µM) prepared in plating medium. Controls and blanks were run simultaneously, and subtracted from the final value to eliminate interference. Samples were analyzed at 550 nm on a UV microplate spectrophotometer (model 7600, version 5.02, Cambridge Technologies Inc.).
Inducible NOS protein expression
Inducible NOS protein expression was determined by immunohistochemistry. Cells were fixed in 4 % paraformaldehyde/permeabilized in 0.1 % triton-X100 in phosphate-buffered saline (PBS) and incubated with anti-iNOS (Schnell et al., 2012), specifically N-Terminal antibody produced in rabbit (Sigma-Aldrich, St. Louis, MO) for 2 h at 37 °C. Samples were washed with PBS and subsequently incubated with anti-rabbit Alexa Fluor® 488 conjugate for 2 h at 37 °C. Samples were counterstained with propidium iodide and photographically collected using a Nikon TE 900 inverted microscope, Nikon PCM 2000 confocal microscope, and data was acquired with C-imaging systems confocal PCI-Simple software (Compix Inc. Cranberry Township, PA, USA).
Inflammatory cytokines multi-analyte ELISArray
Murine inflammatory cytokines were quantified using a Multi-Analyte sandwich-based ELISArray MEM-004A (SABiosciences, QIAGEN Inc, Valencia, CA) with capacity to simultaneously evaluate 12 pro-inflammatory cytokines: IL1α, IL1B, IL2, IL4, IL6, IL10, IL12, IL17α, IFNγ, TNFα, G-CSF, and GM-CSF. Very briefly, cell supernatants were removed after 24 h and stored at −20 °C. Supernatant from each sample was thawed and incubated with capture antibodies for 2 h at RT along with a mixed antigen standard cocktail. After washing, samples were then re-incubated with biotinylated detection antibodies for 1 h at RT. After a 2nd washing process, samples were incubated with avidin–horseradish peroxidase conjugate for 30 min, re-washed, and incubated with a development reagent. Experiments were performed according to the manufacturers guidelines and quantified using a Spectra 190-MAX UV spectrophotometric detector (Molecular devices, Sunnydale, CA, USA) with settings at 450 nm (with corrective wavelength set to 570 nm).
IL-1a and IL-6
OmniKine™ Murine IL-6 (Catalog # OK-0187) and Murine IL-1a (Catalog # OK-0181) quantitative “Sand-wich” Enzyme Linked Immunosorbent Assay (ELISA) (Assay Biotechnology Company Inc. Sunnyvale, CA) were used for detection and quantification of IL-6 and IL1 α produced/released within the range of 62–4,000 pg/ml. Experiments were performed according to the manufacturers guidelines and quantified using a Spectra 190-MAX UV spectrophotometric detector (Molecular devices, Sunnydale, CA, USA) with settings at 450 nm (with corrective wavelength set to 570 nm).
Data analysis
Statistical analysis was performed using Graph Pad Prism (version 3.0; Graph Pad Software Inc. San Diego, CA, USA) with significance of difference between the groups assessed using a one-way ANOVA, followed by Tukey post hoc means comparison test or Student’s t test. IC50s were determined by regression analysis using Origin Software (OriginLab, Northampton, MA).
Results
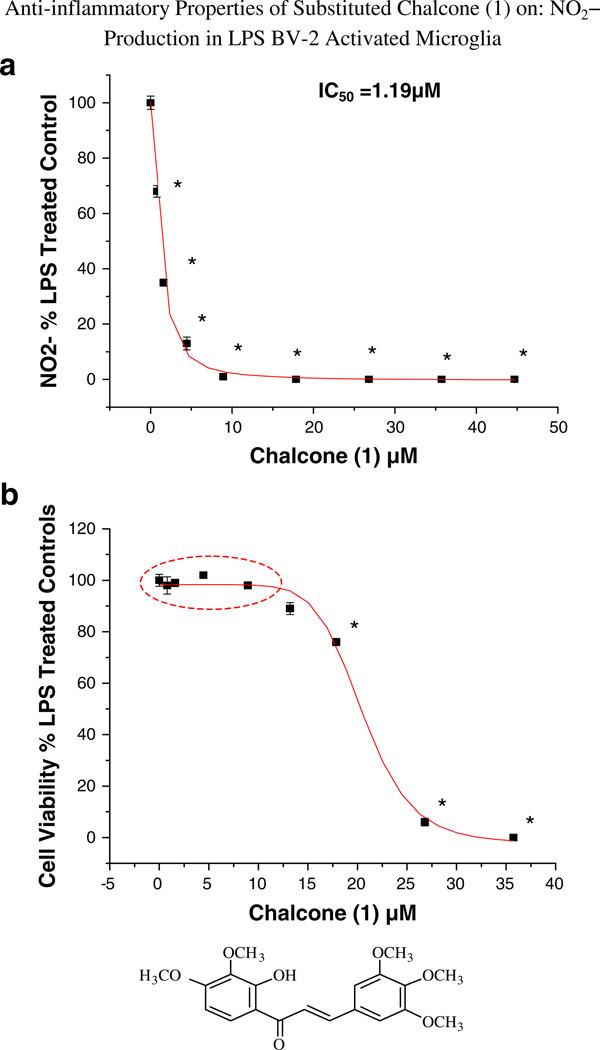
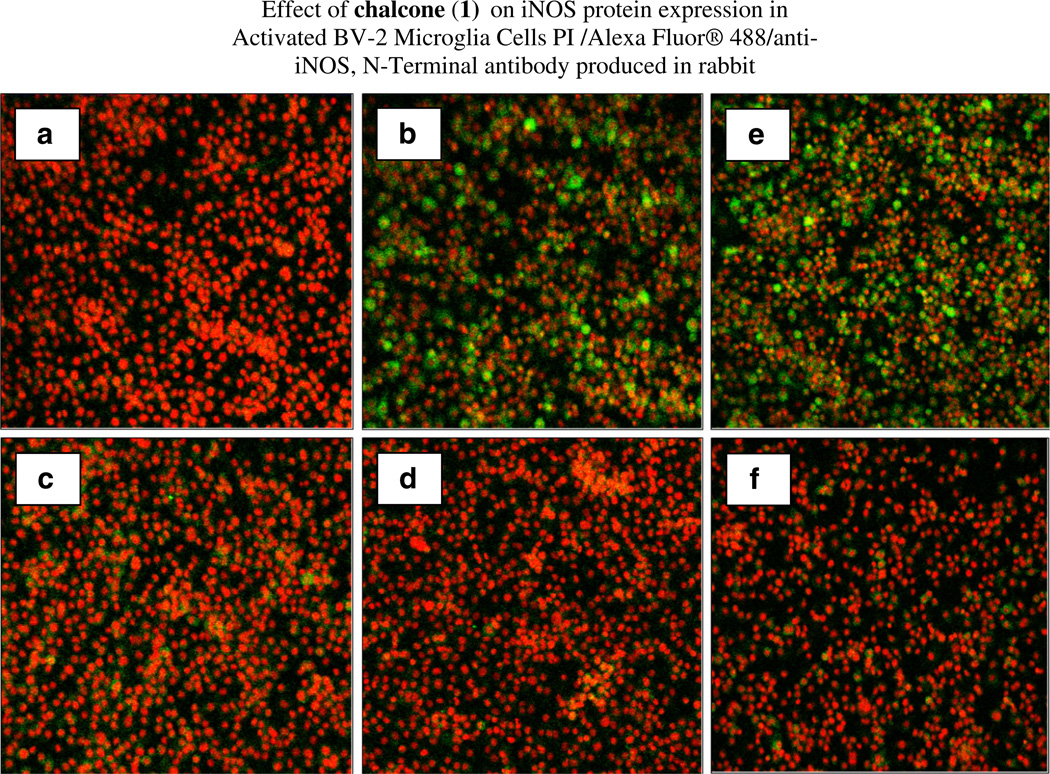
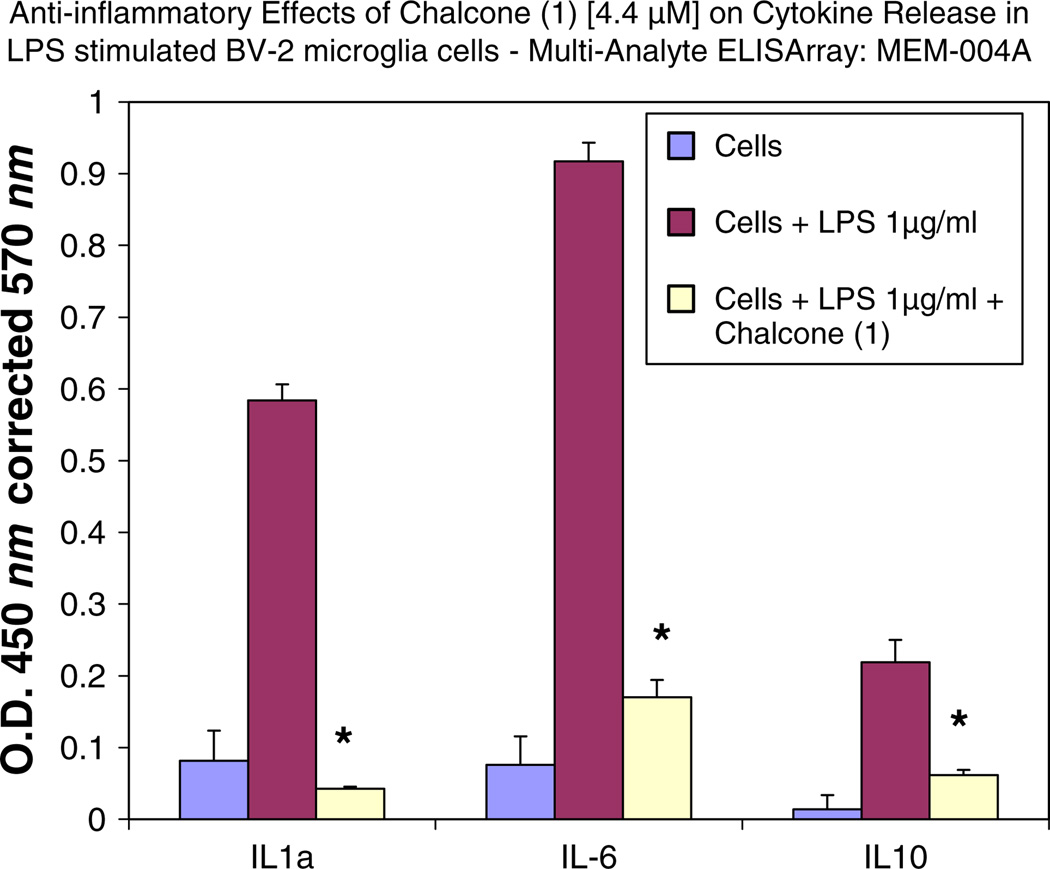
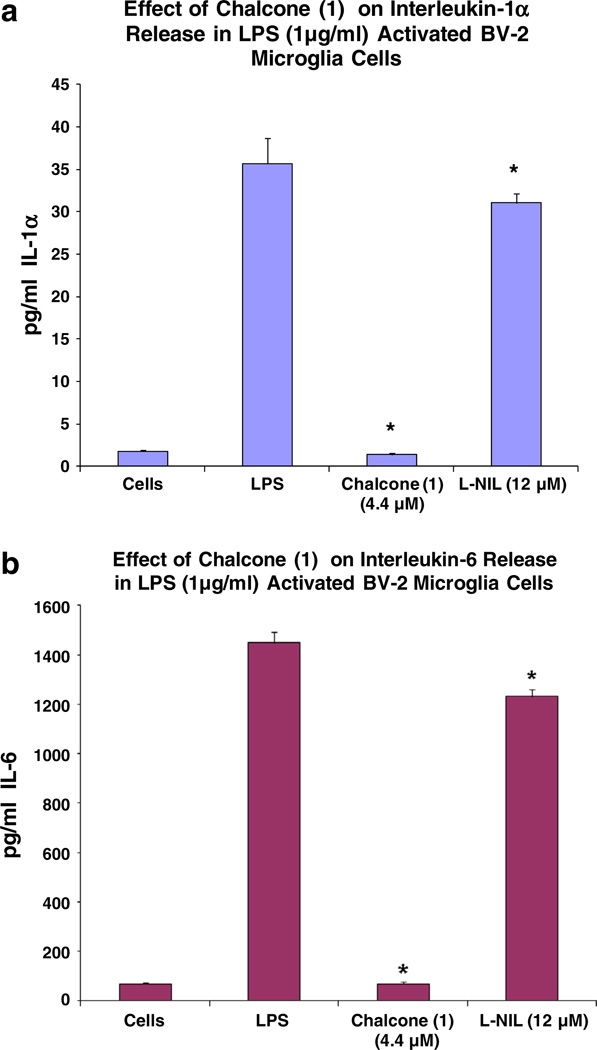
All substituted chalcones and flavones were screened for dose-dependent iNOS inhibition at concentrations at or equal to 40 µM evidenced by a reduction in NO in LPS (1 µg/ml)-treated BV-2 cells (Table 1). IC50 values were calculated based on regression analysis for a minimum of 5 concentrations (n = 4), in a non-toxic range, with little to no effects observed for majority of compounds. Among 24 synthesized chalcones and flavones, two compounds including 2′-hydroxy-3,4,5,3′,4′-pentamethoxychalcone (1), 2′-hydroxy-3,4,5-trimethoxychalcone (2), showed the most potent inhibitory effect on NO production with an IC50 values of 1.1, 2.2 µM, respectively, at concentrations below the threshold for cell toxicity (Fig. 2a, b), also corresponding to marked loss of iNOS (Fig. 3a – d). Moreover, 2′-hydroxy-3,4,5,3′,4′-pentamethoxychalcone Chalcone (1), attenuated LPS-activated production and release of IL-1 α, IL-6, and IL-10 in activated BV-2 cell determined by ELISArray profiling (Fig. 4). Further corroboration of anti-inflammatory properties were acquired for chalcone (1) on IL-α and IL-6 (Fig. 5a, b), respectively.
Table 1.
Evaluation of synthetic chalcones and flavones on NO inhibition in BV-2 LPS-stimulated microglial cells
| Compound | LPS-NO2 IC50 (µM) | LC50 (µM) Tox Ctrl | |
|---|---|---|---|
| Ctrl 1 | Dexamethasone | <200 nM | >50 µm |
| Ctrl 2 | L-NIL-L-N6-(1-iminoethyl)lysine | 3.1 | >50 µm |
| (1) | 1.1 | 18.3 | |
| Lead compound | 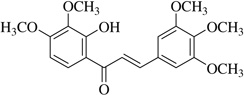 |
||
| (2) | 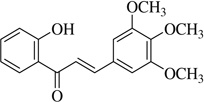 |
2.2 | >40 |
| (3) | 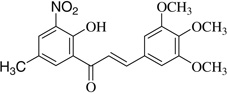 |
6.5 | 34.8 |
| (4) | 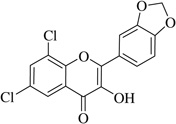 |
8.6 | >40 |
| (5) | 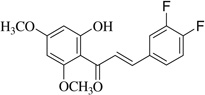 |
10.7 | >40 |
| (6) | 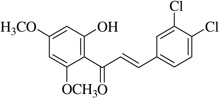 |
11.0 | >40 |
| (7) | 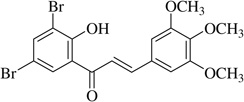 |
14.4 | 37 |
| (8) | 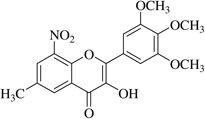 |
20.2 | >40 |
| (9) | 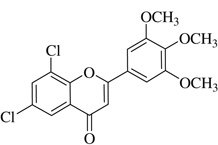 |
22.0 | >40 |
| (10) | 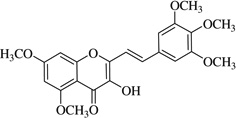 |
24.3 | >40 |
| (11)* | 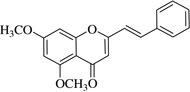 |
>40 | >40 |
| (12)* | 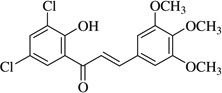 |
>40 | >40 |
| (13)* | 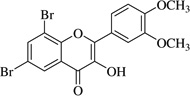 |
>40 | >40 |
| (14)* | 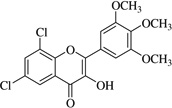 |
>40 | >40 |
| (15)* | 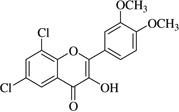 |
>40 | >40 |
| (16)* | 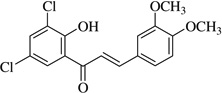 |
>40 | >40 |
| (17)* | 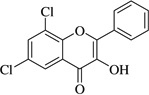 |
>40 | >40 |
| (18)* | 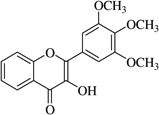 |
>40 | >40 |
| (19)* | 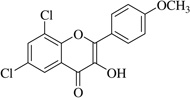 |
>40 | >40 |
| (20)* | 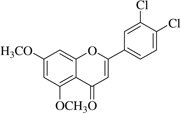 |
>40 | >40 |
| (21)* | 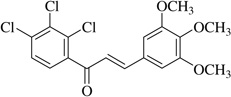 |
>40 | >40 |
| (22)* | 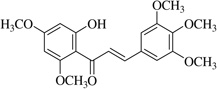 |
>40 | >40 |
| (23)* | 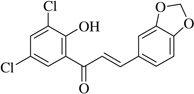 |
>40 | >40 |
| (24)* | 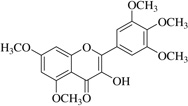 |
>40 | >40 |
IC50s were calculated based on regression analysis for the data obtained using mean ± SEM, n = 4 of a minimum of 5 concentrations below 40 µM
No anti-inflammatory effect
Fig. 2.
a and b The effect of chalcone (1) on NO2− production by LPS-activated BV-2 microglial cells. The data represent NO2− as % control and are presented as the mean ± SEM, n = 4. Statistical differences from the control were evaluated by a one-way ANOVA, followed by a Tukey post hoc test, * p < 0.05
Fig. 3.
The effect of chalcone (1) on reduction of iNOS protein expression in LPS-activated BV-2 microglial cells. Cells were fixed, permeabilized, and stained with Alexa Fluor® 488/anti-iNOS, N-Terminal antibody produced in rabbit and counterstained with PI. a Untreated cells, b LPS, c LPS + 4.4 µM chalcone (1), d LPS + 8.9 µM chalcone (1), dexamethasone control set, and e LPS, f LPS + dexamethasone 1 µM
Fig. 4.
Effect of chalcone (1) on cytokine release in LPS-stimulated BV-2 microglia cells by Multi-Analyte ELISArray: MEM-004A— evaluated for: IL1A, IL1B, IL2, IL4, IL6, IL10, IL12, IL17A, IFNγ, TNFα, G-CSF, and GM-CSF. The data represent cytokines released as % LPS controls for each subset, and are presented as the mean ± STD, n = 2. Statistical differences from the LPS control were evaluated by a student’s t test, * p < 0.05
Fig. 5.
a Effect of chalcone (1) (4.4 µM) on IL-1α release in LPS-stimulated BV-2 microglia cells. The data represent IL-1α released in pg/ml and presented as the mean ± SEM, n = 6. Statistical differences between the treatment and LPS controls were evaluated by a student’s t test, * p < 0.05. b Effect of chalcone (1) (4.4 µM) on IL-6 release in LPS-stimulated BV-2 microglia cells. The data represent IL-6 released as pg/ml and presented as the Mean ± SEM, n = 6. Statistical differences for the treatment groups from the LPS control were evaluated by a student’s t test, * p < 0.05
Discussion
In this study, a preliminary screening of diverse substituted synthetic chalcones and flavones were evaluated for structure/functional anti-inflammatory effects in LPS-activated microglial cells. The elucidation of novel synthetic flavonoids of superior potency could be used to attenuate central nervous system inflammation (Phani et al., 2012; Batool et al., 2013; Pradhan and Andreasson, 2013) an otherwise significant contributor to chronic neurological/ cognitive decline associated with degenerative diseases such as Parkinson’s Disease. (Lopategui et al., 2012).
In this work, an initial screening elucidated that 2′-hydroxy-3,4,5,3′,4′-pentamethoxychalcone (1) exemplified a basic structure with the most potent anti-inflammatory effect, evidenced by complete inhibition of NO, reduction of iNOS protein, and the reduction of various pro-inflammatory cytokines. It appears that a presence of electron donating groups viz., OH, and OCH3, in compound (1) and (2) exhibited stronger activity than that of electron withdrawal groups. Furthermore, the chalcones (3), (5), (6), (7), (10), (11) and flavones (4), (8), and (9) also exhibited NO inhibition to lesser extent (Table 1). The opposite situation was found when the electron withdrawing groups were present on the A ring of chalcone and flavones with the IC50 values being greater than 40 µM.
With respect to different substitutions on A- and B-ring, substitution at positions 2′ or 4′ and 3, 4, and 5 positions played an important role in the different IC50 trends against the NO production. As shown in Table 1, the presence of a strong electron withdrawing group like F, Cl in chalcones (5), and (6), the NO inhibition was decreased significantly. In case of flavones, when the electron donating groups are present on B-ring as in compounds (4), (8), and (9) the inhibition was decreased.
Chalcones (synthetic or natural) with capacity to reduce NO or attenuate iNOS expression are known to prevent neurological injury in experimental models (Li et al., 2012; Ojha et al., 2012) and could reduce otherwise elevated levels of neurotoxic molecules including peroxynitrite ONOO(−) directly responsible for invoking neurotoxicity (Kouti et al., 2013). The capacity of specific flavonoids to reduce both NO and superoxide can lessen nitrosative stress-induced protein aggregates and prevent formation of peroxynitrite (Chen et al., 2012; Drechsel et al., 2012; Mandawad et al., 2013) which initiates faulty autophagy, a failure of required degradative processes and greater likelihood of accumulated neurotoxic aggregate-prone proteins (i.e., alpha-synuclein) (Sarkar et al., 2011; Liu et al., 2014).
In brief, the data presented in this work show that novel synthetic 2′-hydroxy-3,4,5,3′,4′-pentamethoxychalcone (1) exerts general anti-inflammatory properties with capability to inhibit protein expression of iNOS, in excess of strength to a selective iNOS inhibitor; L-NIL but less than dexamethasone. Future research will be required to evaluate efficacy in vivo, and establish synthetic derivatives of greater potency from this basic structure.
Acknowledgments
This research was supported by grants received from the National Institute on Minority Health and Health Disparities off the National Institutes of Health through Grant No. G12MD007582-28 and Grant No. P20MD006738. And, gratitude is owed to Dr. Elisabetta Blasi and Dr. Equar Taka for kindly providing the BV-2 cell microglial cell line.
Abbreviations
- CNS
Central nervous system
- COX-2
Cyclooxygenase 2
- CSF
Colony stimulating factor
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethylsulfoxide
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- L-NIL
L-N6-(1-iminoethyl)lysine
- LPS
Lipopolysaccharide
- NO
Nitric oxide
- PBS
Phosphate-buffered saline
Contributor Information
Nelly Mateeva, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL 32307, USA.
Madhavi Gangapuram, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL 32307, USA.
Elizabeth Mazzio, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL 32307, USA.
Suresh Eyunni, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL 32307, USA.
Karam F. A. Soliman, College of Pharmacy and Pharmaceutical Sciences, Florida A&M University, Tallahassee, FL 32307, USA
Kinfe K. Redda, Division of Research, Florida A&M University, 410 Foote-Hilyer Administration Center, Tallahassee, FL 32307, USA kinfe.redda@famu.edu
References
- Batool S, Nawaz MS, et al. Molecular interaction study of N1-p-fluorobenzyl-cymserine with TNF-alpha, p38 kinase and JNK kinase. Antiinflamm Antiallergy Agents Med Chem. 2013;12(2):129–135. doi: 10.2174/1871523011312020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, et al. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Byun EB, Yang MS, et al. Quercetin negatively regulates TLR4 signaling induced by lipopolysaccharide through Tollip expression. Biochem Biophys Res Commun. 2013;431(4):698–705. doi: 10.1016/j.bbrc.2013.01.056. [DOI] [PubMed] [Google Scholar]
- Chen X, Guan T, et al. SOD1 aggregation in astrocytes following ischemia/reperfusion injury: a role of NO-mediated S-nitrosylation of protein disulfide isomerase (PDI) J Neuroinflammation. 2012;9:237. doi: 10.1186/1742-2094-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcia P, Tauber C, et al. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS ONE. 2012;7(12):e52941. doi: 10.1371/journal.pone.0052941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas F, Andres AC, et al. Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features. Cent Nerv Syst Agents Med Chem. 2013;13(1):30–35. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- Dimmock JR, Elias DW, et al. Bioactivities of chalcones. Curr Med Chem. 1999;6(12):1125–1149. [PubMed] [Google Scholar]
- Dou W, Zhang J, et al. Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-kappaB signaling pathway. J Pharmacol Exp Ther. 2013;345(3):473–482. doi: 10.1124/jpet.112.201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel DA, Estevez AG, et al. Nitric oxide-mediated oxidative damage and the progressive demise of motor neurons in ALS. Neurotox Res. 2012;22(4):251–264. doi: 10.1007/s12640-012-9322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Casartelli A, et al. Development of a high throughput in vitro toxicity screen predictive of high acute in vivo toxic potential. Toxicol In Vitro. 2001;15(4–5):579–584. doi: 10.1016/s0887-2333(01)00064-9. [DOI] [PubMed] [Google Scholar]
- Furusawa J, Funakoshi-Tago M, et al. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Lic-ochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway. Int Immunopharmacol. 2009;9(4):499–507. doi: 10.1016/j.intimp.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Hirai S, Kim YI, et al. Inhibitory effect of naringenin chalcone on inflammatory changes in the interaction between adipocytes and macrophages. Life Sci. 2007;81(16):1272–1279. doi: 10.1016/j.lfs.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Jellema RK, Lima Passos V, et al. Cerebral inflammation and mobilization of the peripheral immune system following global hypoxia-ischemia in preterm sheep. J Neuroinflammation. 2013;10:13. doi: 10.1186/1742-2094-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, et al. Brain. Inflammation and white matter degeneration persist for years after a single traumatic brain injury;136(Pt 1):28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SH, Ryu GR, et al. Inducible nitric oxide synthase-nitric oxide plays an important role in acute and severe hypoxic injury to pancreatic beta cells. Transplantation. 2008;85(3):323–330. doi: 10.1097/TP.0b013e31816168f9. [DOI] [PubMed] [Google Scholar]
- Kouti L, Noroozian M, et al. Nitric oxide and peroxynitrite serum levels in Parkinson’s disease: correlation of oxidative stress and the severity of the disease. Eur Rev Med Pharmacol Sci. 2013;17(7):964–970. [PubMed] [Google Scholar]
- Li M, Dai FR, et al. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson’s disease. J Mol Neurosci. 2012;48(1):225–233. doi: 10.1007/s12031-012-9814-5. [DOI] [PubMed] [Google Scholar]
- Lin CC, Chu CL, et al. Immunomodulation of phloretin by impairing dendritic cell activation and function. Food Funct. 2014;5(5):997–1006. doi: 10.1039/c3fo60548e. [DOI] [PubMed] [Google Scholar]
- Liu P, Sun L, et al. PAR2-mediated epigenetic upregulation of alpha-synuclein contributes to the pathogenesis of Parkinsons disease. Brain Res. 2014;1565:82–89. doi: 10.1016/j.brainres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Lopategui Cabezas I, Herrera Batista A, et al. The role of glial cells in Alzheimer’s disease: potential therapeutic implications. Neurologia. 2012 doi: 10.1016/j.nrl.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Mandawad GG, Dawane BS, et al. Trisubstituted thiophene analogues of 1-thiazolyl-2-pyrazoline, super oxidase inhibitors and free radical scavengers. Bioorg Med Chem. 2013;21(1):365–372. doi: 10.1016/j.bmc.2012.09.060. [DOI] [PubMed] [Google Scholar]
- Mateeva NN, Redda N, Kode R. Synthesis of novel flavonoid derivatives as potential HIV-integrase inhibitors. J Heterocycl Chem. 2002;39:1251–1258. [Google Scholar]
- Mills CJ, Redda K. Synthesis of novel substituted flavonoids. J Heterocycl Chem. 2006;43:59–64. [Google Scholar]
- Monif M, Burnstock G, et al. Microglia: proliferation and activation driven by the P2×7 receptor. Int J Biochem Cell Biol. 2010;42(11):1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Nam Y, Choi M, et al. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phytother Res. 2013;27(3):404–411. doi: 10.1002/ptr.4737. [DOI] [PubMed] [Google Scholar]
- Ojha RP, Rastogi M, et al. Neuroprotective effect of curcuminoids against inflammation-mediated dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. J Neuroimmune Pharmacol. 2012;7(3):609–618. doi: 10.1007/s11481-012-9363-2. [DOI] [PubMed] [Google Scholar]
- Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-kappaB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract. 2013;7(6):423–429. doi: 10.4162/nrp.2013.7.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phani S, Loike JD, et al. Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S207–S209. doi: 10.1016/S1353-8020(11)70064-5. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Andreasson K. Commentary: progressive inflammation as a contributing factor to early development of Parkinson’s disease. Exp Neurol. 2013;241:148–155. doi: 10.1016/j.expneurol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Raza SS, Khan MM, et al. Neuroprotective effect of naringenin is mediated through suppression of NF-kappaB signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Korolchuk VI, et al. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43(1):19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheff SW, Ansari MA, et al. Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp Neurol. 2013;239:183–191. doi: 10.1016/j.expneurol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell U, Dijk F, et al. Immunolabeling artifacts and the need for live-cell imaging. Nat Methods. 2012;9(2):152–158. doi: 10.1038/nmeth.1855. [DOI] [PubMed] [Google Scholar]
- Senggunprai L, Kukongviriyapan V, et al. Quercetin and EGCG exhibit chemopreventive effects in cholangiocarcinoma cells via suppression of JAK/STAT signaling pathway. Phytother Res. 2014;28(6):841–848. doi: 10.1002/ptr.5061. [DOI] [PubMed] [Google Scholar]
- Shrivastava K, Llovera G, et al. Temporal expression of cytokines and signal transducer and activator of transcription factor 3 activation after neonatal hypoxia/ischemia in mice. Dev Neurosci. 2013;35(2–3):212–225. doi: 10.1159/000348432. [DOI] [PubMed] [Google Scholar]
- Siriwardhana N, Kalupahana NS, et al. Modulation of adipose tissue inflammation by bioactive food compounds. J Nutr Biochem. 2013;24(4):613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Vafeiadou K, et al. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Stough CK, Pase MP, et al. A randomized controlled trial investigating the effect of Pycnogenol and Bacopa CDRI08 herbal medicines on cognitive, cardiovascular, and biochemical functioning in cognitively healthy elderly people: the Australian Research Council Longevity Intervention (ARCLI) study protocol (ANZCTR12611000487910) Nutr J. 2012;11:11. doi: 10.1186/1475-2891-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Takahashi R. Flavonoids and asthma. Nutrients. 2013;5(6):2128–2143. doi: 10.3390/nu5062128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Qian C, et al. Inhibition of Th1/Th17 responses via suppression of STAT1 and STAT3 activation contributes to the amelioration of murine experimental colitis by a natural flavonoid glucoside icariin. Biochem Pharmacol. 2013;85(6):798–807. doi: 10.1016/j.bcp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Toledo AC, Sakoda CP, et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br J Pharmacol. 2013;168(7):1736–1749. doi: 10.1111/bph.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z, Mah D, et al. Globular adiponectin induces a pro-inflammatory response in human astrocytic cells. Biochem Biophys Res Commun. 2014;446(1):37–42. doi: 10.1016/j.bbrc.2014.02.077. [DOI] [PubMed] [Google Scholar]
- Wong WT. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Front Cell Neurosci. 2013;7:22. doi: 10.3389/fncel.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav VR, Prasad S, et al. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int Immunopharmacol. 2011;11(3):295–309. doi: 10.1016/j.intimp.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Hou J, et al. Baicalin protects rat brain microvascular endothelial cells injured by oxygen-glucose deprivation via anti-inflammation. Brain Res Bull. 2013;97:8–15. doi: 10.1016/j.brainresbull.2013.05.005. [DOI] [PubMed] [Google Scholar]