Abstract
OBJECTIVE
To evaluate if temperature and humidity influenced the etiology of bloodstream infections in a hospital from 2005 to 2010.
METHODS
The study had a case-referent design. Individual cases of bloodstream infections caused by specific groups or pathogens were compared with several references. In the first analysis, average temperature and humidity values for the seven days preceding collection of blood cultures were compared with an overall “seven-days moving average” for the study period. The second analysis included only patients with bloodstream infections. Several logistic regression models were used to compare different pathogens and groups with respect to the immediate weather parameters, adjusting for demographics, time, and unit of admission.
RESULTS
Higher temperatures and humidity were related to the recovery of bacteria as a whole (versus fungi) and of gram-negative bacilli. In the multivariable models, temperature was positively associated with the recovery of gram-negative bacilli (OR = 1.14; 95%CI 1.10;1.19) or Acinetobacter baumannii (OR = 1.26; 95%CI 1.16;1.37), even after adjustment for demographic and admission data. An inverse association was identified for humidity.
CONCLUSIONS
The study documented the impact of temperature and humidity on the incidence and etiology of bloodstream infections. The results correspond with those from ecological studies, indicating a higher incidence of gram-negative bacilli during warm seasons. These findings should guide policies directed at preventing and controlling healthcare-associated infections.
Keywords: Cross Infection, etiology; Temperature; Humidity; Bacterial Infections and Mycoses; microbiology
Abstract
OBJETIVO
Avaliar se temperatura e umidade influenciam a etiologia das infecções na corrente sanguínea em hospital, no período de 2005 a 2010.
MÉTODOS
O estudo teve delineamento caso-referência. Casos individuais de infecções de corrente sanguínea por patógenos ou grupos de interesse foram comparados com diferentes referências. Na primeira etapa, valores médios de temperatura e umidade, para os sete dias que precederam a coleta de culturas de sangue, foram comparados com a “média-móvel de ordem 7” para todos os dias do período do estudo. A segunda etapa incluiu somente os casos com culturas positivas. Foram realizadas análises por regressão logística para avaliar a influência dos parâmetros meteorológicos imediatos sobre a etiologia dessas infecções, ajustando os resultados para dados demográficos, tempo e unidade de internação.
RESULTADOS
Temperatura e umidade mais elevadas foram associadas às infecções de corrente sanguínea causadas por bactérias como um todo (versus fungos) e por bacilos Gram-negativos. Nos modelos multivariados, a temperatura foi positivamente associada com o isolamento nas culturas de bacilos Gram-negativos (OR = 1,14; IC95% 1,10;1,19) ou A. baumannii (OR = 1,26; IC95% 1,16;1,37), mesmo após ajuste para dados demográficos e de internação. Associação inversa foi identificada por umidade.
CONCLUSÕES
O estudo documentou o impacto de temperatura e umidade sobre incidência e etiologia de infecções da corrente sanguínea. Os resultados são coerentes com os relatados em estudos ecológicos, apontando para maior incidência de bacilos Gram-negativos durante as estações quentes. Esses achados devem orientar as estratégias direcionadas à prevenção e controle de infecções relacionadas à assistência à saúde.
INTRODUCTION
Healthcare-associated infections (HAI) pose a threat to hospitalized patients worldwide. 20 Their incidence is higher in developing countries, where problems with human resources, technical expertise, and laboratory support hinder infection control policies. 1 Traditionally, the occurrence of HAI has been attributed to patient fragilities, invasive procedures, use of antimicrobials, and other work processes. 5 However, recent studies indicate the role of climate and weather as additional epidemiological determinants of HAI incidence and etiology. 16 , 20 This novel approach may include those infections in the wide group of seasonal diseases, alongside dengue fever and influenza. 10
Summer peaks of gram-negative bacilli (GNB) infections in healthcare settings have been reported in developed countries with temperate climates. 9 , 14 , 19 These countries have well-defined seasons, and hospitals are artificially climatized. 22 , 24 On the other hand, data on the seasonality of HAI pathogens in developing countries, which generally lack those characteristics, are scarce. 21 In a previous study conducted in a Brazilian hospital, seasonality was identified in the incidence of bloodstream infections caused by overall GNB and specific pathogens such as Acinetobacter baumannii. 11
In all the above mentioned studies, an ecological approach was applied and aggregate data were analyzed. Therefore, it remains to be identified how and to what extent does the weather influence the likelihood of acquiring HAI on an individual basis.
This study was aimed at evaluating if temperature and humidity influence the etiology of bloodstream infections (BSI) in a hospital from 2005 to 2010.
METHODS
This study was conducted at the Hospital das Clínicas of Faculdade de Medicina de Botucatu (HCFMB). The hospital is located in a semi-urban area in the city of Botucatu, Sao Paulo State, Brazil (22°53′09″S, 48°26′42″W). The hospital has 450 beds, 90.0% of which are distributed among non-climatized units. There are several balconies, large windows, and wide corridors with openings to the external environment. HCFMB is a teaching hospital for medical and nursing graduate students as well as for interns, residents, and postgraduates. It is also a facility for tertiary care for several municipalities in the surrounding area, which comprises a million inhabitants.
The hospital has its own microbiology laboratory and an infection control committee, constituted in accordance with Brazilian sanitary regulations.
The study had a case-referent design, and its sample group consisted of subjects who had a laboratory-confirmed, healthcare-associated BSI (HA-BSI) from 2005 to 2010. For typical bacterial pathogens (e.g., Staphylococcus aureus, Enterococci, Klebsiella spp., A. baumannii) or fungi, a single positive blood culture defined BSI. For coagulase-negative Staphylococci (CoNS), positivity in two blood cultures from the same day was required. 13 As recommended by the Society for Healthcare Epidemiology of America, 3 in association with healthcare, the three-midnight rule was applied, i.e., only BSI diagnosed based on culture collection after the third day of admission were defined as HA-BSI.
All cultures were collected based on the attending physicians’ request. Although there was no strict decision-making protocol for requesting microbiological tests, doctors were encouraged to collect blood cultures from patients with suspected HAI. This was in accordance with the United States Centers for Diseases Control and Prevention (CDC) diagnostic guidelines in effect during the study period, including their most recent version. 13 For the purposes of the present study, primary or catheter-related BSI were not differentiated from those secondary to other sites of infection.
Data from positive cultures were obtained in files from the microbiology laboratory in HCFMB. A database for HA-BSI was created based on the definitions described above. Duplications, defined as positive cultures for the same pathogen in the same subject within a 30-day period, were excluded. All other patient data such as sex, age, hospital unit, and length of stay were recovered from administrative files. Data on temperature and humidity for the city of Botucatu were obtained in the Department of Soil Science and Natural Resources from the Faculdade de Agronomia de Botucatu.
Average temperature and humidity data from the seven days preceding the collection of cultures (comprising the day of collection and the six previous days) were calculated for each case subject. An overall reference was obtained from the moving average of the seventh order of daily weather parameters (i.e., the average for every seven-day period) for the whole study period. In the first phase of this study, the parameters for subjects groups were compared based on etiology [fungi, bacteria, Gram-positive cocci (GPC), GNB, or A. baumannii] with the period reference, using the Student’s t-test. The second study phase consisted of the fitting of logistic regression models, including HA-BSI subjects. The outcomes of interest were etiological agents: bacteria (versus fungi), GNB (versus GPC), and A. baumannii (versus other GNB). The immediate seven-day weather parameters were analyzed in an initial non-adjusted (univariate) step, in multivariable models adjusting temperature and humidity for each other, as well as for subject demographics (age, sex, etc.) and admission data (unit of admission and length of stay previous to BSI diagnosis). All tests, models and graphics were performed or built with SPSS 19.0 (IBM, Armonk, NY, USA). The level of significance was p < 0.05.
The study was approved by the Committee for Ethics in Human Research of Faculdade de Medicina de Botucatu, Brazil, Process 15949613.6.0000.5411, approved in 5/7/2013.
RESULTS
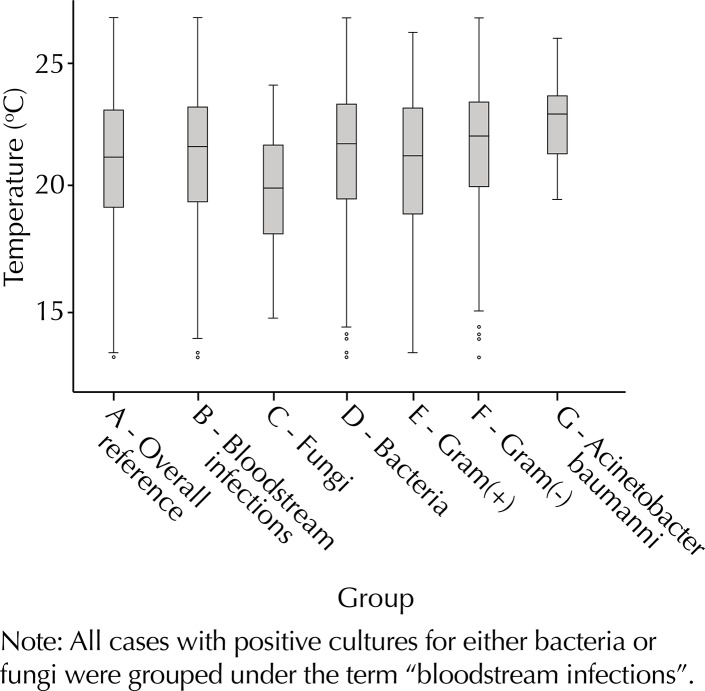
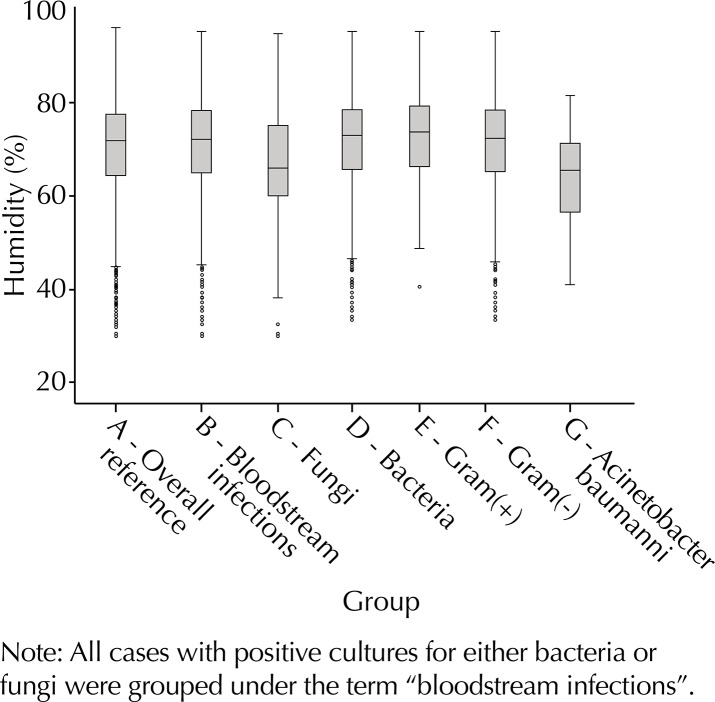
A total of 1,619 HA-BSI were included in the study. Of these, 1,417 had bacterial etiology (55.8% GNB, 44.2% GPC). Among GNB infections, 22.4% were caused by A. baumannii. When all groups were compared to the overall period reference (Table 1, Figures 1 and 2), higher temperature parameters were found for bacteria GNB and A. baumannii, whereas fungi were associated with lower temperatures. Humidity parameters were higher than the reference for bacteria and GPC and lower for fungi and A. baumannii. On the other hand, lower temperatures were identified in the days preceding HA-BSI of fungal etiology.
Table 1. Comparison of the immediate meteorological parameters preceding the diagnosis of bloodstream infection with overall data from the study period. Hospital das Clínicas from Faculdade de Medicina de Botucatu. January 2005 – December 2010.
| Category | Number of observations | Temperature (°C) | Relative humidity (%) | ||
|---|---|---|---|---|---|
| Average | Standard error | Average | Standard error | ||
| Overall data (reference) | 2,815 | 20.99 | 2.66 | 70.20 | 10.76 |
| Fungi | 204 | 19.66 | 2.34 | 66.74 | 12.37 |
| Bacteria | 1,417 | 21.84 | 2.74 | 71.29 | 10.27 |
| Gram-positive cocci | 627 | 20.93 | 2.83 | 73.40 | 8.55 |
| Gram-negative bacilli | 790 | 21.76 | 2.51 | 69.91 | 11.18 |
| Acinetobacter baumannii | 177 | 22.99 | 2.62 | 63.82 | 10.23 |
Note: For the purpose of this study, immediate parameters indicate temperature and relative humidity for the seven days preceding the collection of blood cultures. The overall data consisted of the moving average to seventh order for each day of the study period.
Bold values indicate statistical significance.
Figure 1. Boxplot of seven-day average temperature parameters for the overall period and all study groups.
Figure 2. Boxplot of seven-day average relative humidity parameters for the overall period and all study groups.
Table 2 presents the results from logistic regression models aimed at identifying weather predictors of HA-BSI etiology. Temperature was positively associated with bacterial etiology as well as with infections caused by GNB as a whole or A. baumannii. The recovery of overall bacteria was likely to occur in periods of greater relative humidity. However, humidity was negatively associated with GNB and A. baumannii. These results were unaffected by the adjustment for subject demographics and admission parameters.
Table 2. Univariate and multivariable logistic regression models for the association of immediate meteorological parameters and the etiology of bloodstream infections.
| Predictive factors | Crude OR | 95%CI | Adjusted OR1 | 95%CI | Adjusted OR2 | 95%CI | Adjusted OR3 | 95%CI | Adjusted OR3 | 95%CI |
|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria 1,417 versus Fungi 204 | ||||||||||
| Temperature (°C) | 1.25 | 1.19;1.32 | 1.25 | 1.18;1.31 | 1.25 | 1.19;1.32 | 1.26 | 1.19;1.33 | 1.26 | 1.19;1.33 |
| Relative humidity (%) | 1.04 | 1.02;1.05 | 1.04 | 1.02;1.05 | 1.04 | 1.02;1.05 | 1.03 | 1.02;1.05 | 1.03 | 1.02;1.05 |
| Intensive care unit | – | – | 0.71 | 0.52;0.99 | 0.88 | 0.63;1.24 | 0.88 | 0.63;1.24 | ||
| Time since admission | – | – | 1.00 | 0.99;1.01 | 1.00 | 0.99;1.01 | 1.00 | 0.99;1.01 | ||
| Age (years) | – | – | – | 1.02 | 1.01;1.03 | 1.02 | 1.01;1.03 | |||
| Sex feminine | – | – | – | 1.03 | 0.75;1.40 | 1.03 | 0.75;1.40 | |||
| GNB 790 versus GPC 627 | ||||||||||
| Temperature (°C) | 1.12 | 1.08;1.17 | 1.14 | 1.10;1.19 | 1.14 | 1.10;1.19 | 1.14 | 1.10;1.19 | 1.14 | 1.10;1.19 |
| Relative humidity (%) | 0.96 | 0.95;0.97 | 0.96 | 0.95;0.97 | 0.96 | 0.95;0.97 | 0.96 | 0.95;0.97 | 0.96 | 0.95;0.97 |
| Intensive care unit | – | – | 0.79 | 0.62;1.00 | 0.75 | 0.59;0.96 | 0.75 | 0.59;0.96 | ||
| Time since admission | – | – | 1.00 | 0.99;1.00 | 1.00 | 0.99;1.00 | 1.00 | 0.99;1.00 | ||
| Age (years) | – | – | – | 1.00 | 0.99;1.00 | 1.00 | 0.99;1.00 | |||
| Sex feminine | – | – | – | 1.21 | 0.98;1.51 | 1.21 | 0.98;1.51 | |||
| Acinetobacter baumannii 177 versus other GNB 613 | ||||||||||
| Temperature (°C) | 1.23 | 1.14;1.33 | 1.26 | 1.16;1.37 | 1.26 | 1.16;1.38 | 1.26 | 1.16;1.37 | 1.26 | 1.16;1.37 |
| Relative humidity (%) | 0.95 | 0.93;0.96 | 0.94 | 0.93;0.96 | 0.94 | 0.93;0.96 | 0.94 | 0.93;0.96 | 0.94 | 0.93;0.96 |
| Intensive care unit | – | – | 0.73 | 0.49;1.11 | 0.75 | 0.49;1.13 | 0.75 | 0.49;1.13 | ||
| Time since admission | – | – | 1.00 | 0.99;1.00 | 1.00 | 0.99;1.00 | 1.00 | 0.99;1.00 | ||
| Age (years) | – | – | – | 1.00 | 0.99;1.01 | 1.00 | 0.99;1.01 | |||
| Sex feminine | – | – | – | 0.89 | 0.62;1.28 | 0.89 | 0.62;1.28 | |||
GNB: Gram-negative bacilli; GPC: Gram-positive cocci
Note: For the purpose of this study, immediate parameters indicate temperature and relative humidity for the seven days preceding the collection of blood cultures. The number of cases in each category is stated in parenthesis. Crude odds ratio (OR) refers to univariate analysis. The following columns present the adjusted OR in multivariable models, including, the meteorological parameters, admission data, and demographics of cases, in a cumulative fashion.
Bold values indicate statistical significance.
DISCUSSION
There is now compelling evidence that the incidence of HAI caused by GNB is increased during warm seasons. However, although results from ecological studies have a value of their own, they should be complemented by an analysis conducted on an individual basis. This is an important strategy to address the “ecological fallacy”, i.e., the possibility that findings that are valid for aggregated data do not reflect relations on the individual level. 8 In epidemiological studies, the assessment of ecological fallacy is of practical importance for the recommendation of interventions targeting both the collectivity and individuals. This study focused on individual outcomes as a means of reducing bias that could arise from ecological fallacy. 11
Our findings generally corresponded with those from previous ecological studies; higher immediate temperature values were associated with gram-negative etiology. Even higher temperatures were found in the days preceding HA-BSI caused by A. baumannii. These findings echo results from the ecological analysis performed previously. In that analysis, increases of 4.0% and 13.0% were identified in monthly incidences of GNB and A. baumannii HA-BSI for each additional centigrade degree of average temperature. 11 Data concerning A. baumannii is of utmost interest. Those agents are particularly common in regions with a tropical climate. 2 , 7 They are widely disseminated among hospitals in Brazil, and recent reports describe an increase in both overall prevalence and resistance to antimicrobials. 18 , 23 In addition, they are the nosocomial pathogen for which most evidence of seasonality has been accumulated. 4 , 17 , 24 Hence, A. baumannii was highlighted, and a separate analysis of weather determinants was included for HA-BSI caused by this agent. The results, together with those from previous studies, strongly suggest that infection control policies aimed at controlling the dissemination of A. baumannii within hospitals should give special attention to the increase in its prevalence during warm months.
Although the recognition of seasonality in human diseases dates back to Hippocrates, the mechanisms underlying this epidemiological behavior are not completely understood. Small changes in seasonal behavior of either the pathogen or its host may give rise to significant fluctuations in the incidence of infectious diseases. 10 It can be presumed that a similar phenomenon accounts for seasonality of healthcare-associated GNB infections.
Previous studies raised interesting explanatory hypotheses. First, the ability of GNB to invade human tissues may increase in response to temperature because of the expression of virulence factors. 9 Second, the increase in bacterial growth in inanimate reservoirs. These findings correspond with the recent recognition of the importance of environmental contamination in the epidemiology of HAI. 25 They are also consistent with the impact of weather in hospitals from tropical countries, including our study hospital. These hospitals generally lack artificial climatization and have several openings to the external environment. However, the findings fail to explain why seasonality of GNB has been reported even in climatized units. 22
In our study hospitals, the 24 wards varied widely with respect to location, contact with the external environment, and climatization (which exists only in intensive care units). However, the association of temperature and humidity with the etiology of HA-BSI was not affected when data on the admission ward was included in the multivariable model. Although a more refined subgroup analysis and longer time span of data collection are necessary to clarify this issue, it seems that GNB prevalence increased throughout the hospital in warm periods.
Studies have suggested that environmental reservoirs outside the hospital increase during summer months. According to this hypothesis, healthcare workers are responsible for the introduction of seasonal GNB into the wards. 22 Some authors reported that A. baumannii recovered from clinical cultures during warm months are likely to be multidrug susceptible and/or present a polyclonal profile on strain typing methods. 4 , 12 While these findings strengthen the hypothesis of a community-associated origin, other studies found a significant increase in infections caused by multidrug-resistant isolates of both A. baumannii and Pseudomonas a erugino sa during periods of higher temperature. 9 Because multidrug-resistance is a proxy of nosocomial origin, those results point to an increased spread of GNB within hospital units.
A particularly puzzling finding from the present study was the negative association between humidity and GNB or A. baumannii etiology. The association was not found in the previous ecological study conducted in the same hospital. 11 This aspect of the result emphasizes the differences in the analysis of aggregate and individual data. They may also be due to the paradoxical relation of temperature and humidity during very short time spans. 15 Interestingly, the results did not change when the analysis was repeated using only temperature and humidity values from the day of collection of cultures instead of the seven-day average (data not shown).
Finally, another hypothesis that has been raised to explain the summer peaks in GNB infections within hospitals is related to understaffing. Summer vacations can decrease the nurse/patient ratio, thus increasing workload and failure to comply with infection control rules. 22 Although understaffing has been consistently associated with an increased risk of HAI, 6 no studies have focused on the association between understaffing and seasonal increases of GNB infections in hospitals. Furthermore, in HCFMB, as in many healthcare services in developing countries, understaffing is a phenomenon that persists throughout the entire year.
This study has some limitations. First, it has a retrospective design, and we analyzed data from administrative and microbiology files. Thus, many risk factors for HAI, such as invasive procedures (including central venous catheter) and severity of illness, were not addressed. Second, possible seasonal changes in patient profiles (e.g., demographics, admission diagnosis) or physician behavior were not addressed. Therefore, the selection bias in the study cannot be completely ruled.
However, these analyses were beyond the scope of our objectives, which focused specifically on the impact of environmental factors, including spatial distribution, time of exposure to the hospital environment, and weather data. We did not analyze clonality or antimicrobial resistance among HA-BSI agents. Finally, it was not possible to completely rule out ecological fallacy because the data regarding exposure to weather conditions was measured for the whole city and does not represent the temperature and humidity in each patient room. However, the analysis of individual outcomes increases the consistency of previous purely ecological analyses. 11 This study also has strengths, including the number of HA-BSI subjects and the stability of risk factors in all steps of the multivariable analysis.
The results raise new questions regarding GNB seasonality in tropical healthcare settings: what is the relation between seasonality and resistance to antimicrobials? Do specific risk factors (severity of illness, procedures, antimicrobial use, etc.) vary among patients who acquired HAI in different seasons? Is seasonality equal among hospitals located in different regions? We intend to address these questions in future studies.
In conclusion, we found that high temperatures in the seven days preceding the collection of blood cultures were associated with the recovery of bacteria rather than fungi and GNB rather than GPC. Among gram-negatives, higher immediate temperature values were associated with HA-BSI caused by A. baumannii. These findings not only reflect a change in the relative incidence among different pathogens but also result (as shown in previous analyses 11 ) from an absolute increase in the incidence of GNB and A. baumannii during warm seasons, which contrasted with the stability in the occurrence of other pathogens. Together, these findings suggest that infection control policies should be reinforced during periods of high temperature. In addition, guidelines for empirical antimicrobial therapy should consider the higher risk for GNB infections during those periods. Moreover, these findings pose new questions for research to contribute to the elucidation of the complex association between climate, weather and HAI.
Footnotes
Based on the doctoral thesis of Silvia Maria Caldeira titled: “Impacto das condições climáticas sobre a etiologia das bacteremias nosocomiais no Hospital das Clínicas da Faculdade de Medicina de Botucatu-UNESP”, presented to the Postgraduate Program in Tropical Diseases of the Faculdade de Medicina de Botucatu, in 2013.
REFERENCES
- 1.Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. 10.1016/S0140-6736(10)61458-4Lancet. 2011;377(9761):228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 2.Chim H, Tan BH, Song C. Five-year review of infections in a burn intensive care unit: High incidence of Acinetobacter baumannii in a tropical climate. 10.1016/j.burns.2007.03.003Burns. 2007;33(8):1008–1014. doi: 10.1016/j.burns.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AL, Calfee D, Fridkin SK, Huang SS, Jernigan JA, Lautenbach E, et al. Society for Healthcare Epidemiology of America and the Healthcare Infection Control Practices Advisory Committee. Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/HICPAC Position paper. 10.1086/591741Infect Control Hosp Epidemiol. 2008;29(10):901–903. doi: 10.1086/591741. [DOI] [PubMed] [Google Scholar]
- 4.Christie C, Mazon D, Hierholzer W, Jr, Patterson JE. Molecular heterogeneity of Acinetobacter baumanii isolates during seasonal increase in prevalence. 10.2307/30141099Infect Control Hosp Epidemiol. 1995;16(10):590–594. doi: 10.1086/647013. [DOI] [PubMed] [Google Scholar]
- 5.Curtis LT. Prevention of hospital-acquired infections: review of non-pharmacological interventions. 10.1016/j.jhin.2008.03.018J Hosp Infect. 2008;69(3):204–219. doi: 10.1016/j.jhin.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daud-Gallotti RM, Costa SF, Guimarães T, Padilha KG, Inoue EN, Vasconcelos TN, et al. Nursing workload as a risk factor for healthcare associated infections in ICU: a prospective study. 10.1371/journal.pone.0052342PLoS One. 2012;7(12):19. doi: 10.1371/journal.pone.0052342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doughari HJ1, Ndakidemi PA, Human IS, Benade S. The ecology, biology and pathogenesis of Acinetobacter spp: an overview. 10.1264/jsme2.ME10179Microbes Environ. 2011;26(2):101–112. doi: 10.1264/jsme2.me10179. [DOI] [PubMed] [Google Scholar]
- 8.Dufault B, Klar N. The quality of modern cross-sectional ecologic studies: a bibliometric review. 10.1093/aje/kwr241Am J Epidemiol. 2011;174(10):1101–1107. doi: 10.1093/aje/kwr241. [DOI] [PubMed] [Google Scholar]
- 9.Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. 10.1371/journal.pone.0025298PLoS One. 2011;6(9):19. doi: 10.1371/journal.pone.0025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisman DN. Seasonality of infectious diseases. 10.1146/annurev.publhealth.28.021406.144128Annu Rev Public Health. 2007;28:127–143. doi: 10.1146/annurev.publhealth.28.021406.144128. [DOI] [PubMed] [Google Scholar]
- 11.Fortaleza CM, Caldeira SM, Moreira RG, Akazawa RT, Corrente JE, Souza LR, et al. Tropical healthcare epidemiology: weather determinants of the etiology of bloodstream infections in a Brazilian hospital. 10.1086/674392Infect Control Hosp Epidemiol. 2014;35(1):85–88. doi: 10.1086/674392. [DOI] [PubMed] [Google Scholar]
- 12.Fukuta Y, Clarke LG, Shields RK, Wagener MM, Pasculle AW, Doi Y. Lack of seasonality in the occurrence of multidrug-resistant Acinectobacter baumannii complex. 10.1086/667741Infect Control Hosp Epidemiol. 2012;33(10):1051–1052. doi: 10.1086/667741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. 10.1016/j.ajic.2008.03.002Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Kaier K, Frank U, Conrad A, Meyer E. Seasonal and ascending trends in the incidence of carriage of extended-spectrum ß-lactamase-producing Escherichia coli and Klebsiella species in 2 German hospitals. 10.1086/656748Infect Control Hosp Epidemiol. 2010;31(11):1154–1159. doi: 10.1086/656748. [DOI] [PubMed] [Google Scholar]
- 15.Kimball JS, Running SW, Nemani R. An improved method for estimating surface humidity from daily minimum temperature. 10.1016/S0168-1923(96)02366-0Agric Forest Meteorol. 1997;85(1):87–98. [Google Scholar]
- 16.Leekha S, Diekema DJ, Perencevich EN. Seasonality of staphylococcal infections. 10.1111/j.1469-0691.2012.03955.xClin Microbiol Infect. 2012;18(10):927–933. doi: 10.1111/j.1469-0691.2012.03955.x. [DOI] [PubMed] [Google Scholar]
- 17.McDonald LC, Banerjee SN, Jarvis WR. Seasonal variation of Acinetobacter infections: 1987-1996. Nosocomial Infections Surveillance System. 10.1086/313441Clin Infect Dis. 1999;29(5):1133–1137. doi: 10.1086/313441. [DOI] [PubMed] [Google Scholar]
- 18.Padoveze MC, Assis DB, Freire MP, Madalosso G, Ferreira SA, Valente MG, et al. Surveillance Programme for Healthcare Associated Infections in the State of São Paulo, Brazil. Implementation and the first three years’ results. 10.1016/j.jhin.2010.07.005J Hosp Infect. 2010;76(4):311–315. doi: 10.1016/j.jhin.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Perencevich EN, McGregor JC, Shardell M, Furuno JP, Harris AD, Morris JG, et al. Summer Peaks in the incidences of gram-negative bacterial infection among hospitalized patients. 10.1086/592698Infect Control Hosp Epidemiol. 2008;29(12):1124–1131. doi: 10.1086/592698. [DOI] [PubMed] [Google Scholar]
- 20.Pittet D, Allegranzi B, Storr J, Bagheri Nejad S, Dziekan G, Leotsakos A, et al. Infection control as a major World Health Organization priority for developing countries. 10.1016/j.jhin.2007.12.013J Hosp Infect. 2008;68(4):285–292. doi: 10.1016/j.jhin.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Ramos GP, Rocha JL, Tuon FF. Seasonal humidity may influence Pseudomonas aeruginosa hospital-acquired infection rates. 10.1016/j.ijid.2013.03.002Int J Infect Dis. 2013;17(9):e757–e761. doi: 10.1016/j.ijid.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Richet H. Seasonality in gram-negative and healthcare-associated infections. 10.1111/j.1469-0691.2012.03954.xClin Microbiol Infect. 2012;18(10):934–940. doi: 10.1111/j.1469-0691.2012.03954.x. [DOI] [PubMed] [Google Scholar]
- 23.Rossi F. The challenges of antimicrobial resistance in Brazil. 10.1093/cid/cir120Clin Infect Dis. 2011;52(9):1138–1143. doi: 10.1093/cid/cir120. [DOI] [PubMed] [Google Scholar]
- 24.Schwab F, Gastmeier P, Meyer E. The warmer the weather, the more gram-negative bacteria - impact of temperature on clinical isolates in intensive care units. 10.1371/journal.pone.0091105Plos One. 2014;9(3):19. doi: 10.1371/journal.pone.0091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. 10.1097/QCO.0b013e3283630f04Curr Opin Infect Dis. 2013;26(4):338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]