Abstract
The heme prosthetic group in hemoglobins is most often attached to the globin through coordination of either one or two histidine side chains. Those proteins with one histidine coordinating the heme iron are called “pentacoordinate” hemoglobins, a group represented by red blood cell hemoglobin and most other oxygen transporters. Those with two histidines are called “hexacoordinate hemoglobins”, which have broad representation among eukaryotes. Coordination of the second histidine in hexacoordinate Hbs is reversible, allowing for binding of exogenous ligands like oxygen, carbon monoxide, and nitric oxide. Research over the past several years has produced a fairly detailed picture of the structure and biochemistry of hexacoordinate hemoglobins from several species including neuroglobin and cytoglobin in animals, and the nonsymbiotic hemoglobins in plants. However, a clear understanding of the physiological functions of these proteins remains an elusive goal.
Keywords: Hexacoordinate hemoglobin, Structure, Kinetics, Ligand binding, Evolution, Plant hemoglobin, Neuroglobin
The term “hemoglobin” has spent decades at the forefront of biochemical research, including the eras of “grind-and-find”, protein structure determination, and the relationship between protein structure and function. It has also served as a familiar target in genomic annotation, and as a guidepost to study the evolution of primary structure and protein function. We have learned over the past fifteen years that most organisms contain genes with homology to globins, and that not all globins are oxygen transport proteins [1–4]. In fact, it is presumptuous to assume that all globins use a heme prosthetic group as part of their physiological functions [5]. Most of what has been learned during this period originated from wide spread sequencing of genomes representing all kingdoms of life, followed by biochemical analysis of proteins resulting from select members of these new-found sequences, in most cases using recombinant methods for their production.
The infrastructure for detailed biophysical research with hemoglobins (Hbs) has been in place for decades, established in an effort to understand how the structures of oxygen transport Hbs confer this function. All of the newly discovered Hbs have been naturally welcomed into this framework, which has produced a wealth of structures, spectroscopic characterizations, and biochemical investigations of recombinant Hbs from the three kingdoms of life. The downside of all the ready physical analysis is that such work, when not guided by knowledge of a clear physiological function, sometimes digresses from biology and can mislead functional hypotheses by asking leading questions. Thus, organizing globins based on function is currently difficult. Nevertheless, irrespective of functional hypotheses, structural and chemical studies of newly discovered Hbs have revealed unusual behavior that has challenged some of the principles distilled from the wealth of research on oxygen transport Hbs.
The chemistry of heme proteins and hemoglobins is dictated to a large degree by the manner in which the heme group is coordinated to the globin, and the environment of the surrounding heme pocket. The range of coordination states and axial ligands exhibited by heme proteins in general is extensive compared to the subset that have globin folds [6]. The degree of coordination ranges from the NO-bound conformations of soluble guanylate cyclase [7], some H-NOX proteins [8], and cytochrome c [9], which completely lack coordination to the protein, to cytochromes b5, which are bis-histidyl in coordination and unreactive with exogenous ligands. Within the globin class of heme proteins, the unique properties of nitric oxide [10] have been shown to form pentacoordinate NO complexes with myoglobin and blood cell alpha chains [11,12] akin to that of soluble guanylate cyclase, but these conformations are significantly populated only at lower pH and are probably not common in vivo. There is however at least one hexacoordinate complex of alpha chains that is considered to be of potential physiological importance. Binding of oxy-alpha Hb to alpha-Hb stabilizing protein (AHSP) prior to holo-Hb formation leads to the rapid formation of an oxidized (ferric) bis-histidyl complex [13]. The hemichrome structure is thought to be relatively stable, and could provide a mechanism for an inert folding reaction followed by rapid heme iron reduction.
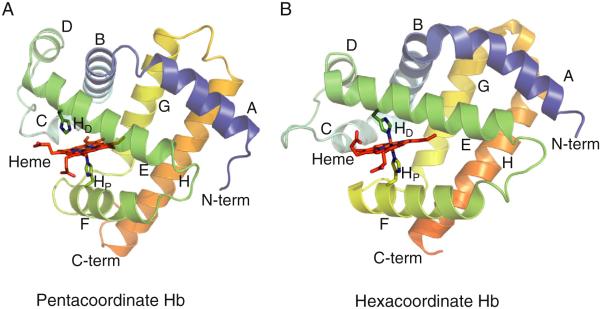
The familiar oxygen transport Hbs of plants and animals use pentacoordinate heme iron (Fig. 1A) to reversibly bind oxygen in the Fe2+ (ferrous) oxidation state. In all cases, a single histidine side chain coordinates an axial site on the heme iron to help hold the prosthetic group in place, leaving the other axial site open for oxygen binding. Only the ferrous oxidation state will reversibly bind oxygen, and the tissues in which these proteins function have mechanisms to prevent or reverse the spontaneous oxidation to the Fe3+ (ferric) oxidation state. In newly discovered Hbs, absent the knowledge of physiological function, there is no way to know what oxidation state is biologically relevant or how heme coordination relates to function. The heme iron could potentially exist (or cycle through) in the Fe2+ (ferrous), Fe3+ (ferric), or Fe4+ (ferryl) oxidation state, attachment to the protein could potentially result from a number of amino acids capable of donating a pair of electrons to form a coordination bond [6], and attachment to the protein could result from one or two coordinate bonds (two if both axial binding sites are filled by proteinaceous amino acids).
Fig. 1.
Pentacoordinate and hexacoordinate hemoglobins. A) The structure of ferric sperm whale myoglobin (2MBW.pdb) shows a pentacoordinate, low spin heme with an open distal binding site. B) The structure of Neuroglobin (1QIF.pdb) demonstrates hexacoordinate hemoglobin with the binding site occupied by the side chain of the distal histidine. In each structure, eight alpha-helices are labeled (A through H) along with showing the N-terminus (N-term), the C-terminus (C-term) and the distal (HD) and proximal (HP) histidines.
In practice, recombinant Hbs in the laboratory will readily adopt the ferrous and ferric oxidations states, and can be pushed into the ferryl state by exposure to hydrogen peroxide [14–16]. But without knowledge of function in vivo, it is difficult to judge the objective importance of these observations. However, the number of bonds coordinating the heme group can be objectively measured spectroscopically and by observation of protein structure, and has provided an important distinguishing classification for Hbs. In fact, the first discoveries of non-oxygen transport Hbs in plants and animals revealed recombinant proteins with coordination states distinct from penta-coordinate oxygen transporters [17,18] (Fig. 1B). These Hbs share the features of having their heme groups coordinated by two histidine side chains, one of which is capable of reversible dissociation to allow the stable binding of exogenous ligands like oxygen, carbon monoxide, and nitric oxide. While these proteins do not necessarily share sequence homology, and sequence alone has not yet been used to predict coordination state, the shared structural similarity of six coordinate bonds to the heme iron has resulted in these proteins being collectively referred to as “hexacoordinate” Hbs (hxHbs). The purpose of this review is to describe hexacoordinate Hbs by comparing their structures, ligand reactivities and biochemical activities.
1. In what organisms are hexacoordinate hemoglobins found?
1.1. Plant hemoglobins
HxHbs have been found in plants, animals, and cyanobacteria [18–20]. They were first noted in the plant “nonsymbiotic” hemoglobins (nsHbs), which were discovered during a search for globins in plants that are unrelated to oxygen transport [18]. Prior to the discovery of nsHbs, it was thought that Hbs in plants were limited to nitrogen fixing legumes, where these “leghemoglobins” (Lbs) scavenge oxygen and deliver it to respiring symbiotic bacteria in the root nodules [21,22]. The identification of globin genes in many other plants helped to explain the evolutionary origin of the Lbs, and brought about the continuing question of the physiological function of nsHbs [23]. Since the discovery and characterization of nsHbs in barley [18] and rice [24] nearly 15 years ago, dozens of plant globin genes have been sequenced and can be grouped into three different clades, corresponding to groups of plant Hbs derived from a common ancestor (Fig. 2), that exhibit distinct physical behavior [4].
Fig. 2.
Maximum likelihood phylogram of select plant globin sequences. Plant globins can be classified into three “classes”, each containing hexacoordinate members (red lines). The term “nonsymbiotic hemoglobin” (nsHb) is in deference to the previously discovered symbiotic “leghemoglobins”, which are pentacoordinate oxygen transporters.
With the exception of the Lbs, all plant Hbs show some degree of hexacoordinate character. The Class 2 nsHbs have the highest affinities for distal histidine coordination in the ferrous oxidation state (KH in Table 1), whereas the degree of coordination in Class 1 nsHbs is much less [4,25]. The Class 3 nsHbs share greater sequence similarity (40–45%) with bacterial Hbs of the “2-on-2” structural motif [26–32] than with the other nsHbs (b 25%) [33], and likely result from horizontal gene transfer from bacteria [34]. Many of the bacterial Hbs are also shorter in primary structure in which the antiparallel helix pairs B/E and G/H are arranged in a “2-on-2” sandwich, but the Class 3 nsHbs are actually longer than the other nsHbs and typical globins. The only reported bacterial hxHb is found in the cyanobacterium Synechocystis (SynHb) [20,35,36], which contains a “2-on-2” Hb that is hexacoordinate in both the ferrous and ferric oxidation states [37,38].
Table 1.
Rates and equilibrium constants for hexacoordination by the distal histidine.
| Kinetic and equilibrium constants for reversible distal histidine coordination | |||||
|---|---|---|---|---|---|
| Protein | Emid (mv) | kH2 (s−1) | k–H2 (s−1) | K H2 | Reference |
| Mb | 50 | ~0 | ~0 | [53] | |
| Mb H64V/V68H | −128 | >20,000 | >200 | ~100 | [53]a |
| Ngbhuman | −115 | 1900 | 1.5 | 2000 | |
| Ngbmouse | −129 | 1000 | 0.5 | 2000 | |
| Ngbzebrafish | 2500 | 2 | 1250 | [39], [46,48]b | |
| Cgb | −28 | 315 | 1.3 | ~400 | [40,47] |
| DrosHb | 550 | 30 | 18 | [49] | |
| Mollusk nHb | 14,000 | 1000 | 14 | [41] | |
| Plant nsHb1 (average) | 130 | 75 | 1.7 | ||
| Rice nsHb1 | −143 | 75 | 40 | 1.9 | |
| Plant nsHb2 (average) | 1500 | 25 | 84 | ||
| Tomato nsHb2 | 1400 | 30 | 60 | ||
| SynHb | −195 | 4200 | 14 | 300 | [48] |
Average values for members of each class are in bold. The value of KH2 for Mb H64V/V68H is unpublished, and is from a personal communication from John S. Olson; other values are from [53].
For Ngb, KH is average of values from these references.
1.2. Animal hemoglobins
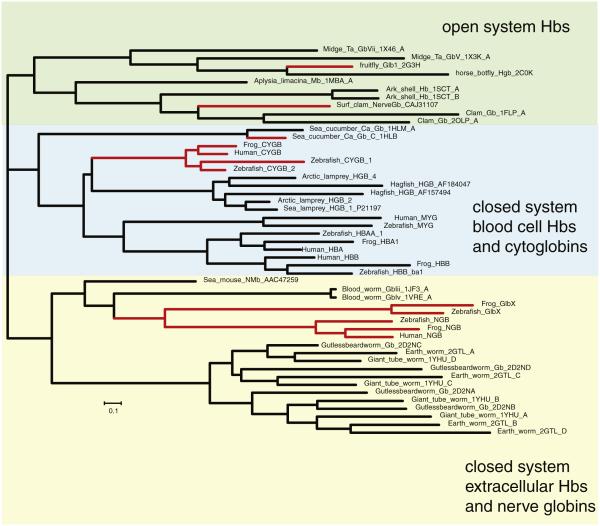
Concurrent with the discovery of hxHbs in plants was the identification of new globin sequences in the genomes of hundreds of species spanning all kingdoms of life [2,19,39–44]. Examination of recombinant proteins resulting from many of these gene sequences has identified the presence of hxHbs within each of the three major groups of animal Hbs (Fig. 3). Animal Hbs can be grouped into three separate clades in a manner that is consistent with the nature of the circulatory system of the corresponding organism and with how the oxygen transport Hbs are packaged within the circulatory system. In the phylogeny shown in Fig. 3, for example, the top-most clade corresponds to arthropod and mollusk Hbs, two groups that have open circulatory systems. The middle clade consists of intracellular oxygen transport Hbs from organisms that have closed circulatory systems. The bottom-most clade consists of extracellular Hbs from organisms that have closed circulatory systems.
Fig. 3.
Maximum likelihood phylogram of select metazoan globin sequences. Multicellular animal globin sequences segregate into three major clades, and there are hexacoordinate members of each clade (shown in red). In each case, the phylogeny indicates that hxHbs have evolved from pentacoordinate progenitors.
The animal hxHbs that are most closely related to the cell-bound Hbs in closed circulatory systems are the “cytoglobins” (Cgb) found in vertebrates and “Hb Chain C” from the sea cucumber Caudina arenicola [2,40,45]. The two other vertebrate hxHbs, “neuroglobins” (Ngb) and “globin X” (GlbX), are more closely related to extracellular oxygen transport Hbs present in animals with closed circulatory systems [19,43,44]. In general, the Ngbs are very strongly coordinated by the distal histidine in both the ferrous and ferric oxidation states, Cgbs are intermediate in this regard, and hxHbs from branch 1 of the tree are most weakly hexacoordinate, on par with the nsHbs from plants [40,41,46–49] (Table 1).
2. The chemistry of hexacoordinate hemoglobins
Because our knowledge of hxHbs is based mainly on in vitro reactions with recombinant proteins, our knowledge of their chemistry and reactions with ligands is limited to what they can do under controlled experimental conditions. And because the experimental questions asked are often influenced by our knowledge of the function and reactivity of oxygen transporters, much of what we know about hxHbs is derived from similar experiments. Thus, the data presented in Tables 1 and 2 and the following discussion of the chemistry and reactivity of hxHbs is grounded in a comparison to their pentacoordinate oxygen transport counterparts.
Table 2.
Kinetic and equilibrium constants for ligand binding to ferrous hexacoordinate hemoglobins.
| Proteins | k′ co,pent (μM−1 s−1) | kco, (s−1) | Kco (μM−1) | k′O2 (μM−1 s−1) | kO2, (s−1) | KO2 (μM−1) |
P50 (torr) from kinetics |
P50 (torr) from EQ |
kautox (s−1) |
|---|---|---|---|---|---|---|---|---|---|
| Mb | 0.51 | 0.02 | 25.5 | 17 | 15 | 1.1 | 0.5 | 0.33 | |
| Mb H64V/V68H | 0.1 | 0.005 | 15 | Fast | |||||
| Ngbhuman | 39 | 0.01 | 2 | 150 | 0.6 | 0.13 | 5 | 5 | 0.18(25) 5.4 (37) |
| Ngbmouse | 72 | 0.013 | 2.7 | 200 | 0.4 | 0.25 | 2.4 | 2.2 | 19 (37) |
| Ngbzebrafish | 70 | na | na | 250 | 0.3 | 0.7 | 0.9 | 0.7 | |
| Cgb | 5 | 0.003 | 4 | 30 | 0.35 | 3 | 1 | ||
| DrosHb | 13 | na | na | 64 | 1 | 3.3 | 0.2 | 0.1 | |
| Mollusk nHb | 75 | na | na | 130 | 30 | 0.3 | 1.9 | 0.6 | |
| nsHb1 (average) | 8.4 | na | na | 67 | 0.14 | 410 | 0.002 | ||
| Rice nsHb1 | 6.8 | 0.001 | 2300 | 60 | 0.038 | 540 | 0.08 (20, pH7) | ||
| nsHb2 average | 39 | 0.001 | 460 | 76 | 1.1 | 2.9 | 0.2 | ||
| Tomato nsHb2 | 26 | na | na | 45 | 0.4 | 1.8 | 0.3 | ||
| SynHb | 90 | na | na | 240 | 0.014 | 57 | 0.01 |
References for these values are as follows: Mb [50,154], Mb H64V/V68H (average values from human and pig [53]), Ngbhuman (CO values [17,39,46], O2 (average values from [17,39,155]), Ngbmouse (O2 values [155], CO values [46]), Ngbzebrafish [39,156], Cgb [40], O2 values [40,47], DrosHb [49], Mollusk Hb [41], nsHb1 (average values [24]), nsHb2 [4] (CO off values [157]), SynHb [35].
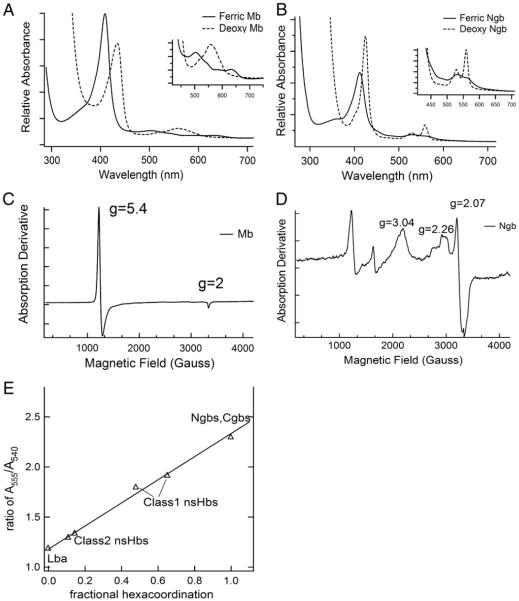
Absent any bound ligands, myoglobin (Mb) is pentacoordinate in both the ferrous and ferric oxidation states. This is readily evident from electronic absorbance spectra of both oxidation states, and from electron paramagnetic resonance spectroscopy for the ferric protein (Fig. 4). The characteristic visible-region absorption bands are weak and broad, with peaks near 500 and 635 nm for the ferric proteins, and a single asymmetric absorbance band near 555 nm for the ferrous proteins, indicating that the heme iron is in the high-spin electronic configuration in both oxidation states (Fig. 4A) [50]. On the contrary, histidine coordination to the sixth axial position converts the heme iron of hxHbs to the low spin electronic configuration in both oxidation states giving rise to stronger visible absorbance in the ferric state, and splitting of the ferrous visible absorbance band into two peaks near 560 and 530 nm (Fig. 4B). EPR is a particularly sensitive measure of the spin state in ferric Hbs, where high spin (usually pentacoordinate) Hbs exhibit a strong axial signal at g = 5.4 and 2 demonstrating a single species in the sample (Fig. 4C), and low spin Hbs (like the hxHbs) exhibit weaker and more complex spectra dominated by a rhombic signal with features at g values of 3, 2.2 and 2 (Fig. 4D) [51].
Fig. 4.
Electronic and paramagnetic spectral characteristics of hxHbs. A) Absorbance spectra of ferric and ferrous sperm whale Mb demonstrate characteristics of high spin, pentacoordinate hemoglobins. B) Absorbance spectra of Ngb in the ferric and ferrous oxidation states demonstrate characteristics typical of low spin hxHbs. C) The EPR spectrum of ferric sperm whale Mb shows the axial high-spin signal of iron at g = 5.4 and 2 D) The EPR spectrum of a low spin heme with g values of 3.04, 2.26 and 2.07, characteristic of a low spin iron as depicted by Ngb. EPR spectra were collected at 10 K with amplitude modulation of 10 G and frequency of 9.26 GHz.
While all ferric hxHbs bind the distal histidine with equilibrium constants ≫10 [52], the strength of coordination in the ferrous state is variable (Fig. 4E, Table 2) [48]. Ferrous Ngbs, Cgbs, and SynHb are very tightly coordinated compared to the plant nsHbs, drosophila Hb (DrosHb), and mollusk nerve Hb (Mollusk nHb). The “Class 3” nsHbs are low spin in the ferric form, and transition to the high-spin, pentacoordinate state upon reduction [33]. The differential strength of coordination is observed in the lower midpoint reduction potentials of hxHbs (Table 2), which are all well below 0, and typically near −130 mV [46,52,53].
Reversible binding of ligands to pentacoordinate Hbs is a bimolecular process often carried out under pseudo first order reaction conditions, with the ligand in excess of the protein [54]. Under these conditions, the observed rate of binding is linearly dependent on ligand concentration, with the slope of the line equal to the bimolecular rate constant. The first observations of ligand binding to hxHbs revealed limiting rates of bimolecular binding resulting from coordination of the distal histidine, which blocks the ligand binding site [24,40,46,54]. This complicates ligand binding reactions by preceding bimolecular interactions with a reversible first order event, as shown in Eq. (1) [54–56].
| (1) |
The degree to which distal histidine coordination affects binding time courses for exogenous ligands is influenced by two factors [48,57]: 1) the speed with which the coordinating histidine associates and dissociates from the heme iron, and 2) the equilibrium fraction of protein in the hexacoordinate state. If the speed of coordination (kH) and dissociation (k−H) is very rapid compared to the association of exogenous ligands (k′L[L]), then the hexacoordination reactions are at fast exchange on the time scale of the ligand binding reaction, and the observed time course will described by a single rate (kobs,L) influenced by the time-average of all three reactions.
| (2) |
If the speed of coordination is much slower than exogenous ligand binding, the observed time course will be limited by histidine dissociation and will potentially exhibit two phases, one for the fraction of Hb in the hexacoordinate state, and one for the fraction that is pentacoordinate.
| (3) |
Regardless of the kinetics of the reaction, the influence of hexacoordination on equilibrium affinity constants is directly related to the affinity constant for histidine coordination [17,40].
| (4) |
Eq. (4) demonstrates how hexacoordination could augment the innate equilibrium affinity constant of a pentacoordinate Hb by lowering the effective strength of binding [17,40,46].
The reaction central to the function of oxygen transport Hbs such as Mb and red blood cell Hb is the reversible binding of oxygen. The ferrous form of these proteins reacts with oxygen reversibly to form a stable protein usually referred to as the “oxy-ferrous” complex, although the exact electronic state of the oxygen and heme iron are still in question. There is evidence to support the oxygen bound complex existing mainly as , but upon reversible dissociation of oxygen, the heme iron is left in the Fe2+ state [50]. Occasionally, O2− will dissociate leaving the Fe3+ heme iron in one mechanism of oxidation (or “autooxidation”) of the Hb [58]. This process is relatively slow in oxygen transport Hbs due to stabilization of bound oxygen by the distal histidine [59], but there are many features of Hb structure that can affect rates of autooxidation by allowing solvent access to the heme pocket, and in ways that are not completely understood [60,61]. The mechanisms used to establish appropriate oxygen binding kinetics (and thus affinities) in oxygen transport proteins involve the combined efforts of the proximal and distal histidines [22,59]. In general, to facilitate oxygen diffusion, the oxygen dissociation rate constant must be at least 1 s− 1, affinities must be appropriate to maintain fractional saturation between the source and sink, and the Hb concentration must be higher than that of oxygen in solution [4,62–64].
Due to the augmentation of affinity by hexacoordination (Eq. (4)), several hxHbs have affinity constants appropriate for oxygen transport [4,17,40]. However, rates of oxygen dissociation from hxHbs are generally too slow for oxygen transport, with the exception of the mollusk nHb, DrosHb, and the Class 2 nsHbs [4,24,41,42,48]. Of the hxHbs for which autooxidation has been measured, rates are generally much faster than the pentacoordinate Hbs [46]. The reasons for the rapid rates of oxidation are not well understood, but could be related to rates of electron transfer, which is generally faster in hxHbs [65]. The rate constants for association and dissociation of oxygen and other diatomic ligands (like CO) are not exceptional in hxHbs, and fall within the range observed for the variety of pentacoordinate Hbs that have been reported [63]. Due to the increased strength of coordination by the distal histidine in the ferric oxidation state, ferric hxHbs are generally less reactive than their ferrous counterparts. This is indicated by generally slow and weak binding to ferric ligands such as cyanide and azide (Table 3).
Table 3.
Rates and equilibrium constants for ligand binding to ferric hexacoordinate hemoglo-bins, and for the NOD reaction.
| Kinetic and equilibrium constants for ligand binding to ferric hexacoordinate hemoglobins | ||||||
|---|---|---|---|---|---|---|
| Protein |
k′CN
(M−1 s−1) |
KDCN
(mM) |
k′azide
(M−1 s−1) |
KDazide
(mM) |
k′NO
(μM−1 s−1) |
kNOD,
(μM−1 s−1) |
| Mb | 320 | 0.001 | 2900 | 0.034 | 22 | 34 s−1 |
| Ngb | 1.6 | 300 s−1 | ||||
| Cgb | 500 s−1 | |||||
| DrosHb | 180 | |||||
| nsHb1 | 1.8 | 0.625 | 100 s−1 | |||
| SynHb | 0.7 | 2 | 200 | ~100 s−1 | ||
Reaction of Hbs with NO and other nitrogenous compounds of various oxidation states have been reported since the discovery of the role of NO as a biological signaling molecule that acts through binding the heme group of guanylyl cyclase [66,67]. It has been demonstrated that blood cell Hb and Mb are likely scavengers of NO in vivo [68–70], and that bacterial and fungal “flavohemoglobins” (flavoHbs) are scavengers of NO in those organisms [71]. While there is still some discussion of the mechanisms of these reactions [72], the most likely is the reaction of NO with oxy-ferrous Hb, yielding nitrate and ferric Hb (known as the nitric oxide dioxygenase reaction, or NOD) [68,70,71,73]. The possibility of a similar function has been tested in several hxHbs. In all cases, ferrous hxHbs will bind reversibly to NO [15], the ferric forms will react with NO [74] in some cases showing slow reduction [15,75–78], and the oxyferrous hxHbs will scavenge NO resulting in their oxidation (Table 3) [75,79–83]. In spite of the fact that the efficiency of these reactions is at best on-par with Mb and red blood cell Hb, and is certainly limited in vivo by as-of-yet unknown mechanisms for re-reduction of the heme iron [4], observation of the NOD reaction has been proposed as support for an NO scavenging function in nsHbs [80,84], Ngbs [79], and Cgbs [85]. However, unlike Mb and red blood cell Hb, whose high concentrations allow them to serve as effective NO scavengers in vivo in spite of relatively slow reduction mechanisms, hxHbs are present in very low concentrations, and only one specific reductase (for a nsHb [86]) has been identified that might support catalytic NO scavenging. Thus, there is still little direct chemical evidence supporting hxHbs functioning as NO scavengers to a degree greater than Mb and red blood cell Hb.
3. Structures of hexacoordinate hemoglobins
There are ten crystal structures of unbound and ligand-bound hxHbs published to date [37,42,87–93] (The PDB entries are listed in Fig. 6). These include structures of hexacoordinate representatives of each of the three branches of the animal phylogenetic tree, one of the three “classes” of plant hxHbs, and prokaryotic SynHb. In addition, the structure of a hexacoordinate globin domain from the multi-domain sensor GLB-6 of C. elegans has been solved [94]. Evaluation of quaternary structure from these crystal structures suggests that mouse Ngb and DrosHb are monomeric [46,49], and that rice nsHb and Cgb are dimeric [95–97]. Direct measurement of quaternary structure in solution by analytical ultracentrifugation has shown that rice nsHb dimerizes with a dissociation equilibrium constant of 86 μM [98], and that mouse Ngb is in fact monomeric [46]. Attempts to measure cooperativity in ligand binding studies with Ngb and Cgb by Weber and coworkers have shown Hill coefficients of ~ 1 for Ngb consistent with it being monomeric, and 0.63–1.63 for Cgb, implying possible heme–heme interactions for a dimer [99].
Fig. 6.
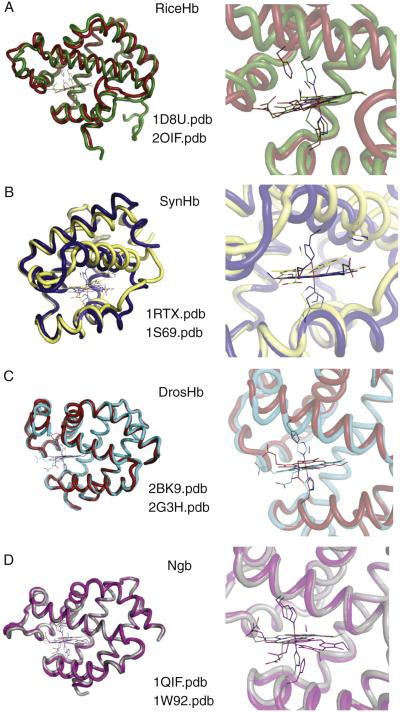
Structural changes upon ligand binding in hxHbs. These are structural overlays of A) Rice hxHb, B) Drosophila hxHb, C) SynHb, and D) Mouse Ngb in the hexacoordinate and exogenous ligand-bound states. E) Plot of root mean square deviation between hexacoordinate and ligand-bound crystal structures for each of the proteins above.
In general, the monomeric units of each structure of the eukaryotic hxHbs share the characteristic globin fold of eight helices (labeled A through H, Fig. 1) with a “3-on-3” helix assembly (Fig. 5A). The antiparallel sets of helices A/E/F and B/G/H are positioned into a 3-on-3 “sandwich” arrangement. This is in contrast to SynHb, which has the “2-on-2” α-helical “sandwich” fold common to many bacterial Hbs [100] (Fig. 5B). Among the eukaryotic hxHbs, the presence of a conspicuous D helix is variable [101], and the folding and stability of this helix has been implicated in the control of reversible distal histidine coordination [91,102]. Each hxHb crystal structure shows clear coordination by both the distal and proximal histidines. Additionally, there have been NMR, EPR, and vibrational spectroscopic characterizations to test heme iron hexacoordination in solution. Resonance Raman and EPR have confirmed heme iron hexacoordination in the ferric and the ferrous states of mouse Ngb [103], but NMR studies of reveal axial histidine orientations that are not consistent with those seen in crystal structures [14,88,104–106]. It is believed that this inconsistency may be due to the higher solvent content in solution, and fewer hydrogen bond acceptors N–H protons of the coordinating histidines [105]. EPR spectroscopic investigations of mouse Ngb by Vinck and coworkers are consistent with the X-ray diffraction data of the ferric protein [107,108].
Fig. 5.

Structural features of hxHbs. A) Rice nsHb1 overlayed with DrosHb shows a “3-on-3” globin core composed the A, E, and F helices stacking on top of the B, G, and H helices. B) The structure of SynHb belongs to structural class of globins with a “2-on-2” helical core consisting of the G and H helices stacking against the B and E helices. The N-terminal end of Helix A is largely truncated and CD loop and D-helix region shortened to three residues.
One of the most unusual properties of hxHbs is their ability to bind exogenous ligands. Other heme proteins with bis-histidyl coordination do not bind ligands [109,110], and a hexacoordinate Mb mutant protein is structurally constrained, and limited in its ability to bind exogenous ligands [53]. Thus, there has been interest in observing the structural changes accompanying ligand binding in the context of naturally occurring hxHbs. Structures of hexacoordinate and ligand-bound hxHbs have been solved for Ngb [88,89,111], class 1 plant nsHbs [91,92], DrosHb [42,90], and SynHb [37,93]. The structural rearrangements revealed for each protein are surprisingly distinct. In the case of class 1 plant nsHbs, distal histidine dissociation from the heme iron is accompanied by rotation and translation of the E helix, formation of a D helix through contacts that span several helices, and development of several new trans-helical contacts in the EF turn (Fig. 6A). Fig. 6E shows the RMS deviation between ligand-bound and hexacoordinate nsHb, indicating that most of the changes are associated with the E helix and its termini. The structured D helix and the stabilization of the EF turn are believed to support the pentacoordinate E-helix position. The structure of ligand-bound rice nsHb is consistent with the structures of Lbs, which evolved from class 2 nsHbs to become fixed in the pentacoordinate configuration [92,112].
In the case of cyanide-bound SynHb, the heme is tilted by ~ 6 degrees from the orientation seen in the unliganded structure. The A and B helices swing inward towards the heme, and the A helix lies in close proximity to the E helix (Fig. 6B). Additionally, the E helix and EF loop shift outward away from the heme cofactor in ligand-bound SynHb [37,93]. In general, ligand binding in plant nsHbs and bacterial Hbs involves large-scale motions of the E helix. This is evident from the root mean square deviation between unbound and bound ligand states for Rice Hb1 and SynHb (Fig. 6E). Structures of DrosHb reveal translation and flattening of the heme inside the heme pocket, and heme rotation accompanying ligand binding. A rearrangement of the CD loop and amino terminal half of the E-helix region is also observed upon ligand binding [42,90]. However, these conformational changes do not involve large movements of E helix, as seen in nsHbs (Fig. 6C).
In contrast to the examples above, the structural transition exhibited by mouse Ngb upon ligand binding is very modest (Fig. 6D, E). The heme slides deeper into the heme pocket away from the distal histidine to produce a binding site, but the overall protein structure is not significantly altered [89]. However, the ligand-bound crystal was prepared by reduction and carbon monoxide (CO) exposure to ferric hexacoordinate crystals, and the crystals had to be frozen prior to data collection to avoid cracking [89]. In fact, many hxHb crystal forms shatter when exogenous ligands are introduced, consistent with a structural change incompatible with the crystal lattice. Thus, it is possible that the CO-bound murine Ngb structure is not at equilibrium and that a larger structural change might be observed in crystals grown in the presence of a bound exogenous ligand.
4. Physiological functions of hexacoordinate hemoglobins
4.1. Neuroglobin
Because Ngb is found in the human brain, retina, and other nervous system tissues, the discovery of its physiological function has received a disproportionate amount of attention [113]. 1) Based on sequence similarity between Ngb and G-protein regulators, Wakasugi and coworkers have proposed a role for Ngb in G-protein mediated signaling [14,114,115]. Antibody pull-down assays and surface plasmon resonance experiments have demonstrated binding of Ngb to human Gαi/o, one of the many Gα proteins associated with heterotrimeric G protein signaling in animals. Binding occurs only to the GDP-bound (inactive) form of Gαi/o, which prevents Gαi/o from being recycled. As Gαi/o inactivation has been linked to protection of neurons during oxidative stress [116], Ngb could serve as a mechanism to limit ischemic damage by mitigating the deleterious effects of Gαi/o:GDP [117]. Although there is significant sequence conservation among Ngb sequences from various species, this activity is not exhibited by other Ngbs, such as that from zebrafish [115]. Thus, these observations tend to question the importance of this interaction in vivo.
2) The first hypothesis proposed for Ngb was that of oxygen transport. This was based on it being a hemoglobin [118], its presence in the retina (which consumes a lot of oxygen) [96,119,120], its intercellular location near mitochondria [19], and up-regulation of Ngb mRNA in response to hypoxia [121,122], although other studies refute this observation [123,124]. However, this hypothesis is not supported by the low concentrations of Ngb found in vivo, slow oxygen dissociation rate constants, and high oxygen equilibrium affinity constants observed for these proteins [19,125].
3) Currently, the most popular hypothesis for Ngb function is protection against hypoxia and oxidative stress, probably through a mechanism involving NO scavenging. Reperfusion of tissues following hypoxia is a well-known cause of damage resulting from reactive oxygen species, and Ngb is suggested to have a neuroprotective effect under such conditions [121,126–129]. Greenberg and coworkers have shown that expression of Ngb increases after ischemic stroke and that Ngb might be a novel target in stroke therapy [130]. A cognate reductase is required for NO scavenging, and there has been a lot of research directed toward finding one, but none has been identified yet [46,75,131,132]. Thus, there is currently no biochemical mechanism to explain how Ngb increases cell survival following ischemia.
Fago and coworkers have proposed a different molecular mechanism for the protective effect of Ngb in cell death induced by hypoxia, based on a fast electron transfer between ferrous Ngb and ferric cytochrome c [125,133]. In this mechanism, Ngb causes rapid reduction of ferric cytochrome c, thereby maintaining levels of non-apoptotic ferrous cytochrome c and simultaneously generating ferric Ngb. Raychaudhuri et al. have reported a similar neuroprotective role of Ngb by way of inhibiting apoptosis [134]. This hypothesis also requires a mechanism for reduction of Ngb, and a candidate reductase, Apoptosis Inducing Factor (AIF), was proposed for this function. However, a direct test of this activity in vitro failed to support a role for AIF in Ngb reduction [131].
4.2. Cytoglobin
Cgb is expressed in almost all types of human tissues [2,40], but was first identified in a proteomic screen of conditions activating hepatic stellate cells [135]. Although all other hxHbs have very high oxygen affinity, Cgb possesses an oxygen affinity comparable to that of Mb [40,136], and is up-regulated following hypoxia [95,137]. Thus, it is possible that Cgb replaces Mb function in tissues where the latter is not expressed [138]. In a role similar to one proposed for Ngb, Cgb is a possible NO scavenger for the purpose of fibroblast proliferation [85], or cytoprotection under oxidizing conditions such as ischemic reperfusion injury [139]. Alternatively, others have proposed roles for Cgb in collagen synthesis [95], and tumor suppression [140], but distinct mechanisms for these roles remain to be established.
4.3. Drosophila hemoglobin
DrosHb is a monomeric globin discovered in the fruit fly, Drosophila melanogaster [49,141]. It is present in low concentrations in the tracheal system and the fat body of both larval and adult fly. Early research speculated a role in facilitated oxygen diffusion across tracheal walls for the protein. However, the Hb expression levels decreased under limiting oxygen conditions making it unlikely to function as an oxygen storage protein [49]. Like Ngb and Cgb, DrosHb is also a candidate for protection against reactive oxygen species produced by oxygen diffused via the trachea, but there is no clear evidence or mechanism supporting this role [142].
4.4. Mollusk nerve Hb
The nerve Hb from the bivalve mollusk, S. solidissima is believed to be an oxygen transport protein [41]. Such glial nerve Hbs are common in species exposed to hypoxia, and enable neuronal function under these conditions [143]. These globins enable mollusk neuronal function and survival under low levels of oxygen, either prevalent in natural habitats or under ischemic conditions [144,145]. This globin has oxygen binding rate constants very similar to the leghemoglobins, and could seemingly transport oxygen effectively as a pentacoordinate Hb. Thus, the role of hexacoordination in its function could be simply to lower the overall affinity to that needed in this specific environment.
4.5. Globin X
GlbX is weakly expressed in amphibia and fish [44]. Its primary sequence is longer than other HxHbs, possessing 25–30 extra amino acids at both the N- and C-termini. However, the proximal and distal histidines along with phenylalanine at CD1 position are conserved [43]. GlbX is distantly related to Ngb, but mRNA expression analysis in goldfish has shown that it is not a neuronal protein [44]. The function of this globin is still unknown (hence the name “Globin X”).
4.6. Caudina arenicola Chain C
The sea cucumber Caudina arenicola has four different globin chains, which together facilitate cooperative oxygen transport. The mechanism of cooperativity is not completely understood, but appears to involve changes in heteromeric quaternary structure linked to ligand binding [45]. In spite of very high sequence similarity among these chains, only the “Chain C” subunit is hexacoordinate. When first discovered in the absence of other known hxHbs, this observation could have been considered an aberrant conformation resulting from experimental conditions, or an alternative conformation rarely occupied in vivo (such as the case with low-pH human α chains). However, it is also possible that hexacoordination plays a role in these systems, as proposed for human α chains, where it could help to maintain ferrous heme iron by facilitating reduction [146].
4.7. Plant nsHbs
Class 1 nsHbs are expressed at low levels in root tissues and have high oxygen affinities with low dissociation rate constants, indicating that they are unlikely to serve as oxygen transport proteins [4,22]. Robert Hill and coworkers have proposed a function for Class 1 nsHbs in the maintenance of redox and energy status in plant cells under hypoxia [147,148]. Cells expressing Class 1 nsHb display elevated ATP levels, low accumulation of NO, and decreased cell death under hypoxia [149]. NOD function has also been explored for these Hbs [75,82,150], and the rate of NADH-dependent reduction is enhanced by cytosolic monodehydroascorbate reductase, a likely cognate reductase in vivo [86]. Much less is known about Class 2 and Class 3 nsHbs. It has been shown that, similar to Class 1 nsHb, over-expression of Class 2 nsHbs increases cell survival during hypoxia. Class 2 Hbs exhibit tighter hexacoordination than Class 1 nsHbs and thus have lower oxygen affinities, increasing the likelihood of possible roles in sensing sustained low levels of oxygen [25,151].
Class 3 nsHbs are found in most plant genomes, with expression reported to be in root and shoot tissues, and down-regulated during hypoxia [33,152]. A Class 3 nsHb from Arabidopsis shows transient hexacoordination upon reduction, and has unusual ligand binding kinetics [33]. However, these properties have not yet been examined in much detail, and there has been little discussion of potential physiological roles for this class of proteins. Further characterization is needed to determine if the “2-on-2” structural motif observed in their prokaryotic homologous is present in the plant proteins, even though their primary sequences are longer than the “truncated” versions found in bacteria.
4.8. SynHb
SynHb has been shown to have a nitric oxide scavenging function in vitro [75]. However, a reductase remains to be characterized in order to sustain the reaction in vivo. Large structural motions on ligand binding suggest that SynHb might play a role in signaling mechanisms, as has been proposed for human Ngb [114,115]. An enzymatic role in oxidation–reduction chemistry has also been proposed based on the hydrogen bond networks in the crystal structure [93].
5. Which came first, pentacoordinate or hexacoordinate Hbs?
The globins found in prokaryotes, eukaryotes, and archaea are believed to have evolved from a common ancestor with a function unrelated to oxygen transport [143]. Whether this primordial Hb was of the “3-on-3” or the “2-on-2” structural variety, and whether its coordination state was pentacoordinate or hexacoordinate is unknown. In fact, examination of Figs. 2 and 3 suggests a difference in coordination state for the progenitors of plant and animal Hbs, respectively. In plants, pentacoordinate oxygen transporters have evolved from hexacoordinate Hbs in both Class 1 and Class 2 nsHbs [4,153], and there are no examples of hxHbs evolving from pentacoordinate proteins. By contrast, all animal hxHbs evolved from a pentacoordinate ancestral state. In fact, phylogenetic evidence indicates that evolutionary transitions from an ancestral pentacoordinate state to a derived hexacoordinate state have occurred four times independently (Fig. 3). As the tree in Fig. 3 illustrates, DrosHb, nHb of mollusks, Cgb of gnathostome vertebrates, and the Ngbs of all animals have each independently evolved hexacoordination from different ancestral starting points. There is no evidence of pentacoordinate Hbs arising from hxHbs in the metazoan phylogeny. However, the predominance of pentacoordinate Hbs in bacteria suggests that this coordination state has existed far longer than it has served as a scaffold for oxygen transport. Therefore, hxHbs could very well have evolved from pentacoordinate Hbs in contrast to earlier suggestions [22,106].
6. Conclusions
The discovery of reversible histidine coordination and exogenous ligand binding in Hbs was surprising in light of the relative inertness of cytochrome b5. The structures of hxHbs in the hexacoordinate and ligand-bound states have revealed different mechanisms for achieving histidine dissociation from the ligand binding site, ranging from the large conformational changes observed in SynHb to modest repositioning of the heme in Ngb. The conformational changes accompanying ligand binding in SynHb and rice nsHb are relatively large, indicating a degree of flexibility in the globin fold that was not anticipated from previous globin structures. These conformational changes could be a component of the mechanism of action of hxHbs, or a structural necessity for reversible ligand binding; an answer to this question must await identification of physiological function(s).
It was first thought that, in general, pentacoordinate Hbs evolved from hxHbs. This makes sense from a structural perspective; a distal histidine nearby but not coordinating the heme iron would be difficult to stabilize in a flexible globin. It was logical to consider that pentacoordinate Hbs evolved from hxHbs by stabilizing the penta-coordinate conformation of the hxHb, and reducing the flexibility of the globin to lock it into only this conformation. However, the animal Hb phylogeny does not support this conclusion, showing instead that pentacoordinate Hbs predate hxHbs in animals. This is an important consideration as it suggests selection for a hexacoordinate heme center and the accompanying chemistry and potential conformational variability. It is thus likely that these properties will be linked to function, and should be carefully considered as these physiological activities are identified.
Based on the current description of hxHbs, it seems likely that their function(s) involve 1) exogenous ligand binding, 2) a change in heme iron oxidation state, and 3) a role in signaling. These conclusions are based on the following observations, respectively. 1) Hexacoordination and affinity for exogenous ligands is conserved across each group of hxHbs, and even across the classes of plant nsHbs. 2) Hexacoordination facilitates electron transfer. If the goal were simply to bind and release ligands, a pentacoordinate heme would be preferred (as in oxygen transport Hbs). 3) HxHbs are present in very low concentrations, and ligand binding could trigger conformational and redox changes that regulate interactions with other signaling molecules.
Identification of the function of proteins is the next frontier of biochemistry, and is certainly the rate-limiting step in our understanding of hxHbs. The magnitude of this problem is realized by considering the difficulty that would face researchers trying to discover the function of the red blood cell Hb subunits using only recombinant proteins in the laboratory. The behavior of these isolated chains reflects that of native Hb in some ways, but could also lead down many false paths. This is the situation with hxHbs, where the results from in vitro experiments are certainly telling us something about physiological function, but are also providing much more information than can be assimilated into clear hypothesis in the background of a much smaller number of physiological studies. A confident interpretation of biochemistry will only come from its ability to explain a clear physiological function.
Abbreviations
- Hb
hemoglobin
- Hxhb
hexacoordinate hemoglobins
- nsHb
non-symbiotic hemoglobins
- Lbs
leghemoglobins
- SynHb
Synechocystis sp hemoglobin
- Ngb
neuroglobin
- Cgb
cytoglobin
- GlbX
globin X
- Mb
myoglobin
- DrosHb
drosophila hb
- Mollusk nhb
mollusk nerve hemoglobin
- CO
carbon monoxide
- NO
nitric oxide
- EPR
electron paramagnetic resonance
- NMR
nuclear magnetic resonance
References
- [1].Hankeln T, Ebner B, Fuchs C, Gerlach F, Haberkamp M, Laufs T, Roesner A, Schmidt M, Weich B, Wystub S, Saaler-Reinhardt S, Reuss S, Bolognesi M, De Sanctis D, Marden M, Kiger L, Moens L, Dewilde S, Nevo E, Avivi A, Weber R, Fago A, Burmester T. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J. Inorg. Biochem. 2005;99:110–119. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- [2].Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol. Biol. Evol. 2002;19:416–421. doi: 10.1093/oxfordjournals.molbev.a004096. [DOI] [PubMed] [Google Scholar]
- [3].Wittenberg J, Bolognesi M, Wittenberg B, Guertin M. Truncated hemoglobins: a new family of hemoglobins widely distributed in bacteria, unicellular eukaryotes, and plants. J. Biol. Chem. 2002;277:871–874. doi: 10.1074/jbc.R100058200. [DOI] [PubMed] [Google Scholar]
- [4].Smagghe B, Hoy J, Percifield R, Kundu S, Hargrove M, Sarath G, Hilbert J, Watts R, Dennis E, Peacock W, Dewilde S, Moens L, Blouin G, Olson J, Appleby C. Review: correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers. 2009;91:1083–1096. doi: 10.1002/bip.21256. [DOI] [PubMed] [Google Scholar]
- [5].Murray J, Delumeau O, Lewis R. Structure of a nonheme globin in environmental stress signaling. Proc. Natl. Acad. Sci. U. S. A. 2005;102:17320–17325. doi: 10.1073/pnas.0506599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Smith L, Kahraman A, Thornton J. Heme proteins — diversity in structural characteristics, function, and folding. Proteins. 2010;78:2349–2368. doi: 10.1002/prot.22747. [DOI] [PubMed] [Google Scholar]
- [7].Derbyshire E, Marletta M. Biochemistry of soluble guanylate cyclase. Handb. Exp. Pharmacol. 2009:17–31. doi: 10.1007/978-3-540-68964-5_2. [DOI] [PubMed] [Google Scholar]
- [8].Boon E, Marletta M. Ligand discrimination in soluble guanylate cyclase and the H-NOX family of heme sensor proteins. Curr. Opin. Chem. Biol. 2005;9:441–446. doi: 10.1016/j.cbpa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [9].Lawson D, Stevenson C, Andrew C, Eady R. Unprecedented proximal binding of nitric oxide to heme: implications for guanylate cyclase. EMBO J. 2000;19:5661–5671. doi: 10.1093/emboj/19.21.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Traylor TG, Sharma VS. Why NO? Biochemistry. 1992;31:2847–2849. doi: 10.1021/bi00126a001. [DOI] [PubMed] [Google Scholar]
- [11].Hille R, Olson JS, Palmer G. Spectral transitions of nitrosyl hemes during ligand binding to hemoglobin. J. Biol. Chem. 1979;254:12110–12120. [PubMed] [Google Scholar]
- [12].Duprat A, Traylor T, Wu G, Coletta M, Sharma V, Walda K, Magde D. Myoglobin-NO at low pH: free four-coordinated heme in the protein pocket. Biochemistry. 1995;34:2634–2644. doi: 10.1021/bi00008a030. [DOI] [PubMed] [Google Scholar]
- [13].Zhou S, Olson J, Fabian M, Weiss M, Gow A. Biochemical fates of alpha hemoglobin bound to alpha hemoglobin-stabilizing protein AHSP. J. Biol. Chem. 2006;281:32611–32618. doi: 10.1074/jbc.M607311200. [DOI] [PubMed] [Google Scholar]
- [14].Hua S, Antao S, Corbett A, Witting P. The significance of neuroglobin in the brain. Curr. Med. Chem. 2010;17:160–172. doi: 10.2174/092986710790112611. [DOI] [PubMed] [Google Scholar]
- [15].Herold S, Fago A, Weber R, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J. Biol. Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- [16].Witting P, Douglas D, Mauk A. Reaction of human myoglobin and H2O2. Involvement of a thiyl radical produced at cysteine 110. J. Biol. Chem. 2000;275:20391–20398. doi: 10.1074/jbc.M000373200. [DOI] [PubMed] [Google Scholar]
- [17].Trent JR, Watts R, Hargrove M. Human neuroglobin, a hexacoordinate hemoglobin that reversibly binds oxygen. J. Biol. Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- [18].Duff S, Wittenberg J, Hill R. Expression, purification, and properties of recombinant barley (Hordeum sp.) hemoglobin. Optical spectra and reactions with gaseous ligands. J. Biol. Chem. 1997;272:16746–16752. doi: 10.1074/jbc.272.27.16746. [DOI] [PubMed] [Google Scholar]
- [19].Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- [20].Scott N, Lecomte J. Cloning, expression, purification, and preliminary characterization of a putative hemoglobin from the cyanobacterium Synechocystis sp. PCC 6803. Protein Sci. 2000;9:587–597. doi: 10.1110/ps.9.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Appleby C, Tjepkema J, Trinick M. Hemoglobin in a nonleguminous plant, parasponia: possible genetic origin and function in nitrogen fixation. Science. 1983;220:951–953. doi: 10.1126/science.220.4600.951. [DOI] [PubMed] [Google Scholar]
- [22].Kundu S, Trent J.r., Hargrove M. Plants, humans and hemoglobins. Trends Plant Sci. 2003;8:387–393. doi: 10.1016/S1360-1385(03)00163-8. [DOI] [PubMed] [Google Scholar]
- [23].Bogusz D, Appleby C, Landsmann J, Dennis E, Trinick M, Peacock W. Functioning haemoglobin genes in non-nodulating plants. Nature. 1988;331:178–180. doi: 10.1038/331178a0. [DOI] [PubMed] [Google Scholar]
- [24].Arredondo-Peter R, Hargrove M, Sarath G, Moran J, Lohrman J, Olson J, Klucas R. Rice hemoglobins. Gene cloning, analysis, and O2-binding kinetics of a recombinant protein synthesized in Escherichia coli. Plant Physiol. 1997;115:1259–1266. doi: 10.1104/pp.115.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bruno S, Faggiano S, Spyrakis F, Mozzarelli A, Abbruzzetti S, Grandi E, Viappiani C, Feis A, Mackowiak S, Smulevich G, Cacciatori E, Dominici P. The reactivity with CO of AHb1 and AHb2 from Arabidopsis thaliana is controlled by the distal HisE7 and internal hydrophobic cavities. J. Am. Chem. Soc. 2007;129:2880–2889. doi: 10.1021/ja066638d. [DOI] [PubMed] [Google Scholar]
- [26].Yamauchi K, Tada H, Usuki I. Structure and evolution of Paramecium hemoglobin genes. Biochim. Biophys. Acta. 1995;1264:53–62. doi: 10.1016/0167-4781(95)00114-v. [DOI] [PubMed] [Google Scholar]
- [27].Iwaasa H, Takagi T, Shikama K. Protozoan myoglobin from Paramecium caudatum. Its unusual amino acid sequence. J. Mol. Biol. 1989;208:355–358. doi: 10.1016/0022-2836(89)90395-1. [DOI] [PubMed] [Google Scholar]
- [28].Iwaasa H, Takagi T, Shikama K. Protozoan hemoglobin from Tetrahymena pyriformis. Isolation, characterization. and amino acid sequence. J. Biol. Chem. 1990;265:8603–8609. [PubMed] [Google Scholar]
- [29].Takagi T, Iwaasa H, Yuasa H, Shikama K, Takemasa T, Watanabe Y. Primary structure of Tetrahymena hemoglobins. Biochim. Biophys. Acta. 1993;1173:75–78. doi: 10.1016/0167-4781(93)90245-9. [DOI] [PubMed] [Google Scholar]
- [30].Potts M, Angeloni S, Ebel R, Bassam D. Myoglobin in a cyanobacterium. Science. 1992;256:1690–1691. doi: 10.1126/science.256.5064.1690. [DOI] [PubMed] [Google Scholar]
- [31].Couture M, Das T, Lee H, Peisach J, Rousseau D, Wittenberg B, Wittenberg J, Guertin M. Chlamydomonas chloroplast ferrous hemoglobin. Heme pocket structure and reactions with ligands. J. Biol. Chem. 1999;274:6898–6910. doi: 10.1074/jbc.274.11.6898. [DOI] [PubMed] [Google Scholar]
- [32].Couture M, Yeh S, Wittenberg B, Wittenberg J, Ouellet Y, Rousseau D, Guertin M. A cooperative oxygen-binding hemoglobin from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11223–11228. doi: 10.1073/pnas.96.20.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Watts R, Hunt P, Hvitved A, Hargrove M, Peacock W, Dennis E. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10119–10124. doi: 10.1073/pnas.191349198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vinogradov S, Hoogewijs D, Bailly X, Arredondo-Peter R, Guertin M, Gough J, Dewilde S, Moens L, Vanfleteren J. Three globin lineages belonging to two structural classes in genomes from the three kingdoms of life. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11385–11389. doi: 10.1073/pnas.0502103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hvitved A, Trent J.r., Premer S, Hargrove M. Ligand binding and hexacoordination in synechocystis hemoglobin. J. Biol. Chem. 2001;276:34714–34721. doi: 10.1074/jbc.M105175200. [DOI] [PubMed] [Google Scholar]
- [36].Couture M, Das T, Savard P, Ouellet Y, Wittenberg J, Wittenberg B, Rousseau D, Guertin M. Structural investigations of the hemoglobin of the cyanobacterium Synechocystis PCC6803 reveal a unique distal heme pocket. Eur. J. Biochem. 2000;267:4770–4780. doi: 10.1046/j.1432-1327.2000.01531.x. [DOI] [PubMed] [Google Scholar]
- [37].Hoy J, Kundu S, Trent J.r., Ramaswamy S, Hargrove M. The crystal structure of Synechocystis hemoglobin with a covalent heme linkage. J. Biol. Chem. 2004;279:16535–16542. doi: 10.1074/jbc.M313707200. [DOI] [PubMed] [Google Scholar]
- [38].Vu B, Jones A, Lecomte J. Novel histidine-heme covalent linkage in a hemoglobin. J. Am. Chem. Soc. 2002;124:8544–8545. doi: 10.1021/ja026569c. [DOI] [PubMed] [Google Scholar]
- [39].Fuchs C, Heib V, Kiger L, Haberkamp M, Roesner A, Schmidt M, Hamdane D, Marden M, Hankeln T, Burmester T. Zebrafish reveals different and conserved features of vertebrate neuroglobin gene structure, expression pattern, and ligand binding. J. Biol. Chem. 2004;279:24116–24122. doi: 10.1074/jbc.M402011200. [DOI] [PubMed] [Google Scholar]
- [40].Trent J.r., Hargrove M. A ubiquitously expressed human hexacoordinate hemoglobin. J. Biol. Chem. 2002;277:19538–19545. doi: 10.1074/jbc.M201934200. [DOI] [PubMed] [Google Scholar]
- [41].Dewilde S, Ebner B, Vinck E, Gilany K, Hankeln T, Burmester T, Kreiling J, Reinisch C, Vanfleteren J, Kiger L, Marden M, Hundahl C, Fago A, Van Doorslaer S, Moens L. The nerve hemoglobin of the bivalve mollusc Spisula solidissima: molecular cloning, ligand binding studies, and phylogenetic analysis. J. Biol. Chem. 2006;281:5364–5372. doi: 10.1074/jbc.M509486200. [DOI] [PubMed] [Google Scholar]
- [42].de Sanctis D, Dewilde S, Vonrhein C, Pesce A, Moens L, Ascenzi P, Hankeln T, Burmester T, Ponassi M, Nardini M, Bolognesi M. Bishistidyl heme hexacoordination, a key structural property in Drosophila melanogaster hemoglobin. J. Biol. Chem. 2005;280:27222–27229. doi: 10.1074/jbc.M503814200. [DOI] [PubMed] [Google Scholar]
- [43].Fuchs C, Burmester T, Hankeln T. The amphibian globin gene repertoire as revealed by the Xenopus genome. Cytogenet. Genome Res. 2006;112:296–306. doi: 10.1159/000089884. [DOI] [PubMed] [Google Scholar]
- [44].Roesner A, Fuchs C, Hankeln T, Burmester T. A globin gene of ancient evolutionary origin in lower vertebrates: evidence for two distinct globin families in animals. Mol. Biol. Evol. 2005;22:12–20. doi: 10.1093/molbev/msh258. [DOI] [PubMed] [Google Scholar]
- [45].Mitchell D, Kitto G, Hackert M. Structural analysis of monomeric hemichrome and dimeric cyanomet hemoglobins from Caudina arenicola. J. Mol. Biol. 1995;251:421–431. doi: 10.1006/jmbi.1995.0445. [DOI] [PubMed] [Google Scholar]
- [46].Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden M, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J. Biol. Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- [47].Hamdane D, Kiger L, Dewilde S, Green B, Pesce A, Uzan J, Burmester T, Hankeln T, Bolognesi M, Moens L, Marden M. The redox state of the cell regulates the ligand binding affinity of human neuroglobin and cytoglobin. J. Biol. Chem. 2003;278:51713–51721. doi: 10.1074/jbc.M309396200. [DOI] [PubMed] [Google Scholar]
- [48].Smagghe B, Sarath G, Ross E, Hilbert J, Hargrove M. Slow ligand binding kinetics dominate ferrous hexacoordinate hemoglobin reactivities and reveal differences between plants and other species. Biochemistry. 2006;45:561–570. doi: 10.1021/bi051902l. [DOI] [PubMed] [Google Scholar]
- [49].Hankeln T, Jaenicke V, Kiger L, Dewilde S, Ungerechts G, Schmidt M, Urban J, Marden M, Moens L, Burmester T. Characterization of Drosophila hemoglobin. Evidence for hemoglobin-mediated respiration in insects. J. Biol. Chem. 2002;277:29012–29017. doi: 10.1074/jbc.M204009200. [DOI] [PubMed] [Google Scholar]
- [50].Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Reactions with Ligands. North-Holland Publishing Company; Amsterdam: 1971. [Google Scholar]
- [51].Peisach J, Blumberg W, Wittenberg B, Wittenberg J, Kampa L. Hemoglobin A: an electron paramagnetic resonance study of the effects of interchain contacts on the heme symmetry of high-spin and low-spin derivatives of ferric alpha chains. Proc. Natl. Acad. Sci. U. S. A. 1969;63:934–939. doi: 10.1073/pnas.63.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Halder P, Trent J.r., Hargrove M. Influence of the protein matrix on intramolecular histidine ligation in ferric and ferrous hexacoordinate hemoglobins. Proteins. 2007;66:172–182. doi: 10.1002/prot.21210. [DOI] [PubMed] [Google Scholar]
- [53].Dou Y, Admiraal S, Ikeda-Saito M, Krzywda S, Wilkinson A, Li T, Olson J, Prince R, Pickering I, George G. Alteration of axial coordination by protein engineering in myoglobin. Bisimidazole ligation in the His64–NVal/Val68–NHis double mutant. J. Biol. Chem. 1995;270:15993–16001. doi: 10.1074/jbc.270.27.15993. [DOI] [PubMed] [Google Scholar]
- [54].Hargrove M. A flash photolysis method to characterize hexacoordinate hemoglobin kinetics. Biophys. J. 2000;79:2733–2738. doi: 10.1016/S0006-3495(00)76512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Brancaccio A, Cutruzzolá F, Allocatelli C, Brunori M, Smerdon S, Wilkinson A, Dou Y, Keenan D, Ikeda-Saito M, Brantley RJ. Structural factors governing azide and cyanide binding to mammalian metmyoglobins. J. Biol. Chem. 1994;269:13843–13853. [PubMed] [Google Scholar]
- [56].Coletta M, Angeletti M, De Sanctis G, Cerroni L, Giardina B, Amiconi G, Ascenzi P. Kinetic evidence for the existence of a rate-limiting step in the reaction of ferric hemoproteins with anionic ligands. Eur. J. Biochem. 1996;235:49–53. doi: 10.1111/j.1432-1033.1996.00049.x. [DOI] [PubMed] [Google Scholar]
- [57].Smagghe B, Halder P, Hargrove M. Measurement of distal histidine coordination equilibrium and kinetics in hexacoordinate hemoglobins. Methods Enzymol. 2008;436:359–378. doi: 10.1016/S0076-6879(08)36020-0. [DOI] [PubMed] [Google Scholar]
- [58].Brantley RJ, Smerdon S, Wilkinson A, Singleton E, Olson J. The mechanism of autooxidation of myoglobin. J. Biol. Chem. 1993;268:6995–7010. [PubMed] [Google Scholar]
- [59].Olson J, Phillips GJ. Kinetic pathways and barriers for ligand binding to myoglobin. J. Biol. Chem. 1996;271:17593–17596. doi: 10.1074/jbc.271.30.17593. [DOI] [PubMed] [Google Scholar]
- [60].Liong E, Dou Y, Scott E, Olson J, Phillips GJ. Waterproofing the heme pocket. Role of proximal amino acid side chains in preventing hemin loss from myoglobin. J. Biol. Chem. 2001;276:9093–9100. doi: 10.1074/jbc.M008593200. [DOI] [PubMed] [Google Scholar]
- [61].Aranda R.t., Cai H, Worley C, Levin E, Li R, Olson J, Phillips GJ, Richards M. Structural analysis of fish versus mammalian hemoglobins: effect of the heme pocket environment on autooxidation and hemin loss. Proteins. 2009;75:217–230. doi: 10.1002/prot.22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Murray J, Wyman J. Facilitated diffusion. The case of carbon monoxide. J. Biol. Chem. 1971;246:5903–5906. [PubMed] [Google Scholar]
- [63].Gibson Q, Wittenberg J, Wittenberg B, Bogusz D, Appleby C. The kinetics of ligand binding to plant hemoglobins. Structural implications. J. Biol. Chem. 1989;264:100–107. [PubMed] [Google Scholar]
- [64].Wittenberg J, Appleby C, Wittenberg B. The kinetics of the reactions of leghemoglobin with oxygen and carbon monoxide. J. Biol. Chem. 1972;247:527–531. [PubMed] [Google Scholar]
- [65].Weiland T, Kundu S, Trent JR, Hoy J, Hargrove M. Bis-histidyl hexacoordination in hemoglobins facilitates heme reduction kinetics. J. Am. Chem. Soc. 2004;126:11930–11935. doi: 10.1021/ja046990w. [DOI] [PubMed] [Google Scholar]
- [66].Russwurm M, Koesling D. NO activation of guanylyl cyclase. EMBO J. 2004;23:4443–4450. doi: 10.1038/sj.emboj.7600422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ignarro L. Haem-dependent activation of guanylate cyclase and cyclic GMP formation by endogenous nitric oxide: a unique transduction mechanism for transcellular signaling. Pharmacol. Toxicol. 1990;67:1–7. doi: 10.1111/j.1600-0773.1990.tb00772.x. [DOI] [PubMed] [Google Scholar]
- [68].Eich R, Li T, Lemon D, Doherty D, Curry S, Aitken J, Mathews A, Johnson K, Smith R, Phillips GJ, Olson J. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- [69].Brunori M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem. Sci. 2001;26:21–23. doi: 10.1016/s0968-0004(00)01698-4. [DOI] [PubMed] [Google Scholar]
- [70].Gardner P. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J. Inorg. Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- [71].Gardner P, Gardner A, Martin L, Salzman A. Nitric oxide dioxygenase: an enzymic function for flavohemoglobin. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10378–10383. doi: 10.1073/pnas.95.18.10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gow A, Luchsinger B, Pawloski J, Singel D, Stamler J. The oxyhemoglobin reaction of nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1999;96:9027–9032. doi: 10.1073/pnas.96.16.9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gardner P, Gardner A, Brashear W, Suzuki T, Hvitved A, Setchell K, Olson J. Hemoglobins dioxygenate nitric oxide with high fidelity. J. Inorg. Biochem. 2006;100:542–550. doi: 10.1016/j.jinorgbio.2005.12.012. [DOI] [PubMed] [Google Scholar]
- [74].Van Doorslaer S, Dewilde S, Kiger L, Nistor S, Goovaerts E, Marden M, Moens L. Nitric oxide binding properties of neuroglobin. A characterization by EPR and flash photolysis. J. Biol. Chem. 2003;278:4919–4925. doi: 10.1074/jbc.M210617200. [DOI] [PubMed] [Google Scholar]
- [75].Smagghe B, Trent J.r., Hargrove M. NO dioxygenase activity in hemoglobins is ubiquitous in vitro, but limited by reduction in vivo. PLoS One. 2008;3:e2039. doi: 10.1371/journal.pone.0002039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ford P, Fernandez B, Lim M. Mechanisms of reductive nitrosylation in iron and copper models relevant to biological systems. Chem. Rev. 2005;105:2439–2455. doi: 10.1021/cr0307289. [DOI] [PubMed] [Google Scholar]
- [77].Fernandez B, Lorkovic I, Ford P. Mechanisms of ferriheme reduction by nitric oxide: nitrite and general base catalysis. Inorg. Chem. 2004;43:5393–5402. doi: 10.1021/ic049532x. [DOI] [PubMed] [Google Scholar]
- [78].Hoshino M, Masahiro M, Reiko K, Seki H, Ford Peter C. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J. Am. Chem. Soc. 1996;118:5702–5707. [Google Scholar]
- [79].Brunori M, Giuffrè A, Nienhaus K, Nienhaus G, Scandurra F, Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Perazzolli M, Romero-Puertas M, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. J. Exp. Bot. 2006;57:479–488. doi: 10.1093/jxb/erj051. [DOI] [PubMed] [Google Scholar]
- [81].Minning D, Gow A, Bonaventura J, Braun R, Dewhirst M, Goldberg D, Stamler J. Ascaris haemoglobin is a nitric oxide-activated ‘deoxygenase’. Nature. 1999;401:497–502. doi: 10.1038/46822. [DOI] [PubMed] [Google Scholar]
- [82].Perazzolli M, Dominici P, Romero-Puertas M, Zago E, Zeier J, Sonoda M, Lamb C, Delledonne M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell. 2004;16:2785–2794. doi: 10.1105/tpc.104.025379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dordas C, Hasinoff B, Rivoal J, Hill R. Class-1 hemoglobins, nitrate and NO levels in anoxic maize cell-suspension cultures. Planta. 2004;219:66–72. doi: 10.1007/s00425-004-1212-y. [DOI] [PubMed] [Google Scholar]
- [84].Delledonne M. NO news is good news for plants. Curr. Opin. Plant Biol. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [85].Halligan K, Jourd'heuil F, Jourd'heuil D. Cytoglobin is expressed in the vasculature and regulates cell respiration and proliferation via nitric oxide dioxygenation. J. Biol. Chem. 2009;284:8539–8547. doi: 10.1074/jbc.M808231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Igamberdiev A, Bykova N, Hill R. Nitric oxide scavenging by barley hemoglobin is facilitated by a monodehydroascorbate reductase-mediated ascorbate reduction of methemoglobin. Planta. 2006;223:1033–1040. doi: 10.1007/s00425-005-0146-3. [DOI] [PubMed] [Google Scholar]
- [87].de Sanctis D, Dewilde S, Pesce A, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Crystal structure of cytoglobin: the fourth globin type discovered in man displays heme hexa-coordination. J. Mol. Biol. 2004;336:917–927. doi: 10.1016/j.jmb.2003.12.063. [DOI] [PubMed] [Google Scholar]
- [88].Vallone B, Nienhaus K, Brunori M, Nienhaus G. The structure of murine neuroglobin: novel pathways for ligand migration and binding. Proteins. 2004;56:85–92. doi: 10.1002/prot.20113. [DOI] [PubMed] [Google Scholar]
- [89].Vallone B, Nienhaus K, Matthes A, Brunori M, Nienhaus G. The structure of carbonmonoxy neuroglobin reveals a heme-sliding mechanism for control of ligand affinity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17351–17356. doi: 10.1073/pnas.0407633101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].de Sanctis D, Ascenzi P, Bocedi A, Dewilde S, Burmester T, Hankeln T, Moens L, Bolognesi M. Cyanide binding and heme cavity conformational transitions in Drosophila melanogaster hexacoordinate hemoglobin. Biochemistry. 2006;45:10054–10061. doi: 10.1021/bi060462a. [DOI] [PubMed] [Google Scholar]
- [91].Hargrove M, Brucker E, Stec B, Sarath G, Arredondo-Peter R, Klucas R, Olson J, Phillips GJ. Crystal structure of a nonsymbiotic plant hemoglobin. Structure. 2000;8:1005–1014. doi: 10.1016/s0969-2126(00)00194-5. [DOI] [PubMed] [Google Scholar]
- [92].Hoy J, Robinson H, Trent J.r., Kakar S, Smagghe B, Hargrove M. Plant hemoglobins: a molecular fossil record for the evolution of oxygen transport. J. Mol. Biol. 2007;371:168–179. doi: 10.1016/j.jmb.2007.05.029. [DOI] [PubMed] [Google Scholar]
- [93].Trent JR, Kundu S, Hoy J, Hargrove M. Crystallographic analysis of synechocystis cyanoglobin reveals the structural changes accompanying ligand binding in a hexacoordinate hemoglobin. J. Mol. Biol. 2004;341:1097–1108. doi: 10.1016/j.jmb.2004.05.070. [DOI] [PubMed] [Google Scholar]
- [94].Yoon J, Herzik M, Winter M, Tran R, Olea C, Marletta M. Structure and properties of a bis-histidyl ligated globin from Caenorhabditis elegans. Biochemistry. 2010;49:5662–5670. doi: 10.1021/bi100710a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Schmidt M, Gerlach F, Avivi A, Laufs T, Wystub S, Simpson J, Nevo E, Saaler-Reinhardt S, Reuss S, Hankeln T, Burmester T. Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J. Biol. Chem. 2004;279:8063–8069. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- [96].Ostojić J, Sakaguchi D, de Lathouder Y, Hargrove M, Trent J.r., Kwon Y, Kardon R, Kuehn M, Betts D, Grozdanić S. Neuroglobin and cytoglobin: oxygen-binding proteins in retinal neurons. Invest. Ophthalmol. Vis. Sci. 2006;47:1016–1023. doi: 10.1167/iovs.05-0465. [DOI] [PubMed] [Google Scholar]
- [97].Schmidt M, Laufs T, Reuss S, Hankeln T, Burmester T. Divergent distribution of cytoglobin and neuroglobin in the murine eye. Neurosci. Lett. 2005;374:207–211. doi: 10.1016/j.neulet.2004.10.071. [DOI] [PubMed] [Google Scholar]
- [98].Goodman M, Hargrove M. Quaternary structure of rice nonsymbiotic hemoglobin. J. Biol. Chem. 2001;276:6834–6839. doi: 10.1074/jbc.M009254200. [DOI] [PubMed] [Google Scholar]
- [99].Fago A, Hundahl C, Dewilde S, Gilany K, Moens L, Weber R. Allosteric regulation and temperature dependence of oxygen binding in human neuroglobin and cytoglobin. Molecular mechanisms and physiological significance. J. Biol. Chem. 2004;279:44417–44426. doi: 10.1074/jbc.M407126200. [DOI] [PubMed] [Google Scholar]
- [100].Pesce A, Couture M, Dewilde S, Guertin M, Yamauchi K, Ascenzi P, Moens L, Bolognesi M. A novel two-over-two alpha-helical sandwich fold is characteristic of the truncated hemoglobin family. EMBO J. 2000;19:2424–2434. doi: 10.1093/emboj/19.11.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Laberge M, Yonetani T. Common dynamics of globin family proteins. IUBMB Life. 2007;59:528–534. doi: 10.1080/15216540701222914. [DOI] [PubMed] [Google Scholar]
- [102].de Sanctis D, Pesce A, Nardini M, Bolognesi M, Bocedi A, Ascenzi P. Structure-function relationships in the growing hexa-coordinate hemoglobin sub-family. IUBMB Life. 2004;56:643–651. doi: 10.1080/15216540500059640. [DOI] [PubMed] [Google Scholar]
- [103].Van Doorslaer S, Vinck E, Trandafir F, Ioanitescu I, Dewilde S, Moens L. Tracing the structure–function relationship of neuroglobin and cytoglobin using resonance Raman and electron paramagnetic resonance spectroscopy. IUBMB Life. 2004;56:665–670. doi: 10.1080/15216540500037877. [DOI] [PubMed] [Google Scholar]
- [104].Du W, Syvitski R, Dewilde S, Moens L, La Mar G. Solution 1 h NMR characterization of equilibrium heme orientational disorder with functional consequences in mouse neuroglobin. J. Am. Chem. Soc. 2003;125:8080–8081. doi: 10.1021/ja034584r. [DOI] [PubMed] [Google Scholar]
- [105].Walker F. The heme environment of mouse neuroglobin: histidine imidazole plane orientations obtained from solution NMR and EPR spectroscopy as compared with X-ray crystallography. J. Biol. Inorg. Chem. 2006;11:391–397. doi: 10.1007/s00775-006-0095-8. [DOI] [PubMed] [Google Scholar]
- [106].Brunori M, Vallone B. Neuroglobin, seven years after. Cell. Mol. Life Sci. 2007;64:1259–1268. doi: 10.1007/s00018-007-7090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vinck E, Van Doorslaer S, Dewilde S, Mitrikas G, Schweiger A, Moens L. Analyzing heme proteins using EPR techniques: the heme-pocket structure of ferric mouse neuroglobin. J. Biol. Inorg. Chem. 2006;11:467–475. doi: 10.1007/s00775-006-0100-2. [DOI] [PubMed] [Google Scholar]
- [108].Vinck E, Van Doorslaer S, Dewilde S, Moens L. Structural change of the heme pocket due to disulfide bridge formation is significantly larger for neuroglobin than for cytoglobin. J. Am. Chem. Soc. 2004;126:4516–4517. doi: 10.1021/ja0383322. [DOI] [PubMed] [Google Scholar]
- [109].Durley R, Mathews F. Refinement and structural analysis of bovine cytochrome b5 at 1.5 A resolution. Acta Crystallogr. D Biol. Crystallogr. 1996;52:65–76. doi: 10.1107/S0907444995007827. [DOI] [PubMed] [Google Scholar]
- [110].Xu Z, Farid R. Design, synthesis, and characterization of a novel hemoprotein. Protein Sci. 2001;10:236–249. doi: 10.1110/ps.30801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Pesce A, Dewilde S, Nardini M, Moens L, Ascenzi P, Hankeln T, Burmester T, Bolognesi M. Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity. Structure. 2003;11:1087–1095. doi: 10.1016/s0969-2126(03)00166-7. [DOI] [PubMed] [Google Scholar]
- [112].Hargrove M, Barry J, Brucker E, Berry M, Phillips GJ, Olson J, Arredondo-Peter R, Dean J, Klucas R, Sarath G. Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J. Mol. Biol. 1997;266:1032–1042. doi: 10.1006/jmbi.1996.0833. [DOI] [PubMed] [Google Scholar]
- [113].Burmester T, Hankeln T. What is the function of neuroglobin? J. Exp. Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- [114].Wakasugi K, Nakano T, Morishima I. Oxidized human neuroglobin acts as a heterotrimeric Galpha protein guanine nucleotide dissociation inhibitor. J. Biol. Chem. 2003;278:36505–36512. doi: 10.1074/jbc.M305519200. [DOI] [PubMed] [Google Scholar]
- [115].Wakasugi K, Morishima I. Identification of residues in human neuroglobin crucial for Guanine nucleotide dissociation inhibitor activity. Biochemistry. 2005;44:2943–2948. doi: 10.1021/bi0477539. [DOI] [PubMed] [Google Scholar]
- [116].Lewerenz J, Letz J, Methner A. Activation of stimulatory heterotrimeric G proteins increases glutathione and protects neuronal cells against oxidative stress. J. Neurochem. 2003;87:522–531. doi: 10.1046/j.1471-4159.2003.02019.x. [DOI] [PubMed] [Google Scholar]
- [117].Wakasugi K, Kitatsuji C, Morishima I. Possible neuroprotective mechanism of human neuroglobin. Ann. N.Y. Acad. Sci. 2005;1053:220–230. doi: 10.1196/annals.1344.020. [DOI] [PubMed] [Google Scholar]
- [118].Moens L, Dewilde S. Globins in the brain. Nature. 2000;407:461–462. doi: 10.1038/35035181. [DOI] [PubMed] [Google Scholar]
- [119].Burmester T, Hankeln T. Neuroglobin: a respiratory protein of the nervous system. News Physiol. Sci. 2004;19:110–113. doi: 10.1152/nips.01513.2003. [DOI] [PubMed] [Google Scholar]
- [120].Schmidt M, Giessl A, Laufs T, Hankeln T, Wolfrum U, Burmester T. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J. Biol. Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- [121].Sun Y, Jin K, Mao X, Zhu Y, Greenberg D. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Schmidt-Kastner R, Haberkamp M, Schmitz C, Hankeln T, Burmester T. Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture. Brain Res. 2006;1103:173–180. doi: 10.1016/j.brainres.2006.05.047. [DOI] [PubMed] [Google Scholar]
- [123].Mammen P, Shelton J, Goetsch S, Williams S, Richardson J, Garry M, Garry D. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J. Histochem. Cytochem. 2002;50:1591–1598. doi: 10.1177/002215540205001203. [DOI] [PubMed] [Google Scholar]
- [124].Hundahl C, Stoltenberg M, Fago A, Weber R, Dewilde S, Fordel E, Danscher G. Effects of short-term hypoxia on neuroglobin levels and localization in mouse brain tissues. Neuropathol. Appl. Neurobiol. 2005;31:610–617. doi: 10.1111/j.1365-2990.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- [125].Fago A, Mathews A, Brittain T. A role for neuroglobin: resetting the trigger level for apoptosis in neuronal and retinal cells. IUBMB Life. 2008;60:398–401. doi: 10.1002/iub.35. [DOI] [PubMed] [Google Scholar]
- [126].Sun Y, Jin K, Peel A, Mao X, Xie L, Greenberg D. Neuroglobin protects the brain from experimental stroke in vivo. Proc. Natl. Acad. Sci. U. S. A. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang X, Liu J, Zhu H, Tejima E, Tsuji K, Murata Y, Atochin D, Huang P, Zhang C, Lo E. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke. 2008;39:1869–1874. doi: 10.1161/STROKEAHA.107.506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Liu J, Yu Z, Guo S, Lee S, Xing C, Zhang C, Gao Y, Nicholls D, Lo E, Wang X. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J. Neurosci. Res. 2009;87:164–170. doi: 10.1002/jnr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Khan A, Wang Y, Sun Y, Mao X, Xie L, Miles E, Graboski J, Chen S, Ellerby L, Jin K, Greenberg D. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jin K, Mao Y, Mao X, Xie L, Greenberg D. Neuroglobin expression in ischemic stroke. Stroke. 2010;41:557–559. doi: 10.1161/STROKEAHA.109.567149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Moschetti T, Giuffrè A, Ardiccioni C, Vallone B, Modjtahedi N, Kroemer G, Brunori M. Failure of apoptosis-inducing factor to act as neuroglobin reductase. Biochem. Biophys. Res. Commun. 2009;390:121–124. doi: 10.1016/j.bbrc.2009.09.078. [DOI] [PubMed] [Google Scholar]
- [132].Trandafir F, Hoogewijs D, Altieri F, Rivetti di Val Cervo P, Ramser K, Van Doorslaer S, Vanfleteren J, Moens L, Dewilde S. Neuroglobin and cytoglobin as potential enzyme or substrate. Gene. 2007;398:103–113. doi: 10.1016/j.gene.2007.02.038. [DOI] [PubMed] [Google Scholar]
- [133].Fago A, Mathews A, Moens L, Dewilde S, Brittain T. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006;580:4884–4888. doi: 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [134].Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411. doi: 10.1007/s10495-009-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Kawada N, Kristensen D, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J. Biol. Chem. 2001;276:25318–25323. doi: 10.1074/jbc.M102630200. [DOI] [PubMed] [Google Scholar]
- [136].Springer BA, Sliger SG, Olson JS, Phillips GNJ. Mechanisms of ligand recognition in myoglobin. Chem. Rev. 1994;94:699–714. [Google Scholar]
- [137].Fordel E, Geuens E, Dewilde S, Rottiers P, Carmeliet P, Grooten J, Moens L. Cytoglobin expression is upregulated in all tissues upon hypoxia: an in vitro and in vivo study by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;319:342–348. doi: 10.1016/j.bbrc.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [138].Xi Y, Obara M, Ishida Y, Ikeda S, Yoshizato K. Gene expression and tissue distribution of cytoglobin and myoglobin in the Amphibia and Reptilia: possible compensation of myoglobin with cytoglobin in skeletal muscle cells of anurans that lack the myoglobin gene. Gene. 2007;398:94–102. doi: 10.1016/j.gene.2007.01.040. [DOI] [PubMed] [Google Scholar]
- [139].Hodges N, Innocent N, Dhanda S, Graham M. Cellular protection from oxidative DNA damage by over-expression of the novel globin cytoglobin in vitro. Mutagenesis. 2008;23:293–298. doi: 10.1093/mutage/gen013. [DOI] [PubMed] [Google Scholar]
- [140].Shivapurkar N, Stastny V, Okumura N, Girard L, Xie Y, Prinsen C, Thunnissen F, Wistuba I, Czerniak B, Frenkel E, Roth J, Liloglou T, Xinarianos G, Field J, Minna J, Gazdar A. Cytoglobin, the newest member of the globin family, functions as a tumor suppressor gene. Cancer Res. 2008;68:7448–7456. doi: 10.1158/0008-5472.CAN-08-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Burmester T, Hankeln T. A globin gene of Drosophila melanogaster. Mol. Biol. Evol. 1999;16:1809–1811. doi: 10.1093/oxfordjournals.molbev.a026093. [DOI] [PubMed] [Google Scholar]
- [142].Gleixner E, Abriss D, Adryan B, Kraemer M, Gerlach F, Schuh R, Burmester T, Hankeln T. Oxygen-induced changes in hemoglobin expression in Drosophila. FEBS J. 2008;275:5108–5116. doi: 10.1111/j.1742-4658.2008.06642.x. [DOI] [PubMed] [Google Scholar]
- [143].Weber R, Vinogradov S. Nonvertebrate hemoglobins: functions and molecular adaptations. Physiol. Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- [144].Kraus D, Colacino J. Extended oxygen delivery from the nerve hemoglobin of Tellina alternata (Bivalvia) Science. 1986;232:90–92. doi: 10.1126/science.232.4746.90. [DOI] [PubMed] [Google Scholar]
- [145].Choi D, Zea C, Do Y, Semrau J, Antholine W, Hargrove M, Pohl N, Boyd E, Geesey G, Hartsel S, Shafe P, McEllistrem M, Kisting C, Campbell D, Rao V, de la Mora A, Dispirito A. Spectral, kinetic, and thermodynamic properties of Cu(I) and Cu(II) binding by methanobactin from Methylosinus trichosporium OB3b. Biochemistry. 2006;45:1442–1453. doi: 10.1021/bi051815t. [DOI] [PubMed] [Google Scholar]
- [146].Robinson V, Smith B, Arnone A. A pH-dependent aquomet-to-hemichrome transition in crystalline horse methemoglobin. Biochemistry. 2003;42:10113–10125. doi: 10.1021/bi030059t. [DOI] [PubMed] [Google Scholar]
- [147].Sowa A, Duff S, Guy P, Hill R. Altering hemoglobin levels changes energy status in maize cells under hypoxia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10317–10321. doi: 10.1073/pnas.95.17.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Nie X, Hill R. Mitochondrial respiration and hemoglobin gene expression in barley aleurone tissue. Plant Physiol. 1997;114:835–840. doi: 10.1104/pp.114.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Dordas C, Hasinoff B, Igamberdiev A, Manac'h N, Rivoal J, Hill R. Expression of a stress-induced hemoglobin affects NO levels produced by alfalfa root cultures under hypoxic stress. Plant J. 2003;35:763–770. doi: 10.1046/j.1365-313x.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- [150].Igamberdiev A, Seregélyes C, Manac'h N, Hill R. NADH-dependent metabolism of nitric oxide in alfalfa root cultures expressing barley hemoglobin. Planta. 2004;219:95–102. doi: 10.1007/s00425-003-1192-3. [DOI] [PubMed] [Google Scholar]
- [151].Bruno S, Faggiano S, Spyrakis F, Mozzarelli A, Cacciatori E, Dominici P, Grandi E, Abbruzzetti S, Viappiani C. Different roles of protein dynamics and ligand migration in non-symbiotic hemoglobins AHb1 and AHb2 from Arabidopsis thaliana. Gene. 2007;398:224–233. doi: 10.1016/j.gene.2007.02.042. [DOI] [PubMed] [Google Scholar]
- [152].Vieweg M, Hohnjec N, Küster H. Two genes encoding different truncated hemoglobins are regulated during root nodule and arbuscular mycorrhiza symbioses of Medicago truncatula. Planta. 2005;220:757–766. doi: 10.1007/s00425-004-1397-0. [DOI] [PubMed] [Google Scholar]
- [153].Sturms R, Kakar S, Trent J, Hargrove M. Trema and Parasponia hemoglobins reveal convergent evolution of oxygen transport in plants. Biochemistry. 2010;49:4085–4093. doi: 10.1021/bi1002844. [DOI] [PubMed] [Google Scholar]
- [154].Olson J, Mathews A, Rohlfs R, Springer B, Egeberg K, Sligar S, Tame J, Renaud J, Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988;336:265–266. doi: 10.1038/336265a0. [DOI] [PubMed] [Google Scholar]
- [155].Kiger L, Uzan J, Dewilde S, Burmester T, Hankeln T, Moens L, Hamdane D, Baudin-Creuza V, Marden M. Neuroglobin ligand binding kinetics. IUBMB Life. 2004;56:709–719. doi: 10.1080/15216540500037711. [DOI] [PubMed] [Google Scholar]
- [156].Hundahl C, Fago A, Dewilde S, Moens L, Hankeln T, Burmester T, Weber R. Oxygen binding properties of non-mammalian nerve globins. FEBS J. 2006;273:1323–1329. doi: 10.1111/j.1742-4658.2006.05158.x. [DOI] [PubMed] [Google Scholar]
- [157].Watts R. Characterization of Nonsymbiotic Hemoglobins from Dicotyledonous Plants. Australian National University; 1999. Division of Biochemistry and Molecular Biology, vol. Ph.D. [Google Scholar]