Abstract
CD34+ stem cells play an important role during liver development and regeneration. Thus, we hypothesized that some human liver carcinomas (HLCs) might be derived from transformed CD34+ stem cells. Here, we determined that a population of CD34+ cells isolated from PLC/PRF/5 hepatoma cells (PLC) appears to function as liver cancer stem cells (LCSCs) by forming HLCs in immunodeficient mice with as few as 100 cells. Moreover, the CD34+ PLC subpopulation cells had an advantage over CD34− PLCs at initiating tumors. Three types of HLCs were generated from CD34+ PLC: hepatocellular carcinomas (HCCs); cholangiocarcinomas (CC); and combined hepatocellular cholangiocarcinomas (CHCs). Tumors formed in mice transplanted with 12 subpopulations and 6 progeny subpopulations of CD34+ PLC cells. Interestingly, progenies with certain surface antigens (CD133, CD44, CD90, or EPCAM) predominantly yielded HCCs. CD34+ PLCs that also expressed OV6 and their progeny OV6+ cells primarily produced CHC and CC. This represents the first experiment to demonstrate that the OV6+ antigen is associated with human CHC and CC. CD34+ PLCs that also expressed CD31 and their progeny CD31+ cells formed CHCs. Gene expression patterns and tumor cell populations from all xenografts exhibited diverse patterns, indicating that tumor-initiating cells (TICs) with distinct antigenic profiles contribute to cancer cell heterogeneity. Therefore, we identified CD34+ PLC cells functioning as LCSCs generating three types of HLCs. Eighteen subpopulations from one origin had the capacity independently to initiate tumors, thus functioning as TICs. This finding has broad implications for better understanding of the multistep model of tumor initiation and progression. Our finding also indicates that CD34+ PLCs that also express OV6 or CD31 result in types of HLCs. This is the first report that PLC/PRF/5 subpopulations expressing CD34 in combination with particular antigens defines categories of HLCs, implicating a diversity of origins for HLC.
Introduction
Over 90% of human liver carcinomas (HLCs) are hepatocellular carcinomas (HCCs), which is the fifth most common cancer worldwide [1], with a median survival of 6–16 months despite advances in the detection and treatment of the disease [2]. Moreover, the chemotherapy/radiation-resistant nature of these cancers means that there is often no effective cure and a very poor prognosis. Understanding the mechanism of liver carcinogenesis is essential for the treatment of this malignancy. An emerging concept being employed to help in the understanding of tumorgenicity is that only a small subset of the cancer cell population, designated cancer stem cells (CSCs), is capable of initiating and sustaining tumor formation [3]. HCCs appear to represent heterogeneous populations and genetic/genomic profiles [4], suggesting that HCCs can initiate and develop from different cell lineages [5].
There are two major nonexclusive hypotheses of the cellular origin of liver cancers: from stem cells due to maturational arrest or from dedifferentiation of mature cells. It appears that 40% of HCCs are clonal and therefore potentially arise from progenitor/stem cells [2]. Reports indicate that some CSCs derive from their corresponding adult stem cells [6], and a recent report has suggested that liver CSCs (LCSCs) are derived from enhanced self-renewal of liver stem cells [6]. Therefore, it appears that stem cells may not only be responsible for the development and regeneration of tissues and organ systems, but they are also targets of carcinogenesis. In this study, we investigated whether liver cancers were initiated and developed from transformed hepatic stem cells.
A number of investigators have apparently isolated and characterized LCSC by putative CSC markers such as CD90+ [7], CD133+ [8–10], CD44+ [7,10], or EpCAM+ [11]. However, the origins of these LCSCs are still unknown. CD34+ stem cells play an important role during liver development and regeneration [12–14]. We hypothesized that some HLCs might be derived from oncogenically mutated or epigenetically aberrant CD34+ hepatic stem cells. Our aims in this study were to identify whether there are any transformed CD34+ hepatic stem cells that function as LCSCs, and to explain the heterogeneity of tumor cells that originated from a monoclonal origin.
To undertake these aims, we evaluated the CD34+ population in seven existing hepatoma cell lines, and found that the percentage of CD34+ cells in PLC/PRF/5 hepatoma cells (PLC) was higher when compared to the six other hepatoma cell lines, and characterized them as LCSCs (Fig. 1A).
FIG. 1.
Isolation and characterization of CD34+ liver cancer stem cells. (A) A carton shows the procedure of isolation and characterization of CD34+ liver cancer stem cells. (B) Mouse with tumor after injection of CD34+ cells (upper panel), and the tumor was isolated from mouse (lower panel). (C–I) Hematoxylin and eosin staining of human xenografts presents the typical histologic features of human hepatocellular carcinomas (HCCs), there are polygonal cells with pleomorphic nuclei in cells with distinct cell borders, nucleus:cytoplasm (N:C) ratios are increased and nuclei are hyperchromatic with prominent nucleoli (C–I), atypical mitotic figures are present (D, white arrow). Another hallmark histopathologic feature of HCC is the presence of endothelial cells lining the sinusoids (E, F, H, black arrow) and dilated blood vessels (F). As with any high-grade malignancy, HCC may contain small and large foci of necrosis (G). The neoplastic cells in HCC can synthesize and store various components of hepatocytes such as lipids (H, black arrowhead), and other cytoplasmic constituents. HCCs typically grow in a nested pattern with large tumor nodules separated by thick fibrous bands (D, I). (J, K) Immunostaining showed that the tissues of human xenografts expressed human liver-specific proteins, albumin and alpha fetoprotein (AFP) (J), and alpha 1-antitrypsin (α1-AT) and EpCAM (K). The specificity of primary antibodies was checked by employing isotype controls (J, K). (C–F) Magnification: 200×. (J, K) Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
Materials and Methods
Cell culture and determination of CD34+ population
Hepatoma cell lines, Hep G2 cells, Hep 3B cells, PLC/PRF/5 cells, and SK Hep-1 cells, were purchased from ATCC; hepatoma cell lines, HLE and HLF, were purchased from Health Science Research Resources Bank, Tokyo, Japan; and hepatoma cell line, Huh 7, was a gift from Dr. Mark Feitelson, Temple University. The cell culture conditions for growing and expanding these lines were according to the instructions per provider. These hepatoma cells were stained with mouse anti-human CD34 antibody conjugated with PE (BD), and the CD34+ population was analyzed by BD FACScan (BD).
Transplantation of the cells into mice
To evaluate the tumorigenicity of CD34+ cells, CD34+ cells were sorted and injected into NOD/SCID/IL2rg mice (Jackson Laboratory) by subcutaneous injection of 100, 200, 500, 1,000, 5,000, or 10,000 cells. Parental PLC, and parental PLC after removing CD34+ cells (CD34− PLC), were also injected into the same mouse models with 10,000, 25,000, 50,000, 100,000, 200,000, 400,000, 500,000, or 1,000,000 cells. To determine the diversity of the tumors produced by different subpopulations of CD34+ PLC cells, 12 subpopulations (CD34+CD133± cells, CD34+CD44± cells, CD34+CD90± cells, CD34+CD31± cells, CD34+EpCAM± cells, and CD34+OV6± cells), were sorted from PLCs and injected into the same mouse model with 1,000 cells. To evaluate whether the progeny of CD34+ PLC cells with putative CSC markers have the capacity to form the tumors, CD34+ cells were sorted from PLC, reseeded, and cultured under the same conditions for growing PLCs. Then six subpopulations of progenies from CD34+ cells (CD34−CD133+ cells, CD34−CD44+ cells, CD34−CD90+ cells, CD34−CD31+ cells, CD34−EpCAM+ cells, and CD34−OV6+ cells), were sorted from cultured CD34+ PLC cells, and injected into the same mouse model at 1,000 cells per mice. Surgical procedures for transplantation and monitoring the tumor formation and subsequent tumor collection were approved by the Animal Care and Use Administrative Advisory Committee of the University of California Davis.
The isolation and reculture of the tumor cells
The tumors (human xenografts) were cut into small pieces after collection under sterile conditions, and treated with collagenase type IV (1 mg/mL), and dispase (1 mg/mL) and incubated at 37°C for 20 min; then, the tumor tissue was homogenized with a serological pipette, and supernatants with single cell suspensions were collected. The remaining tissue was treated with the same solution for an additional two to four times until almost all tissue was digested. The supernatants were spun at 300 g for 5 min; and the cell pellet was resuspended with MEM medium after discarding the supernatant; then they were treated with fixative-free lysing solution (Invitrogen) for 15 min in the dark to destroy the blood cells, and filtered with a 100 μm cell strainer; and spun again. Finally, the cells were resuspended and seeded onto collagen I-coated six-well plates.
Generation of cDNA and quantitative reverse transcription-polymerase chain reaction
RNA was extracted from the cultured cells of xenografts, hepatoma cell lines using the Qiagen mini RNA kit, and cDNA was generated, and quantitative polymerase chain reaction was performed as preciously described [15]. Primers/probes used are listed in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/scd).
Cryosection of human xenografts
The xenograft tissue was cut into small pieces, and fixed with 4% PFA for 4 h, then embedded in Tissue-Tek O.C.T, and stored at −80°C as previously described [16]. Slides with cryosections with a 5 μm thickness were cut and immunostained with antibodies against human liver proteins.
Immunohistochemistry and flow cytometry analysis
Tumor tissues, cultured tumor cells, and hepatoma cells were fixed with 4% PFA, and stained with different primary and secondary antibodies as previously described [16]. The cultured cells of human xenografts, hepatoma cell lines, and freshly isolated human primary hepatocytes were stained with antibodies conjugated with PE against surface markers, then analyzed by the BD FACScan (BD). All antibodies used are listed in the Supplementary Table S2.
Gene expression analysis
The cDNAs from cultured xenografted cells or from parental PLC cells were used to evaluate and measure the expression of liver genes and liver cancer markers using quantitative PCR. Primers/probes used are listed in Supplementary Table S1.
Human primary hepatocytes
Freshly isolated human primary hepatocytes from donor livers were provided by the Liver Tissue Cell Distribution System of NIH (University of Pittsburg, PA), and were used in this study with the approval of the IRB of the University of California, Davis.
Statistics
All data are summarized as mean±SEM from at least three independent measurements. An unpaired Student t-test was used to analyze the data. P<0.05 was considered statistically significant.
Results
Evaluation of tumorigenicity by CD34+ cells
Employing flow cytometry, we found that the percentage of CD34+ cells in PLCs was 3% to 6%, which was higher than in six other cell lines where the range in most of the lines was less than 1% (Supplementary Fig. S1). To evaluate tumorigenicity, CD34+ PLC cells were sorted and injected into NOD/SCID/IL2rg mice. Parental PLCs and parental CD34− PLC were also used to inject into the same mouse model, and tumors formed within 3 months in transplanted mice (Fig. 1A, B and Table 1). Compared to the tumorigenicity of parental PLCs, the tumors also were formed in the mice injected by parental CD34− PLC with 100,000 cells and more. However, the timing needed to form the tumors took longer in mice injected with parental CD34− PLC, suggesting that PLCs expressing CD34+ had an advantage over those that were CD34− in initiating tumors (Table 1). Hematoxylin and eosin (H and E) staining (Fig. 1C–I) of human xenografts showed the typical histologic features of human HCCs. There are polygonal cells with pleomorphic nuclei in cells with distinct cell borders. The nucleus:cytoplasm (N:C) ratios are increased, and nuclei are hyperchromatic with prominent nucleoli (Fig. 1C–I). Atypical mitotic figures shown in advanced HCC are present (Fig. 1D, white arrow). Another hallmark histopathologic feature of HCC is the presence of plump endothelial cells lining the sinusoids (Fig. 1E, F, H, black arrow) and dilated blood vessels (Fig. 1F). As with any high-grade malignancy, HCC may contain small and large foci of necrosis (Fig. 1G). The neoplastic cells in HCC can synthesize and store various components of hepatocytes such as lipids (Fig. 1H, black head arrow), bile, alpha-1 antitrypsin, alpha fetoprotein (AFP), and other cytoplasmic constituents. HCCs typically grow in a nested pattern with large tumor nodules separated by thick fibrous bands (Fig. 1D, I). The details of the histological and pathological features of these human xenografts are provided in Supplementary Figs. S2–S4. Immunochemistry results showed that the tissues of human xenografts expressed human liver specific proteins, albumin, AFP (Fig. 1J), and alpha-1antitrypsin and EpCAM (Fig. 1K), further confirming that CD34+ PLC cells could produce HLCs. Remarkably, xenografts were formed with the inoculation of as few as 100 cells, indicating that these CD34+ PLC cells are more tumorigenic, functioning as LCSCs.
Table 1.
Tumorigenicity by Injection of Different Cells with Different Cell Numbers
| Cell types | No. injected cells | No. of mice with tumors | Time range of forming tumors (days) |
|---|---|---|---|
| CD34+ PLC | 100 | 2/4 | 80–88 |
| 200 | 4/4 | 76–85 | |
| 500 | 4/4 | 50–65 | |
| 1,000 | 8/8 | 44–50 | |
| 5,000 | 6/6 | 28–46 | |
| 10,000 | 8/8 | 28–44 | |
| PLC | 25,000 | 0/8 | 120 |
| 50,000 | 0/8 | 120 | |
| 100,000 | 2/8 | 45–55 | |
| 200,000 | 4/8 | 41–47 | |
| 400,000 | 4/4 | 34–39 | |
| CD34− PLC | 25,000 | 0/8 | 120 |
| 50,000 | 0/8 | 120 | |
| 100,000 | 2/8 | 62–69 | |
| 200,000 | 4/8 | 54–69 | |
| 500,000 | 4/4 | 52–62 | |
| 1,000,000 | 4/4 | 38–60 | |
| CD34+CD133− | 1,000 | 4/4 | 51–55 |
| CD34+CD133+ | 1,000 | 4/4 | 44–49 |
| CD34−CD133+ | 1,000 | 4/4 | 39–42 |
| CD34+EpCAM− | 1,000 | 4/4 | 48–55 |
| CD34+EpCAM+ | 1,000 | 4/4 | 44–50 |
| CD34−EpCAM+ | 1,000 | 4/4 | 43–50 |
| CD34+CD44− | 1,000 | 4/4 | 49–58 |
| CD34+CD44+ | 1,000 | 4/4 | 44–56 |
| CD34−CD44+ | 1,000 | 4/4 | 41–47 |
| CD34+CD90− | 1,000 | 4/4 | 50–55 |
| CD34+CD90+ | 1,000 | 4/4 | 50–76 |
| CD34−CD90+ | 1,000 | 4/4 | 48–75 |
| CD34+CD31− | 1,000 | 4/4 | 50–55 |
| CD34+CD31+ | 1,000 | 4/4 | 49–53 |
| CD34−CD31+ | 1,000 | 4/4 | 39–54 |
| CD34+OV6− | 1,000 | 4/4 | 50–64 |
| CD34+OV6+ | 1,000 | 6/8 | 49–80 |
| CD34−OV6+ | 1,000 | 7/8 | 48–67 |
The capacity to form the tumor was evaluated by the injection of different cell numbers, and the tumorigenicity by cell number was defined as the lowest cell number of injected cells that was capable of producing tumor formation within 4 months. The tumor xenografts were formed with the injection of as few as 100 cells of CD34+ cells, whereas 100,000 parental PCL cells were required to form tumor xenografts.
The range of the time for the tumor formation observed in the mice injected with 1,000 CD34+ PLCs was within 1 week (Table 1). It suggested that this cell number might be the appropriate one to use to characterize the tumor formation by CD34+ PLC subpopulations and their progeny. We found that the time range required for tumor formation in most groups was similar to those in the mice injected with 1,000 CD34+ PLCs (Table 1). Interestingly, we found that the progeny from CD34+ PLC appeared to be more tumorigenic when compared with their ancestors, for example, the progeny CD34−CD133+ cells formed tumors earlier than CD34+CD133+ cells did (Table 1).
Characterization of the cells from human xenografts
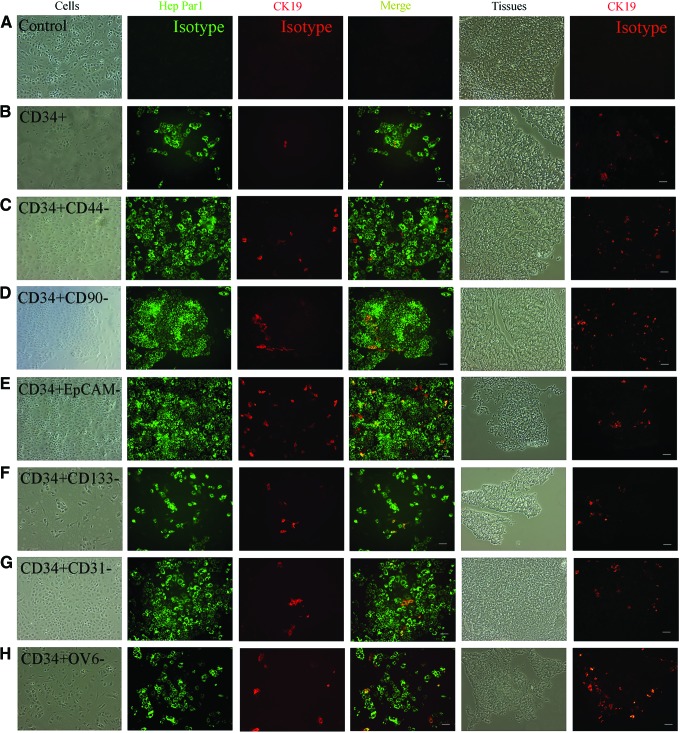
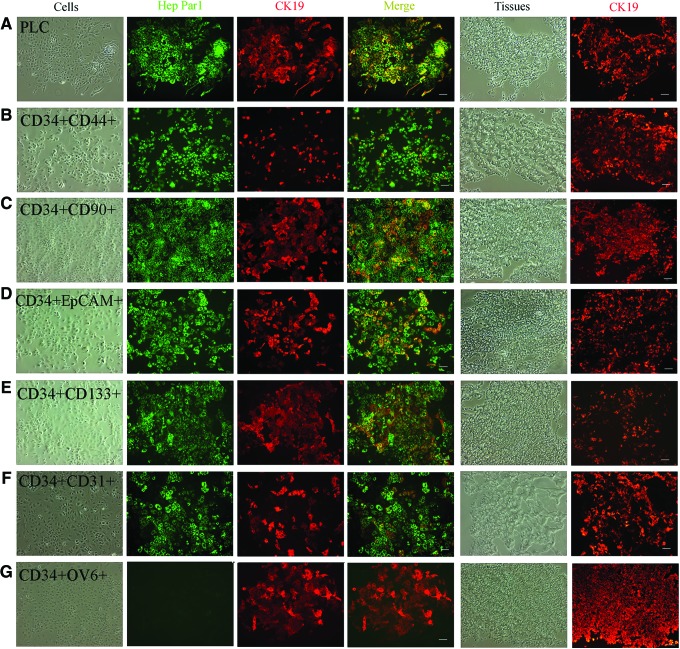
Human xenografts produced by CD34+ PLCs, 12 subpopulations of CD34+ PLCs, 6 subpopulations of progeny from CD34+ PLCs, parental PLCs, and parental PLCs after removing CD34+ PLCs, were fixed with formalin for H and E staining, or embedded in O.C.T and cut into 5 μm-thick sections for immunohistochemistry (IHC), or dissociated and recultured for further analysis. H and E staining showed that these tumor cells from human xenografts had pathological and histological features of HLCs (Fig. 1 and Supplementary Figs. S2–S4), and human xenograft tissue expressed liver-specific proteins (Fig. 1). The recultured cells were further double stained with antibodies against human Hep Par1, a marker for HCC and CK19, a marker of cholangiocarcinoma (CC). In the xenografts produced by all 21 groups (except some xenografts produced by CD34+OV6+ PLCs and their progeny CD34−OV6+ PLCs), the xenografted cells expressed Hep Par1 as a relatively homogenous population, whereas CK19 was expressed variably, from a low percentage to a relatively homogenous population, depending on the subpopulation being injected. CK19 was expressed at a higher percentage in xenografts produced by the corresponding double positive groups than in those by CD34+ PLCs; but negative for six markers in xenografts by 12 subpopulations of CD34+ PLCs (Figs. 2 and 3). For example, the percentage of CK19+ cells was higher in the tumors produced by PLCs expressing CD34 and CD133 than in those produced by PLCs expressing CD34 but not CD133. Some xenografts produced by CD34+OV6+ PLCs did not express Hep Par1, but expressed CK19 as a relatively homogenous population (Fig. 3G).
FIG. 2.
Immunohistochemistry of tumor cells produced by subpopulations of CD34+ positive negative for six markers. The reculture of tumor cells and tumor tissues from the tumors produced by the injection of CD34+ cells (CD34+, A, B), CD34+CD44− cells (CD34+CD44−, C), CD34+CD90− cells (CD34+CD90−, D), CD34+EpCAM− cells (CD34+EpCAM−, E), CD34+CD133− cells (CD34+CD133−, F), CD34+CD31− cells (CD34+CD31−, G), CD34+OV6− cells (CD34+OV6−, H), were used to evaluate liver gene expression. Left four columns: the recultured tumor cells were double stained with antibodies against human Hep Par1 and CK19, and merged with each other; right two columns: tumor tissues on slides were stained with anti-human CK19 antibody. The specificity of primary antibodies was checked by isotype controls (A). (B–H) Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
FIG. 3.
Immunohistochemistry of tumor cells produced by subpopulations of CD34 double positive for six markers. The reculture of tumor cells and tumor tissues from the tumors produced by the injection of parental PLC cell line (PLC, A), CD34+CD44+ cells (CD34+CD44+, B), CD34+CD90+ cells (CD34+CD90+, C), CD34+EpCAM+ cells (CD34+EpCAM+, D), CD34+CD133+ cells (CD34+CD133+, E), CD34+CD31+ cells (CD34+CD31+, F), CD34+OV6+ cells (CD34+OV6+, G), were used to evaluate liver gene expression. Left four columns: the recultured tumor cells were double stained with antibodies against human Hep Par1 and CK19, and merged with each other; right two columns: tumor tissues on slides were stained with antihuman CK19 antibody. (A–G) Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
In xenografts produced by six subpopulations of progeny from CD34+ PLCs, the tumors from all xenografts except some produced by CD34−OV6+ PLCs expressed Hep Par1 as a relatively homogenous population, and CK19 was expressed as a relatively homogenous population (Fig. 4G), or at a high percentage only in the xenografts produced by the progeny CD34−OV6+ PLCs and CD34−CD31+ PLCs, and at very low percentage in the xenografts produced by another 4 subpopulations of progenies of PLCs: CD34−CD133+ cells, CD34−CD44+ cells, CD34−CD90+ cells, and CD34−EpCAM+ cells (Fig. 4). Interestingly, some xenografts produced by CD34−OV6+ PLCs did not express Hep Par1, but expressed CK19 as a relatively homogenous population (Fig. 4G).
FIG. 4.
Immunohistochemistry of tumor cells produced by subpopulations of progeny from CD34+ cells. The reculture of tumor cells and tumor tissues from the tumors produced by the injection of parental PLC cell line after removing CD34+ cells (CD34− PLC, A), CD34−CD44+ cells (CD34−CD44+, B), CD34−CD90+ cells (CD34−CD90+, C), CD34−EpCAM+ cells (CD34−EpCAM+, D), CD34−CD133+ cells (CD34−CD133+, E), CD34−CD31+ cells (CD34−CD31+, F), CD34−OV6+ cells (CD34−OV6+, G), were used to evaluate liver gene expression. Left four columns: the recultured tumor cells were double stained with antibodies against human Hep Par1 and CK19, and merged with each other; right two columns: tumor tissues on slides were stained with anti-human CK19 antibody. (A-G) Scale bar: 100 μm. Color images available online at www.liebertpub.com/scd
In xenografts produced by parental PLCs and by parental CD34− PLCs, all xenografts expressed Hep Par1 as a relatively homogenous populations, and CK19 was expressed at high percentages in xenografts produced by parental PLCs (Fig. 3A), and at very low percentages in xenografts produced by parental CD34− PLCs (Fig. 4A).
In xenografts produced by total CD34+ PLCs, all xenografts expressed Hep Par1 as a relatively homogenous population, but CK19 was expressed at low percentages in most of the xenografts, and at high percentages in a few xenografts (Fig. 2B). No xenograft was found to be negative for Hep Par1, and to express CK19 as a relatively homogenous population in these xenografts.
The xenografts that expressed both Hep Par1 and CK19 at high percentages were designated as a combined hepatocellular cholangiocarcinoma (CHC) phenotype, those expressing Hep Par1 at high percentages and CK19 at low percentages as the HCC phenotype, and those negative for Hep Par1 but expressing CK19 as a relatively homogenous population as CC phenotype. Thus, the xenografts produced by the subpopulations of CD34 double positive PLCs yielded CHCs (Fig. 3). The xenografts produced by CD34+ PLCs but negative for the other six markers exhibited the HCC phenotype (Fig. 2). The CC phenotype of the xenografts was found in the tumors produced by CD34+OV6+ PLCs and their progeny of CD34−OV6+ PLCs (Figs. 3G and 4G). The cells from xenografts produced by total CD34+ PLCs demonstrated CK19 positive cells at low to high percentages, showing both the HCC (major, 80%) and CHC phenotypes. However, the CC phenotype (Hep Par1 negaitve) was not found in any of the 11 mice analyzed from this group (Fig. 2B). All of the cells from xenografts produced by parental PLCs showed the CHC phenotypes, and Hep Par1-negative cells were also not found in the nine mice that were analyzed (Fig. 3A). Xenografts produced by parental CD34− PLCs exhibited HCC phenotypes in the eight mice analyzed (Fig. 4A). CK19 expression in the tissues on slides from cryosections was consistent with the corresponding in vitro reculture results (Figs. 2–4)
Flow cytometric assays of human xenografts
By using nine surface markers, we found that the parental PLC line and the xenografted cells from all 21 populations showed a similar phenotype of relatively homogenous populations of CD54+ and CD13+ cells. They showed in most cases also a high percentage of CD133+ and EpCAM+ cells, and elevated levels of CD44+ and OV6+ in cells from xenografts (Table 2). CD54 and CD13 were found in a high percentage of freshly isolated human primary hepatocytes. Expression of cancer markers (CD133, CD44, CD90, and EpCAM) were at very low levels in human primary hepatocytes. The major difference among the phenotypes of all xenografts was the varied percentages of PLCs positive for CD31 and CD44. These differences were associated with the different subpopulations that were injected. Interestingly, this is the first report of a human liver cancer with a high percentage of CD31+ cells. CD31 is normally present on endothelial cells, platelets, macrophages, granulocytes, and blood leucocytes [17,18]. This suggests that the liver cancer from which PLC was derived was initiated and developed from a CD31 cell lineage [19]. As expected, in the xenografts produced by the 12 subpopulations of CD34+ PLCs, the percentage of all of the six markers (CD44, CD90, CD31, CD133, OV6, and EpCAM) in the xenografts was higher in the positive subpopulations than those in the negative subpopulations. For example, the percentage of CD44+ cells was higher in the xenografts produced by the injection of cells expressing CD34+CD44+ than those produced by the subpopulation with a CD34+CD44− expression pattern. Cancer cells are a pool of different cell populations, and the combination of xenograft cells produced by these 12 subpopulations of CD34+ PLCs demonstrates a markedly heterogeneous population. The percentage of CD31+ cells, CD44+, and OV6+ cells were found to be concurrently higher in xenografts produced by six subpopulations (CD34±CD44+ cells, CD34±CD31+ cells, and CD34±OV6+ cells). Interestingly, the highest percentages of CD31+ cells were only found in xenografts produced by three progeny (CD34−CD44+ cells, CD34−CD31+ cells, and CD34−OV6+ cells), whereas the lowest percentages of CD133+ cells and EpCAM+ cells were shown in xenografts produced by the same three progeny. Thus, this indicated the diverse phenotypes of tumors produced by different tumor-initiating cells (TICs). Of note, all of the xenograft cells produced by the 21 populations and parental PLC line cells presented a number of cells positive for OV6, a marker of hepatobiliary stem/progenitor cells (HSPCs; oval cells in rodents)
Table 2.
Flow Cytometry Analysis of Surface Markers of Xenograft Cells, PLC Cell Line, and Human Primary Hepatocytes
| Tumor cells by subpopulations | Control | CD34 | CD31 | CD54 | CD90 | EpCAM | CD44 | CD133 | CD13 | OV6 |
|---|---|---|---|---|---|---|---|---|---|---|
| CD34+CD133− | 0.5±0.1 | 3.0±0.1 | 3.0±0.7 | 99±0.1 | 4.4±1.6 | 51±19 | 12±0.8 | 66±14 | 89±2.3 | 6.9±0.8 |
| CD34+CD133+ | 0.4±0.1 | 2.7±0.1 | 1.1±0.3 | 99±0.2 | 1.6±0.5 | 74±15 | 12±2.4 | 77±6.4 | 94±0.4 | 4.9±0.3 |
| CD34−CD133+ | 0.7±0.3 | 1.3±0.7 | 4.8±0.1 | 92±4.9 | 7.5±3.4 | 85±6.5 | 7.4±4.6 | 76±14 | 99±0.8 | 5.2±0.3 |
| CD34+CD44− | 0.5±0.2 | 5.2±1.1 | 5.0±0.2 | 99±0.6 | 5.4±0.3 | 83±4.5 | 9.0±0.7 | 62±2.4 | 85±4.3 | 7.0±0.7 |
| CD34+CD44+ | 0.5±0.2 | 6.6±1.9 | 14±1.4 | 99±0.1 | 3.3±1.2 | 71±4.8 | 31±7.1 | 29±8.6 | 93±0.9 | 15±0.6 |
| CD34−CD44+ | 0.4±0.2 | 1.1±0.3 | 62±3.1 | 99±0.8 | 1.7±0.6 | 30±6.2 | 38±6.8 | 8.7±2.5 | 91±4.4 | 17±1.1 |
| CD34+CD90− | 0.6±0.1 | 4.3±1.7 | 10±4.6 | 98±0.5 | 4.6±0.6 | 33±10 | 30±16 | 71±13 | 68±23 | 4.1±0.3 |
| CD34+CD90+ | 0.5±0.2 | 4.7±0.3 | 3.7±1.5 | 99±0.8 | 11±6.6 | 92±5.8 | 7.9±1.9 | 49±26 | 89±1.7 | 8.7±1.3 |
| CD34−CD90+ | 0.7±0.2 | 1.9±0.2 | 3.6±1.1 | 99±0.9 | 5.4±1.3 | 69±8.9 | 14±2.2 | 76±6.5 | 97±1.1 | 6.7±1.0 |
| CD34+CD31− | 0.5±0.1 | 5.3±2.9 | 3.0±0.5 | 99±0.1 | 4.2±0.8 | 81±12 | 13±5.4 | 84±16 | 95±1.7 | 5.3±1.1 |
| CD34+CD31+ | 0.5±0.1 | 3.2±0.8 | 23±7.8 | 99±0.2 | 4.3±0.9 | 66±7.2 | 30±6.3 | 77±4.5 | 92±1.8 | 18±0.6 |
| CD34−CD31+ | 0.6±0.1 | 1.9±0.1 | 89±11 | 95±3.5 | 3.6±1.4 | 27±9.8 | 65±4.8 | 21±8.8 | 99±0.2 | 18±0.8 |
| CD34+EpCAM− | 0.6±0.2 | 3.6±1.1 | 8.2±3.6 | 99±0.1 | 3.5±0.7 | 40±9.0 | 11±0.3 | 64±11 | 90±4.4 | 8.1±1.8 |
| CD34+EpCAM+ | 0.5±0.1 | 4.2±2.0 | 6.2±3.6 | 99±0.2 | 4.5±1.1 | 97±1.8 | 19±1.7 | 78±1.6 | 94±2.6 | 8.9±2.2 |
| CD34−EpCAM+ | 0.6±0.1 | 1.8±1.5 | 3.6±1.2 | 99±0.9 | 2.8±0.3 | 86±9.0 | 13±5.3 | 74±5.1 | 90±0.8 | 11±0.6 |
| CD34+OV6− | 0.6±0.2 | 3.6±0.5 | 8.2±1.7 | 98±0.9 | 3.6±0.5 | 78±9.5 | 15±3.2 | 59±5.8 | 96±1.1 | 6.2±0.3 |
| CD34+OV6+ | 0.6±0.1 | 2.6±0.6 | 15±7.4 | 98±1.0 | 6.2±1.3 | 88±8.6 | 28±3.1 | 42±7.6 | 95±2.1 | 18±0.6 |
| CD34−OV6+ | 0.7±0.1 | 1.1±0.5 | 71±3.1 | 87±2.0 | 4.5±0.7 | 7.5±1.6 | 30±2.5 | 7.2±1.8 | 97±1.6 | 15±1.8 |
| CD34+ cells | 0.6±0.1 | 5.4±0.7 | 12±4.5 | 98±0.6 | 5.0±1.2 | 73±11 | 22±5.7 | 61±9.3 | 97±0.7 | 8.4±1.7 |
| PLC | 0.5±0.1 | 6.4±0.4 | 15±2.4 | 99±0.1 | 3.5±0.3 | 37±2.9 | 13±1.5 | 69±1.7 | 95±0.7 | 8.9±0.9 |
| CD34− PLC | 0.5±0.3 | 1.7±0.4 | 8.5±2.5 | 97±2.1 | 2.1±0.4 | 59±14 | 18±3.2 | 48±10 | 85±5.4 | 5.5±1.2 |
| PLC cell line | 0.4±0.1 | 6.8±0.9 | 4.8±2.0 | 99±0.3 | 1.1±0.1 | 14±3.2 | 3.3±1.2 | 76±3.0 | 97±0.4 | 12±3.1 |
| HPH | 0.4±0.1 | 1.1±0.3 | 1.6±0.5 | 64±2.7 | 0.5±0.1 | 1.4±0.3 | 2.6±0.3 | 0.6±0.1 | 78±4.3 | 16±5.4 |
The cells from xenografts produced by 21 cell populations, parental PLC line and human primary hepatocytes were stained with antibodies against nine surface markers, and the percentage of these markers were measured by flow cytometry. Data represent mean±SEM (4≤n≤11).
Expression of liver genes and cancer markers by human xenografts
Three types of HLC xenografts were derivatives of CD34+ PLCs. The expression of human liver genes and the stem cell marker, CD34, were evaluated in these xenograft cells of three types and in the parental PLC, and compared to those in CD34+ PLCs. Hepatocyte markers, albumin and AFP were highly expressed in HCC xenografts; cholangiocyte or cholagiocarcinoma markers, CK7 or CK19, were highly expressed in CC and CHC xenografts. Interestingly, hepatocyte marker, α1-antitrypsin, was even slightly increased in CC and CHC xenografts when compared to those in CD34+ cells. This phenomenon might be associated with a high percentage of CD31+ cells in some CHC and CC xenografts, since this CD31+ cell population is related to macrophages, and α1-antitrypsin is also expressed by macrophage. CD34 expression was significantly downregulated during the differentiation of CD34+ cells into these HLC xenografts (Fig. 5A). Human liver genes, cancer-related markers (CD133, CD44, CD90, and EpCAM) and CD31, were further evaluated by quantitative reverse transcription-polymerase chain reaction in human xenografts produced by the 21 cell populations plus the parental PLC cells (Table 3). The major phenotypes in the xenografts produced by CD34+ PLCs that are negative for six markers (CD133 or CD44 or CD90 or CD31 or EpCAM or OV6) showed HCC phenotypes, determined by IHC. Gene expressions in these xenografts were used as calibrators. We found that the xenograft cells produced by six double positive subpopulations expressed higher levels of cholangiocyte markers, thus representing CHC phenotypes; they generated well-differentiated CHC with very high expression of CK19. These results further confirmed the findings determined by IHC (Fig. 3). Second, the cells from xenografts produced by five progenies except progeny CD34−OV6+ PLCs expressed high levels of hepatocyte markers, albumin and alpha-fetoprotein when compared with those produced by their ancestors, showing HCC phenotypes. This also was consistent with the findings determined by IHC. Third, the xenografts produced by CD34+OV6+ PLCs and its progeny, CD34−OV6+ PLCs expressed high levels of cholangiocyte markers, and very low levels of hepatocyte markers, suggesting that these two populations predominantly produced CHC and CC xenografts. Fourth, the xenografts produced by parental PLCs expressed higher levels of cholangiocyte or CC markers, whereas the xenografts produced by parental CD34− PLC expressed higher levels of hepatocyte markers, indicating that two different phenotypes of xenografts were produced by these two populations. Fifth, greater expression of CD31 and CD44 were found in the xenografts produced by six PLC subpopulations (CD34±CD44+, CD34±CD31+, and CD34±OV6+). The highest expression of CD31 and CD44 was found in the xenografts produced by three subpopulation progeny of CD34+ PLCs (CD34−CD31+ cells, CD34−CD44+ cells, and CD34−OV6+ cells), whereas the lowest expression of EpCAM and CD133 was found in the xenografts produced by the same three subpopulations. This finding was consistent with those in flow cytometric analysis. Finally, expression of both hepatocyte markers and cholangiocyte markers in the parental PLC line was at higher levels when compared with those in HCC xenograft cells produced by CD34+ cells negative for six markers or CD34+ cells, suggesting that the patient's primary liver carcinoma from which the PLC line was derived might be a mix of HCC and CC, or CHC. In summary, HCC was the major phenotype of xenografts produced by the combined population of CD34+ PLCs, by CD34+ populations that were negative for six markers, by four progeny except the progeny CD34−OV6+ PLCs and CD34−CD31+ PLCs, and by parental CD34− PLC; the xenografts produced by CD34 double positive subpopulations, two progeny (CD34−CD31+ cells and CD34−OV6+ cells), and parental PLC showed CHC as the major phenotype; the phenotype of CC was only found in the xenografts produced by CD34+OV6+ subpopulations and its progeny CD34−OV6+ cells. Of note, one xenograft produced by the CD34+OV6+ subpopulation exhibited the HCC phenotype, meaning that three types of HLCs could be found in the xenografts produced by the injection of CD34+OV6+ PLCs.
FIG. 5.
Characterization of liver gene expression in tumor cells and illustration of formation of CD34+ CSCs. (A) Expression levels of human liver genes and CD34 was determined by quantitative polymerase chain reaction among CD34+ cells (CD34+), the tumor cells of combined hepatocellular cholangiocarcinomas (CHCs), the tumor cells of HCCs, the tumor cells of cholangiocarcinomas (CC), and the parental PLC cell line (PLC). (B) The recultured CC tumor cells were double stained with antibodies against Hep Par 1 and CK19, CK19+ cells exhibited a cuboidal to columnar morphology with round central nuclei, resembling bile duct cells, not hepatocytes. (C) The recultured CHC tumor cells were double stained with antibodies against Hep Par 1 and AE1/AE3, a CC marker. Hep Par1+ cells and AE1/AE3+ cells well overlapped. (D) The tissues of CC tumors were double stained with antibodies against Hep Par1, and Mucin 1, a CC marker. The cells only expressed mucin 1. (E) The tissues of CHC tumors were double stained with antibodies against Hep Par1 and CK20, a CC marker. Hep Par1+ cells and CK20+ cells well overlapped. The specificity of primary antibodies was checked by isotype controls (C–E). (B–E) Scale bar: 100 μm. (F) A cartoon illustrates the putative transformation of CD34+ cancer stem cells (CSC) and their differentiation to mature cancer cells with the decrease of multipotency, and the increase of heterogeneity of cancer cells: Normal CD34+ stem cells received an oncogenic hit and acquired oncogenicity, then were transformed into CD34+ CSC with cancer markers. Normal CD34+ stem cells and CD34+ CSC have the capacity to self-renew. With the differentiation, the progeny of CD34+ CSC lose CD34+ marker and self-renewal characteristics, and become different committed progenitors to differentiate to mature cancer cells. During the differentiation, CD34+ CSC decreased its multipotency by differentiating into different progenitors and increased the heterogeneity of cancer cells produced by these progenitors derived from CD34+ CSC. Color images available online at www.liebertpub.com/scd
Table 3.
Gene Expression Analysis of Xenograft Cells and PLC Cell Line
| Tumor cells by subpopulations | ALB | AFP | α1AT | CK19 | CK7 | CD31 | CD133 | EpCAM | CD44 | CD90 | Major phenotype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD34+CD133− | 1.0±0.2 | 1.0±0.2 | 1.0±0.1 | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | HCC |
| CD34+CD133+ | 0.7±0.1 | 0.9±0.2 | 2.6±0.3 | 4.5±0.8 | 1.5±0.1 | 0.4±0.1 | 2.9±0.1 | 2.0±0.2 | 1.1±0.1 | 0.4±0.1 | CHC |
| CD34−CD133+ | 145±23 | 1.3±0.1 | 1.3±0.1 | 0.5±0.1 | 0.3±0.1 | 2.4±0.1 | 3.1±0.3 | 4.5±0.4 | 0.2±0.1 | 1.4±0.1 | HCC |
| PLC cell line | 7.6±1.8 | 1.7±0.2 | 2.4±0.2 | 1.0±0.2 | 7.6±1.1 | 2.7±0.5 | 2.1±0.1 | 0.2±0.1 | 0.1±0.1 | 0.3±0.1 | CHC |
| CD34+CD44− | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | HCC |
| CD34+CD44+ | 0.5±0.1 | 0.6±0.1 | 0.5±0.2 | 10±1.8 | 18±1.1 | 16±3.0 | 0.3±0.1 | 0.7±0.1 | 15±1.4 | 1.1±0.1 | CHC |
| CD34−CD44+ | 196±49 | 1.4±0.2 | 1.6±0.2 | 2.0±0.2 | 10±0.8 | 118±11 | 0.1±0.1 | 0.2±0.1 | 19±1.8 | 0.1±0.1 | HCC |
| PLC cell line | 3.7±0.9 | 1.6±0.1 | 1.4±0.2 | 2.8±1.3 | 10±1.5 | 1.7±0.3 | 1.1±0.1 | 0.1±0.1 | 0.4±0.1 | 0.1±0.1 | CHC |
| CD34+CD90− | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 1.0±0.2 | 1.0±0.1 | HCC |
| CD34+CD90+ | 0.6±0.1 | 0.7±0.1 | 1.6±0.3 | 149±28 | 3.5±0.9 | 2.2±0.2 | 0.8±0.1 | 31±4.6 | 0.3±0.1 | 3.9±0.7 | CHC |
| CD34−CD90+ | 2.2±0.2 | 2.0±0.1 | 1.1±0.2 | 0.4±0.1 | 0.6±0.1 | 1.1±0.1 | 4.7±0.8 | 9.2±1.4 | 0.4±0.1 | 1.3±0.2 | HCC |
| PLC cell line | 2.4±0.1 | 0.9±0.1 | 1.2±0.1 | 37±9.4 | 3.1±0.5 | 2.9±0.2 | 1.0±0.2 | 0.1±0.1 | 0.1±0.1 | 0.2±0.1 | CHC |
| CD34+CD31− | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | HCC |
| CD34+CD31+ | 0.2±0.1 | 0.7±0.1 | 1.0±0.1 | 60±10 | 4.0±0.7 | 28±3.9 | 0.8±0.1 | 1.0±0.1 | 6.1±0.2 | 1.0±0.1 | CHC |
| CD34−CD31+ | 73±11 | 9.3±1.2 | 2.7±0.8 | 21±3.3 | 10±0.8 | 488±69 | 0.1±0.1 | 0.2±0.1 | 11±0.5 | 0.7±0.1 | CHC |
| PLC cell line | 1.5±0.2 | 3.1±0.9 | 2.6±0.4 | 5.6±1.7 | 2.0±0.1 | 8.2±1.5 | 0.7±0.1 | 0.1±0.1 | 0.1±0.1 | 0.3±0.1 | CHC |
| CD34+EpCAM− | 1.0±0.2 | 1.0±0.1 | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | HCC |
| CD34+EpCAM+ | 0.7±0.1 | 0.6±0.1 | 0.8±0.1 | 35±2.9 | 2.4±0.1 | 0.6±0.1 | 1.8±0.3 | 1.6±0.2 | 1.9±0.1 | 3.4±0.1 | CHC |
| CD34−EpCAM+ | 12±1.4 | 8.1±1.6 | 0.6±0.1 | 0.5±0.1 | 0.2±0.1 | 0.3±0.1 | 1.5±0.2 | 1.3±0.3 | 1.1±0.1 | 1.0±0.1 | HCC |
| PLC cell line | 1.8±0.4 | 2.6±0.8 | 0.9±0.1 | 7.4±2.5 | 3.0±0.6 | 0.5±0.1 | 1.1±0.1 | 0.1±0.1 | 0.2±0.1 | 0.4±0.1 | CHC |
| CD34+OV6− | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 1.0±0.2 | 1.0±0.1 | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | HCC |
| CD34+OV6+ | 0.1±0.1 | 0.1±0.1 | 1.0±0.1 | 42±6.9 | 9.6±0.5 | 13±2.1 | 0.4±0.1 | 1.6±0.1 | 14±0.5 | 2.2±0.2 | CHC, CC |
| CD34−OV6+ | 0.1±0.1 | 0.1±0.1 | 2.0±0.1 | 42±5.7 | 48±4.0 | 142±7.6 | 0.1±0.1 | 0.1±0.1 | 62±12 | 1.4±0.3 | CHC, CC |
| PLC cell line | 2.1±0.1 | 2.8±0.1 | 3.9±0.5 | 7.9±1.6 | 8.4±1.2 | 1.5±0.2 | 1.5±0.2 | 0.1±0.1 | 0.4±0.1 | 0.3±0.1 | CHC |
| CD34+ cells | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.2 | 1.0±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | HCC |
| PLC | 1.1±0.2 | 0.4±0.4 | 1.0±0.1 | 7.5±1.2 | 4.5±0.7 | 1.9±0.3 | 2.0±0.2 | 0.9±0.1 | 0.4±0.1 | 0.8±0.2 | CHC |
| CD34− PLC | 4.1±0.6 | 4.9±1.4 | 1.8±0.4 | 0.6±0.1 | 0.9±0.2 | 0.8±0.1 | 0.2±0.1 | 0.7±0.2 | 0.6±0.1 | 0.5±0.1 | HCC |
| PLC cell line | 1.6±0.3 | 1.2±0.2 | 2.5±0.1 | 2.0±0.2 | 4.9±1.0 | 0.5±0.1 | 1.6±0.1 | 0.1±0.1 | 0.1±0.1 | 0.2±0.1 | CHC |
cDNAs were generated from the cells of xenografts produced by 21 cell populations, and the parental PLC line, and the expression of human liver genes (ALB, AFP, α1-AT, CK19, CK7), liver cancer-related markers (CD133, EpCAM, CD44, CD90), and CD31, were determined by TaqMan PCR. The relative expression levels in each subgroup were normalized to the cells of xenografts produced by the injection of CD34+ negative for CD133, or CD44, or CD31, or CD90, or EpCAM, or OV6 respectively, or by CD34+ cells (bottom of the table). The major phenotype of xenografts produced by each subpopulation is listed at the far right column. Data represent mean±SEM (4≤n≤11).
CC, cholangiocarcinomas; CHC, combined hepatocellular cholangiocarcinoma; HCC, hepatocellular carcinoma.
IHC analysis for CC markers
Recultured cells from CC xenografts were double stained with antibodies against Hep Par 1 and CK19, and xenograft cells were negative for Hep Par1, and CK19+ cells showed a cuboidal to columnar appearance with round central nuclei, resembling bile duct cells, not hepatocytes (Fig. 5B). Recultured cells from CHC xenografts were double stained with antibodies against Hep Par 1 and AE1/AE3, a specific marker of CC [20]. Xenograft cells were positive for both markers, indicating that they co-expressed markers of both HCC and CC (Fig. 5C). Another two markers, CK20, and mucin 1 (MUC1), have also been used to determine CHC/CC [21,22]. Tissues on slides from CHC and CC xenografts were double stained with antibodies against Hep Par1 and CK20, in addition to Hep Par1 and MUC1 respectively, and the results showed that these two markers also were expressed in CC and CHC xenografts (Fig. 5D, E).
Discussion
Many treatment modalities have been developed; however, we are still far from finding a cure for most cancers. An emerging concept of CSC helps in our understanding of tumorigenicity [1]. A number of putative LCSCs have apparently been isolated and characterized by others based on putative CSC markers [7–11]; however, the origin of LCSCs remains elusive. CD34+ stem cells are an important cell population during liver development and regeneration [12–14]. We hypothesized that some HLCs might be transformed from normal CD34+ stem cells. After evaluating seven hepatoma cell lines, we found that the percentage of CD34+ cells was highest in PLC. After injecting as few as 100 CD34+ cells, HLC xenografts were formed in NOD/SCID/Il2rg mice. One hundred thousand parental PCL cells were required to form HLC in NOD/SCID/IL2rg mice during the same period; thus, this demonstrated that the CD34+ cells were more tumorigenic, indicating that CD34+ cells functioned as LCSCs.
We then attempted to characterize xenografts produced by this LCSC. Twelve subpopulations of CD34+ PLC and 6 progenies isolated from differentiated CD34+ PLC could form HLC xenografts with three phenotypes (HCC, CHC, and CC) in mice. This is the first time that CD34+ cells have been shown to have the characteristics of an LCSC showing the capacity to differentiate into HLC xenografts. This is also the first report that LCSC can produce three types of HLC in an animal model, indicating its multipotency. These 18 subpopulations from one origin have the capacity to independently initiate the tumors, indicating that they are TICs. Clinically, 40% of HCCs are clonal, and therefore potentially arise from progenitor/stem cells [2]. The multicentric nature of many HCCs strongly suggests that all lesions were not initiated by the same single stem/progenitor cell. Thus, clinically HLCs are a pool of heterogeneous populations produced by different TIC. This new finding may have broad implications for the multistep model of tumor initiation and progression, and for developing novel strategies of anticancer therapies (Fig. 5F).
There are several interesting findings in this study. First, the percentages of any of six markers (CD44, CD133, CD31, CD90, EpCAM, and OV6) were higher in xenografts produced by these six positive subpopulations of CD34+ PLC and their progenies when compared to those in the xenografts produced by these six corresponding negative subpopulations of CD34+ PLC. This phenomenon might be the reason why the cancer population initiated by diverse ITC represents heterogeneity, and these diverse ITC were derived from one origin with multipotency. Second, in 21 injected subgroups, 12 subgroups generated HCC xenografts as the major phenotype, 9 subgroups produced CHC xenografts as the major phenotype; and 2 subgroup generated CC (Table 3). Thus, our results would suggest a better chance of developing HCC by these ITC in this case. Interestingly, we also determine that CD31+ cells were found at high levels in some xenografts, normally neither human liver stem cells nor liver cancer cells express CD31; this suggests that CD31+ cells might be involved in the origination or transformation of this CD34+ LCSC. Thus, this is also the first report that CD31+ cells could form a human liver cancer. Third, six double positive subpopulations of CD34+ cells generated CHC and CC xenografts, and their progenies generated HCC, CHC, and CC xenografts, but the expression of four cancer markers (CD133, CD44, CD90, and EpCAM) was not uniform, a further representation of the heterogeneity of cancer cells. Finally, the period of tumor formation varied among these TICs (Table 1); six progenies (progenitors) from CD34+ cells showed more tumorigenic, especially CD34−CD133+ cells, CD34−CD44+ cells, and CD34−CD31+ cells, when compared to their ancestors. Four progeny with cancer markers (CD133, or CD90, or CD44, or EpCAMP) predominantly produced HCC xenografts as their major phenotypes, and these four markers are found in all known well-, and moderately differentiated hepatoma cells [7–11], and they have been used to isolate LCSCs [7–11]. Importantly, all progeny with these cancer markers were more tumorigenic and produced HCC xenografts with high expression levels of hepaptocyte markers (Table 3). Thus, if liver cancer progenitor cells with these markers widely exhibit more tumorigenic capacity as they do in this study, this may also explain why HCC is the clinically predominant phenotype in human primary liver cancers.
Although CD133+ cells, CD90+ cells, CD44+ cells, EpCAM+ cells, and OV6+ cells were isolated from hepatoma cell lines and showed tumorigenicity in mice [7–11,23], the origin of these putative liver cancer stem/progenitor cells still remain unknown. In adult liver, HSPCs (oval cells in rodents) are thought to be liver stem/progenitor cells, which differentiate into hepatocytes and biliary duct cells and are responsible for liver regeneration and repair if the liver damage is extensive and proliferation of hepatocytes is inhibited. HSPCs/Oval cells are not a homogeneous, well-defined population, but represent a complex mixture of different cell types, all of which are activated during progenitor-dependent regeneration [14]. In mice and rats, some HCC and CC have been proposed to be of oval cell origin [24,25]. The phenotype and important markers of rodent oval cells are well defined [26–28]. HSPCs include intra- and extra-hepatic stem/progenitor cells, and the intrahepatic compartment most likely derives primarily from the biliary tree, particularly the most proximal branches, that is, the canals of Hering and smallest ductules. The extrahepatic compartment is at least in part derived from diverse populations of cells from the bone marrow (BM) [29–31]. Kupffer cells (KC) are BM-derived components of the hepatic sinusoid, and express CD34 and CD31, and KCs are implicated in the pathophysiological process of liver injury [32]. BM-derived hematopoietic stem cells (BM-HSC) can migrate to and engraft into injured adult livers [33,34]. Moreover, BM-HSC have been shown to directly differentiate into hepatocytes in vivo [35–38], and it has been shown by other investigations that BM-HSC-derived cells can be converted into a hepatocyte phenotype by fusion with hepatocytes in the livers [39–43]. These CD34+ cell types represent the potential cell source of our CD34+ LCSC. The origin, formation mechanism, pathophysiology, and clinical relevance of this CD34+ LCSC are under investigation.
Conclusions
Our results demonstrated that CD34+ PLCs function as an LCSC. Subpopulations expressing specific antigenic traits indicate the types of HLC that will form in HLC xenografts. Thus, our study provides evidence to support the hypothesis that some HLCs might be derived from transformed CD34+ stem cells, indicating that stem cells not only are responsible for organ regeneration and tissue repair, but they are also targets of carcinogenesis. Eighteen subpopulations from CD34+ cells from one origin function as TIC, and we further determined that all progenies from CD34+ cells appeared to be more tumorigenic when compared to their ancestors, especially progeny with cancer markers (CD133, or CD44, or CD90, or EPCAM), which predominantly produced HCC xenografts with high levels of hepatocyte markers. Thus, if this phenomenon widely exists, this may explain why HCC are the clinically predominant phenotype in human primary liver cancers. In addition, the multicentric nature of many HCCs strongly suggests that all lesions were not initiated by the same TIC, and that clinically HLC are a pool of heterogeneous populations produced by different TIC. This new finding may have broad implications for the multistep model of tumor initiation and progression, and for developing novel strategies of anticancer therapies (Fig. 5F). In our study, we also revealed that OV6+ cells were associated with the formation of human CHC and CC. Of note, CD34+ PLC cells co-expressed OV6 and CD31; moreover CD34+OV6+ cells, and CD34+CD31+ cells, and their progeny OV6+ cells and CD31+ cells formed HLC xenografts, implying that the formation of this CD34+ LCSC may be associated with OV6+ cells and CD31 lineage cells. This is the first report that HLC appear to be initiated and developed from CD34+ cells, thus revealing a diversity of origins for human liver cancers.
Supplementary Material
Acknowledgments
We gratefully acknowledge the NIH-supported Liver Tissue Cell Distribution System for providing human primary hepatocytes. This work was supported by NIH grant DK075415 (to M.A.Z.), and the GlaxoSmithKline Research Fund of the Korean Association for the Study of the Liver (to S.C.P., and J.R.E.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Llovet JM, Burroughs A. and Bruix J. (2003). Hepatocellular carcinoma. Lancet 362:1907–1917 [DOI] [PubMed] [Google Scholar]
- 2.Yao Z. and Mishra L. (2009). Cancer stem cells and hepatocellular carcinoma. Cancer Biol Ther 8:1691–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reya T, Morrison SJ, Clark MF. and Weissman IL. (2001). Stem cell, cancer and cancer stem cells. Nature 414:105–111 [DOI] [PubMed] [Google Scholar]
- 4.Thorgeirsson SS. and Grisham JW. (2002). Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31:339–346 [DOI] [PubMed] [Google Scholar]
- 5.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, et al. (2006). A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med 12:410–416 [DOI] [PubMed] [Google Scholar]
- 6.Chiba T, Zheng Y, Kita K, Yokosuka O, Saisho H, Onodera M, Miyoshi H, Nakano M, Zen Y, et al. (2007). Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology 133:937–950 [DOI] [PubMed] [Google Scholar]
- 7.Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PWK, Lam CT, Poon RTP, et al. (2008). Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 13:153–166 [DOI] [PubMed] [Google Scholar]
- 8.Ma S, Chan K, Hu L, Lee TK, Wo JY, Ng IO, Zheng B. and X Guan. (2007). Identification and characterization of tumorigenic liver stem/progenitor cells. Gastroenterology 132:2542–2556 [DOI] [PubMed] [Google Scholar]
- 9.Ding W, Mouzaki M, You H, Laird JC, Mato J, Lu SC. and Rountree CB. (2009). CD133+ liver cancer stem cells from methionine adenosyl transferase 1A-deficient mice demonstrate resistance to transforming growth-factor (TGF)-beta-induced apoptosis. Hepatology 49:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J. and Li J. (2009). Cancer stem/progenitor cells are highly enriched in CD133(+) CD44(+) population in hepatocellular carcinoma. Int J Cancer 126:2067–2078 [DOI] [PubMed] [Google Scholar]
- 11.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang H, Jia H, Ye Q, Qin L, et al. (2009). EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 136:1012–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X. and Chen D. (2006). Origin of hepatocellular carcinoma: role of stem cells. J Gastroenterol Hepatol 21:1093–1098 [DOI] [PubMed] [Google Scholar]
- 13.Eckersley-Maslin MA, Warner FJ, Grzelak CA, McCaughan GW. and Shackel NA. (2009). Bone marrow stem cells and the liver: are they relevant? J Gastroenterol Hepatol 24:1608–1616 [DOI] [PubMed] [Google Scholar]
- 14.Duncan AW, Dorrell C. and Grompe M. (2009). Stem cells and liver regeneration. Gastroenterology 137:466–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, Tolstikov V. and Zern MA. (2010). Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells 28:674–686 [DOI] [PubMed] [Google Scholar]
- 16.Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, Gambhir SS. and Zern MA. (2007). Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells 25:3058–3068 [DOI] [PubMed] [Google Scholar]
- 17.Van Mourik JA, Leeksma OC, Reinders JH, de Groot PG. and Zandbergen-Spaargaren J. (1985). Vascular endothelia cells synthesize a plasma membrane protein indistinguishable from the platelet membrane glycoprotein lla. J Biol Chem 260:11300–11306 [PubMed] [Google Scholar]
- 18.Stockinger H, Gadd SJ, Eher R, Majdic O, Schreiber W, Kasinrerk W, Strass B, Schnabl E. and Knapp W. (1990). Molecular characterization and functional analysis of the leukocyte surface protein CD31. J Immunol 145:3889–3897 [PubMed] [Google Scholar]
- 19.Alexander JJ, Bey EM, Geddes EW. and Lecatsas G. (1976). Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J 50:2124–2148 [PubMed] [Google Scholar]
- 20.Lau SK, Prakash S, Geller SA. and R Alsabeh. (2002). Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol 33:1175–1181 [DOI] [PubMed] [Google Scholar]
- 21.Chu PG, Schwarz RE, Lau SK, Yen Y. and Weiss LM. (2005). Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol 29:359–367 [DOI] [PubMed] [Google Scholar]
- 22.Rullier A, Le Bail B, Fawaz R, Blanc JF, Saric J. and Bioulac-Sage P. (2000). Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol 24:870–876 [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Wang C, Lin Y, Liu Q, Yu L, Tang L, Yan H, Fu J, Chen Y, et al. (2012). OV6+ tumor-initiating cells contribute to tumor progression and invasion in human hepatocellular carcinoma. J Hepatol 57:613–620 [DOI] [PubMed] [Google Scholar]
- 24.Sell S. and Dunsford HA. (1989). Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol 134:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- 25.Sell S. and Leffert HL. (1982). An evaluation of cellular lineage in the pathogenesis of experimental hepatocellular carcinoma. Hepatology 2:77–86 [DOI] [PubMed] [Google Scholar]
- 26.Alison MR, Golding M, Sarraf CE, Edwards RJ. and Lalani EN. (1996). Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroentrology 110:1182–1190 [DOI] [PubMed] [Google Scholar]
- 27.Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S, Canaday PS, Streeter PR. and Grompe M. (2008). Surface markers for the murine oval cell response. Hepatology 48:1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M. and Grompe M. (2003). The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci U S A 100:11881–11888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A. and Crawford JM. (1999). The canals of Hering and hepatic stem cells in human. Hepatology 30:1425–1433 [DOI] [PubMed] [Google Scholar]
- 30.Baumann U, Crosby HA, Ramani P, Kelly DA. and Strain AJ. (1999). Expression of the stem cell factor receptor c-kit in normal and disease pediatric liver: identification of a human hepatic progenitor cell? Hepatology 30:112–117 [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Alvarez N, Soto-Gutierrez A. and Kobayashi N. (2010). Hepatic stem cells and liver development. Methods Mol Biol 640:181–236 [DOI] [PubMed] [Google Scholar]
- 32.Arii S. and Imamura M. (2007). Physiological role of sinusoidal endothelial cells and Kupffer cells and their implication in the pathogenesis of liver injury. J Hepatobiliary Pancreat Surg 7:40–48 [DOI] [PubMed] [Google Scholar]
- 33.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS. and Goff JP. (1999). Bone marrow as a potential source of hepatic oval cells. Science 284:1168–1170 [DOI] [PubMed] [Google Scholar]
- 34.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM. and Krause DS. (2000). Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology 31:235–240 [DOI] [PubMed] [Google Scholar]
- 35.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, et al. (2000). Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 6:1229–1234 [DOI] [PubMed] [Google Scholar]
- 36.Jang Y, Collector MI, Baylin SB, Diehl AM. and Sharkis S. (2004). Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol 6:532–539 [DOI] [PubMed] [Google Scholar]
- 37.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS. and Krause DS. (2004). Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 305:90–93 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, et al. (2002). Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 109:1291–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willenbring H, Bailey AS, Foster M, Akkari Y, Dorrell C, Olson S, Finegold M, Fleming WH. and Grompe M. (2004). Myelomonocytic cells are sufficient for therapeutic cell fusion in the liver. Nat Med 10:744–748 [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, et al. (2003). Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422:897–901 [DOI] [PubMed] [Google Scholar]
- 41.Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, et al. (2000). Hepatocytes from non-hepatic adult stem cells. Nature 406:257. [DOI] [PubMed] [Google Scholar]
- 42.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O. and Krause DS. (2000). Liver from bone marrow in humans. Hepatology 32:11–16 [DOI] [PubMed] [Google Scholar]
- 43.Thorgeirsson SS. and Grisham JW. (2006). Hematopoietic cells as hepatocyte stem cells: a critical review of the evidence. Hepatology 43:2–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.