Abstract
Purpose
Clinical guidelines recommend oncologists prescribe exercise to their patients with colorectal cancer (CRC). However, 84% of oncologists do not prescribe exercise citing concerns of safety and feasibility. Data are inadequate regarding the proportion of CRC survivors that could be safely prescribed the dose of exercise recommended by the American College of Sports Medicine (ACSM), American Cancer Society (ACS), or National Comprehensive Cancer Network (NCCN), in an unsupervised setting.
Methods
We reviewed published guidelines for exercise prescription among cancer survivors and extracted health-factors that may necessitate referral to trained personnel (physical therapist, exercise professional) for an individualized exercise program, or supervision of exercise as recommended by ACSM/ACS/NCCN. We applied these health-factors to a cohort of non-metastatic CRC survivors, six-months after completing curative care. The primary outcome was the proportion of CRC survivors for whom oncologists could prescribe unsupervised exercise at the dose recommended by ACSM/ACS/NCCN.
Results
Among 351 CRC survivors, six-months after curative care, 21% to 42% of patients could be prescribed the dose of exercise recommended by ACSM/ACS/NCCN. Estimates varied as a function of the inclusion or exclusion of several prevalent comorbid health conditions including hypertension, diabetes, arthritis, obesity, and hyperlipidemia.
Conclusion
Our data are consistent with the clinical observation that a large proportion of CRC survivors may be unable to participate in unsupervised exercise six-months after curative care. These data underscore the need for continued research to clarify the safety and feasibility of prescribing exercise to CRC survivors.
Keywords: Safety, Adverse Event, Physical Activity, Efficacy, Survivorship
INTRODUCTION
Exercise training has similar risks and benefits of pharmacological therapy (18, 39, 40). Exercise is a distinct subset of physical activity that is structured, repetitive, and performed to sustain or improve health and fitness (4). An appropriately prescribed dose of exercise engages cardiopulmonary, metabolic, and musculoskeletal tissues. Long-term participation in exercise yields numerous health-benefits that include risk-reduction for premature mortality, and primary and secondary prevention of several chronic conditions (39). The benefits of exercise have been documented among cancer survivors (31). The American College of Sports Medicine (ACSM), American Cancer Society (ACS), and National Comprehensive Cancer Network (NCCN) recommend all cancer survivors engage in 150-minutes of moderate-intensity or 75-minutes of vigorous-intensity aerobic exercise per week, perform two to three muscle strengthening sessions per week, and perform flexibility activities on days of exercise (23, 27, 31). Exercise for colorectal cancer (CRC) survivors reduces cancer recurrence, cancer-specific mortality, and all-cause mortality in dose-response fashion (21, 22). Therefore, it is of clinical interest to determine how to safely increase the dose of exercise prescribed to CRC survivors to meet the recommended guidelines for exercise and cancer survivorship.
Despite risks and benefits similar to pharmacological therapy (18, 39, 40), the integration of exercise into the standard of cancer care has not followed the regulatory process of drug approval (6). Such an approval process would require the indications, dose escalation, contraindications, and adverse events associated with exercise to be systematically reported from clinical trials and made known prior to being implemented for use in the oncology clinic. When indicated, the benefits of exercise among cancer survivors have been well-characterized, and include improvements in clinical and patient-reported outcomes (31). However, the dose escalation, contraindications, and adverse events associated with exercise have been poorly characterized (15, 16). Current guidelines ask oncologists to prescribe exercise without knowing the risk to benefit ratio, a ratio that would otherwise be available if the intervention were a drug (29). This recommendation is worrisome as the current infrastructure to provide exercise training for cancer survivors is such that the majority of patients engage in unsupervised exercise (17). To minimize potential risk to patients, oncologists are reluctant to prescribe exercise (8, 28). More specifically, among CRC survivors, the risk to benefit ratio of exercise may seem equivocal, given that CRC survivors are older, have multiple comorbid conditions, and frequently report late-effects of cancer treatment (8). Many oncologists believe their patients are unable to tolerate or successfully complete an unsupervised exercise program (8, 28, 29). Subsequently, 84% of oncologists do not recommend any exercise to their patients with CRC (28), which may partially explain why 68% of CRC survivors are physically inactive after completing curative care (19). This is troubling, given that physical activity is associated with improvements in clinical outcomes among CRC survivors (21, 22).
The proportion of CRC survivors for whom oncologists could prescribe unsupervised exercise at the dose recommended by the ACSM/ACS/NCCN clinical guidelines is unknown. Herein, and consistent with the current infrastructure of most cancer center settings (17), exercise is assumed to be unsupervised. To this end, we synthesized published guidelines of exercise prescription for cancer survivors and extracted health-factors that may necessitate referral to trained personnel (physical therapist, exercise professional) for an individualized exercise program, or for supervision of exercise (23). We applied these synthesized health-factors to a sample of CRC survivors treated in a university health system. The primary outcome of this study was the proportion of CRC survivors for whom oncologists could prescribe unsupervised exercise at the dose suggested by the ACSM/ACS/NCCN clinical guidelines, six-months after curative care.
METHODS
Cohort Inclusion
Men and women with a diagnosis of CRC (International Classification of Disease, 9th Revision [ICD-9]: 153, 154) in the University of Pennsylvania Health System (UPHS) between 30 October 2008 and 30 November 2011 were considered for cohort inclusion. Cohort members were required to have all of their curative care in the UPHS system. All cohort members were required to have a follow-up visit approximately six-months after completing curative care. Patients with metastatic disease at diagnosis were excluded from cohort inclusion. This study was approved by the Institutional Review Board of the University of Pennsylvania. The Institutional Review Board determined that informed consent could be waived on the basis that this study did not involve any direct patient interaction.
Synthesis of Exercise Guidelines
We conducted a targeted search to obtain peer-reviewed guidelines for exercise prescription among cancer survivors. Our search identified nine documents and ranged in publication year from 2006–2013. Three documents were published from the ACSM, including the ACSM’s Guidelines for Exercise Testing and Prescription 8th Edition (37), the ACSM roundtable on exercise guidelines for cancer survivors (31), and the ACSM’s Guide to Exercise and Cancer Survivorship (30). Additional documents were published by the ACS (27), NCCN (23), Australian Association for Exercise and Sport Science (12), and the National Research Council Canada (16). Two documents were published by independent investigators, one as a supplement to the ACSM roundtable on exercise guidelines for cancer survivors (15), and one that served as a foundation for the ACSM’s guidelines (20). These guidelines were reviewed by the two authors, and health-factors described as requiring referral to trained personnel for an individualized exercise program, or supervision of exercise were abstracted into a categorized list. All guidelines were considered of equal importance, and health-factors identified in any of the nine documents were included in this analysis. There was a high degree of consistency across guidelines as 86% of health-factors were described in two or more documents. Health-factors were classified into one of the following system-specific categories: hematologic, musculoskeletal, systemic, gastrointestinal, cardiovascular symptoms, cardiovascular disease history, pulmonary, neurologic, comorbidities, and implanted medical device (see Table, Supplemental Digital Content 1, derivation of outcomes). The presence of one or more health-factors indicated the need to refer to trained personnel for an individualized exercise program, or supervised the ACSM/ACS/NCCN recommended dose of exercise. This approach is consistent with a variety of pre-exercise screening questionnaires (1).
Outcome Assessment Time Point
The optimal time for lifestyle intervention and provision of lifestyle recommendations is six-months after curative care (7). Six-months allows time for the acute symptoms of cancer treatment to subside, while still in the interval for the teachable moment to be in effect, a time when cancer survivors are most likely to adopt lifestyle and behavioral recommendations before regressing back to pre-diagnostic behaviors and lifestyle (7). Qualitative research has concluded CRC survivors prefer to start exercise six-months after completing curative care, when they feel physically and psychologically ready for exercise (3).
Abstraction of Outcomes & Application of Exercise Guidelines to Participant Cohort
We abstracted data from the electronic medical record at the clinical visit most proximal to six-months after curative care. Abstracted data included standard measures for cancer care follow-up in UPHS consisting of blood chemistries, resting pulse and blood pressure, oral temperature, physician-diagnosed symptoms and side-effects, patient-reported symptoms and side-effects present at the time of the clinical visit, and any ICD-9 or procedure codes used to classify conditions or procedures in our abstracted list. Demographic information, including age, sex, race, and tobacco use, were abstracted. Clinical information including type of cancer, stage (AJCC 7th edition), and treatment (chemotherapy/radiation) were abstracted. We calculated the Charlson age-comorbidity index to estimate 10-year mortality (5).
Statistical Analysis
Using the data abstracted at the six-month follow-up visit, we generated binary variables (yes/no) to indicate if each cohort member had health-factors sufficient to refer to trained personnel for an individualized exercise program, or supervise the ACSM/ACS/NCCN recommended dose of exercise. We generated a composite outcome as the sum of all health-factors, and then dichotomized that variable between CRC survivors that had zero versus one or more health-factor(s). Values of zero indicate no need for referral or supervision of exercise. Values of one or more would indicate potential need to refer to trained personnel for an individualized exercise program, or supervised the ACSM/ACS/NCCN recommended dose of exercise. We estimated this proportion and calculated 95% confidence intervals (95% CI) using the binomial exact method. This outcome was modeled as our dependent variable in exploratory logistic regression analyses. We calculated the odds ratio (OR) and 95% Confidence Intervals (95% CI) to estimate the magnitude of association of demographic and clinical variables with the likelihood to refer to trained personnel for an individualized exercise program, or supervision of exercise. For all covariates we had ≥80% statistical power to detect an odds ratio of 2.0, indicating a two-fold increase in the need for referral for an individualized exercise program or supervised exercise. Lastly, we conducted pre-specified sensitivity analyses excluding common comorbidities among CRC survivors from the composite outcome including hypertension, diabetes, arthritis, obesity, and hyperlipidemia (8, 38, 42).
RESULTS
Patient Selection for Cohort Inclusion
Between 30 October 2008 and 30 November 2011, UPHS diagnosed 700 patients with CRC. Among the 700 diagnosed, 223 had metastatic CRC and were excluded. Among the remaining 477 patients with non-metastatic CRC, 52 received a diagnosis but no further treatment in the UPHS system and 74 were excluded on the basis of receiving curative treatments outside of UPHS, not having a documented six-month follow-up visit, having recurrent cancer, or death. To this end, 351 patients met all inclusion criteria and were included in this analysis. The median time since completing curative care was 6.5 months [interquartile range: 3.5–9.9 months]; patients who underwent surgery only, generally had their follow-up visits more proximal to completing curative therapy (i.e., <6 months), as compared to those who received chemotherapy and/or radiation in addition to surgery.
Cohort Demographic and Clinical Characteristics
Among the 351 cohort members, age ranged from 18–99 (Table 1). Colon cancer survivors constituted 63% of the cohort, and the remaining 37% were rectal cancer survivors. The Charlson age-comorbidity index ranged from zero to seven, 78% of CRC survivors had an index score ≤1, and the predicted median 10-year survival was 39% [interquartile range: 10–68%].
Table 1.
Demographic and clinical variables (N=351)
| Variable | Valuea |
|---|---|
| Age - yr | 61.5±13.7 |
| Sex | |
| Male | 180 (51%) |
| Female | 171 (49%) |
| Race | |
| White | 243 (69%) |
| Black | 64 (18%) |
| Other | 44 (13%) |
| Tobacco Use | |
| Never | 163 (46%) |
| Former | 125 (36%) |
| Current | 63 (18%) |
| Type of Cancer | |
| Colon | 222 (63%) |
| Rectal | 129 (37%) |
| Stage | |
| I | 110 (31%) |
| II | 77 (22%) |
| III | 153 (44%) |
| Unknown/Missing | 11 (3%) |
| Chemotherapy - % | 176 (50%) |
| Radiation - % | 90 (26%) |
| Charlson Comorbidity Index | |
| 0 | 226 (64%) |
| 1 | 48 (14%) |
| ≥2 | 77 (22%) |
Variables are mean ± standard deviation or n (%), unless otherwise noted
Application of Exercise Guidelines to Participant Cohort: Primary Outcome
The prevalence of individual and system-specific health-factors recommended for referral to trained personnel for an individualized exercise program, or supervision of exercise varied widely (Table 2). The cumulative number of health-factors is depicted graphically (Figure 1A). The median number of health-factors was two [interquartile range: 1–3] and ranged from zero to 11. In our primary outcome analysis, 21% (95% CI: 16–25%) of CRC survivors were able to be prescribed unsupervised exercise of the dose recommended by ACSM/ACS/NCCN, six-months after curative care.
Table 2.
Number and percentage of patients with health related factors that may require referral to trained personnel for an individualized exercise program, or supervision of exercise (N=351)
| Health Related Factor | n (%) |
|---|---|
| Hematologic - any of the following | 26 (7%) |
| Platelets <50,000 | 1 (<1%) |
| White Blood Cells <3,000 | 8 (2%) |
| Hemoglobin <10g/dl | 18 (5%) |
| Musculoskeletal - any of the following | 19 (5%) |
| Bone, Back, or Neck Pain | 15 (4%) |
| Unusual Muscular Weakness | 6 (2%) |
| Systemic - any of the following | 11 (3%) |
| Fever >100°F | 9 (3%) |
| Malaise | 5 (1%) |
| Gastrointestinal - any of the following | 15 (4%) |
| Nausea | 1 (<1%) |
| Vomiting or Diarrhea | 5 (1%) |
| Inadequate Food/Fluid Intake | 11 (3%) |
| Fecal or Urinary incontinence | 4 (1%) |
| Cardiovascular - any of the following | 81 (23%) |
| Chest Pain | 3 (1%) |
| Pulse >100 or <50 beats.min−1 | 6 (2%) |
| SBP >145 or <85 mmHg or DBP>95 mmHga | 50 (14%) |
| Irregular Pulse | 5 (1%) |
| Ankle Edema | 25 (7%) |
| Cardiovascular Disease History - any of the following | 43 (12%) |
| Ventricular Ectopy | 5 (1%) |
| Myopericarditis | 0 (0%) |
| Cardiomyopathy | 0 (0%) |
| Congestive Heart Failure | 11 (3%) |
| Aortic Stenosis | 7 (2%) |
| Heart Attack | 12 (3%) |
| Cardiac Catheterization | 3 (1%) |
| Coronary Angioplasty | 24 (7%) |
| Heart Valve Disease | 9 (3%) |
| Pulmonary - any of the following | 39 (11%) |
| Dyspnea | 3 (1%) |
| Coughing or Wheezing | 14 (4%) |
| Chest Pain with Deep Breath | 3 (1%) |
| Asthma or Bronchospasm | 22 (6%) |
| Neurologic - any of the following | 3 (1%) |
| Blurred Vision | 1 (<1%) |
| Neuropathy | 2 (<1%) |
| Concussion | 0 (0%) |
| Comorbidities - any of the following | 243 (69%) |
| Hypertension | 152 (43%) |
| Heart Murmur | 5 (1%) |
| Unspecified Cardiac Disease | 5 (1%) |
| Diabetes | 51 (15%) |
| Arthritis | 13 (4%) |
| Osteoporosis | 18 (5%) |
| COPD | 17 (5%) |
| Depression | 22 (6%) |
| Obesity | 22 (6%) |
| Hernia | 35 (10%) |
| Kidney Disease | 8 (2%) |
| Hyperlipidemia | 133 (38%) |
| Hyperthyroidism | 4 (1%) |
| Liver Disease | 9 (3%) |
| HIV/AIDS | 1 (<1%) |
| Implanted Medical Devices - any of the following | 26 (7%) |
| Ostomy/Stoma | 19 (5%) |
| Cardiac Pacemaker | 7 (2%) |
SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure
Figure 1.
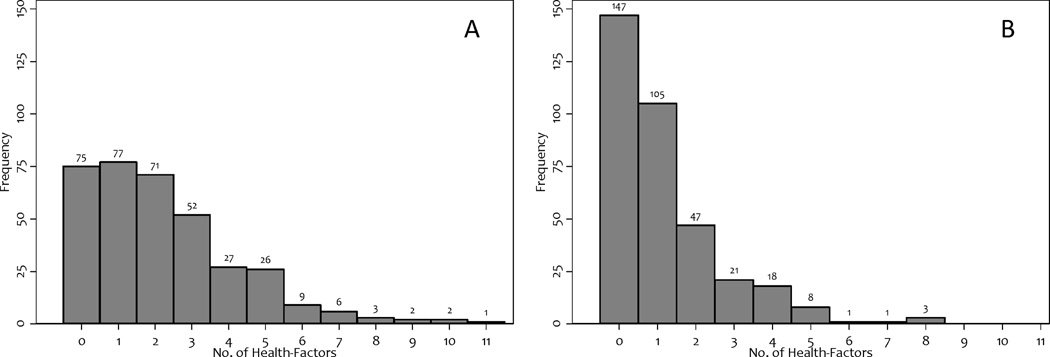
Distribution of health-factors that may require referral to trained personnel, or supervision of exercise in: (a) the primary outcome analysis, and; (b) the sensitivity analysis that excluded hypertension, diabetes, arthritis, obesity, and hyperlipidemia.
Application of Exercise Guidelines to Participant Cohort: Sensitivity Analysis
In sensitivity analysis, we excluded common comorbidities documented among CRC survivors including hypertension, diabetes, arthritis, obesity, and hyperlipidemia (8, 38, 42). The median number of health-factors was one [interquartile range: 0–2] and ranged from zero to eight (Figure 1B). After exclusion of those five comorbidities, 42% (95% CI: 37–47%) of CRC survivors were able to be prescribed unsupervised exercise of the dose recommended by ACSM/ACS/NCCN, six-months after curative care.
Factors Associated with Needing to Modify Exercise Guidelines
In multivariable logistic regression, increasing age (Ptrend=0.007), and increasing number of comorbidities (Ptrend<0.001), associated with greater need for referral to trained personnel for an individualized exercise program, or supervision of exercise (Table 3).
Table 3.
Association between demographic and clinical variables and need to refer to trained personnel for an individualized exercise program, or supervision of exercise
| Variable | Univariable OR (95% CI)a | P | Multivariable OR (95% CI)a | P |
|---|---|---|---|---|
| Age | ||||
| Quintile 1 – 45±6 yr. | 1.00 | 1.00 | ||
| Quintile 2 – 56±3 yr. | 2.16 (1.10–4.25) | 0.026 | 1.84 (0.85–3.99) | 0.124 |
| Quintile 3 – 66±3 yr. | 3.12 (1.51–6.43) | 0.002 | 2.96 (1.29–6.77) | 0.010 |
| Quintile 4 – 80±6 yr. | 3.64 (1.70–7.78) | 0.001 | 2.79 (1.11–7.02) | 0.029 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.97 (0.58–1.61) | 0.904 | 1.11 (0.61–2.04) | 0.726 |
| Race | ||||
| White | 1.00 | 1.00 | ||
| Black | 1.22 (0.59–2.50) | 0.594 | 0.99 (0.43–2.27) | 0.974 |
| Other | 0.49 (0.24–0.98) | 0.044 | 0.45 (0.20–1.01) | 0.053 |
| Tobacco Use | ||||
| Never | 1.00 | 1.00 | ||
| Former | 1.87 (1.01–3.46) | 0.046 | 1.12 (0.56–2.25) | 0.740 |
| Current | 0.79 (0.41–1.51) | 0.471 | 0.50 (0.23–1.08) | 0.077 |
| Type of Cancer | ||||
| Colon | 1.00 | 1.00 | ||
| Rectal | 1.04 (0.61–1.77) | 0.879 | 0.82 (0.33–2.02) | 0.660 |
| Stage | ||||
| I | 1.00 | 1.00 | ||
| II | 1.52 (0.73–3.20) | 0.265 | 0.92 (0.39–2.21) | 0.853 |
| III | 1.12 (0.63–2.10) | 0.692 | 0.77 (0.31–1.91) | 0.569 |
| Chemotherapy | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.04 (0.62–1.74) | 0.874 | 1.57 (0.60–4.12) | 0.362 |
| Radiation | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.23 (0.67–2.24) | 0.506 | 1.56 (0.51–4.82) | 0.437 |
| Charlson Comorbidity Index | ||||
| 0 | 1.00 | 1.00 | ||
| 1 | 4.94 (1.71–14.27) | 0.003 | 4.27 (1.38–13.20) | 0.012 |
| ≥2 | 34.10 (4.65–250.19) | 0.001 | 32.14 (4.26–242.28) | 0.001 |
Odds Ratio (OR) from Logistic Regression and 95% Confidence Interval (95% CI).
DISCUSSION
Our data are consistent with the clinical observation that six-months after completing curative care 21–42% CRC survivors are able to be prescribed unsupervised exercise of the dose recommended by ACSM/ACS/NCCN. The true proportion of CRC survivors who could successfully complete the recommended dose of exercise may be overestimated. After determining safety of exercise, CRC survivors must possess the knowledge and resources to complete exercise. CRC survivors report uncertainty about what type exercise they should engage in and from whom they should seek information (8). Our data are consistent with oncologist prescribing patterns (28), and suggest that exercise guidelines may require other supportive services such as supervision, and staff with the knowledge necessary to appropriately tailor exercise for the majority of CRC survivors, such as physical therapists and exercise professionals (32).
The major limitation of this investigation was the retrospective cohort design. While all necessary information required for the ACSM/ACS/NCCN guidelines for exercise prescription were available in this cohort, our information was limited to what was recorded in the medical chart. Similar to prior studies (38, 42), our study utilized ICD-9 codes, but also leveraged a variety of other clinical data gathered at the six-month follow-up including results of blood chemistries, physician exam, patient self-report, and resting physiologic measures of pulse, blood pressure, and oral temperature. The hospitals in the UPHS system are large tertiary care centers, and the characteristics of CRC survivors seen in these two metropolitan hospitals may not reflect characteristics of CRC survivors seen in the community setting. Additional data are necessary to confirm whether our findings can be replicated in other CRC populations. Our exploratory logistic regression models had sufficient statistical power to detect a doubling of the odds ratio. It is plausible that smaller odds ratios may be clinically meaningful. For example, in the current analysis radiation and chemotherapy increased the need for referral for an individualized exercise program or supervised exercise by 50%, but did not reach statistical significance due to the limited sample size.
Implications for the Physician
Oncologists are hesitant to prescribe exercise to their patients with CRC on the basis that the risks may outweigh the potential benefits (8, 28). Our data are consistent with this clinical observation, as 21–42% of CRC survivors are able to be prescribed unsupervised exercise of the dose recommended by ACSM/ACS/NCCN. Oncologists have acknowledged their reluctance to prescribe exercise relates to multiple factors. Oncologists acknowledge they have inadequate understanding about the specific dose of exercise necessary to safely improve outcomes, and are unsure how to tailor the ACSM/ACS/NCCN guidelines for older patients with multiple comorbid conditions. Furthermore, oncologists lack the necessary time to discuss topics like exercise with their patients, given the competing need to address late and long-term complications from treatment, and monitor on-going surveillance for recurrent disease (8). While oncologists may be reluctant to prescribe exercise, data suggests that primary care physicians may serve as a source of information about post-treatment health and wellness for cancer survivors. Primary care physicians have frequent encounters with cancer survivors (34), and express willingness to prescribe exercise (33). Several studies in non-cancer survivorship populations have confirmed that exercise counseling by the primary care physician is feasible and improves patient behavior (9, 10). Patients view their physician as a decision-maker for their health, and the physician recommendation is possibly the biggest catalyst to initiate behavior change (14). Patients are likely to remember recommendations about exercise from their physician, and patients will consider engaging in exercise if there is the perception that the physician values such behaviors (8). Given this role of authority, educating oncologists and primary care physicians about the importance of exercise in CRC survivorship is a priority.
To further aid oncologists and primary care physicians in prescribing exercise as a routine part of CRC care, there is a need for empirically-derived risk-stratification algorithms to identify individuals who may require need for referral to trained personnel for an individualized exercise program or supervised exercise. There is currently inadequate safety data from clinical trials to allow the development of a risk-stratification algorithm. The NCCN created a risk-stratification algorithm for cancer survivors (23), based on expert-opinion (16). The NCCN risk-stratification algorithm considers nearly all CRC survivors as high-risk because of a history of abdominal surgery for the resection of their CRC. High-risk patients are required to undergo a medical evaluation, have physician clearance, and be referred to a physical therapist or exercise professional for a tailored exercise prescription (23). In the absence of empirical data, it is unknown if the NCCN risk-stratification algorithm sufficiently discriminates patients who are more (versus less) likely to have an exercise-induced adverse event. The derivation of a risk-stratification algorithm informed from clinical trial data should be considered a future research priority. Resources such as risk-stratification algorithms will equip physicians with the ability to request the participation of the patient in the secondary prevention of cancer and maintenance of their overall health. This approach is consistent with the shared care model in which collaboration among a variety of healthcare providers allows the delivery of information by the most qualified provider (24).
Implications for the Physical Therapist and Exercise Professional
Allied healthcare professionals, such as physical therapists and exercise professionals, will garner increasing responsibility as part of the CRC survivorship team if physicians increase their prescription of exercise to CRC survivors. CRC survivors have more comorbid health conditions than the general population (13), and compared to other cancer sites (26). It is important that allied healthcare professionals who prescribe exercise to CRC survivors coordinate with physicians to comprehensively assess the risks and benefits of a progressive exercise program. For example, 23% of CRC survivors in our study had acute cardiovascular symptoms, such as uncontrolled hypertension, chest pain, or ankle edema. Consequently, knowledge of the relative and absolute contraindications for exercise prescription is critical when working with CRC survivors (37). Allied healthcare professionals must possess the ability to tailor of the frequency, intensity, time, and type of exercise to maximize health, fitness, and safety outcomes. For example, patients with existing cardiovascular disease are at greatest risk of experiencing an exercise-induced adverse event upon the initiation of a progressive exercise program (36). Therefore, supervision and tailored progression of exercise intensity and duration may be important safety considerations for CRC survivors with pre-existing cardiovascular disease. Patients with conditions such as arthritis or morbid obesity will derive numerous health-benefits from exercise, but these patients may require tailoring the mode of exercise to non-weight bearing activities to reduce the risk of joint strain and musculoskeletal injury (37). The overarching goal for allied healthcare professional will be to promote participation in exercise, provide exercise programs that are tailored to maximize health, fitness, and safety outcomes, while minimizing unnecessary barriers to exercise. For this overarching goal to be realized, allied healthcare professionals working with CRC survivors must have expertise in the principles of exercise prescription, complemented by ongoing physician communication. Despite the challenges of prescribing exercise to CRC survivors in clinical practice, the promise of exercise as an efficacious intervention to improve the quantity and quality-of-life among CRC survivors make this an exciting time for the field of exercise-oncology.
Implications for the Colorectal Cancer Survivor
Over 68% of CRC survivors are not regularly physically active after diagnosis (19). CRC survivors decrease their volume of moderate or vigorous intensity aerobic exercise during treatment, and increase to pre-diagnostic levels after treatment (2). This may help to explain why CRC survivors believe they already engage in sufficient levels of exercise to reap health-benefits (8), as they are as active as they were before diagnosis, but not to the levels recommended by the ACSM/ACS/NCCN. Furthermore, CRC survivors experience late and long-term effects of cancer therapy, which act synergistically with pre-existing comorbidity to impair the physical ability and psychological readiness to exercise. Our data suggest a large proportion of CRC survivors have multiple health conditions which may influence ability to exercise. These conditions may act collectively to impair the intention and ability to exercise, two behavioral determinants of exercise participation and adherence among cancer survivors (35). CRC survivors report poor attitudes about exercise, perhaps as a result of having inadequate knowledge about the health-benefits associated with exercise, and being unsure who to ask for recommendations about exercise (8). These issues have been illuminated by the difficulty of randomized controlled exercise trials to successfully accrue participants with CRC. For example, a 12-week home-based walking trial accrued 34% of its targeted recruitment goal despite implementing multiple recruitment strategies, and providing telephone counseling and home-based exercise (25). The use of telephone contact and home-based exercise were two modes of intervention that were expressed as being desirable characteristics by CRC survivors (25). It is plausible that a lack of awareness of the health-benefits of exercise adversely affected the willingness of CRC survivors to participate in this exercise trial. Addressing this knowledge gap through education is important, as higher levels of post-diagnosis physical activity are associated with reductions in recurrence, cancer-specific mortality, and all-cause mortality (8).
A Paradigm Shift
We have reached a critical juncture in the translation of exercise to the standard of cancer care. There are 25-million cancer survivors worldwide, and this population will grow to 75-million over the next three decades (11). There are numerous potential health-benefits associated with regular exercise among cancer survivors. However, placing these health-benefits into context requires knowledge of the risks or harms associated with exercise training in this population. It has been noted that the rigor of adverse event monitoring in exercise training studies among cancer survivors has been of poor methodological quality and underestimates the risks associated with exercise training (16). As noted in the introduction, exercise is a potent intervention, much like a drug (39). However, few of the clinical trials of exercise training among cancer survivors have systematically collected and reported all serious and non-serious adverse events among all study participants, including the control group, such as that in drug trials. This information would help to delineate the most frequent adverse events, their severity, and the excess risk attributable to the exercise training (16). It is unlikely that an oncologist would prescribe a drug to a patient without knowing the risk to benefit ratio. Therefore, it is reasonable that oncologists have been reluctant to prescribe exercise (28). Some guidelines have suggested that the benefits of exercise outweigh its associated risks (31), and others have explicitly stated that specific exercises such as stationary cycling are safe for nearly all cancer survivors (23). Additional research regarding the safety of exercise is necessary to empirically support these conclusions.
Conclusion
Our data are consistent with the clinical observation that a large proportion of CRC survivors may be unable to participate in unsupervised exercise of the dose recommended by ACSM/ACS/NCCN, six-months after curative care. These data may help to explain the discordance between clinical guidelines that recommend all oncologists prescribe exercise, and the 84% of oncologists that make no mention of exercise in clinical practice. We acknowledge that the prescription of exercise to cancer survivors is complex and multifactorial, beyond that of the presence or absence of comorbid health conditions. However, these data are the first step in providing support for continued research to empirically determine what efforts are necessary to safely prescribe exercise to CRC survivors. To promote the prescription of exercise in oncology, efforts to delineate the risk to benefit ratio of exercise are needed. For example, mandating that all exercise trials systematically collect and report serious and non-serious adverse event data on all study participants, such as that in drug trials, would help to describe the most common events that occur with exercise in this population. These data would help to inform the empirical development and validation of risk stratification tools to identify patients for which the benefits of exercise outweigh the risks. For these methods to merit integration into practice they must be easily accessed and quickly implemented in clinical care. Educating oncologists, primary care physicians, and patients about the risks and benefits of exercise may help to raise awareness about the role of exercise in oncology and inform decision making. However, if exercise is to become a standard component of cancer care, the development of an infrastructure where physicians can refer patients may be warranted (41), as it is possible that asking physicians to assume more responsibility over patient care is unsustainable. Given myriad benefits associated with exercise, endorsing the safe prescription of this intervention to CRC survivors will help to promote quality and length of life.
Supplementary Material
Acknowledgements
Funding
KH Schmitz has received funding from the National Institutes of Health unrelated to this work. The results of this study do not constitute endorsement by ACSM. This work was completed without funding.
Footnotes
Conflicts of Interest
JC Brown declares no conflicts of interest.
REFERENCES
- 1.American College of Sports Medicine Position Stand and American Heart Association. Recommendations for cardiovascular screening, staffing, and emergency policies at health/fitness facilities. Med Sci Sports Exerc. 1998;30(6):1009–1018. [PubMed] [Google Scholar]
- 2.Anderson AS, Caswell S, Wells M, Steele RJ, Macaskill S. "It makes you feel so full of life" LiveWell, a feasibility study of a personalised lifestyle programme for colorectal cancer survivors. Support Care Cancer. 2010;18(4):409–415. doi: 10.1007/s00520-009-0677-4. [DOI] [PubMed] [Google Scholar]
- 3.Anderson AS, Steele R, Coyle J. Lifestyle issues for colorectal cancer survivors-perceived needs, beliefs and opportunities. Support Care Cancer. 2012 doi: 10.1007/s00520-012-1487-7. [DOI] [PubMed] [Google Scholar]
- 4.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Baciu Alina, Stratton Kathleen, Burke Sheila P., editors. The Future of Drug Safety: Promoting and Protecting the Health of the Public. The National Academies Press; 2007. Committee on the Assessment of the US Drug Safety System. [Google Scholar]
- 7.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denlinger CS, Engstrom PF. Colorectal cancer survivorship: movement matters. Cancer Prev Res (Phila) 2011;4(4):502–511. doi: 10.1158/1940-6207.CAPR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eakin EG, Brown WJ, Marshall AL, Mummery K, Larsen E. Physical activity promotion in primary care: bridging the gap between research and practice. Am J Prev Med. 2004;27(4):297–303. doi: 10.1016/j.amepre.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Eakin EG, Glasgow RE, Riley KM. Review of primary care-based physical activity intervention studies. J Fam Pract. 2000;49(2):158–168. [PubMed] [Google Scholar]
- 11.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 12.Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12(4):428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman C, Rice D, Sung HY. Persons with chronic conditions. Their prevalence and costs. JAMA. 1996;276(18):1473–1479. [PubMed] [Google Scholar]
- 14.Jones LW, Courneya KS, Peddle C, Mackey JR. Oncologists' opinions towards recommending exercise to patients with cancer: a Canadian national survey. Support Care Cancer. 2005;13(11):929–937. doi: 10.1007/s00520-005-0805-8. [DOI] [PubMed] [Google Scholar]
- 15.Jones LW, Eves ND, Peppercorn J. Pre-exercise screening and prescription guidelines for cancer patients. Lancet Oncol. 2010;11(10):914–916. doi: 10.1016/S1470-2045(10)70184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones LW. Evidence-based risk assessment and recommendations for physical activity clearance: cancer. Appl Physiol Nutr Metab. 2011;36(S1):S101–S112. doi: 10.1139/h11-043. [DOI] [PubMed] [Google Scholar]
- 17.Karvinen KH, Carr LJ, Stevinson C. Resources for Physical Activity in Cancer Centers in the United States. Clin J Oncol Nurs. 2013;17(6):E71–E76. doi: 10.1188/13.CJON.E71-E76. [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch BM, Cerin E, Owen N, Hawkes AL, Aitken JF. Prospective relationships of physical activity with quality of life among colorectal cancer survivors. J Clin Oncol. 2008;26(27):4480–4487. doi: 10.1200/JCO.2007.15.7917. [DOI] [PubMed] [Google Scholar]
- 20.McNeely ML, Peddle CJ, Parliament MB, Courneya KS. Cancer Rehabilitation: Recommendations for Integrating Exercise Programming in the Clinical Practice Setting. Curr Cancer Ther Rev. 2006;2(4) [Google Scholar]
- 21.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 22.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 23.NCCN Clinical Practice Guidelines in Oncology: Survivorship. [Internet] [cited 2013. Available from: https://subscriptions.nccn.org/gl_login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. [Google Scholar]
- 24.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24(32):5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 25.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–64. doi: 10.1002/pon.2047. [DOI] [PubMed] [Google Scholar]
- 26.Read WL, Tierney RM, Page NC, Costas I, Govindan R, Spitznagel EL, Piccirillo JF. Differential prognostic impact of comorbidity. Journal of Clinical Oncology. 2004;22(15):3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012 doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 28.Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. Journal of Clinical Oncology. 2007;25(15):2100–2106. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]
- 29.Santa Mina D, Alibhai SM, Matthew AG, Guglietti CL, Steele J, Trachtenberg J, Ritvo PG. Exercise in clinical cancer care: a call to action and program development description. Curr Oncol. 2012;19(3):e136–e144. doi: 10.3747/co.19.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz KH. Exercise prescription and programming adaptations: based on surgery, treatment, and side effects. In: Irwin ML, editor. ACS's Guide to Exercise and Cancer Survivorship. 1st ed. Human Kinetics; 2012. p. 87. [Google Scholar]
- 31.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, VON Gruenigen VE, Schwartz AL. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz KH. Exercise for secondary prevention of breast cancer: moving from evidence to changing clinical practice. Cancer Prev Res (Phila) 2011;4(4):476–480. doi: 10.1158/1940-6207.CAPR-11-0097. [DOI] [PubMed] [Google Scholar]
- 33.Smith S, Wai E, Alexander C, Singh–Carlson S. Caring for survivors of breast cancer: perspective of the primary care physician. Current Oncology. 2011;18(5):e218. doi: 10.3747/co.v18i5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith GF, Toonen TR. Primary care of the patient with cancer. Am Fam Physician. 2007;75(8):1207–1214. [PubMed] [Google Scholar]
- 35.Speed-Andrews AE, Rhodes RE, Blanchard CM, Culos-Reed SN, Friedenreich CM, Belanger LJ, Courneya KS. Medical, demographic and social cognitive correlates of physical activity in a population-based sample of colorectal cancer survivors. Eur J Cancer Care (Engl) 2012;21(2):187–196. doi: 10.1111/j.1365-2354.2011.01290.x. [DOI] [PubMed] [Google Scholar]
- 36.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NM, Fulton JE, Gordon NF, Haskell WL, Link MS. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- 37.Thompson WR, Gordon NF, Pescatello LS. ACSM's Guidelines for Exercise Testing and Prescription. 2010 doi: 10.1249/JSR.0b013e31829a68cf. [DOI] [PubMed] [Google Scholar]
- 38.van Leersum NJ, Janssen-Heijnen ML, Wouters MW, Rutten HJ, Coebergh JW, Tollenaar RA, Lemmens VE. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995–2010. Int J Cancer. 2013;132(9):2157–2163. doi: 10.1002/ijc.27871. [DOI] [PubMed] [Google Scholar]
- 39.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167(1):1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, Kumanyika S, Schmitz KH, Diewald LK, Barg R. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365(21):1969–1979. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–177. doi: 10.1016/j.suponc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, Yates JW. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82(11):2123–2134. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


