Abstract
Introduction
Screening of substance use may prove useful to prevent readmission after the first episode of psychosis. The aim of the present study was to evaluate the influence of drug use on readmission risk in a first-episode psychosis sample, and to determine whether the cannabis/cocaine subscale of the Dartmouth Assessment of Lifestyle Inventory (DALI) is a better predictive instrument than urinary analysis.
Methods
After admission, first-episode psychotic patients were interviewed for substance use and assessed with the DALI scale. They also underwent blood and urine sampling. Time to readmission was studied as a dependent outcome. The Kaplan–Meier estimator was applied to estimate the survival curves for bivariate analysis. The Cox proportional hazards model for multivariate analysis was assessed in order to control for potential confounders. ROC curve and validity parameters were used to assess validity to detect readmission.
Results
Fifty-eight patients were included. The DALI cannabis/cocaine subscale and urinalysis were associated with increased readmission risk in survival curves, mainly the first five years of follow-up. After controlling for potential confounding variables for readmission, only the DALI cannabis/cocaine subscale remained as a signifi-cant risk factor. In terms of validity, the DALI cannabis/cocaine subscale was more sensitive than urinalysis. Alcohol assessments were not related to readmission.
Conclusions
The findings demonstrated that a quick screening self-report scale for cannabis/cocaine use disorders is superior to urinary analysis for predicting readmission. Future research should consider longitudinal assessments of brief validated screening tests in order to evaluate their benefits in preventing early readmission in first-episode psychosis.
Keywords: Screening, Substance use, Cannabis, First-episode psychosis, Readmission
1. Introduction
Identifying modifiable prognostic factors for preventing recurrent psychotic episodes is an extremely important issue (Lambert et al., 2005). Misuse of tobacco, alcohol, cannabis and other illicit substances is common among people with psychotic illnesses (Regier et al., 1990; Kavanagh et al., 2002; Margolese et al., 2004). A high prevalence of substance misuse is also characteristic of patients with first-episode psychosis, with rates varying from 22% to over 50% (Cantwell et al., 1999; Van Mastrigt et al., 2004; Lambert et al., 2005; Larsen et al., 2006; Addington and Addington, 2007; Wade et al., 2007; Baeza et al., 2009; Kamali et al., 2009). Drug misuse, especially cannabis in the early stages of psychosis, has been associated with younger age of onset (Cantwell et al., 1999; Van Mastrigt et al., 2004; Addington and Addington, 2007; Sugranyes et al., 2009), increased symptoms (Lambert et al., 2005; Addington and Addington, 2007; Baeza et al., 2009), poorer treatment compliance (Buhler et al., 2002; Green et al., 2004; Zammit et al., 2008), higher rates of relapses and more hospitalizations (Linszen et al., 1994; Cantor-Graae et al., 2001; Salyers and Mueser, 2001; Sorbara et al., 2003; Zammit et al., 2008). Therefore, good screening for substance use during this phase of the illness may prove useful as a predictor of relapse. In spite of this, few longitudinal studies have investigated the impact of substance use on readmission to hospital. Detection and screening of substance use are typically undertaken through clinical interviews, patients' self-reports or toxicological tests. Urinalysis, though reliable and valid, has a narrow window of detection; for their part, structured diagnostic procedures are able to identify a high prevalence of drug use disorders but they are not practical on a day-to-day basis (Bennett, 2009). Research on screeners suggests that brevity is essential for an instrument to be adopted for regular use (Tiet et al., 2008). Although several screening scales are available (Tiet et al., 2008), they are not routinely studied in longitudinal cohorts involving psychotic patients, since these cohorts usually use self-report measures (Grech et al., 2005; Stirling et al., 2005; Hides et al., 2006; Degenhardt et al., 2007), structured interviews (Coldham et al., 2002; Green et al., 2004; Pencer et al., 2005; Wade et al., 2006) or urine drug screening (Grace et al., 2000; Hides et al., 2006). Therefore, their potential influence on outcome measures such as readmission is not frequently considered. Furthermore, screening measures may miss many diagnoses due to their having been developed in the general population or in primary substance abusing samples, with the result that their relevance to people with severe mental illness is doubtful (Bennett, 2009). One potential solution may be the use of screening measures specifically developed for people with psychiatric disorder (Bennett, 2009), such as the Dartmouth Assessment of Lifestyle Inventory (DALI), an 18-item screening questionnaire designed to identify substance use and abuse in people with severe mental illness. The scale contains two subscales: one for assessing the risk of alcohol use disorders and the second for assessing the risk of cannabis and/or cocaine use disorders. The main strengths of the scale are its brevity, as the mean time of administration is approximately 6 min, and its high classificatory accuracy for alcohol, cannabis and cocaine use disorders (Rosenberg et al., 1998; Ford, 2003). However, it has not yet been used to evaluate outcome measures in first-episode psychosis cohorts such as risk for readmission, and its predictive validity has not been explored.
The aim of the present study was to evaluate the influence of drug use on readmission risk in a first-episode psychosis sample, and to establish whether the DALI cannabis/cocaine subscale is a better predictive instrument than a positive urine sample.
2. Methods
2.1. Subjects
Non-affective first-episode psychotic patients were consecutively recruited at the time of their first clinical contact for psychotic symptoms at a general academic hospital (Hospital Clinic, Barcelona). As part of the Spanish National Health System, the hospital offers inpatient and outpatient services to the 560,000 inhabitants who live in the surrounding catchment area. The area is a relatively homogeneous middle/upper-middle class neighborhood in the center of the city, in which Hospital Clinic is the regional referral center for psychosis. The patients met criteria for schizophrenia, schizophreniform disorder, brief psychotic disorder, delusional disorder or psychosis not otherwise specified and had a maximum cumulative (lifetime) anti-psychotic exposure of one week and no antipsychotic use in the 30 days prior to the study (although in this particular study, all subjects were drug naïve). Subjects were allowed to receive antianxiety medication (lorazepam) the night before blood was drawn, up to a maximum of 3 mg, but not on the day of the assessment. Additional inclusion and exclusion criteria for all subjects were: 1) age from 18 to 64 years, 2) no history of diabetes or other serious medical or neurological condition associated with glucose intolerance or insulin resistance (e.g. Cushing's disease), and 3) not taking medication associated with insulin resistance (hydrochlorothiazide, furosemide, ethacrynic acid, metolazone, chlorthalidone, beta blockers, glucocorticoids, phenytoin, nicotinic acid, cyclosporine, pentamidine, or narcotics).
One hundred and seven eligible patients were admitted during the study period. After excluding patients who did not have an address in the hospital catchment area (n = 39; 36.4%), patients not discharged during the recruitment period (n = 3; 2.8%) and patients whose blood/urine sample was not collected within 48 h (n = 7; 6.5%), the final sample consisted of 58 patients. There were no differences in baseline socio-demographic or clinical data between the excluded group and the study group: the variables assessed were age, gender, race, marital status, level of education and psychiatric history in first-degree relatives, scores on the Spanish version of the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) (Peralta and Cuesta, 1994) and duration of untreated psychosis (DUP). DSM-IV diagnoses for the subjects included were schizophrenia (n = 40; 69.0%), brief psychotic disorder (n = 5; 8.6%), schizophreniform disorder (n = 4; 6.9%), and psychosis not otherwise specified (n = 9; 15.5%).
2.2. Procedures
Patients experiencing non-affective psychotic symptoms were consecutively admitted to the inpatient unit after their first contact with one of the hospital's psychiatric services. The recruitment period was from 1st January 2004 to 31st October 2010. All patients and their close relatives were carefully interviewed to ensure that inclusion and exclusion criteria were met. After discharge, the patients were followed up by outpatient services. All the interviews, assessments and follow-ups were performed by two fully trained psychiatrists in adult psychiatry (CGR and EFE). The main outcome was the time until first readmission to the hospital's inpatient unit. The follow-up time period was defined as days since discharge from the index admission until readmission or censoring from the study. The end of the study was set at 30th April 2011.
All subjects were interviewed using the Spanish version of the Structured Clinical Interview for DSM-IV Axis I Disorders, clinician version (SCID-I) (First and Spitzer, 1999). They were also administered the Spanish version of the PANSS (Peralta and Cuesta, 1994) and the DALI (Rosenberg et al., 1998). The DALI, which is based on 18 items—three non-scored used to establish the frame for the interview, and 15 scored—focuses on detecting substance use disorders in people with severe mental illness, and includes alcohol and drug screen subscales. The items of the scale were selected from ten instruments, and the scale was validated against the Structured Clinical Interview for DSM-III-R (SCID) (Spitzer et al., 1988) and the Clinician Rating Scale (Drake et al., 1990). The DALI drug screen had a sensitivity = 1.0, specificity = 0.80, positive predictive value (PPV) = 0.56 and negative predictive value (NPV) = 1.0, accuracy rate = 88%, kappa = 0.98, and area under the receiver operating characteristic (ROC) curve (AUC) = 0.93 for cannabis and cocaine disorders (Rosenberg et al., 1998). Among the nine questions related to alcohol, item 7, for example, assesses whether close friends or relatives have shown concern about the subject's alcohol use; and item 9 whether the subject sometimes drinks alcohol soon after getting up. Among the eight questions in the drug scale, item 13 assesses whether marijuana has caused the subject to lose a job; and item 16 whether cocaine use has caused the subject problems with close relatives. The socio-demographic variables recorded included: age, gender, race, marital status, level of education and psychiatric history in first-degree relatives. Self-reported drug use was recorded with a systematic ad hoc protocol which assessed whether tobacco, alcohol, cannabis, cocaine, amphetamines, LSD or ecstasy had been taken in the last three months. DUP was defined as the interval from first psychotic symptom to first psychiatric hospitalization.
All subjects underwent blood and urine sampling as soon as possible after admission. Admissions during which at least one sample was obtained within 48 h were included in this study. All urine samples were screened for the following substances: benzodiazepines, cannabis, cocaine, amphetamines (amphetamines, methamphetamines and ecstasy), opiates, methadone and lysergic acid diethylamide (LSD), using an enzyme immunoassay method on the Siemens ADVIA automated chemistry analyzer. Broadly, urine samples show evidence of drug use between one and four days, although this timeframe may vary according to the chronicity of use and type of drug: for instance, chronic cannabis use may be detected up to three weeks after the last use (Verstraete, 2004). Blood samples were screened for alcohol using an enzymatic assay of alcohol dehydrogenase. Positive screening results were confirmed by gas chromatography (GC-FID). All subjects gave informed consent prior to participating. The study was conducted under the supervision of the ethics committee, and is part of a larger study of metabolic abnormalities and glucose dysregulation in neuropsychiatric disorders (Fernandez-Egea et al., 2009; Garcia-Rizo et al., 2012) and a gene–environment study in first-episode psychosis (Bernardo et al., 2012).
2.3. Statistical analysis
Time to readmission was studied as a dependent outcome. The Kaplan–Meier estimator (using log-rank test) was applied to estimate the survival curves for bivariate analysis. Patients were censored if they moved out of the hospital's recruitment area, died, were lost to follow-up or had not been readmitted by the end of the study. The Cox proportional hazards model for multivariate analysis was assessed to control for potential confounders.
Sensitivity, specificity, positive and negative predictive values of the DALI cannabis/cocaine subscale and urine test were calculated and related to future readmissions. ROC curves were also constructed between the DALI cannabis/cocaine subscale score and future readmission. The area under the curve (AUC) was calculated by means of the trapezoidal rule with 95% CI to find the best cutoff. ROC curves allow the examination of the entire range of sensitivities and specificities at each possible cutoff score. Statistical significance was set at p = 0.05. All analyses were performed using SPSS version 19.0 (SPSS version 19.0, for Windows, SPSS, Inc., Chicago, Ill).
3. Results
3.1. Descriptive analysis
Socio-demographic and clinical descriptive data are summarized in Table 1. Of the 58 admissions, psychoactive substances (excluding benzodiazepines) were detected in 25 patients (43.1%; 95% CI = 31.2% to 55.9%) on urine/blood tests. Cannabis was found in 22 patients (37.9%) and alcohol in four (6.9%). No other psychoactive substances were detected in urine/blood samples, although 65.5% (n = 38) of the patients reported having taken at least one substance of abuse (excluding tobacco) in the last three months: 32.8% (n = 19) alcohol, 50% (n = 29) cannabis, 24.1% (n = 14) cocaine, 5.2% (n = 3) amphetamines and 10.3% (n = 6) other substances (LSD or ecstasy). 53.4% (n = 31) reported having taken cannabis and/or cocaine. The DALI cannabis/cocaine subscale classified 29 patients (50%) as being at high risk of cannabis and/or cocaine use disorders and 11 (19.0%) as at high risk of alcohol use disorders. Eight of the eleven patients classified as being at high risk for alcohol use disorder were also classified as at high risk for cannabis/cocaine disorder.
Table 1.
Sample characteristics and bivariate survival analysis (Kaplan-Meier).
| Variable | Descriptive | Probability to be readmitted | 95% CI | p | |
|---|---|---|---|---|---|
| Age: Mean (SD; range) | 27.6 (6.6; 18-45) | 0.03 | |||
| 18-23 years old: N (%) | 19 (32.8) | 0.54 | 0.48 to 0.60 | ||
| 24-29 years old: N (%) | 20 (34.5) | 0.32 | 0.25 to 0.39 | ||
| >29 years old: N (%) | 19 (32.8) | 0.13 | 0.09 to 0.16 | ||
| Gender | 0.04 | ||||
| Male: N (%) | 39 (67.2) | 0.60 | 0.55 to 0.65 | ||
| Female: N (%) | 19 (32.8) | 0.19 | 0.13 to 0.25 | ||
| Caucasian: N (%) | 51 (87.9) | 0.51 | 0.47 to 0.55 | 0.97 | |
| Single: N (%) | 46 (79.3) | 0.53 | 0.49 to 0.57 | 0.48 | |
| Level of education: N (%) | 0.83 | ||||
| Primary education | 13 (23.2) | 0.67 | 0.54 to 0.80 | ||
| High school certificate | 21 (37.5) | 0.29 | 0.24 to 0.34 | ||
| Vocational training | 9 (16.1) | 0.33 | 0.19 to 0.47 | ||
| University graduate | 13 (23.2) | 0.35 | 0.25 to 0.45 | ||
| First-degree relatives with psychiatric | 7 (12.1) | 0.33 | 0.30 to 0.36 | 0.93 | |
| history: N (%) | |||||
| DUP: Mean (SD; range) | 14.7 (19.8; 01-83) | 0.77 | |||
| ≤12 months: N (%) | 36 (62.1) | 0.43 | 0.38 to 0.48 | ||
| >12 months: N (%) | 22 (37.9) | 0.53 | 0.45 to 0.61 | ||
| PANSS Positive subscale: Mean (SD) | 26.0 (5.7) | <0.001 | |||
| ≤25 | 11 (19.0) | 0.39 | 0.30 to 0.48 | ||
| Percentile N (%): | 25-75 | 38 (65.5) | 0.47 | 0.40 to 0.54 | |
| ≥75 | 9 (15.5) | 0.80 | 0.69 to 0.91 | ||
| Cannabis urine analysis | 0.021 | ||||
| Positive: N (%) | 22 (37.9) | 0.55 | 0.49 to 0.61 | ||
| Negative: N (%) | 38 (62.1) | 0.49 | 0.42 to 0.56 | ||
| Alcohol blood/urine analysis | 0.773 | ||||
| Positive: N (%) | 4 (6.9%) | 0.46 | 0.41 to 0.51 | ||
| Negative: N (%) | 54 (93.1) | 0.51 | 0.44 to 0.58 | ||
| DALI cannabis/cocaine subscale | 0.002 | ||||
| Positive: N (%) | 29 (50.0) | 0.60 | 0.55 to 0.65 | ||
| Negative: N (%) | 29 (50.0) | 0.55 | 0.42 to 0.68 | ||
| DALI alcohol subscale | 0.330 | ||||
| Positive: N (%) | 11 (19.0) | 0.41 | 0.32 to 0.50 | ||
| Negative: N (%) | 47 (81.0) | 0.49 | 0.44 to 0.54 | ||
CI: confidence interval; DUP: duration of untreated psychosis.
The median (P25–P75) length of follow-up was 888 (348–1556) days in the total sample, 409 (105–861) days in patients readmitted and 1180 (508–1753) days in patients not readmitted. Reasons for censoring from the study were moving/lost to follow-up (n = 7; 12.1%) and end of the study period (n = 35; 60.3%). No patients died. Sixteen patients (27.6%) were readmitted during the whole follow-up period.
3.2. Bivariate analysis
Regarding drug use, bivariate survival analysis of time to first readmission following the first psychotic episode was significant both for urine analyses for cannabis and for the DALI cannabis/cocaine subscale (Table 1, Fig. 1). Younger age, male gender and high scores in the PANSS positive subscale were also significantly associated with readmission during the follow-up period (Table 1). In terms of alcohol use, neither positivity for alcohol urine/blood analysis nor DALI alcohol subscale was associated with readmission (p = 0.773 and p = 0.330, respectively).
Fig. 1.
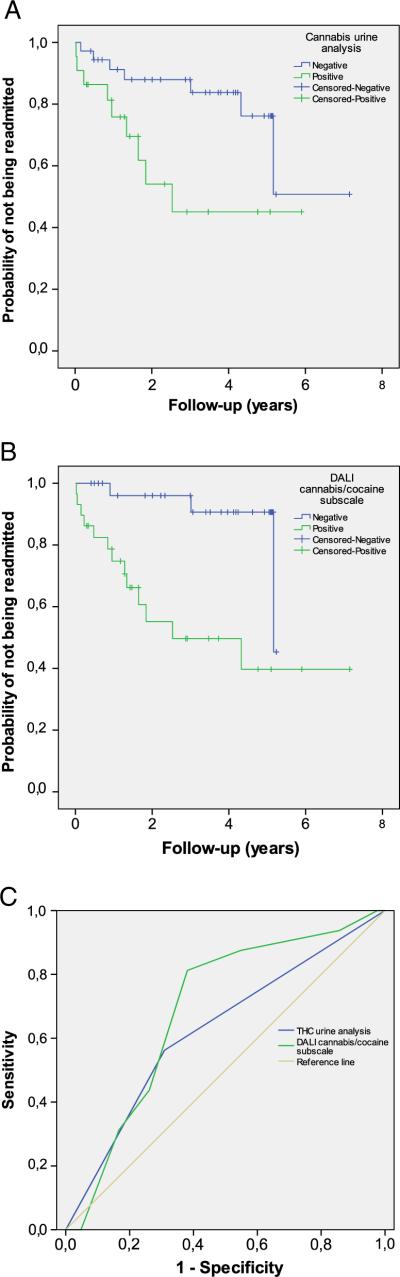
(A) & (B) Survival plot of cannabis urine analysis and DALI cannabis/cocaine subscale, respectively. (C) ROC curves of DALI cannabis/cocaine subscale compared with positive urine analysis for cannabis for readmission during the whole study period.
3.3. Multivariate analysis
In the multivariate analysis (using Cox regression), the DALI cannabis/cocaine subscale at baseline was a significant predictor of readmission over the total study period, after controlling for gender, age, DUP and PANSS positive subscale (Hazard Ratio; HR = 4.5; 95% CI = 1.1 to 18.7; p = 0.036) while urine analysis for cannabis was not (HR = 2.0; 95% CI = 0.7 to 5.7; p = 0.20) (Table 2).
Table 2.
Multivariate analysis (Cox regression).
| Adjusted readmission model | Crude HR | 95% CI | Adjusted HR | 95% CI | p |
|---|---|---|---|---|---|
| Cannabis urine analysis | 3.08 | 1.13 to 8.39 | 1.99 | 0.69 to 5.72 | 0.20 |
| Male gender | 4.16 | 0.94 to 18.39 | 2.90 | 0.61 to 13.84 | 0.18 |
| Age | 0.90 | 0.81 to 1.00 | 0.95 | 0.85 to 1.06 | 0.35 |
| DUP | 0.84 | 0.61 to 1.17 | 0.90 | 0.62 to 1.30 | 0.55 |
| PANSS positive subscale | 1.03 | 0.93 to 1.14 | 1.02 | 0.93 to 1.11 | 0.74 |
| DALI cannabis/cocaine subscale | 6.09 | 1.72 to 21.54 | 4.55 | 1.11 to 18.72 | 0.036 |
| Male gender | 4.16 | 0.94 to 18.39 | 2.63 | 0.55 to 12.47 | 0.22 |
| Age | 0.90 | 0.81 to 1.00 | 0.99 | 0.88 to 1.11 | 0.89 |
| DUP | 0.84 | 0.61 to 1.17 | 0.82 | 0.58 to 1.17 | 0.27 |
| PANSS positive subscale | 1.03 | 0.93 to 1.14 | 1.02 | 0.93 to 1.12 | 0.67 |
CI: confidence interval.
3.4. Validity of screening tests
Regarding the 58 initial admissions, only three (18.8%) readmissions were not recognized by the algorithm-based DALI cannabis/cocaine subscale (false negatives). ROC curve showed a greater AUC for the DALI cannabis/cocaine subscale (0.716; 95% CI = 0.572 to 0.860) than the positive urine analysis for cannabis (0.626; 95% CI = 0.462 to 0.791) (Fig. 1). The optimum cutoff point for DALI cannabis/cocaine subscale to predict readmission was above minus one. Using this cutoff in our sample, sensitivity and specificity for the DALI cannabis/cocaine subscale [0.81 (CI = 0.57–0.93) and 0.62 (0.47–0.75), respectively] showed better validity than those for the urine test [0.56 (CI = 0.33– 0.77) and 0.69 (CI = 0.54–0.81), respectively], suggesting that this subscale is appropriate to predict readmission in this population (Table 3). Other measures to describe the validity of both screening tests are presented in Table 3.
Table 3.
Predictive validity of the screening tests.
| Screening test | Se (95% CI) | Sp (95% CI) | PPV (95% CI) | NPV (95% CI) | AUC (95% CI) |
|---|---|---|---|---|---|
| DALI cannabis/cocaine subscale | 0.81 (0.57-0.93) | 0.62 (0.47-0.75) | 0.45 (0.28-0.62) | 0.90 (0.74-0.96) | 0.72 (0.57-0.86) |
| Positive urine sample for cannabis | 0.56 (0.33-0.77) | 0.69 (0.54-0.81) | 0.41 (0.23-0.61) | 0.81 (0.65-0.90) | 0.63 (0.46-0.79) |
Se: sensitivity; Sp: specificity; PPV: positive predictive value; NPV: negative predictive value; AUC: area under curve; CI: confidence interval.
4. Discussion
This study compared the efficacy of the DALI cannabis/cocaine subscale and urinalysis as predictors of readmission among adults with first-episode psychosis. Overall, both assessments were associated with increased risk of readmission, especially during the first five years of follow-up. However, after controlling for potential confounding variables for readmission, only the DALI cannabis/cocaine subscale remained a significant predictor. In terms of validity, the DALI cannabis/cocaine subscale was more sensitive than urinalysis. Alcohol assessments (DALI subscale and blood samples) were not related to readmission.
We found that nearly two thirds of our sample reported having taken at least one substance of abuse (apart from tobacco) in the last three months, while just under half recorded a positive result in the urine/blood analysis (excluding benzodiazepines). In agreement with other recent European studies in first-episode psychosis samples (Cantwell et al., 1999; Barnes et al., 2006; Larsen et al., 2006; Kamali et al., 2009; Van Dorn et al., 2012), cannabis was the most frequently reported substance of abuse, followed by alcohol and cocaine. The DALI cannabis/cocaine subscale showed that 50% of individuals with first-episode psychosis were at risk of a cannabis and/or cocaine use disorder and 19.0% at risk of alcohol use disorders, a rate that is in the upper range for these studies (Cantwell et al., 1999; Barnes et al., 2006; Larsen et al., 2006; Kamali et al., 2009; Van Dorn et al., 2012). This may be explained by local and national differences in the pattern of substance misuse, as Spain is among the countries with the highest prevalence of alcohol, cannabis and cocaine use (European Monitoring Centre for Drugs and Drug Addiction, 2011). The finding that urinary analysis and blood samples under-detected cannabis, cocaine and alcohol use compared with the self-report supports the validity of self-report data among first-episode psychosis patients (Van Dorn et al., 2012). On the other hand, the self-report over-detected the risk of substance use compared with the DALI subscales, although it was only slightly higher for cannabis/cocaine use. In fact, most patients who reported recent cannabis and/or cocaine use obtained a positive result on the DALI subscale (80%). Taking this into consideration, these findings indicate that the presence of alcohol use in first-episode psychosis may be a poor proxy for the risk of alcohol use disorder, and that the use of other illicit drugs may represent a better approach in this population. However, another study concluded that self-reported illicit drug use was a poor proxy for disordered drug use in a sample of adults with schizophrenia from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial (Van Dorn et al., 2012). These discrepancies may in fact reflect contextual and sample differences, as Van Dorn et al.'s sample was recruited from over fifty sites across the United States and was much older on average (~15 years) than our sample.
In agreement with other literature reports (Addington et al., 2010), we found that younger age, male gender and higher scores on the PANSS positive subscale were associated with readmission throughout the study period. We did not find associations between other socio-demographic or clinical variables and readmission. Nevertheless, considering the significant heterogeneity across studies regarding the influence of DUP on relapses and readmission (Cougnard et al., 2006; Alvarez-Jimenez et al., 2011; Alvarez-Jimenez et al., 2012), we included DUP as a potential confounding factor in our multivariate analysis. Significantly, both positive urine analyses for cannabis and the DALI cannabis/cocaine subscale were associated with readmission, highlighting the importance of drug use in relapses and readmissions (Alvarez-Jimenez et al., 2012). However, after controlling for potential confounding variables, such as gender, age, PANSS positive subscale and DUP, only the DALI cannabis/cocaine subscale remained as a predictor of readmission, a finding that supports the utility of this screening test over laboratory parameters. Our results suggest an overall 4.5-fold increase in risk of readmission for patients at a high risk for cannabis/cocaine disorders, in agreement with other studies which have reported three to five-fold increases in the risk of relapse also when controlling for potential confounders (Wade et al., 2006; Malla et al., 2008; Turkington et al., 2009).
It is interesting that survival plots (Fig. 1) showed the greatest difference in readmission rates during the first five years of the follow-up. Considering that relapse prevention during the first years of illness has a critical impact on life-long outcomes in schizophrenia, avoidance of this modifiable risk factor should be a priority for clinicians and intervention programs. Several studies have reported that comorbid diagnosis of a drug use disorder may enhance the risk of relapse, particularly during the early stages of the illness (Hides et al., 2006; Wade et al., 2006; Malla et al., 2008), and that abstaining from use after the first psychotic episode may contribute to a clear improvement in outcome (Sorbara et al., 2003; Grech et al., 2005; Baeza et al., 2009; Turkington et al., 2009; Gonzalez-Pinto et al., 2011). In fact, cohort studies involving subjects with first-episode psychosis reported that approximately half the subjects become abstinent or significantly reduce their alcohol and drug use, in most cases in a stable manner (Wisdom et al., 2011). Furthermore, while those who become abstinent reduce their rates of relapse and hospitalization, those with persistent substance use disorders present increased rates (Wisdom et al., 2011).
Cannabis use is frequently associated with alcohol consumption (Cantwell et al., 1999), which itself has been associated with deleterious effect and worse outcome in first-episode psychosis and schizophrenia (Wade et al., 2007; Turkington et al., 2009). However, alcohol assessments (DALI subscale and blood samples) were not related to readmission when studied separately. One explanation may be the differences in the severity of substance use, since it has been reported that heavy, but not mild, substance use disorders may be associated with poorer functional outcome (Wade et al., 2007). As the DALI scale does not assess the severity of substance use, such differences cannot be excluded. In any case, the contribution of alcohol to the overall findings cannot be ruled out as most of the patients who were at risk for alcohol use disorder were also at risk for cannabis/ cocaine use disorder. However, despite the mentioned overlap, the limited number of positive results obtained in both the alcohol subscale and the blood tests does not allow us to reach any firm conclusion.
As the predictive validity of the DALI scale for readmission risk was not assessed in the original validation (Rosenberg et al., 1998), we deemed it essential to establish the optimum cutoff point in our sample since the use of an incorrect cutoff would lead to misclassification and an inaccurate prediction of the readmission risk. Our results showed that DALI has good psychometric properties for predicting readmission. Compared to urinalysis, the DALI cannabis/cocaine subscale showed a greater AUC due to its higher sensitivity. Sensitivity assesses the proportion of readmitted subjects who are correctly identified as having a condition. False negatives assess the proportion of readmitted subjects whom the subscale is not able to identify. Therefore, the scale's higher predictive validity may indicate that it is a better detector of patients at risk of readmission than urine samples. In addition to its significant reduction in costs and its efficiency of administration, a positive result on this screening scale may be more reliable for detecting current use and misuse, and even for predicting readmission, than a urine sample. The availability of a brief and practical screening test means that more patients with drug-related problems can be identified and appropriately managed and treated, either within the psychiatric care system, in dual diagnosis programs, or in substance use disorder specialty care (Tiet et al., 2008).
Our study has several limitations, including a relatively small sample size, limited generalizability to non-affective psychosis, and the inability to quantify drug use precisely as we had only self-reported information on drug use in the last three months. With regard to the perceived problems related to non-disclosure, especially among patients with severe mental illness, it is interesting that studies rely, in the main, on self-reports (Van Dorn et al., 2012). In this regard, our results favor the use of self-reports of drug use over laboratory tests. However, given the implications for research and clinical practice, further work is needed to evaluate the accuracy of reported substance use in subjects with severe mental illness, and to assess whether biological measures provide more accurate data. Another limitation is the fact that drug assessment was only conducted at baseline; as a result, we were unable to obtain a clear picture of the temporal relationship between substance misuse and readmission during the follow-up. Longitudinal studies with periodical drug assessments may prove useful in the search for a convergent and standardized methodology for recruitment, assessment and treatment strategies (Wisdom et al., 2011). Another limitation is that the DALI scales have been validated for the most prevalent drugs only (alcohol, cannabis and cocaine), and their performance in patients with other drug disorders is unknown at present. In addition, we compared a subscale that measures cannabis and cocaine consumption with positive urinary analysis for cannabis alone, as no positive results were detected for cocaine. In this regard, it might have been more illuminating to assess each drug separately in order to establish its individual effect. Finally, other well known factors related to relapse, such as medication adherence (Alvarez-Jimenez et al., 2012; Caseiro et al., 2012), were not assessed in the current study. As such, the influence of these variables on the current results cannot be ruled out.
The findings of this study demonstrate that a quick screening self-report scale for cannabis and cocaine use disorders is more useful than urinary analysis for predicting readmission. Indeed, scoring in the “at risk” range for these drug disorders at admission was found to increase the readmission risk in first-episode psychosis by 4.5 times. This finding has direct clinical implications for preventing readmission during the early course of psychosis, when intervention may have the greatest impact on long-term outcomes. After patients are screened, they can be referred to specialty substance use disorder or dual diagnosis integrative care, which may decrease readmission and improve outcome. Future research should consider longitudinal assessment of brief validated screening tests in order to evaluate their benefits in prevention of early readmission in first-episode psychosis.
Acknowledgments
Government of Catalonia, Comissionat per Universitats I Recerca del Departament d'Innovació, Universitats I Empresa (DIUE) 2009SGR1295, 2009SGR1435, Esther Koplowitz Center (Barcelona), Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM, and Ministerio de Sanidad y Consumo, PNSD: PI101/2006 and PNSD: PI041731/2011.
Role of funding source
This work was supported by the Government of Catalonia, Comissionat per Universitats I Recerca del Departament d'Innovació, Universitats I Empresa (DIUE) 2009SGR1295, and 2009SGR1435, by the Esther Koplowitz Center (Barcelona), by the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM, and by the Ministerio de Sanidad y Consumo, PNSD: PI101/2006 and PNSD: PI041731/2011. Prof Yücel is supported by a NHMRC fellowship (#1021973). The founding agencies played no role in the direction of this project.
Footnotes
Contributors
AB, CGR, EFE and MB contributed substantially to conception and design; AB, PC and MY contributed to analysis and interpretation of data; AB, CGR and MY drafted the article; EP, BK, RMS and MB revised it critically for important intellectual content. All authors gave final approval of the version to be published.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Addington J, Addington D. Patterns, predictors and impact of substance use in early psychosis: a longitudinal study. Acta Psychiatr. Scand. 2007;115(4):304–309. doi: 10.1111/j.1600-0447.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- Addington DE, Beck C, Wang J, Adams B, Pryce C, Zhu H, Kang J, McKenzie E. Predictors of admission in first-episode psychosis: developing a risk adjustment model for service comparisons. Psychiatr. Serv. 2010;61(5):483–488. doi: 10.1176/ps.2010.61.5.483. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez M, Parker AG, Hetrick SE, McGorry PD, Gleeson JF. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr. Bull. 2011;37(3):619–630. doi: 10.1093/schbul/sbp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jimenez M, Priede A, Hetrick SE, Bendall S, Killackey E, Parker AG, McGorry PD, Gleeson JF. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr. Res. 2012;139(1–3):116–128. doi: 10.1016/j.schres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Baeza I, Graell M, Moreno D, Castro-Fornieles J, Parellada M, Gonzalez-Pinto A, Paya B, Soutullo C, de la Serna E, Arango C. Cannabis use in children and adolescents with first episode psychosis: influence on psychopathology and short-term outcome (CAFEPS study). Schizophr. Res. 2009;113(2–3):129–137. doi: 10.1016/j.schres.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Barnes TR, Mutsatsa SH, Hutton SB, Watt HC, Joyce EM. Comorbid substance use and age at onset of schizophrenia. Br. J. Psychiatry. 2006;188:237–242. doi: 10.1192/bjp.bp.104.007237. [DOI] [PubMed] [Google Scholar]
- Bennett ME. Assessment of substance use and substance-use disorders in schizophrenia. Clin. Schizophr. Relat. Psychoses. 2009;3(1):50–63. [Google Scholar]
- Bernardo M, Bioque M, Parellada M, Saiz RJ, Cuesta MJ, Llerena A, Sanjuan J, Castro-Fornieles J, Arango C, Cabrera B. Assessing clinical and functional outcomes in a gene–environment interaction study in first episode of psychosis (PEPs). Rev. Psiquiatr. Salud Ment. 2012 Nov 30;6(1):4–16. doi: 10.1016/j.rpsm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Buhler B, Hambrecht M, Loffler W, an der HW, Hafner H. Precipitation and determination of the onset and course of schizophrenia by substance abuse—a retrospective and prospective study of 232 population-based first illness episodes. Schizophr. Res. 2002;54(3):243–251. doi: 10.1016/s0920-9964(01)00249-3. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, Nordstrom LG, McNeil TF. Substance abuse in schizophrenia: a review of the literature and a study of correlates in Sweden. Schizophr. Res. 2001;48(1):69–82. doi: 10.1016/s0920-9964(00)00114-6. [DOI] [PubMed] [Google Scholar]
- Cantwell R, Brewin J, Glazebrook C, Dalkin T, Fox R, Medley I, Harrison G. Prevalence of substance misuse in first-episode psychosis. Br. J. Psychiatry. 1999;174:150–153. doi: 10.1192/bjp.174.2.150. [DOI] [PubMed] [Google Scholar]
- Caseiro O, Perez-Iglesias R, Mata I, Martinez-Garcia O, Pelayo-Teran JM, Tabares-Seisdedos R, de la Foz Ortiz-Garcia, Vazquez-Barquero JL, Crespo-Facorro B. Predicting relapse after a first episode of non-affective psychosis: a three-year follow-up study. J. Psychiatr. Res. 2012;46(8):1099–1105. doi: 10.1016/j.jpsychires.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Coldham EL, Addington J, Addington D. Medication adherence of individuals with a first episode of psychosis. Acta Psychiatr. Scand. 2002;106(4):286–290. doi: 10.1034/j.1600-0447.2002.02437.x. [DOI] [PubMed] [Google Scholar]
- Cougnard A, Parrot M, Grolleau S, Kalmi E, Desage A, Misdrahi D, Brun-Rousseau H, Verdoux H. Pattern of health service utilization and predictors of readmission after a first admission for psychosis: a 2-year follow-up study. Acta Psychiatr. Scand. 2006;113(4):340–349. doi: 10.1111/j.1600-0447.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Tennant C, Gilmour S, Schofield D, Nash L, Hall W, McKay D. The temporal dynamics of relationships between cannabis, psychosis and depression among young adults with psychotic disorders: findings from a 10-month prospective study. Psychol. Med. 2007;37(7):927–934. doi: 10.1017/S0033291707009956. [DOI] [PubMed] [Google Scholar]
- Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS. Diagnosis of alcohol use disorders in schizophrenia. Schizophr. Bull. 1990;16(1):57–67. doi: 10.1093/schbul/16.1.57. [DOI] [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction [2-2-2012];The state of the drugs problem in Europe. EMCDDA, Lisbon. 2011 Available from http:// www.emcdda.europa.eu/publications/annual-report/2011.
- Fernandez-Egea E, Bernardo M, Donner T, Conget I, Parellada E, Justicia A, Esmatjes E, Garcia-Rizo C, Kirkpatrick B. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br. J. Psychiatry. 2009;194(5):434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R. In: SCID-I Structured Clinical Interview for the DSM-IV Axis I Disorders [Spanish] Masson edn. Blanch J, Andreu I, editors. 1999. [Google Scholar]
- Ford P. An evaluation of the Dartmouth Assessment of Lifestyle Inventory and the Leeds Dependence Questionnaire for use among detained psychiatric inpatients. Addiction. 2003;98(1):111–118. doi: 10.1046/j.1360-0443.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Rizo C, Fernandez-Egea E, Oliveira C, Justicia A, Parellada E, Bernardo M, Kirkpatrick B. Prolactin concentrations in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr. Res. 2012;134(1):16–19. doi: 10.1016/j.schres.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Pinto A, Alberich S, Barbeito S, Gutierrez M, Vega P, Ibanez B, Haidar MK, Vieta E, Arango C. Cannabis and first-episode psychosis: different long-term outcomes depending on continued or discontinued use. Schizophr. Bull. 2011;37(3):631–639. doi: 10.1093/schbul/sbp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace RF, Shenfield G, Tennant C. Cannabis and psychosis in acute psychiatric admissions. Drug Alcohol Rev. 2000;19(3):287–290. [Google Scholar]
- Grech A, van OJ, Jones PB, Lewis SW, Murray RM. Cannabis use and outcome of recent onset psychosis. Eur. Psychiatry. 2005;20(4):349–353. doi: 10.1016/j.eurpsy.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Green AI, Tohen MF, Hamer RM, Strakowski SM, Lieberman JA, Glick I, Clark WS. First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr. Res. 2004;66(2–3):125–135. doi: 10.1016/j.schres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Hides L, Dawe S, Kavanagh DJ, Young RM. Psychotic symptom and cannabis relapse in recent-onset psychosis. Prospect. Study Br. J. Psychiatry. 2006;189:137–143. doi: 10.1192/bjp.bp.105.014308. [DOI] [PubMed] [Google Scholar]
- Kamali M, Mctigue O, Whitty P, Gervin M, Clarke M, Browne S, Larkin C, O'callaghan E. Lifetime history of substance misuse in first-episode psychosis: prevalence and its influence on psychopathology and onset of psychotic symptoms. Early Interv. Psychiatry. 2009;3(3):198–203. doi: 10.1111/j.1751-7893.2009.00133.x. [DOI] [PubMed] [Google Scholar]
- Kavanagh DJ, McGrath J, Saunders JB, Dore G, Clark D. Substance misuse in patients with schizophrenia: epidemiology and management. Drugs. 2002;62(5):743–755. doi: 10.2165/00003495-200262050-00003. [DOI] [PubMed] [Google Scholar]
- Lambert M, Conus P, Lubman DI, Wade D, Yuen H, Moritz S, Naber D, McGorry PD, Schimmelmann BG. The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr. Scand. 2005;112(2):141–148. doi: 10.1111/j.1600-0447.2005.00554.x. [DOI] [PubMed] [Google Scholar]
- Larsen TK, Melle I, Auestad B, Friis S, Haahr U, Johannessen JO, Opjordsmoen S, Rund BR, Simonsen E, Vaglum P, McGlashan TH. Substance abuse in first-episode non-affective psychosis. Schizophr. Res. 2006;88(1–3):55–62. doi: 10.1016/j.schres.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Linszen DH, Dingemans PM, Lenior ME. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch. Gen. Psychiatry. 1994;51(4):273–279. doi: 10.1001/archpsyc.1994.03950040017002. [DOI] [PubMed] [Google Scholar]
- Malla A, Norman R, Bechard-Evans L, Schmitz N, Manchanda R, Cassidy C. Factors influencing relapse during a 2-year follow-up of first-episode psychosis in a specialized early intervention service. Psychol. Med. 2008;38(11):1585–1593. doi: 10.1017/S0033291707002656. [DOI] [PubMed] [Google Scholar]
- Margolese HC, Malchy L, Negrete JC, Tempier R, Gill K. Drug and alcohol use among patients with schizophrenia and related psychoses: levels and consequences. Schizophr. Res. 2004;67(2–3):157–166. doi: 10.1016/S0920-9964(02)00523-6. [DOI] [PubMed] [Google Scholar]
- Pencer A, Addington J, Addington D. Outcome of a first episode of psychosis in adolescence: a 2-year follow-up. Psychiatry Res. 2005;133(1):35–43. doi: 10.1016/j.psychres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res. 1994;53(1):31–40. doi: 10.1016/0165-1781(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–2518. [PubMed] [Google Scholar]
- Rosenberg SD, Drake RE, Wolford GL, Mueser KT, Oxman TE, Vidaver RM, Carrieri KL, Luckoor R. Dartmouth Assessment of Lifestyle Instrument (DALI): a substance use disorder screen for people with severe mental illness. Am. J. Psychiatry. 1998;155(2):232–238. doi: 10.1176/ajp.155.2.232. [DOI] [PubMed] [Google Scholar]
- Salyers MP, Mueser KT. Social functioning, psychopathology, and medication side effects in relation to substance use and abuse in schizophrenia. Schizophr. Res. 2001;48(1):109–123. doi: 10.1016/s0920-9964(00)00063-3. [DOI] [PubMed] [Google Scholar]
- Sorbara F, Liraud F, Assens F, Abalan F, Verdoux H. Substance use and the course of early psychosis: a 2-year follow-up of first-admitted subjects. Eur. Psychiatry. 2003;18(3):133–136. doi: 10.1016/s0924-9338(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Spitzer R, Williams JBW, Gibbon M, First M. Instruction Manual for the Structured Clinical Interview for DSM-III-R. State Psychiatric Institute, Biometrics Research; New York: 1988. [Google Scholar]
- Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr. Res. 2005;75(1):135–137. doi: 10.1016/j.schres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Sugranyes G, Flamarique I, Parellada E, Baeza I, Goti J, Fernandez-Egea E, Bernardo M. Cannabis use and age of diagnosis of schizophrenia. Eur. Psychiatry. 2009;24(5):282–286. doi: 10.1016/j.eurpsy.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Tiet QQ, Finney JW, Moos RH. Screening psychiatric patients for illicit drug use disorders and problems. Clin. Psychol. Rev. 2008;28(4):578–591. doi: 10.1016/j.cpr.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Turkington A, Mulholland CC, Rushe TM, Anderson R, McCaul R, Barrett SL, Barr RS, Cooper SJ. Impact of persistent substance misuse on 1-year outcome in first-episode psychosis. Br. J. Psychiatry. 2009;195(3):242–248. doi: 10.1192/bjp.bp.108.057471. [DOI] [PubMed] [Google Scholar]
- Van Dorn RA, Desmarais SL, Scott YM, Sellers BG, Swartz MS. Assessing illicit drug use among adults with schizophrenia. Psychiatry Res. 2012 Dec 30; doi: 10.1016/j.psychres.2012.05.028. http://dx. doi.org/10.1016/j.psychres.2012.05.028. [DOI] [PMC free article] [PubMed]
- Van Mastrigt S, Addington J, Addington D. Substance misuse at presentation to an early psychosis program. Soc. Psychiatry Psychiatr. Epidemiol. 2004;39(1):69–72. doi: 10.1007/s00127-004-0713-0. [DOI] [PubMed] [Google Scholar]
- Verstraete AG. Detection times of drugs of abuse in blood, urine, and oral fluid. Ther. Drug Monit. 2004;26(2):200–205. doi: 10.1097/00007691-200404000-00020. [DOI] [PubMed] [Google Scholar]
- Wade D, Harrigan S, Edwards J, Burgess PM, Whelan G, McGorry PD. Substance misuse in first-episode psychosis: 15-month prospective follow-up study. Br. J. Psychiatry. 2006;189:229–234. doi: 10.1192/bjp.bp.105.017236. [DOI] [PubMed] [Google Scholar]
- Wade D, Harrigan S, McGorry PD, Burgess PM, Whelan G. Impact of severity of substance use disorder on symptomatic and functional outcome in young individuals with first-episode psychosis. J. Clin. Psychiatry. 2007;68(5):767–774. doi: 10.4088/jcp.v68n0517. [DOI] [PubMed] [Google Scholar]
- Wisdom JP, Manuel JI, Drake RE. Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiatr. Serv. 2011;62(9):1007–1012. doi: 10.1176/appi.ps.62.9.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br. J. Psychiatry. 2008;193(5):357–363. doi: 10.1192/bjp.bp.107.046375. [DOI] [PubMed] [Google Scholar]


