Abstract
Antipsychotic medications are associated with an increased risk of diabetes. Previous studies have also found an increased risk of type 2 diabetes mellitus in the relatives of schizophrenia probands. The aim of this study was to explore the metabolic adverse effects of olanzapine in a cohort of patients with newly diagnosed psychosis and minimal or no exposure to antipsychotics. Patients with newly diagnosed psychosis (n = 30) were enrolled in a 16-week open trial of olanzapine. Body mass index, fasting glucose, hemoglobin A1c, fasting insulin, IL-6, and a fasting lipid profile were measured at baseline and at 4-week intervals. There was a significant, linear increase over time in fasting glucose (P = 0.043), weight (P < 0.001), body mass index (P < 0.001), total cholesterol (P = 0.005), triglycerides (P = 0.003), and low-density lipoprotein (P = 0.013), but not hemoglobin A1c (P = 0.691), fasting insulin (P = 0.690), IL-6 (P = 0.877), or high-density lipoprotein (P = 0.446). An abnormal baseline IL-6 was a significant predictor of a greater increase in both total cholesterol (P < 0.01) and low-density lipoprotein (P < 0.01). Otherwise, neither parental history of type 2 diabetes mellitus nor baseline IL-6 predicted changes in metabolic measures. Changes in metabolic measures with olanzapine treatment can be detected early in the treatment of patients who are previously antipsychotic naive. The absence of a change in fasting insulin suggests a failure of pancreatic islet cells to compensate for the increase in fasting glucose.
Keywords: olanzapine, psychosis, diabetes, family history
Many of the atypical antipsychotic medications are associated with an increased risk of new-onset diabetes. However, the strength of this association varies among individual medications, with olanzapine and clozapine seeming to have a great risk.1,2 There is also considerable variation in the risk of subsequent diabetes among patients with psychosis who take these medications. Pharmacogenetic studies have provided some leads in this regard, but the relationships have not been strong, and there are few replications.3–5 An association study found that among 21 different receptors, muscarinic M3 receptor-binding affinity was the best predictor of antipsychotic-induced type 2 diabetes mellitus (DM2).6 Research on baseline clinical predictors of diabetes in patients treated with antipsychotic is also limited. An analysis of 2 multicenter 6-month trials of olanzapine or ziprasidone in schizophrenia found that (1) high-density lipoprotein (HDL) cholesterol less than 28 mg/dL and (2) being 58 years or older if HDL cholesterol is 28 mg/dL or greater were significant baseline predictors of diabetes.7
Choice of antipsychotic can be problematic in patients with newly diagnosed psychosis, and consideration of metabolic adverse effects is an important factor in this decision. Three randomized, double-blind, controlled trials support the efficacy of olanzapine in patients with newly diagnosed psychosis.8–10 However, metabolic data from these trials showed that olanzapine was also associated with significant increases in weight, body mass index (BMI), and treatment-emergent metabolic syndrome. In previous reports, we have described an increased family prevalence of diabetes or prediabetic states in subjects diagnosed with schizophrenia11,12 and increased blood levels of interleukin 6 (IL-6) in drug-naive, first episode of nonaffective psychosis.13 Interleukin 6 is a proinflammatory marker associated with diabetes and with risk of developing diabetes.14
The aim of this study was to explore the metabolic adverse effects of olanzapine in a cohort of patients with newly diagnosed psychosis and minimal or no exposure to antipsychotics. We focused on a description of early metabolic adverse effects and clinical and biochemical features that might predict these adverse effects.
METHODS
Patients
Thirty consecutive antipsychotic-naive patients with newly diagnosed psychosis were enrolled in a 16-week open trial of olanzapine. They were recruited at the time of their first clinical contact for psychosis at a general academic hospital (the Hospital Clinic of Barcelona). All subjects had participated in a larger study of baseline metabolic abnormalities (see Fernandez-Egea et al13 for a more complete description) and agreed to participate in the olanzapine trial. The hospital provides psychiatric services for people who live in the surrounding catchment area, Esquerra Eixample, in the city of Barcelona, as part of the Spanish national health system, and most (n = 25, 83%) of the patients resided in that neighborhood. The study was conducted between August 2005 and December 2009.
All subjects gave informed consent for participation in the study, which was conducted under the supervision of the institutional review boards of the Hospital Clinic, University of Barcelona, and the Medical College of Georgia.
Inclusion/Exclusion Criteria
Patients had a maximum cumulative (lifetime) antipsychotic exposure of 1 week and no antipsychotic use in the 30 days before enrolling in the study. Most patients were antipsychotic naive. They were allowed to receive antianxiety medication (lorazepam) the night before blood was drawn at baseline, to a maximum of 3 mg, but not on the day of assessment. Additional inclusion and exclusion criteria were as follows: (1) age 18 to 64 years, (2) no history of diabetes or other serious medical or neurological condition associated with glucose intolerance or insulin resistance (eg, Cushing disease), (3) not taking a medication associated with insulin resistance (eg, hydrochlorothiazide, furosemide, ethacrynic acid, metolazone, chlorthalidone, β-blockers, glucocorticoids, phenytoin, nicotinic acid, cyclosporine, pentamidine, or narcotics), (4) no history of cocaine use in the previous 30 days, and (5) no laboratory evidence of diabetes at baseline (fasting glucose <126 mg/dL or 2-hour glucose <200 mg/dL on a glucose tolerance test).15
Assessments
All subjects were interviewed using the Spanish translation of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Axis I Disorders, clinician version. Socioeconomic status (SES) of the family of origin was assessed with the Hollingshead-Redlich scale.16
Subjects were also interviewed for a parental history of DM2. A parental history of DM2 was scored either “yes” or “no,” based on whether a physician had diagnosed DM2 in either the father, the mother, or both. One case of maternal gestational diabetes was rated as “yes.” Data were confirmed by interviewing the patients’ parents or siblings during a post-discharge follow-up visit. We excluded from further analysis data that were not verifiable because of either adoption or the absence of relatives.
Baseline Metabolic Assessment
All subjects were given a 2-hour, 75-g oral glucose tolerance test (OGTT), which began between 8 and 9 am after an overnight fast. Hemoglobin A1c (HbA1c), fasting insulin, a fasting lipid panel, C-reactive protein, IL-6, leptin, adiponectin, and cortisol blood levels were also recorded at baseline. Height and weight, while wearing underwear and without shoes, were recorded between the blood samplings. Further details of the metabolic assessment have been previously published.11 The determination of IL-6 was performed with a commercial enzyme immunoassay technique (Biosurce, Nivelles, Belgium). Cortisol was measured using a radioimmunoassay (Immuchem, Ivoz-Ramet, Belgium). Serum insulin concentrations were measured in duplicate by monoclonal immunoradiometric assay (Medgenix Diagnostics, Fleunes, Belgium). No cross-reaction with proinsulin was detected. Glycosylated hemoglobin (HbA1c) was determined by high-performance liquid chromatography (HA 8121; Menarini Diagnostici, Firenze, Italy; reference range, 3.4%–5.5%). Cortisol was measured using a radioimmunoassay (Immuchem). Adiponectin was measured using a radioimmunoassay (Millipore Corporation, Billerica, Mass), leptin with enzyme-linked immunosorbent assay (DBC; Diagnostics Biochem Canada Inc, London, Ontario, Canada), and C-reactive protein in serum using Advia 2400 (Siemens, Barcelona, Spain).
Treatment Phase
Following the baseline assessment, patients entered an open treatment trial with olanzapine lasting 16 weeks. They were started on a dose of 15 mg/d by mouth, which could be adjusted to as low as 10 mg/d or as high as 40 mg/d, based on clinical response. The trial began while they were hospitalized and continued after discharge.
Patients were evaluated clinically on weeks 1, 2, 3, 4, 6, 8, 10, 12, 14, and 16. A fasting glucose, fasting insulin, IL-6, fasting lipids, and HbA1c were also measured on weeks 4, 8, 12, and 16. Height and weight were also measured at each of these visits. A Positive and Negative Syndromes Scale was administered at weeks 4, 8, 12, and 16.
Patients were withdrawn from the study if any of the following occurred at any of the time points: (1) a fasting glucose 126 mg/dL or greater, (2) systolic blood pressure greater than 150 mm Hg or diastolic blood pressure greater than 100 mm Hg, (3) significant worsening of mental status (based on clinical judgment), (4) failure of mental status to improve within 8 weeks (based on clinical judgment), (5) low-density lipoprotein (LDL) cholesterol greater than 160 mg/dL, or triglycerides greater than 225 mg/dL, or (6) any other significant adverse response to treatment. Patients were also withdrawn for an increase in BMI as follows: (a) increase of 4 BMI units or greater in subjects with a BMI of less than 20 at study entry, (b) increase of 3 BMI units or greater in subjects with a BMI of 20.1 to 25 at study entry, (c) increase of 2 BMI units or greater in subjects with a BMI of 25.1 to 30 at study entry, or (d) increase of 1 BMI unit or greater and weight gain of greater than 15 lb in subjects with a BMI of 30 or greater at study entry.
Statistical Analysis
We tested for an increase over time in each metabolic measure using univariate linear regression, with time as the independent variable.
In separate analyses, patients were also stratified based on (1) presence or absence of a parental history of DM2 and (2) presence or absence of an abnormal baseline IL-6 concentration (defined as IL-6 ≥5 pg/mL). We used a repeated-measures analysis of variance, controlling for age, race (white yes/no), sex, smoking, and SES of the family of origin, to evaluate parental history of DM2 and baseline IL-6 as predictors of change in metabolic measures. All analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, Ill). All data were analyzed using a last-observation-carried-forward approach.
RESULTS
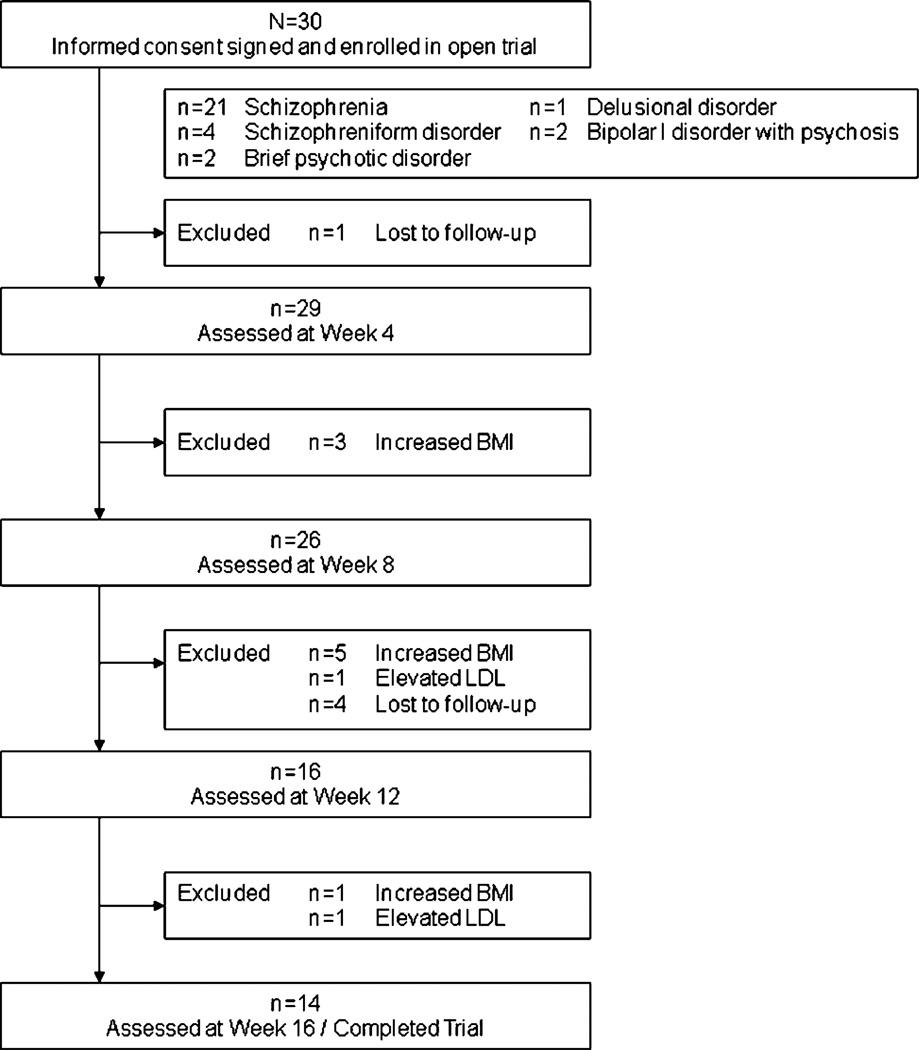
Thirty patients entered the study. They met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for schizophrenia (n = 21), schizophreniform disorder (n = 4), brief psychotic disorder (n = 2), delusional disorder (n = 1), or bipolar I disorder with psychotic features (n = 2). Table 1 provides baseline psychiatric and metabolic characteristics of these patients. The mean olanzapine dose over the trial was 22.9 ± 7.9 mg. Eleven (37%) of the patients were withdrawn from the study during the treatment phase because of the exclusion criteria: n = 9 because of increased BMI (n = 3 at 4 weeks, n = 5 at 8 weeks, and n = 1 at 12 weeks) and n = 2 because of elevated LDL (n = 1 at 4 weeks and n = 1 at 8 weeks). Fourteen patients (47%) completed 16 weeks. The mean weight gain was 7.8 ± 3.6 kg. The flow of patients through the study is described in Figure 1.
TABLE 1.
Baseline Patient Characteristics (n = 30)
| Mean (SD) age, y | 27.5 (7.0) |
| Sex, % male | 66.7 |
| Race, % white | 90 |
| % Residing in catchment area | 83 |
| Mean (SD) no. cigarettes/d | 8.1 (9.7) |
| Mean (SD) SES | 4.9 (1.6) |
| Mean (SD) PANSS positive | 25 (7) |
| Mean (SD) PANSS negative | 23 (9) |
| Mean (SD) PANSS general | 39 (10) |
| Mean (SD) PANSS total | 87 (20) |
| Mean (SD) duration of untreated psychosis, mo | 1.5 (1.4) |
| Mean (SD) BMI | 22.1 (3.0) |
| Mean (SD) total cholesterol, mg/dL | 163 (30) |
| Mean (SD) triglycerides, mg/dL | 80 (26) |
| Mean (SD) LDL, mg/dL | 97 (27) |
| Mean (SD) HDL, mg/dL | 50 (14) |
| Mean (SD) fasting glucose, mg/dL | 82 (8) |
| Mean (SD) HbA1c (%) | 4.4 (0.3) |
| Mean (SD) 2-h glucose, mg/dL | 110 (35) |
| Mean (SD) fasting insulin, mg/dL | 9.7 (4.4) |
| Mean (SD) leptin, mg/dL | 7.3 (10.4) |
| Mean (SD) adiponectin, mg/dL | 12.3 (5.9) |
| Mean (SD) C-reactive peptide, mg/dL | 0.21 (0.34) |
| Mean (SD) IL-6, pg/mL | 3.5 (7.2) |
| Mean (SD) cortisol, mg/dL | 18.3 (5.6) |
| Parental history of DM2, % yes | 23.3 |
PANSS indicates Positive and Negative Syndromes Scale.
FIGURE 1.
Flow of patients through the study.
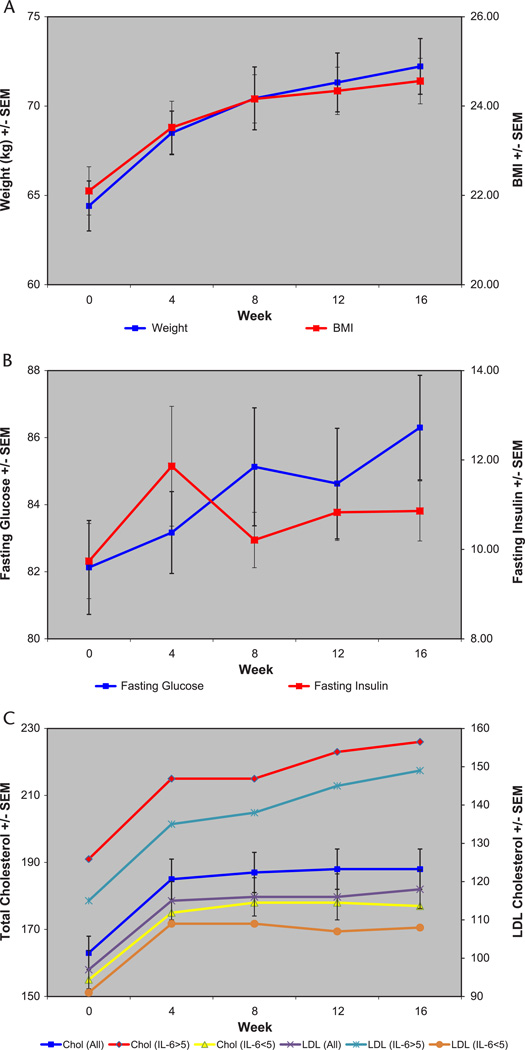
In the univariate linear regression models, there was a significant, linear increase over time in fasting glucose (P = 0.043), weight (P < 0.001), BMI (P < 0.001), total cholesterol (P = 0.005), triglycerides (P = 0.003), and LDL (P = 0.013), but not HbA1c (P = 0.691), fasting insulin (P = 0.690), IL-6 (P = 0.877), or HDL (0.696) (Fig. 2). These results were unchanged when reanalyzed after excluding the 2 subjects with bipolar I disorder with psychotic features (data not shown). The results were also unchanged when the analyses were repeated with only the 14 subjects who completed the trial (data not shown). We did not find any significant correlations between changes in fasting glucose, fasting insulin, 2-hour glucose, weight, or BMI (data not shown). None of the patients met the criteria for metabolic syndrome at the end of the study. A parental history of DM2 (n = 7) was associated with a higher weight, BMI, fasting glucose, IL-6, total cholesterol, triglycerides, and LDL and lower fasting insulin than found in patients without such a history (n = 20; data were missing for 3 subjects), and this difference was sustained throughout the trial. Patients with an abnormal baseline IL-6 (n = 7) had a higher weight, BMI, fasting glucose, total cholesterol, LDL, and HDL than found in patients with normal IL-6, and this difference was sustained throughout the trial. In a repeated-measures ANOVA, parental history of DM2 predicted greater increase in BMI and triglycerides at the trend level (P = 0.07 for BMI and P = 0.10 for triglycerides). Baseline abnormal IL-6 was a significant predictor of a greater increase in both total cholesterol (P < 0.01) and LDL (P < 0.01) (Fig. 2). Otherwise, neither parental history of DM2 nor baseline IL-6 predicted changes in these metabolic measures. The results were unchanged when reanalyzed after excluding the 2 subjects with bipolar I disorder with psychotic features (neither of these subjects had either a parental history of DM2 or an abnormal baseline IL-6; data not shown). In an exploratory analysis, baseline 2-hour glucose in the OGTT was not a significant predictor of changes in these metabolic measures (data not shown).
FIGURE 2.
Changes in metabolic measures over time in all subjects (n = 30). A, Weight and BMI. In the univariate linear regression models, there was a significant, linear increase over time in weight (P < 0.001) and BMI (P < 0.001). B, Fasting glucose and fasting insulin. In the univariate linear regression models, there was a significant, linear increase over time in fasting glucose (P = 0.043), but not in fasting insulin (P = 0.690). C, Total cholesterol and LDL. In the univariate linear regression models, there was a significant, linear increase over time in total cholesterol (P = 0.005) and LDL (P = 0.013). Baseline abnormal IL-6 (≥5) was a significant predictor of a greater increase in both total cholesterol (P < 0.01) and LDL (P < 0.01) in a repeated-measures analysis of variance.
DISCUSSION
We found a significant increase in the first 16 weeks of olanzapine treatment of fasting glucose, weight, BMI, total cholesterol, triglycerides, and LDL in patients with newly diagnosed psychosis, the majority of whom were antipsychotic naive. Fasting insulin, HbA1c, and HDL levels did not change significantly. Baseline abnormal IL-6 was a significant predictor of a greater increase in total cholesterol and LDL cholesterol. Neither a parental history of diabetes nor baseline 2-hour glucose in the OGTT was a significant predictor of changes in metabolic parameters.
The most important limitations in our study were sample size and duration. With a larger sample, some of these measures may have shown significant change within this time frame. There is extensive evidence suggesting that, in patients who receive antipsychotic medications over a period of many months or years, all of the measures presented here change more in those who receive olanzapine and other antipsychotics than in patients who do not take these drugs.
A strength of our study is that we explored parental history of diabetes and baseline IL-6, which have an increased prevalence in nonaffective psychosis,11,12,17–19 as potentially novel clinical predictors of changes in metabolic parameters. To our knowledge, only 1 previous study20 has considered other clinical predictors of metabolic changes in patients treated with olanzapine. In that study, change in BMI was not predicted by age, sex, smoking status, diagnosis, premorbid functioning, age at onset, duration of illness, or history of previous antipsychotic treatment. In our study, patients with an abnormal baseline IL-6 were less likely than patients with normal baseline IL-6 to complete the trial (14% vs 48%, respectively). Thus, our finding that baseline abnormal IL-6 predicted a greater increase in total and LDL cholesterol should be interpreted with caution in light of small numbers and differential dropout. Replication of these results in a larger trial is warranted.
Our results are consistent with other studies that found significant increases in weight21–24 and lipids—particularly total cholesterol and triglycerides22,24,25—in patients with first-episode psychosis treated with olanzapine for 8 to 16 weeks. However, our evidence is in contrast with 2 previous studies that found an increase in fasting insulin but not fasting glucose in patients with first-episode psychosis treated with olanzapine for 8 to 12 weeks.22,25 In one of these studies,22 however, 2 of the 9 patients had been on olanzapine for 5 to 7 weeks before study entry. Another study also found a significant increase in fasting insulin in male but not female patients with first-episode psychosis treated for 1 year with olanzapine, risperidone, or haloperidol.26 This study did not find an increased prevalence of DM2 at end point, but did not present data on mean fasting glucose values. One possible explanation is that our study was underpowered to detect an increase in fasting insulin. However, in the brief time frame of our study, it is possible that insulin production is more of a problem for patients than is insulin sensitivity. The absence of a change in fasting insulin suggests a failure of pancreatic islet cells to compensate for the increase in fasting glucose, although 1 study found increased insulin resistance in patients treated with olanzapine for 8 weeks.25 It is not surprising that no subjects met the criteria for metabolic syndrome, given the young age and low BMI of the patients entering and the short duration of the trial. Moreover, there was a bias against both including and continuing in the study patients with marked metabolic problems.
In summary, parental history of DM2 or baseline IL-6 may predict adverse effects through association with higher BMI, fasting glucose, and fasting lipids. Given the burden of premature cardiovascular disease mortality in psychosis,27 larger studies are needed to identify patients who may be particularly vulnerable to the metabolic adverse effects of antipsychotics, which impact on this risk.
Acknowledgments
Supported in part by NIH grant 5R01DK069265-05 (to B.K.) and the Government of Catalonia, Comissionat per Universitats i Recerca del Departament d’Innovació, Universitats i Empresa (DIUE) 2009SGR1295 (to M.B.).
Dr Miller received support from the University of Oulu and Oy H. Lundbeck. Dr Bernardo received consultant fees from Bristol-Myers Squibb and Wyeth. He also received honoraria from Janssen-Cilag, Eli Lilly Company, Pfizer, Synthelabo, GlaxoSmithKline, and AstraZeneca. Dr Kirkpatrick received consulting and/or speaking fees from Pfizer, Organon, AstraZeneca, Wyeth, Lilly, Bristol-Myers Squibb, Cephalon, Abbott, and Solvay.
Footnotes
AUTHOR DISCLOSURE INFORMATION
Drs Fernandez-Egea and Garcia-Rizo have no conflicts of interest to disclose.
The data have previously been presented as a poster at the 49th Annual NIMH New Clinical Evaluation Drug Unit meeting, June 29–July 2, 2010, Hollywood, Florida (by B.M.).
REFERENCES
- 1.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- 2.Cohen D. Atypical antipsychotics and new onset diabetes mellitus. An overview of the literature. Pharmacopsychiatry. 2004;37(1):1–11. doi: 10.1055/s-2004-815468. [DOI] [PubMed] [Google Scholar]
- 3.Al-Janabi I, Arranz MJ, Blakemore AI, et al. Association study of serotonergic gene variants with antipsychotic-induced adverse reactions. Psychiatr Genet. 2009;19:305–311. doi: 10.1097/YPG.0b013e3283328dcd. [DOI] [PubMed] [Google Scholar]
- 4.Clark D, Skrobot OA, Adebiyi I, et al. Apolipoprotein-E gene variants associated with cardiovascular risk factors in antipsychotic recipients. Eur Psychiatry. 2009;24:456–463. doi: 10.1016/j.eurpsy.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Dickerson F, Rubalcaba E, Viscidi R, et al. Polymorphisms in human endogenous retrovirus K-18 and risk of type 2 diabetes in individuals with schizophrenia. Schizophr Res. 2008;104(1–3):121–126. doi: 10.1016/j.schres.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Silvestre JS, Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find Exp Clin Pharmacol. 2005;27(5):289–304. doi: 10.1358/mf.2005.27.5.908643. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM, Lieberman JA, Sethuraman G. In search of moderators and mediators of hyperglycemia with atypical antipsychotic treatment. J Psychiatr Res. 2009;43(11):997–1002. doi: 10.1016/j.jpsychires.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Sanger TM, Lieberman JA, Tohen M, et al. Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry. 1999;156:79–87. doi: 10.1176/ajp.156.1.79. [DOI] [PubMed] [Google Scholar]
- 9.Green AI, Lieberman JA, Hamer RM, et al. Olanzapine and haloperidol in first episode psychosis: two-year data. Schizophr Res. 2006;86:234–243. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7):1050–1060. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Egea E, Bernardo M, Parellada E, et al. Glucose abnormalities in the siblings of people with schizophrenia. Schizophr Res. 2008;103(1–3):110–113. doi: 10.1016/j.schres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Egea E, Miller B, Bernardo M, et al. Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res. 2008;98(1–3):302–306. doi: 10.1016/j.schres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Egea E, Bernardo M, Donner T, et al. The metabolic profile of antipsychotic-naBve patients with nonaffective psychosis. Br J Psychiatry. 2009;194(95):434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingshead AB, Redlich FC. Social Class and Mental Illness. New York: Wiley; 1958. pp. 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crespo-Facorro B, Perez-Iglesias R, Ramirez-Bonilla M, et al. A practical clinical trial comparing haloperidol, risperidone, and olanzapine for the acute treatment of first-episode nonaffective psychosis. J Clin Psychiatry. 2006;67:1511–1521. doi: 10.4088/jcp.v67n1004. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients [letter] Lancet. 1989;1(8636):495. doi: 10.1016/s0140-6736(89)91392-5. [DOI] [PubMed] [Google Scholar]
- 19.Spelman LM, Walsh PI, Sharifi N, et al. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24(5):481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- 20.Zipursky RB, Gu H, Green AI, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry. 2005;187:537–543. doi: 10.1192/bjp.187.6.537. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry. 2006;67:1253–1260. doi: 10.4088/jcp.v67n0812. [DOI] [PubMed] [Google Scholar]
- 22.Graham KA, Perkins DO, Edwards LJ, et al. Effect of olanzapine on body composition and energy expenditure in adults with first-episode psychosis. Am J Psychiatry. 2005;162:118–123. doi: 10.1176/appi.ajp.162.1.118. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman JA, Tollefson G, Tohen M, et al. HGDH Study Group. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry. 2003;160:1396–1404. doi: 10.1176/appi.ajp.160.8.1396. [DOI] [PubMed] [Google Scholar]
- 24.Sengupta SM, Klink R, Stip E, et al. Weight gain and lipid metabolic abnormalities induced by olanzapine in first-episode, drug-naive patients with psychotic disorders. Schizophr Res. 2005;80:131–133. doi: 10.1016/j.schres.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Wu RR, Zhao JP, Liu ZN, et al. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology (Berl) 2006;186:572–578. doi: 10.1007/s00213-006-0384-5. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Iglesias R, Mata I, Pelayo-Teran JM, et al. Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naBve population. Schizophr Res. 2009;107(2–3):115–121. doi: 10.1016/j.schres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 27.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]