Abstract
Objective: The molecular events that lead to human thyroid cell speciation remain incompletely characterized. It has been shown that overexpression of the regulatory transcription factors Pax8 and Nkx2-1 (ttf-1) directs murine embryonic stem (mES) cells to differentiate into thyroid follicular cells by initiating a transcriptional regulatory network. Such cells subsequently organized into three-dimensional follicular structures in the presence of extracellular matrix. In the current study, human embryonic stem (hES) cells were studied with the aim of recapitulating this scenario and producing functional human thyroid cell lines.
Methods: Reporter gene tagged pEZ-lentiviral vectors were used to express human PAX8-eGFP and NKX2-1-mCherry in the H9 hES cell line followed by differentiation into thyroid cells directed by Activin A and thyrotropin (TSH).
Results: Both transcription factors were expressed efficiently in hES cells expressing either PAX8, NKX2-1, or in combination in the hES cells, which had low endogenous expression of these transcription factors. Further differentiation of the double transfected cells showed the expression of thyroid-specific genes, including thyroglobulin (TG), thyroid peroxidase (TPO), the sodium/iodide symporter (NIS), and the TSH receptor (TSHR) as assessed by reverse transcription polymerase chain reaction and immunostaining. Most notably, the Activin/TSH-induced differentiation approach resulted in thyroid follicle formation and abundant TG protein expression within the follicular lumens. On stimulation with TSH, these hES-derived follicles were also capable of dose-dependent cAMP generation and radioiodine uptake, indicating functional thyroid epithelial cells.
Conclusion: The induced expression of PAX8 and NKX2-1 in hES cells was followed by differentiation into thyroid epithelial cells and their commitment to form functional three-dimensional neo-follicular structures. The data provide proof of principal that hES cells can be committed to thyroid cell speciation under appropriate conditions.
Introduction
With advances in understanding of stem cell biology, human pluripotent stem cells (hPSCs), including human embryonic stem (hES) cells and induced pluripotent stem cells (iPSCs), have been shown to exhibit replication competence and the ability to differentiate into many cell types. hES cells are, therefore, an important potential model for the study of human thyroid cell speciation and a potential source of thyroid cells for research and even for potential cell therapy. Several protocols have been reported to induce thyroid cells from mouse embryonic stem (mES) cells, which attempted to mimic the differentiation process during thyroid development (1–4). It has previously been found that these methods are highly effective for procuring murine thyroid follicles, after differentiation with Activin and thyrotropin (TSH) (3), but this approach has not yet been applied to hES cells.
Human thyroid development is regulated at the transcriptional level involving primarily four different factors—PAX8, NKX2-1, HEX, and FOXE1—and controlled by various morphogens, particularly NODAL factors regulating the endoderm formation at gastrulation (5,6). Thyroid cells are derived from endoderm that develops from pluripotent cells in the early embryo. The endoderm differentiates into gut endoderm containing thyroid progenitors expressing PAX8, NKX2-1, HEX, and FOXE1 that play important roles in thyroid organogenesis. While HEX and FOXE1 are expressed throughout the endoderm, NKX2-1 and PAX8 expression is restricted to the thyroid placode, indicating their crucial role in thyroid cell speciation. Further evidence of this includes the fact that murine Pax8 and Nkx2-1 alone can direct mES cells to differentiate into thyroid follicular cells (1,3). Such cells subsequently organized into three-dimensional follicular structures in the presence of extracellular matrix.
In the present experiment, hES cells (H9) were studied with the aim of producing functional human thyroid cell lines.
Methods
hES cells culture
hES cells (line H9) were maintained in feeder-free culture conditions with mTeSR medium (Stemcell Technologies) on 6-well plates coated with hES cell-qualified gelatin/Matrigel (BD Biosciences). The culture medium was changed daily, and cells were passaged every four to five days at ratios of 1:3 to 1:6. Cells were cultured in a humidified chamber in a 5% CO2–air mixture at 37°C.
Establishment of stable hES cell lines expressing either PAX8 or NKX2-1 or both factors
Two pEZ-lentiviral vectors expressing either PAX8 or NKX2-1 were used to establish individual cell lines. The expression of PAX8 was tagged with eGFP and NKX2-1 tagged with mCherry. Lentiviruses were produced using the Lenti-Pax HIV Expression Packing Kit (GeneCopoeia) according to the manufacturer's manual.
For virus transduction, 5×105 hES cells were seeded into gelatin/Matrigel precoated 6-well plates 24–48 h prior to viral transduction. When the cells reached 60–70% confluence, either one or both virus-containing supernatants diluted in 1 mL hES medium supplemented with polybrene (final concentration: 8 μg/mL) were added. The medium was refreshed daily. The cells were checked under a fluorescence microscope and split when necessary. To obtain stable single or double transformants, the cells were cultured in hygromycin (50 μg/mL) or purimycin (0.5 μg/mL) or both until resistant clones were established. The positive clones were then expanded and characterized for their gene expression. Stable cell lines were chosen for further experiments.
For thyroid cell differentiation, embryoid bodies (EBs) were differentiated as described previously (3). In brief, hES cell suspensions were plated in ultra-low attachment dishes to induce EB formation in RPMI 1640 containing 1% B27 (Life Technologies) supplemented with 100 ng/mL Activin A (R&D Systems, Inc.) for five days for thyroid endoderm induction. Subsequently, the supplements were changed to include 1000 μIU/mL bovine TSH (Sigma-Aldrich 2 IU/mg), and the EBs cells were cultured for 10 days. Then, the cells were embedded in growth factor-restricted Matrigel (BD Biosciences) and placed into 6-well plates in RPMI 1640 containing 1% B27 (Life Technologies) supplemented with 1000 μIU/mL TSH for six days. Cells were harvested for analysis at different time points.
RNA isolation and reverse transcription polymerase chain reaction
Total RNA was extracted from cultured cell lines using the RNeasy Mini Kit Isolation System (Qiagen Ltd.), which included a digestion step with DNase I. RNA quantity and quality were assessed by ultraviolet spectrophotometry. cDNA synthesis was performed using the SuperScript® III First-Strand Synthesis System (Invitrogen Corp.). Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) was carried out using SYBR green qPCR Master Mix (Applied Biosystems) and employing the StepOnePlus Real-time PCR system (Applied Biosystems). Relative expression levels of each gene in real time were analyzed using the 2-ΔΔCT method and normalized to the expression of the housekeeping gene GAPDH. Data presented (mean±standard error of the mean [SEM]) are from three independent experiments in which all sample sets were analyzed in triplicate.
Western blotting
Lysates were prepared from single- and double-transfected cells by treating with 1×RIPA buffer for 20 min on ice. The samples were then centrifuged and treated with 5×sample buffer at 100°C for 5 min and loaded on a 4–15% SDS-PAGE gel. Western blotting was performed according to a standard protocol (LI-COR®; Biosciences) using corresponding antibodies and LI-COR® IRDye® 800CW and IRDye680RD conjugated secondary antimouse or antirabbit antibodies. The primary antibodies used were antithyroglobulin antibody (ab115942), anti-PAX8 (ab97477), and anti-NKX2-1 antibody (ab76013; from Abcam), anti-NIS (sc-134515; Santa Cruz) and anti-TSHR antibody (AB-N16; Advanced Targeting Systems).
Immunodetection
Cells were grown in Delta T culture dishes, fixed for 15 min with 4% paraformaldehyde (PFA), rinsed three times (5 min each) with phosphate-buffered saline (PBS), and blocked for 60 min at room temperature in 3% bovine serum albumin (BSA), 5% horse serum in PBS. Depending on the analysis marker, 0.3% Triton X-100 was added into the blocking buffer for cell permeabilization to detect intracellular antigens. Cells were incubated with appropriate primary antibodies diluted in a solution of PBS containing 1% BSA, 1% horse serum, and 0.1% Triton X-100 with the appropriate secondary antibody for 1 h at room temperature. After that, cells were rinsed three times with PBS and mounted using hard-set mounting media containing DAPI (Vector Laboratories). Additional primary antibodies used were anti-OCT4 (#3576) and anti-NANOG (#3165; BioVision) and anti-SOX2 (#3579; Cell Signaling). The secondary antibodies used were antimouse IgG Alexa Flour 488 Conjugate (#4408) and 555 Conjugate (#4409), and antirabbit IgG Alexa Flour 555 Conjugate (#4413) and 647 Conjugate (#4414) (Cell Signaling).
TSHR functional assessment
Intracellular cAMP generation was measured with a cAMP enzyme immunoassay kit (GE Healthcare Bio-Sciences). Briefly, the differentiated hES cells were seeded in a 24-well tissue culture plate at a density of approximately 1.0×105 cells per dish in 1 mL medium 24 h prior to the experiment. The cells were then stimulated with increasing concentration of TSH for 1 h. The medium was then aspirated. The cells were washed with fresh PBS and lysed for 10 min. A total of 100 μL of each sample was transferred into the appropriate well of the immunoassay microtiter plate, and intracellular cAMP was measured as described by the manufacturer.
Radioactive iodide uptake
The differentiated hES cells were seeded in a 24-well plate at a density of approximately 1.0×105 cells/well for 24–48 h. Cells were then washed with modified Hanks' balanced salt solution, then incubated in the same buffer containing 20 μM sodium iodide supplemented with 10 μCi carrier-free Na125I to give a specific activity of 10 μCi/mmol for 45 min at 37°C in a humidified atmosphere. Parallel wells of cells were incubated in the absence or presence of TSH and/or perchlorate. Aspirating the radioactive solution and washing twice with ice-cold Hanks' balanced salt solution terminated the uptake reactions. To determine the amount of 125I accumulated in the cells, 95% ethanol was added to each well for 20 min at 4°C, then quantitated in a γ-counter, as previously described (7). 125I uptake was expressed as cpm per well per 45 min. The values represent the mean±SEM of two independent experiments performed in triplicate.
Results
Characterization of stable hES cell lines expressing PAX8 and NKX2-1
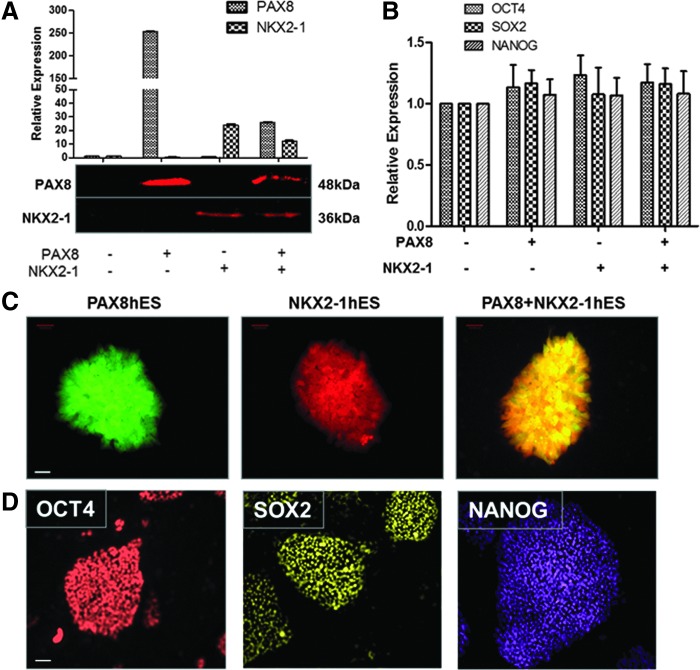
To induce concurrent expression of PAX8 and NKX2-1, pEZ-lentiviral vectors expressing human PAX8-eGFP and NKX2-1-mCherry were used and stable individual hES cell lines expressing either one or both factors were established. The stable lines expressing either PAX8 or NKX2-1, or PAX8+NKX2-1 showed appropriately induced mRNA and protein expression of these transcription factors by real time qPCR, immunoblotting, and fluorescent microscopy (Fig. 1A and C) as well as the induction of FOXE1 and HEX (data not shown). Overexpression of these transcription factors at this stage did not appear to change the pluripotent state of the ES cells, since the stemness markers—OCT4, SOX2, and NANOG—continued to be expressed as evidenced by qPCR and immunostaining of the three individual hES cell lines (Fig. 1B and D).
FIG. 1.
Generation and characterization of stable hES cell lines. (A) Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis (upper panel) and Western blot (lower panel) of selected hES cell lines expressing either PAX8 or NKX2-1, or both, for transfected thyroid transcription factors PAX8 and NKX2-1. Relative expression of each transcript is presented as fold change in mRNA compared to untransfected control ES cells (mean±standard error of the mean [SEM]; n=3). These data represent one of three separate experiments. (B) qRT-PCR analysis of the same transfected hES cell lines as shown in (A) but analyzed for stemness using OCT4, SOX2, and NANOG as read outs. Note the retention of stemness in these cells. (C) Fluorescent images of the individual transfected hES cell colonies growing as monolayers cultured on plastic supports. The expression of PAX8 displays as green (left), NKX2-1 as red (middle), and the combination expresses as yellow (right). Scale: 200 μm. (D) Immunodetection analysis of pluripotent markers OCT4, SOX2, and NANOG expression in the three individual hES transfected cell monlayers. The representative images are also shown with the indicated scale: 200 μm.
Activation of thyroid-specific genes in individual hES cell lines
Analysis of the individual cell lines expressing either one or both factors for activation of the thyroid-specific genes—NIS, TSHR, Tg, and TPO—using RT-qPCR showed thyroid gene expression to a variable degree in all of the transfect cells (Fig. 2A). The expression of NIS and TSHR protein was also detected by Western blot and by immunostaining of the double transfectants (Fig. 2B and C). However, no detectable TG protein expression was seen, although a low level of TG gene expression was evident in the undifferentiated double transfectants.
FIG. 2.
Activation of thyroid-specific genes in stable hES cell lines. (A) qRT-PCR analysis and (B) Western blot for thyroid-specific gene activity in individual transfected hES cell lines. The relative expression of each transcript is presented as fold change in mRNA compared to control untransfected hES cells (mean±SEM; n=3). These data represent one of three separate experiments. (C) Immunodetection analysis of NIS (left) and TSHR (right) expression in the individual hES cell lines. Scale: 100 μm.
Differentiation of PAX8+NKX2-1hES cells
Since Activin A can induce endoderm differentiation (2), the double transfected cells were cultured in ultra-low attachment dishes to form embryoid bodies (EBs; Fig. 3A–C) and the cells were treated with Activin A to induce endoderm differentiation. Five days of culture in Activin A containing medium successfully induced the endoderm markers SOX17 and FOXA2 (Fig. 3D) in comparison to control wild-type hES cells. These Activin A–treated cells demonstrated upregulation of TSHR gene expression but no further induction of TG transcription (data not shown) as seen with mES cells (2,3). However, after further differentiation of these Activin A–induced EBs with TSH, enhanced thyroid-specific gene expression was found (Fig. 4A). While the TSHR was induced in all cell types, NIS, TG, and TPO were most induced in the double transfected cells, although not to the same extent as seen in human thyroid tissue control. Interestingly, almost 50% of the differentiated double transfected hES cells were capable of forming three-dimensional follicles (Fig. 4B and C). Immunostaining with a NIS-specific antibody confirmed abundant NIS expression in the follicular cell membrane (Fig. 4D) and TG expression in the follicular lumen and cytoplasm (Fig. 4E).
FIG. 3.
Induction of endoderm from hES cell embryoid bodies. (A–C) Representative images of individual hES cell embryoid bodies (EBs) in suspension culture in low attachment conditions. (A) PAX8, (B) NKX2-1, and (C) PAX8+NKX2-1. Scale: 20 μm. (D) Representative qRT-PCR analysis of endoderm markers, SOX17 and FOXA2, in EB cells formed from Activin A treated and untreated cells. Data are expressed as mean±SEM and represent one of three separate experiments.
FIG. 4.
Analysis of differentiated hES cells. (A) qRT-PCR analysis of thyroid functional genes (NIS, TSHR, TG, and TPO) in Activin A and thyrotropin (TSH) differentiated cells. The relative expression of each transcript is presented as fold change in mRNA compared to untransfected hES cells (mean±SEM) and represent one out of three separate experiments. (B) Phase contrast image of a human thyroid follicle differentiated from double transfected hES cells. Scale: 100 μm. (C) Fluorescent images of hES-derived thyroid follicles formed from double transfected cells in Matrigel. The picture shows NKX2-1 expression (red), PAX8 expression (green), and double fluorescence (yellow/orange). (D) Immunodetection of NIS (red) expression in a human thyroid neofollicle derived from differentiated hES cells as shown in (B) and (C). Note that the NIS expression is seen on the cell surface. Scale bar: 100 μm. (E) Immunodetection of TG (red) expression in a thyroid neofollicle derived from differentiated hES cells. Note that the TG is seen in the intrafollicluar lumen, and the transcription factor PAX8 (green) is seen within the nuclei. Scale bar: 100 μm.
Functionality of differentiated thyroid cells
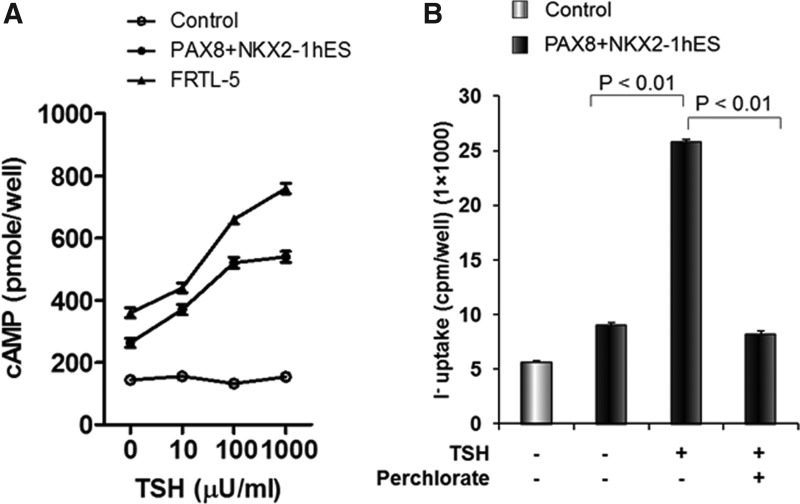
The double transfected and differentiated cells were further analyzed by assessment of TSH-stimulated cAMP generation (Fig. 5A). A dose-dependent cAMP response to TSH was observed with a sensitivity of 10 μIU/mL, similar to the rat thyroid cell controls. No response was seen in the hES control cells. Similarly, when 125I uptake was measured in these differentiated thyroid cells maintained under steady-state conditions without TSH (as described in Materials and Methods), there was significantly increased 125I uptake in response to TSH, and it could be inhibited by sodium perchlorate acting as a NIS inhibitor (Fig. 5B).
FIG. 5.
Demonstration of differentiated hES-derived thyroid cell function. (A) Differentiated hES cells demonstrate cAMP generation in response to increasing concentrations of TSH. This response was not seen in undifferentiated hES cells as control. The sensitivity of rat thyroid cells (FRTL5) is illustrated for comparison within the same experiment. (B) Differentiated hES cells were exposed to 125I as described in Methods, and the radioactive uptake into the differentiated thyroid cells was measured with and without TSH. Sodium perchlorate was used to inhibit the action of NIS and stop radioactive iodine uptake.
Discussion
Since the first hES cell line was derived in 1998, such cells have attracted significant attention because of their potential to enlighten the process of cell speciation and to self-renew human tissues. Application of this approach to the thyroid gland and to thyroid cells has been difficult, and only recently have thyroid follicles been successfully derived from stem cells (1,3).
Structurally, the thyroid gland is composed of two types of cells—the thyroid follicular cells (TFCs) and the parafollicular cells—but the bulk of the gland is composed of TFCs, which originate from the foregut endoderm. This derivation is initiated by the expression of an initial complex of defined transcription factors (5). Although the precise details of human thyroid cell speciation remain unclear, a great deal has been learned from studies in the mouse where it has been possible to knock out a variety of these transcription factors, thus confirming their primary roles (8–11). It is known that during early-to-mid embryogenesis, groups of cells that are destined to become the TFCs are set apart by the co-expression of Pax8, Nkx2-1, Foxe1, and Hex, which form a “thyroid-specific gene expression program” found only in cells designated for TFC formation (5,6). Thus, the signals for thyroid development do not depend on unique transcription factors but rather the simultaneous expression of a specific combination of factors at an appropriate time in cell differentiation.
Initial studies by both the authors and other investigators have focused on committing ES cells into TFCs using recombinant TSH and assessing whether these transformed cells are capable of initiating Tg gene transcription (1,3). The results have been disappointing. The subsequent manipulation of mES cells has taught us that the first step to programming such cells into a TFC pathway successfully is to lead them to form definitive endoderm. There was subsequently a series of experiments with mouse ES cells trying to obtain fully differentiated ES thyroid follicles (12). Initially, it was found that Activin A, TSH, and IGF1 were capable of inducing some TG expression but only at low levels (2,13). This then led to the recapitulation of the molecular events of the in vivo process of speciation by overexpressing two of the critical transcription factors (Pax8 and Nkx2-1) in mES cells (1,3). The induction of thyroid follicle formation required the concomitant overexpression of Pax8 and Nkx2-1 (1,3).
Once a successful approach was found with mES cells, it was logical to apply the same technique to hES cells. However, the culture of hES cells requires greater sophistication in their care, and such cells are less receptive to transfection (14,15). hES cells can, however, be transduced with lentiviral vectors with high efficiency for the maintenance of stable expression (16,17), and there was success in expressing PAX8 and NKX2-1 in the H9 line of hES cells. Exposure to Activin A then induced the development of an endodermal phenotype, as evidenced by the induction of SOX17 and FOXA2 expression (Fig. 3). It was then found that TSH was capable of inducing TG expression in the PAX8+/NKx2-1+ cells, and these cells gained the potential for forming thyroid neofollicles. These cells not only gained the ability to form three-dimensional neofollicles, but also expressed the TSHR and NIS on their basolateral surfaces, and intracytoplasmic and intraluminal TG. These results with hES cells indicate that the developmental stage of the cell and its progenitor program are equally important for full differentiation of thyroid follicles.
The simultaneous expression of PAX8 and NKX2-1 in developing thyroid cells first suggested the existence of a functional interaction between these two transcription factors. Accordingly, it was soon demonstrated that PAX8 and NKX2-1 associate biochemically and synergistically to activate transcription from the TPO and the TG gene promoters (18). The minimal promoter of the TG gene spanning −170 bases upstream of the transcriptional start site has three binding sites for NKX2-1 and one for Pax8. Transcriptional regulation of TG within the TFCs is regulated by the direct binding of these factors to the proximal promoter site(s) and/or by enhancer elements such as the cAMP response element (CRE) located >1 kb distally to the minimal promoter. In addition to their important role in TG and TPO transcriptional regulation, these factors also have an intrinsic role in thyroid follicle formation. In the absence of Nkx2-1, Hhex, Pax8, or Foxe1, the murine thyroid anlage is correctly formed, but subsequent thyroid morphogenesis is severely impaired (8–11). Similarly, no follicle formation was observed in hES cells overexpressing PAX8 or NKX2-1 alone, even after differentiation, although the thyroid-specific markers were upregulated, suggesting that an interaction of these two factors drives precursor cells to form TFCs. As has been observed, using mES cells, it is clear that the hES cells can also be induced to recapitulate the same developmental pathway when transduced with these two transcription factors. The additional extrinsic factor(s) that are secreted by associated cells in vivo or added externally, and which can “jump start” any committed endodermal cells to differentiate into TFCs without the transfection approach, requires further definition. ES cells derived from a Nkx2-1GFP knock-in mouse can be driven into thyroid progenitor cells by inhibition of TGFβ and BMP signaling, followed by combinatorial stimulation of BMP and FGF signaling (19). Thus, it seems clear that once the cells are committed to an endoderm lineage, they can be induced to form TFCs by stimulation of second messengers that converge to activate a combination of endogenous transcription factors, resulting in the thyroid cell phenotype. A clearer understanding of this differentiation requires a systematic dissection of the pathways that control it by using in vitro gene knock out studies (via the CRISP-CAS approach), or well-controlled knockdown studies with siRNAs. However, using the transcriptional approach, it was found that a large majority of human double transfected ES cells are capable of forming thyroid follicles (Fig. 4). The same cells that were committed to form a follicular architecture also had resident, functional TSH receptors and NIS on their cell surface, which responded to TSH with cAMP generation and enhanced radioiodine uptake.
The studies on hES cells described in this report serve as proof of principal that hES calls can be differentiated into functional thyroid follicles by a transcriptional approach similar to mES cells, but such a differentiation response should also be successful without the need for transcription factor overexpression. Further development of the Activin, TSH, IGF1 approach (2,13), or the use of multiple growth factors (19) to induce PAX8 and NKX2-1 expression and function will likely succeed in obviating the need for introducing DNA into the human cells with its likely long-term disadvantages. However, such manipulations may need to vary with the different hES cell lines available.
In conclusion, the current study shows that hES cells, as previously shown for mES cells, can be coaxed to develop into functional TFCs by the simultaneous expression of PAX8 and NKX2-1, thus providing a much-needed resource for research and with therapeutic potential.
Acknowledgments
Supported in part by DK069713 from the National Institutes of Health and the VA Merit Review Program.
Author Disclosure Statement
The authors declare that no competing interests exist.
References
- 1.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M, Costagliola S.2012Generation of functional thyroid from embryonic stem cells. Nature 491:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma R, Latif R, Davies TF.2009Thyrotropin-independent induction of thyroid endoderm from embryonic stem cells by activin A. Endocrinology 150:1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma R, Latif R, Davies TF.2013Thyroid follicle formation and thyroglobulin expression in multipotent endodermal stem cells. Thyroid 23:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY.2006Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology 147:3007–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Felice M, Di Lauro R.2011Minireview: intrinsic and extrinsic factors in thyroid gland development: an update. Endocrinology 152:2948–2956 [DOI] [PubMed] [Google Scholar]
- 6.Fagman H, Nilsson M.2011Morphogenetics of early thyroid development. J Mol Endocrinol 46:R33–42 [DOI] [PubMed] [Google Scholar]
- 7.Dohan O, Gavrielides MV, Ginter C, Amzel LM, Carrasco N.2002Na(+)/I(−) symporter activity requires a small and uncharged amino acid residue at position 395. Mol Endocrinol 16:1893–1902 [DOI] [PubMed] [Google Scholar]
- 8.Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, Gonzalez FJ.1996The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 10:60–69 [DOI] [PubMed] [Google Scholar]
- 9.Mansouri A, Chowdhury K, Gruss P.1998Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet 19:87–90 [DOI] [PubMed] [Google Scholar]
- 10.De Felice M, Ovitt C, Biffali E, Rodriguez-Mallon A, Arra C, Anastassiadis K, Macchia PE, Mattei MG, Mariano A, Scholer H, Macchia V, Di Lauro R.1998A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet 19:395–398 [DOI] [PubMed] [Google Scholar]
- 11.Martinez Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RS.2000The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development 127:2433–2445 [DOI] [PubMed] [Google Scholar]
- 12.Sewell W, Lin RY.2014Generation of thyroid follicular cells from pluripotent stem cells: potential for regenerative medicine. Front Endocrinol (Lausanne) 5:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arufe MC, Lu M, Lin RY.2009Differentiation of murine embryonic stem cells to thyrocytes requires insulin and insulin-like growth factor-1. Biochem Biophys Res Commun 381:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew CG, Draper JS, Walsh J, Moore H, Andrews PW.2007Transient and stable transgene expression in human embryonic stem cells. Stem Cells 25:1521–1528 [DOI] [PubMed] [Google Scholar]
- 15.Yates F, Daley GQ.2006Progress and prospects: gene transfer into embryonic stem cells. Gene Ther 13:1431–1439 [DOI] [PubMed] [Google Scholar]
- 16.Moore JC, van Laake LW, Braam SR, Xue T, Tsang SY, Ward D, Passier R, Tertoolen LL, Li RA, Mummery CL.2005Human embryonic stem cells: genetic manipulation on the way to cardiac cell therapies. Reprod Toxicol 20:377–391 [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA.2003High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells 21:111–117 [DOI] [PubMed] [Google Scholar]
- 18.Altmann A, Schulz RB, Glensch G, Eskerski H, Zitzmann S, Eisenhut M, Haberkorn U.2005Effects of Pax8 and TTF-1 thyroid transcription factor gene transfer in hepatoma cells: imaging of functional protein-protein interaction and iodide uptake. J Nucl Med 46:831–839 [PubMed] [Google Scholar]
- 19.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN.2012Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell 10:398–411 [DOI] [PMC free article] [PubMed] [Google Scholar]