Abstract
Sestrin2 is involved in a different cellular response to stress conditions. However, the function of Sestrin2 in the cardiovascular system remains unknown. In the present study, we tested whether Sestrin2 has a beneficial effect on macrophage cell apoptosis induced by oxidized low-density lipoprotein (oxLDL). We found that oxLDL induces expression of Sestrin2 in RAW264.7 cells in a time-dependent and dose-dependent manner. We also found that knockdown of Sestrin2 using small RNA interference promotes cell apoptosis and reactive oxygen species production induced by oxLDL. In addition, our results show that the c-Jun NH(2)-terminal kinase (JNK)/c-Jun pathway is activated by oxLDL. Inhibiting the activity of the JNK pathway abolishes the increase of Sestrin2 induced by oxLDL. These findings suggest that the inductive effect of Sestrin2 is mediated by the JNK/c-Jun pathway. Our results indicate that the induction of Sestrin2 acts as a compensatory response to oxLDL for survival, implying that stimulating expression of Sestrin2 might be an effective pharmacological target for the treatment of lipid-related cardiovascular diseases.
Introduction
Atherosclerosis (AS) is considered a major cause of cardiovascular diseases. It is characterized by the accumulation of cholesteryl esters, macrophages, and other elements in the intimal region (Parks and Lusis, 2013). Macrophages accumulate in atherosclerotic lesions and contribute to the initiation stage, development, progression, and complication phases of AS (Rousselle et al., 2013). Elevated reactive oxygen species (ROS) production by cells in atherosclerotic plaques, including macrophages, in individuals with hyperlipidemia, smoking, and diabetes mellitus promotes oxidative modification of phospholipids on low-density lipoprotein (LDL) particles, and oxidized LDL (oxLDL) indeed accumulates in arteries (Iwata and Aikawa, 2013). During the past 20 years, apoptosis in atherosclerotic lesions has been broadly reported. Macrophages, smooth muscle cells, and endothelial cells can undergo apoptosis in atherosclerotic lesions. Apoptosis in these cells may play different roles in atherogenesis because of the complexity of atherosclerotic plaque (Lusis, 2000). Although macrophages are relatively long-lived cells, it is considered that a finite incidence of macrophage apoptosis occurs during AS. Some studies indicated that macrophage apoptosis played a protective role in the development of AS by genetic engineering in mice (Liu et al., 2005; Tabas, 2005). Although foam cell apoptosis has long been hypothesized to contribute to the development of AS, the mechanism of macrophage apoptosis in AS remains unclear.
Sestrins are a family of stress-inducible proteins that are conserved across various species and regulate metabolic homeostasis (Budanov et al., 2004; Lee et al., 2013). In mammals, the Sestrin family comprises three members (Sestrin1–3), which can be induced by oxidative stress and DNA damage in a p53-dependent manner. Sestrin2 is most rigorously characterized in the liver and adipose tissue (Lee et al., 2012; Bae et al., 2013). In fact, Sestrin2 plays a key role in p53-dependent antioxidant defenses through regeneration of peroxiredoxins and inhibition of the target of rapamycin complex-1 (TORC1) anabolic pathway (Budanov and Karin, 2008). Additionally, Sestrins have antioxidant properties through inhibition of intracellular ROS. Importantly, Sestrin2 is also expressed in endothelial cells and macrophage cells, where it displays a protective role (Essler et al., 2009; Eid et al., 2013). However, the mechanism of protection by Sestrin2 in macrophages is not clearly understood. The goal of this study is to investigate the role of Sestrin2 on oxLDL-induced cell apoptosis in macrophages and its underlying mechanisms. We report that inhibition of Sestrin2 increases oxLDL-induced apoptosis and ROS production in RAW264.7 macrophages.
Materials and Methods
Materials
Medium and fetal bovine serum were purchased from HyClone. oxLDL was from Alfa Aesar, and the TBARS content of oxLDL was ranging from 5 to 15 nmol MDA/mg cholesterol. The primary antibodies of caspase-3, cleaved caspase-3, phospho-c-Jun NH(2)-terminal kinase (p-JNK) (Tyr185), JNK, p-c-Jun (Ser63), and c-Jun were from Cell Signaling Technology, and the antibodies against Sestrin2 and GAPDH were obtained from Sigma.
Cell culture, treatment, and transfection
The RAW264.7 macrophages were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). RAW264.7 cells were maintained in DMEM (HyClone) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C under 5% CO2. oxLDL was used to treat RAW264.7 cells for various doses and periods of time in an effort to detect alterations in cell apoptosis and Sestrin2 expression in response to oxLDL treatment. RAW264.7 cells were treated with oxLDL at different concentrations and time intervals. The small interfering RNA (siRNA) targeting Sestrin2 RNA sequence purchased from Sigma was found to downregulate Sestrin2 expression as evaluated by western blot. Cells were transfected with either Sestrin2 siRNA or nonspecific siRNA using Lipofectamine 2000 reagent according to the instructions of the manufacturer (Invitrogen).
Sestrin2 expression using western blot analysis
RAW264.7 cells were washed in cold phosphate-buffered saline (PBS) thrice and lysed in RIPA buffer supplemented with complete protease inhibitor for 15 min at 4°C. Protein concentrations in every group were detected using a BCA protein assay kit (Thermo Scientific Pierce). SDS-PAGE immunoblotting was used to quantify expression of Sestrin2, caspase-3, cleaved caspase-3, p-JNK, JNK, p-c-Jun, c-Jun, and GAPDH. Electrophoresis and immunoblotting were employed as described previously (Xie et al., 2011a, 2011b).
Apoptosis detection by FACS
RAW264.7 cells were incubated with oxLDL at different concentrations and time intervals. The cells were then collected and incubated with phycoerythrin (PE)-Annexin-V (BD Biosciences), resuspended in PBS (2% paraformaldehyde), and detected by FACS. The percentage of PE-Annexin-V-positive cells was quantified as the degree of apoptosis.
Caspase-3 activity assay
After incubation with oxLDL, RAW264.7 cells were then lysed with passive lysis buffer (Promega), and the clear supernatant was used for caspase-3 activity assay after centrifugation according to the manufacturer's instructions. The BCA protein assay kit was used to detect protein concentration of the lysate, and the relative caspase-3 activities were normalized to protein concentration.
ROS assay
Intracellular ROS levels were detected using the 5-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) fluorescence probe, which converts to dichlorofluorescein (DCF) after interaction with intracellular ROS. RAW264.7 cells were washed thrice with PBS, incubated with CM-H2DCFDA (25 μM) in PBS for 1 h, and then washed thrice with PBS. The spectrofluorometer was used to detect cell fluorescence intensity.
Quantitative real-time polymerase chain reaction
Total RNA was extracted using TriZol (Invitrogen), and cDNA fragments were generated by reverse transcription. Briefly, a total of 100 ng of cDNA was used in the real-time polymerase chain reaction (RT-PCR) to measure mRNA expression. GAPDH was performed in the same reaction on all samples tested as an internal control for variations in the amount of RNA. The primer sequences used for the amplification were designed using the Primer 5.0 software. The primer sequences (mouse) for Sestrin2 were forward 5′-GGCGGTGGTGATGGGTCTAC-3′ and reverse 5′-GACGACCCGGAAGTGGCCC-3′ (NM_144907.1, 218bp), and for GAPDH were forward 5′-CGGCAAATTCAACGGCACAGT-3′ and reverse 5′-GTTTTCCCAGTAGT AGAGGCGG-3′ (NM_008084.3, 211bp). All reactions followed the typical sigmoidal reaction profile, and the cycle threshold was used as a measurement of amplicon abundance as described previously (Xie et al., 2011a, 2001b).
Statistical analysis
GraphPad Prism software was used for statistical analysis. Numerical variables with normal distribution were compared with the unpaired t-test. Non-normal distribution data were compared with the Wilcoxon rank sum test. Data are expressed as mean±SEM. A value of p<0.05 was considered statistically significant.
Results
The effect of oxLDL on cell apoptosis
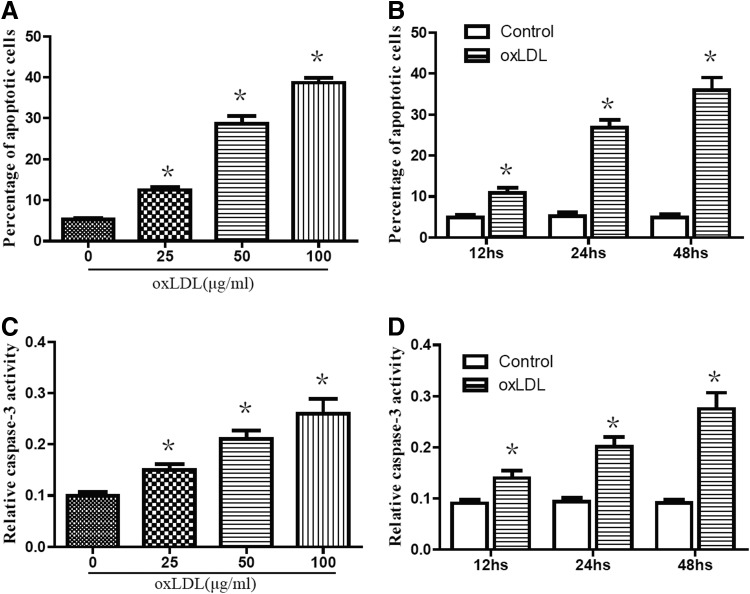
The effect of oxLDL on cell apoptosis was studied using FACS analysis with PE-Annexin-V staining. Treatment of RAW264.7 cells with oxLDL for 24 h at concentrations of 0, 25, 50, and 100 μg/mL resulted in a significant increase in cell apoptosis compared with controls (Fig. 1A). At the same time, cell apoptosis gradually increased after incubation with 50 μg/mL oxLDL for various periods of time ranging from 0 to 48 h (Fig. 1B). In addition, we investigated the change of caspase-3 activity in the presence of oxLDL. Our results indicated that treatment with oxLDL in RAW264.7 cells led to a significant increase in caspase-3 activity (Fig. 1C, D).
FIG. 1.
Oxidized low-density lipoprotein (oxLDL) treatment increased cell apoptosis in RAW264.7 cells. (A, C) RAW264.7 cells were stimulated with oxLDL at various concentrations for 24 h. (B, D) RAW264.7 cells were stimulated with oxLDL at indicated doses (50 μg/mL) for varying periods of time. Cell apoptosis was determined by FACS analysis with PE-Annexin-V staining. Caspase-3 activities were measured as described in the Materials and Methods section. Data are presented as the mean±SEM of at least three independent experiments. *p<0.05 versus nontreated control.
oxLDL induces the expression of Sestrin2 in RAW264.7 cells
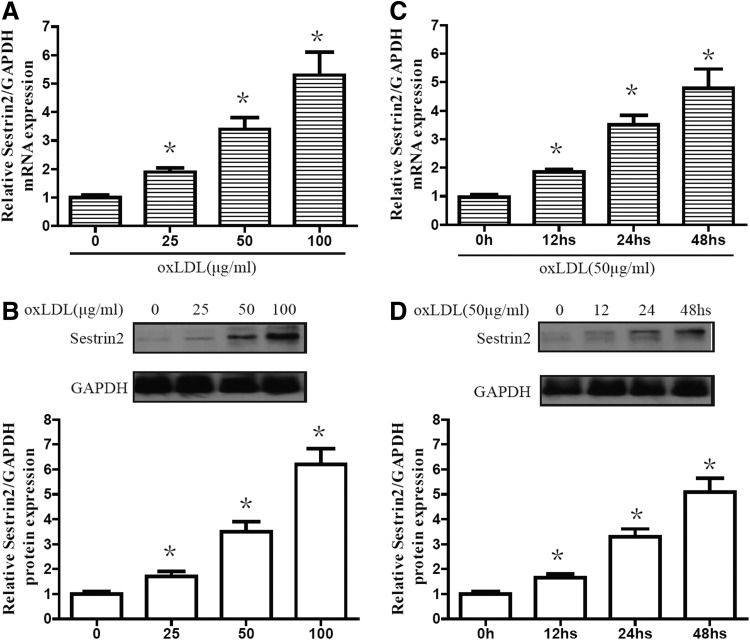
We investigated the effects of oxLDL treatment on Sestrin2 expression in RAW264.7 cells. Cells were incubated with oxLDL for various doses and periods of time. Expression of Sestrin2 at mRNA levels was determined by RT-PCR, and western blot analysis was used to detect Sestrin2 expression at protein levels. As shown in Figure 2A and B, at concentrations of 0, 25, 50, and 100 μg/mL, 24-h oxLDL treatment on RAW264.7 cells led to a sustainable increase in mRNA and protein levels of Sestrin2. Moreover, RAW264.7 cells were incubated with 50 μg/mL oxLDL for various periods of time ranging from 0 to 48 h. The results showed a sustainable increase of Sestrin2 at both mRNA (Fig. 2C) and protein levels in a time-dependent manner (Fig. 2D). The increased levels of Sestrin2 suggest a potential role of Sestrin2 in the treatment of RAW264.7 cells suffering from oxLDL insults.
FIG. 2.
oxLDL induced the expression of Sestrin-2 in RAW264.7 cells. (A, B) RAW264.7 cells were stimulated with oxLDL at various concentrations for 24 h. (C, D) RAW264.7 cells were stimulated with oxLDL at indicated doses (50 μg/mL) for varying periods of time. Protein levels of Sestrin-2 were determined by western blot analysis. mRNA levels of Sestrin-2 were determined by real-time polymerase chain reaction (RT-PCR). Data are presented as the mean±SEM of at least three independent experiments. *p<0.05 versus nontreated control.
Sestrin2 downexpression increases oxLDL-induced apoptosis and ROS production
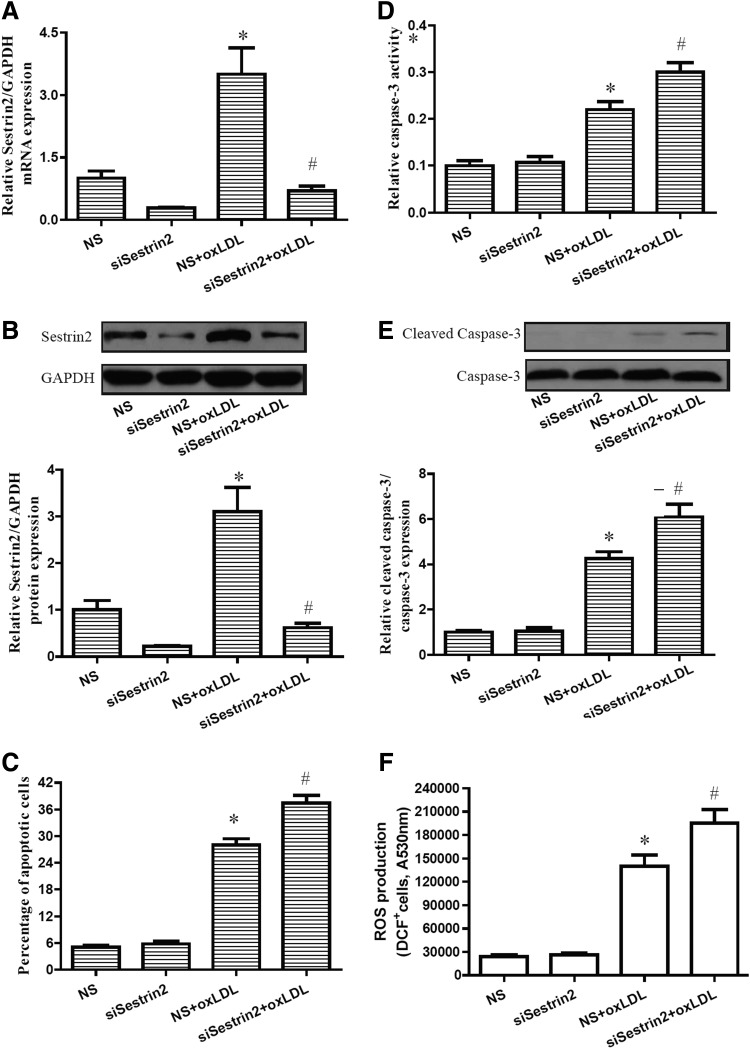
With further investigation, we studied the roles of Sestrin2 in oxLDL insults. The effects of Sestrin2 on cell apoptosis and ROS production induced by oxLDL were examined through inhibition of Sestrin2 using Sestrin2 small RNA interference in RAW264.7 cells. Our results indicate that transfection with Sestrin2 siRNA downregulates expression of Sestrin2 in both control and oxLDL-treated cells (Fig. 3). oxLDL has also been shown to induce apoptosis in multiple cell lines. To determine whether Sestrin2 has a direct effect on apoptosis, cell apoptosis and ROS production were detected following Sestrin2 knockdown and oxLDL treatment. As is shown in Figure 3, inhibition of Sestrin2 significantly promotes apoptosis and ROS production in RAW264.7 cells induced by oxLDL treatment. Caspase-3 is a critical executioner of apoptosis. Our results indicate that inhibition of Sestrin2 exacerbates the effects of oxLDL on the activation of caspase-3 (Fig. 3).
FIG. 3.
Sestrin2 downexpression induced apoptosis and reactive oxygen species (ROS) production of RAW264.7 cells in presence of oxLDL. RAW264.7 cells were transfected with NS or siSestrin2. At 48 h after the transfection, cells were treated with 50 μg/mL oxLDL for 24 h. (A, B) Expression of Sestrin-2 was determined by RT-PCR and western blot analysis. (C) FACS analysis with PE-Annexin-V staining revealed that Sestrin2 downexpression induced apoptosis. (D) Caspase-3 activities were measured as described in the Materials and Methods section. (E) Cleaved caspase-3 expression was detected by western blot analysis. (F) Intracellular ROS production was determined by the fluorescence probe 5-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA). Data are presented as the mean±SEM of at least three independent experiments. NS nonspecific RNA, siSestrin2 Sestrin2 small RNA interference. *p<0.05 versus NS control; #p<0.05 versus NS+oxLDL group.
JNK/c-Jun mediates oxLDL-induced upregulation of Sestrin2
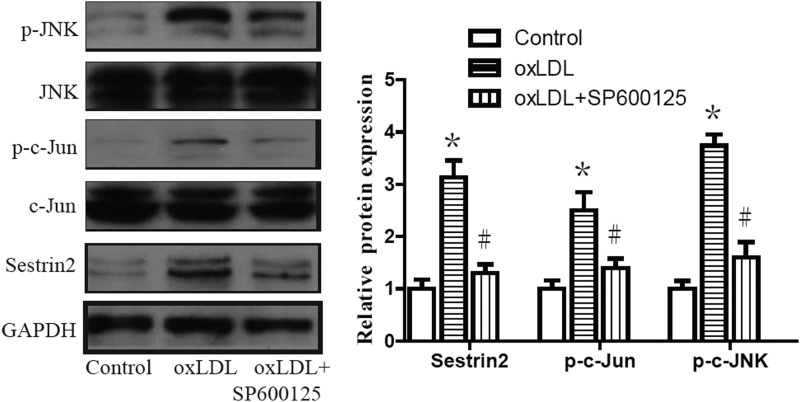
It has been shown that different forms of stress can mediate JNK activation through various cellular pathways. JNK activation has been reported to participate in regulating apoptosis (Hu et al., 2006) and induction of Sestrin2 in the presence of AngII (Yi et al., 2014). To examine whether JNK/c-Jun mediates oxLDL-induced induction of Sestrin2, expression patterns of JNK and c-Jun were investigated in RAW264.7 cells. Our results indicate that exposure to oxLDL significantly increases phospho-c-Jun as well as p-JNK (Fig. 4). To verify whether activated c-Jun is involved in oxLDL-induced induction of Sestrin2 expression, RAW264.7 cells were treated with 25 nM of JNK inhibitor SP600125 (Yi et al., 2014). Our results reveal that oxLDL-induced upregulation of Sestrin2 expression is blunted by SP600125 (Fig. 4), thereby indicating that oxLDL-induced Sestrin2 expression is mediated by activation of the JNK/c-Jun signaling pathway.
FIG. 4.
c-Jun NH(2)-terminal kinase (JNK)/c-Jun mediates oxLDL-induced upregulation of Sestrin2. RAW264.7 cells were treated with oxLDL with or without the presence of 25 nM of JNK inhibitor SP600125 for 24 h. Western blot analysis revealed that the levels of p-JNK, p-c-Jun, and Sestrin2 are increased by oxLDL. Data are presented as the mean±SEM of at least three independent experiments. *p<0.05 versus nontreated control; #p<0.05 versus oxLDL-treated group.
Discussion
Sestrin2 is a member of the Sestrin family of PA26-related proteins. The Sestrin family of proteins was originally identified as critical antioxidant proteins that contribute to the recycling of peroxiredoxins (Budanov et al., 2004; Ro et al., 2014). Sestrins are transcriptionally induced by oxidative stress (Budanov et al., 2002), and Sestrin proteins are important for cell growth and survival under oxidative stress (Budanov et al., 2002, 2004; Lipton, 2008; Papadia et al., 2008). Although Sestrins are not oxidoreductases (Woo et al., 2009), they can regulate antioxidant defense by promoting activities of other oxidoreductases, such as sulfiredoxin, which has been demonstrated in vitro and in vivo (Budanov et al., 2004; Lipton, 2008; Nogueira et al., 2008; Papadia et al., 2008; Bae et al., 2013). Additionally, Sestrin-induced activation of AMPK and inhibition of TORC1 may be involved in reducing ROS production through increasing mitochondrial respiration efficiency (Bonawitz et al., 2007; Budanov and Karin, 2008; Zid et al., 2009). However, its mode of action has yet to be elucidated. Under normal conditions, expression of Sestrin2 is found to take place in macrophage cells, and the decrease of Sestrin2 expression does not affect its survival in normal cells. Sestrin2 may be only involved in cellular responses to different stress conditions and is able to maintain redox homeostasis (Fig. 3). However, alterations in Sestrin2 expression in the process of macrophage dysfunction are not yet understood. The present study demonstrates that induction of Sestrin2 in RAW264.7 cells might act as a compensatory response for cell survival when cells are subjected to oxLDL insult. In this investigation, we find that Sestrin2 levels are increased by oxLDL in both a time-dependent and dose-dependent manner (Figs. 1 and 2). Inhibition of Sestrin2 by small RNA interference promoted cell apoptosis and ROS production induced by oxLDL (Fig. 3). Recent data also indicate that Sestrin2 induction may be as a compensatory response under stressful conditions (Park et al., 2014; Yi et al., 2014). Interestingly, we find that the activation of the JNK/c-Jun pathway involves the upregulation of Sestrin2 induced by oxLDL (Fig. 4).
The JNK pathway can be activated by various stressor signals such as cytokines and oxidative stress. Activation of JNK is implicated in oxLDL-induced cell apoptosis (Schroeter et al., 2001) (Supplementary Fig. S1; supplementary materials are available online at http://www.liebertpub.com/dna). Importantly, activation of JNK has been reported to play an important role in the regulation of vascular tone (Zhou et al., 2010), and the JNK pathway involves the upregulation of Sestrin2 (Zhang et al., 2013; Yi et al., 2014). In this study, we report that activation of JNK/c-Jun mediates induction of Sestrin2, which expands our understanding of the role of JNK in oxLDL-induced apoptosis (Fig. 4). Lee's results indicated that Sestrin2 expression induced upon hypernutrition maintains metabolic homeostasis in the liver of obese mice. Sestrin2 ablation exacerbates obesity-induced mTORC1-S6K activation, glucose intolerance, insulin resistance, and hepatosteatosis, however, all of which are reversed by AMPK activation. Furthermore, concomitant ablation of Sestrin2 and Sestrin3 provokes hepatic mTORC1-S6K activation and insulin resistance even in the absence of nutritional overload and obesity. So, the stress-inducible Sestrin family plays an important homeostatic role in lipid and glucose metabolism (Lee et al., 2012). Importantly, Sestrin2-dependent AMP-activated protein kinase (AMPK) activation has been reported to attenuate high-glucose-induced glomerular mesangial cell fibronectin synthesis through blockade of ROS and peroxynitrite generation, suggesting a potential target for intervention of diabetes (Eid et al., 2013).
In this study, oxLDL treatment was able to upregulate expression of Sestrin2. Furthermore, inhibition of Sestrin2 expression exacerbated oxLDL-induced cell apoptosis and ROS production. These findings suggest that induction of Sestrin2 has a protective effect on oxLDL-induced cell apoptosis and ROS production. Continuing efforts are being made to understand the mechanisms through which oxLDL acts as a mediator of macrophage apoptosis. Prevention of oxLDL toxicity has become an important potential target for the treatment of cardiovascular diseases. Our study implies that changing expression of Sestrin2 might be an effective pharmacological target for the treatment of lipid-related cardiovascular diseases.
Supplementary Material
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (81370974).
Disclosure Statement
No competing financial interests exist.
References
- Bae S.H., Sung S.H., Oh S.Y., Lim J.M., Lee S.K., Park Y.N., Lee H.E., Kang D., and Rhee S.G. (2013). Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab 17,73–84 [DOI] [PubMed] [Google Scholar]
- Bonawitz N.D., Chatenay-Lapointe M., Pan Y., and Shadel G.S. (2007). Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 5,265–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov A.V., and Karin M. (2008). p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 134,451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov A.V., Sablina A.A., Feinstein E., Koonin E.V., and Chumakov P.M. (2004). Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304,596–600 [DOI] [PubMed] [Google Scholar]
- Budanov A.V., Shoshani T., Faerman A., Zelin E., Kamer I., Kalinski H., Gorodin S., Fishman A., Chajut A., Einat P., Skaliter R., Gudkov A.V., Chumakov P.M., and Feinstein E. (2002). Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene 21,6017–6031 [DOI] [PubMed] [Google Scholar]
- Eid A.A., Lee D.Y., Roman L.J., Khazim K., and Gorin Y. (2013). Sestrin 2 and AMPK connect hyperglycemia to Nox4-dependent endothelial nitric oxide synthase uncoupling and matrix protein expression. Mol Cell Biol 33,3439–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler S., Dehne N., and Brune B. (2009). Role of sestrin2 in peroxide signaling in macrophages. FEBS Lett 583,3531–3535 [DOI] [PubMed] [Google Scholar]
- Hu B., Jarzynka M.J., Guo P., Imanishi Y., Schlaepfer D.D., and Cheng S.Y. (2006). Angiopoietin 2 induces glioma cell invasion by stimulating matrix metalloprotease 2 expression through the alphavbeta1 integrin and focal adhesion kinase signaling pathway. Cancer Res 66,775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H., and Aikawa M. (2013). Liver-artery interactions via the plasminogen-CD36 axis in macrophage foam cell formation: new evidence for the role of remote organ crosstalk in atherosclerosis. Circulation 127,1173–1176 [DOI] [PubMed] [Google Scholar]
- Lee J.H., Budanov A.V., and Karin M. (2013). Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab 18,792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Budanov A.V., Talukdar S., Park E.J., Park H.L., Park H.W., Bandyopadhyay G., Li N., Aghajan M., Jang I., Wolfe A.M., Perkins G.A., Ellisman M.H., Bier E., Scadeng M., Foretz M., Viollet B., Olefsky J., and Karin M. (2012). Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab 16,311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S.A. (2008). NMDA receptor activity regulates transcription of antioxidant pathways. Nat Neurosci 11,381–382 [DOI] [PubMed] [Google Scholar]
- Liu J., Thewke D.P., Su Y.R., Linton M.F., Fazio S., and Sinensky M.S. (2005). Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol 25,174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusis A.J. (2000). Atherosclerosis. Nature 407,233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira V., Park Y., Chen C.C., Xu P.Z., Chen M.L., Tonic I., Unterman T., and Hay N. (2008). Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell 14,458–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S., Soriano F.X., Leveille F., Martel M.A., Dakin K.A., Hansen H.H., Kaindl A., Sifringer M., Fowler J., Stefovska V., McKenzie G., Craigon M., Corriveau R., Ghazal P., Horsburgh K., Yankner B.A., Wyllie D.J., Ikonomidou C., and Hardingham G.E. (2008). Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci 11,476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.W., Park H., Ro S.H., Jang I., Semple I.A., Kim D.N., Kim M., Nam M., Zhang D., Yin L., and Lee J.H. (2014). Hepatoprotective role of Sestrin2 against chronic ER stress. Nat Commun 5,4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks B.W., and Lusis A.J. (2013). Macrophage accumulation in atherosclerosis. N Engl J Med 369,2352–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S.H., Nam M., Jang I., Park H.W., Park H., Semple I.A., Kim M., Kim J.S., Einat P., Damari G., Golikov M., Feinstein E., and Lee J.H. (2014). Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc Natl Acad Sci U S A 111,7849–7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle A., Qadri F., Leukel L., Yilmaz R., Fontaine J.F., Sihn G., Bader M., Ahluwalia A., and Duchene J. (2013). CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest 123,1343–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H., Spencer J.P., Rice-Evans C., and Williams R.J. (2001). Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem J 358,547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. (2005). Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol 25,2255–2264 [DOI] [PubMed] [Google Scholar]
- Woo H.A., Bae S.H., Park S., and Rhee S.G. (2009). Sestrin 2 is not a reductase for cysteine sulfinic acid of peroxiredoxins. Antioxid Redox Signal 11,739–745 [DOI] [PubMed] [Google Scholar]
- Xie H., Sun M., Liao X.B., Yuan L.Q., Sheng Z.F., Meng J.C., Wang D., Yu Z.Y., Zhang L.Y., Zhou H.D., Luo X.H., Li H., Wu X.P., Wei Q.Y., Tang S.Y., Wang Z.Y., and Liao E.Y. (2011a). Estrogen receptor alpha36 mediates a bone-sparing effect of 17beta-estrodiol in postmenopausal women. J Bone Miner Res 26,156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Xie P.L., Wu X.P., Chen S.M., Zhou H.D., Yuan L.Q., Sheng Z.F., Tang S.Y., Luo X.H., and Liao E.Y. (2011b). Omentin-1 attenuates arterial calcification and bone loss in osteoprotegerin-deficient mice by inhibition of RANKL expression. Cardiovasc Res 92,296–306 [DOI] [PubMed] [Google Scholar]
- Yi L., Li F., Yong Y., Jianting D., Liting Z., Xuansheng H., Fei L., and Jiewen L. (2014). Upregulation of sestrin-2 expression protects against endothelial toxicity of angiotensin II. Cell Biol Toxicol 30,147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Wu X.Q., Deng R., Sun T., Feng G.K., and Zhu X.F. (2013). Upregulation of sestrin 2 expression via JNK pathway activation contributes to autophagy induction in cancer cells. Cell Signal 25,150–158 [DOI] [PubMed] [Google Scholar]
- Zhou M.S., Schulman I.H., Chadipiralla K., and Raij L. (2010). Role of c-Jun N-terminal kinase in the regulation of vascular tone. J Cardiovasc Pharmacol Ther 15,78–83 [DOI] [PubMed] [Google Scholar]
- Zid B.M., Rogers A.N., Katewa S.D., Vargas M.A., Kolipinski M.C., Lu T.A., Benzer S., and Kapahi P. (2009). 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139,149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.