Abstract
Background
Deregulated Ras/Raf/mitogen-activated protein kinase and PI3 K/AKT/mTOR signaling pathways are significant in hepatocellular carcinoma proliferation (HCC). In this study we evaluated differences in the antiproliferative effect of dual PI3 K/Akt/mTOR and Ras/Raf/mitogen-activated protein kinase inhibition of non liver cancer stem cell lines (PLC and HuH7) and liver cancer stem cell (LCSC) lines (CD133, CD44, CD24, and aldehyde dehydrogenase 1-positive cells).
Materials and methods
Flow cytometry was performed on the resulting tumors to identify the LCSC markers CD133, CD44, CD24, and aldehyde dehydrogenase 1. Methylthiazol tetrazolium assay was used to assess cellular proliferation. Finally, a Western blot assay was used to evaluate for inhibition of specific enzymes in these two signaling pathways.
Results
Using flow cytometry, we found that LCSC contain 64.4% CD133 + cells, 83.2% CD44 + cells, and 96.4% CD24 + cells. PKI-587 and sorafenib caused inhibiton of LCSC and HCC cell proliferation. PLC cells were more sensitive to PKI-587 than LCSC or Huh7 (P < 0.001). Interestingly, HuH7 cells were more sensitive to sorafenib than LCSC or PLC cells. Additionally, combination therapy with PKI-587 and sorafenib caused significantly more inhibition than monotherapy in HuH7, PLC, and LCSC. Using the methylthiazol tetrazolium assay, we found that the LCSC proliferation was inhibited with sorafenib monotherapy 39% at 5 μM (P < 0.001; n = 12) and 67% by PKI-587 at 0.1 μM (P = 0.002, n = 12) compared with control. The combination of PKI-587 and sorafenib, however, synergistically inhibited LCSC proliferation by 86% (P = 0.002; n = 12).
Conclusions
LCSC (CD133+, CD44+, CD24+) were able to develop very aggressive tumors with low cell concentrations at 4 to 6 wk. Cells CD133+, CD44+, CD24+ demonstrated at least moderate resistance to therapy in vitro. The combination of PKI-587 and sorafenib was better than either drug alone at inhibiting of LCSC and on HCC cell proliferation.
Keywords: Liver cancer stem cells, Sorafenib, PKI-587, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver, it has the fifth highest incidence of all cancers, and is the third most common cause of cancer-related deaths worldwide [1].
Recent publications indicate that HCC cell activation by different factors is known to involve several signaling pathways including the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway, the PI3 K/AKT/mTOR pathway, the WNT/β-Catenin pathway, the Hedgehog pathway and the Hippo tumor suppression pathway [2,3]. Among them, Ras/Raf/MAPK and PI3 K/AKT/mTOR pathways appear to be the most critical in development and proliferation of HCC and are the most extensively investigated. The Ras/Raf/MAPK pathway is typically activated in HCC as a result of both (1) increased signaling induced from upstream growth factors and (2) inactivation of tumor suppressor genes [4]. The PI3 K/AKT/mTOR signaling pathway also plays an important role in HCC proliferation and is activated in 30-50% of HCC. Despite numerous efforts, the exact mechanism of HCC tumorigenesis, progression and recurrence remains unclear.
There is an increasing interest among researchers on the cancer stem cells (CSC) concept. This theory suggests that a tumor contains a heterogeneous population of cells that form a distinct cellular hierarchy. Although the existence of CSC was first proposed over 40 years ago, it was not until 20 years ago that John Dick et al. were first able to demonstrate the role of stem cells in hematological malignancies [5]. Since then substantial evidence has emerged to support tumor heterogeneity and cellular hierarchy within a tumor in solid cancers. In recent times, studies have provided convincing evidence that these cells do exist in solid tumors of many types including, brain, breast, colorectal, liver, pancreas and prostate cancers [6].
In 2006, Chiba et al. extended the application of cell sorting to identify CSC in HCC [7]. During the same year, another group from Japan sorted CD133 + subpopulation from Huh7 cell line, in which they described increased proliferative and tumorigenic potential [8]. CD44 is also an important marker used in combination with other stem cell markers to better define the surface phenotype of liver cancer stem cell (LCSC). In fact, it has been reported that cells expressing either CD133 or CD90 in combination with CD44 are more aggressive than cells expressing isolated CD133 or CD90 markers [9].
Sorafenib is a multikinase inhibitor, which has been shown to inhibit tumor cell proliferation by blocking the Ras/Raf/MAPK signaling pathway. It also suppresses angiogenesis by blocking VEGFR and PDGFR signaling. Sorafenib exerts strong inhibitory activity against Raf-1 (C-Raf) kinase, B-Raf (wild-type B-Raf and mutant V600 E B-Raf) serine/threonine kinase, the proangiogenic RTKs VEGFR, PDGFR and FGFR1, and other tyrosine kinases including c-kit, Flt-3 and RET, all of which are involved in tumor progression and overall prognosis.
In the last few years, the novel drug PKI-587, a dual inhibitor of PI3 K and mTOR, has demonstrated potent inhibitory effects on several human cancer cell lines such as melanoma, glioma, lung, colon and breast cancers in pre-clinical studies. PKI-587 has been approved by the FDA and is currently being evaluated in a phase 1 clinical trial in patients with advanced cancer. PKI-587 is a chemically synthesized small molecule that specifically inhibits PI3 K class-IA as well as both mTOR complex 1 (mTORC1) and complex 2 (mTORC2).
Recently our group and others have demonstrated the effects of blocking several pathways, (alone and in combination), involved in activation and proliferation of HCC cells in vitro and in vivo [10]. The aim of this study was to evaluate tumorigenesis, and differences in the anti-proliferative effect of dual PI3 K/AKT/mTOR and Ras/Raf/MAPK inhibition in HCC and LCSC lines.
Materials and methods
Cell culture
The human HCC cell line, Huh7 and PLC, were cultured in Dulbecco’s modified Eagle medium (DMEM) (catalog number 12,100-046) medium (Invitrogen, Carlsbad, CA) +10% heat inactivated fetal bovine serum (FBS) in a 37° C incubator with 5% CO2. The LCSC (CelProgen catalog number 36,116-43, San Pedro, CA) were cultured in CelProgen LCSC growth media with serum.
Chemicals and antibodies
PKI-587 was a gift from Pfizer Inc (New York, NY). Other chemicals and antibodies were purchased as described previously [11].
Methylthiazol tetrazolium (MTT) assay
LCSC were plated in 96-well plates at 2500 cells/well (n = 12) in 100 μL of DMEM + 10% bovine serum and cultured for 24 h. Sorafenib, PKI-587, and the combination of both were then added to the cells and incubated in final volume of 200 μL of DMEM + 10% FBS for another 64 h. Carrier dimethyl sulfoxide (DMSO) was used as a vehicle control (<0.1% final concentration). The MTT assay was performed as previously described [11].
LCSC markers
After the LCSC were cultured in our lab, first passage cells were sent back to CelProgen (stem cell research and therapeutics) lab in California to be characterized by determining the LCSC markers (CD133, CD44, CD24, and aldehyde dehydrogenase-1) using flow cytometry.
Western-blot
Huh7 cells were cultured in DMEM + 10% FBS in 100 x 20 mm tissue culture dishes until about 70% confluence. The cells were treated with sorafenib (10 μM), PKI-587 (1 μM) for 1 h, then treated with epidermal growth factor (EGF) (6.5 nM) for 15 min. DMSO (<0.1%) were used as vehicle control. All the other procedures were performed as previously described [10].
Statistical analysis
All analyses were performed using the software SPSS v. 19 (SPSS Inc, Chicago, IL). Data are presented as mean ± SE. For nominal data, ANOVA followed by Tukey multiple range test was used; for two groups of continuous data, paired t-test was used. The level of statistical significance was set at P < 0.05.
Results
LCSC profile
LCSC markers
Flow cytometry analysis indicated that the LCSC contain 64.4% CD133 positive cells, 83.2% CD44 positive cells, 96.4% CD24 positive cells, and 96.9% aldehyde dehydrogenase-1 (ALDHA-1) positive cells.
PKI-587 and sorafenib monotherapy inhibition of LCSC and HCC cells proliferation detected by MTT assay
PKI-587 and sorafenib proved to be substantial inhibitors of LCSC and HCC proliferation. The results of the MTT assay on LCSC, HuH7, and PLC cells proliferation indicated the IC50 (50% inhibition concentration) of PKI-587 and sorafenib varied among the different cell types.
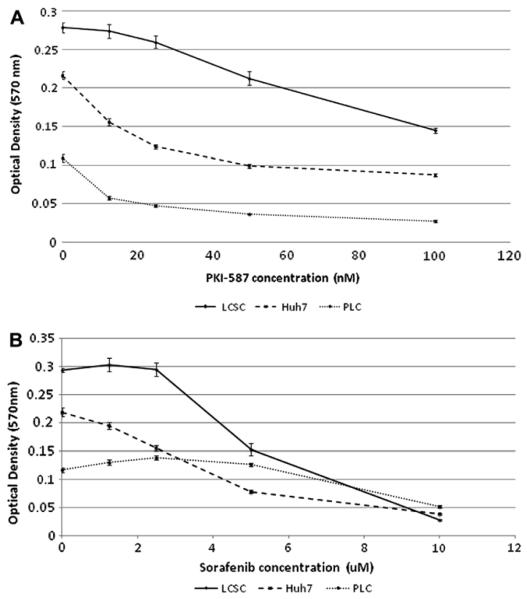
We found LCSC were more resistant to PKI-587 inhibition with an IC50 of 105 nM as compared to HuH7 (IC50 45 nM) and PLC (IC50 16.4 nM) (Fig. 1A). LCSC are more resistant to sorafenib than HuH7 but less resistant to sorafenib than PLC (Fig. 1B). The sorafenib IC50 to LCSC, Huh7, and PLC are 5.7, 3.9, and 9.6 μM, respectively. Sorafenib at 5 μM and PKI-587 at 0.1 μM as single agents significantly inhibited LCSC proliferation (P < 0.001).
Fig. 1.
PKI-587 (A) and sorafenib (B) monotherapy on inhibition of LCSC, Huh7, and PLC cells (MTT assay).
The combination of PKI-587 and sorafenib inhibited LCSC proliferation detected by MTT assay
We used MTT assay to determine if PKI-587 and sorafenib can inhibit LCSC over a longer period of treatment. LCSC were cultured in LCSC growth media with serum.
The cells were plated in a 96-well plate at 2500 cells/well (n = 12) in 100 uL of DMEM + 10% FBS and cultured for 72 h with DMSO (concentration 0.04%, control) and inhibitors at the indicated concentrations. The LCSC proliferation was inhibited (39%) with sorafenib monotherapy at 5 μM (P < 0.001; n = 12) and 67% by PKI-587 at 0.1 μM (P = 0.002) (n = 12) compared with control. We found that the combination of PKI-587 and sorafenib additively inhibited LCSC proliferation by 86% (P = 0.002; n = 12) (Fig. 2).
Fig. 2.
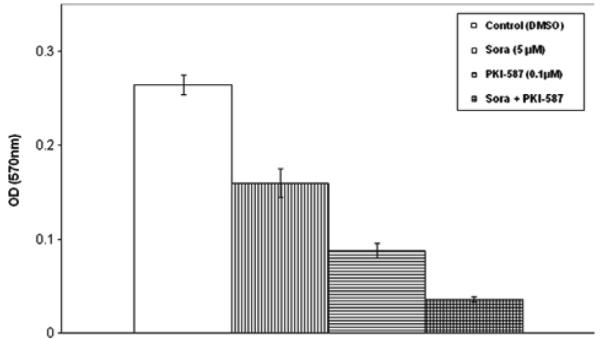
PKI-587 and sorafenib synergistically inhibited LCSC proliferation detected by MTT assay.
Western blot assay demonstrating inhibition of the key enzymes in ras/raf/MAPK and PI3 K/AKT/mTOR signaling pathways
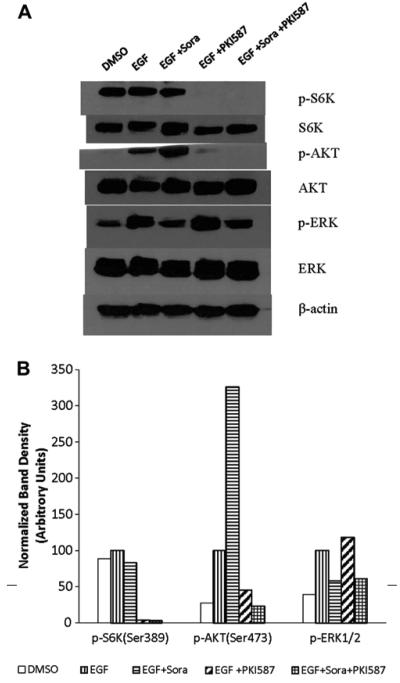
Our Western blot experiment shows that in comparison with DMSO vehicle control, EGF stimulated S6 K (Thr389), AKT (Ser473), and ERK1/2(Thr202/204) phosphorylation 11.3%, 72.8%, 61.3% respectively. Sorafenib at 10 μM inhibited EGF-stimulated ERK1/2 phosphorylation 42%. However sorafenib, as a single agent, slightly inhibited S6 K phosphorylation (16.7%) but did not inhibit EGF-stimulated AKT phosphorylation in the PI3 K/AKT/mTOR signaling pathway. Instead, sorafenib dramatically increased AKT phosphorylation 225.7% compared with EGF treatment. PKI-587 at 1 μM, as a single agent, inhibited EGF stimulated S6 K (Thr389) (marker for mTORC1 activity) and AKT (Ser473) (marker for mTORC2 activity) phosphorylation 96% and 55%, respectively, thus suggesting PKI-587 inhibits both mTORC1 and mTORC2 activity. PKI-587 increased EGF stimulated ERK phosphorylation 18%. The combination of PKI-587 and sorafenib strongly inhibited both Ras/Raf/MAPK and PI3 K/AKT/mTOR signaling pathways (Fig. 3).
Fig. 3.
Sorafenib and PKI-587 differentially inhibited or activated phosphorylation of several key enzymes in the Ras/Raf/MAPK and PI3 K/Akt/mTOR pathways. (A) Western blot for p-S6 K (Thr389), p-AKT (Ser473), and p-ERK1/2(Thr202/204) after different treatments. Also shown are endogenous S6 K, AKT, and ERK1/2. Phosphorylated kinase band densities were analyzed by Scion Image software (Frederick, MD) and normalized by β-actin. (B) A plot generated based on β-actin normalized phosphorylated kinase band densities in Figure 1A. The EGF treatment data are set at arbitrary units of 100 to compare with other treatments.
Discussion
The CSC theory suggests that a tumor contains a heterogeneous population of cells that form a distinct cellular hierarchy. Only a subset of cells within this tumor hierarchy has the ability to self-renew, differentiate into defined progenies and sustain tumor growth [12]. According to this hypothesis, it should be possible to develop new anticancer drugs that target the cells within the tumor responsible for tumor initiation and progression [13]. Substantial efforts have been made to further characterize and define CSC in HCC. CD133 was first found as a marker of primitive hematopoietic progenitor cells [14] it has since been found on endothelial progenitor cells, neuronal and glial stem cells, human fetal liver, and stem cells in cord blood and peripheral blood. CD133 has drawn increased attention as an important LCSC marker in the past 6 y. Suet-sugu et al. first suggested CD133 + HCC cells represent a potential LCSC subpopulation. These authors found that a sorted CD133 + subpopulation from a Huh7 cell line possessed higher proliferative and tumorigenic potential, and expressed lower levels of mature hepatocyte markers, such as glutamine synthetase and cytochrome P450 3A4, compared with their CD133-counterparts [8]. Yin et al. obtained similar findings when isolated a CD133 + fraction of cells from a SMMC-7721 cell line, demonstrating enhanced clonogenicity in vitro and tumorigenicity in vivo of this cell subtype [15].
Subsequent analysis of CD133 expression in a panel of human liver cells lines found that CD133 expression positively correlated with the cells line’s ability to initiate tumor formation in vivo. Compared with CD133-cells, CD133 + liver CSC were more resistant to radiation-induced apoptosis and exhibited greater proliferation in vitro and tumor initiation ability in vivo post-irradiation. In addition, these cells showed higher activation of MAPK/PI3 K signaling pathway and reduction in reactive oxygen species levels following radiation exposure [16].
LCSC are characterized by their tumorigenic potential, the presence of specific stem cell markers, and patterns of resistance to treatment.
Other surface markers, such as EpCAM, CD24, CD44, CD90, CD13, and OV6 have also been used to classify LCSC subsets [17]. Many of these markers have significant overlapping expression with CD133. Zhu et al. found the combination of CD133 and CD44 cell surface markers can more precisely define the LCSC subpopulation [18]. Compared with CD133 + CD44 counterparts, CD133 + CD44 + HCC cells isolated from SMMC-7721, MHCC-LM3, and MHCC-97 L cell lines were found to have even greater ability to initiate tumor formation when injected subcutaneously in immunodeficient mice, to self-renew in serial passages in vivo and to form colonies on anchorage-independent assays. Irene Ng et al. identified a metastatic LCSC subpopulation using CD24 + CD133 + surface phenotypes [19]; this and all the other findings reported to date, highlight the importance of identifying these markers to define a specific primary and metastatic LCSC subpopulation.
In order to determine that these cells still preserve their markers, after being cultured in our laboratory, we re-checked the cells for CD133, CD24, and CD44. The cells were also compared with other cells from the same batch to determine tumor marker expression. These cells continue to demonstrate the same profile and were positive for CD133, CD44, CD24, and aldehyde dehydrogenase-1 on flow cytometry. LCSC with similar profile (CD133+, CD44+, CD24+) have been associated with rapid tumor growth and progression and the presence of metastases [18-20].
Recently, our group demonstrated that blockage/inhibition of only one of the main pathways PI3 K/mTOR or Ras/Raf/MAPK, separately, can result in activation of the other pathway in regular, non-CSC, HCC cell line Huh7 [10,11]. This could at least partially explain why combined inhibition of these two pathways produces a more significant inhibition of HCC cell proliferation.
The implications of dual inhibition of PI3 K and mTOR, (key enzymes of the PI3 K/AKT/mTOR signaling pathway) to treat HCC has been published previously. Our group and others have reported an additive effect using dual PI3 K/mTOR and Ras/Raf/MAPK inhibition (with sorafenib) in other HCC non-stem cell lines. Due to the potency, bioavailability, and biosafety profile of PKI-587 and its recent approval by FDA in a phase 1 clinical trial, we decided to further study this drug and its inhibitory effects in combination with sorafenib on LCSC and compare with other HCC (non-stem cell) cell lines.
Initially, we tested potency by comparing PKI-578 and sorafenib monotherapy on LCSC and HCC cell lines Huh7 and PLC. The results of the MTT assay on LCSC, Huh7, and PLC cells proliferation indicated the IC50 of PKI-587 and sorafenib varied among the different cell types. PLC cells were most sensitive to PKI-587, with an IC50 of 16.40 nM (P < 0.001), compared with LCSC and HuH7 cells, with an IC50 of 105 nM (P < 0.001) and 45 nM (P < 0.001), respectively. Interestingly, HuH7 cells were more sensitive to sorafenib, with an IC50 of 3.8 uM (P < 0.001), compared with LCSC and PLC cells, with an IC50 of 4.5uM (P < 0.001) and 9.6 uM (P < 0.001) respectively. Combination therapy with PKI-587 and sorafenib on LCSC caused significantly stronger inhibition than monotherapy. Using the MTT assay we found that the LCSC proliferation was inhibited 39% with sorafenib monotherapy at 5 μM (P < 0.001; n = 12) and 67% by PKI-587 at 0.1 μM (P = 0.002, n =12) compared with control. The combination of PKI-587 and sorafenib synergistically inhibited LCSC proliferation by 86% (P = 0.002; n = 12). According to the CSC hypothesis, cancer initiation, progression, recurrence, metastasis and therapy resistance are unique properties implicit in CSC subsets. Focusing on targeting CSC could have important therapeutic implications in future HCC treatment. Current strategies are focused on targeting rapidly proliferating tumor cells. Treatment may initially seem to be successful, but often fails to provide a long lasting cure of the disease. We believe these are very heterogeneous cancers with different cell lines with significant different sensitivities. The failure to eradicate the CSC population is believed to be the root of disease recurrence and tumor progression [21]. We demonstrated that although monotherapy is effective, the patterns of response are different between different cell lines, both stem cell lines and non-LCSC lines. We showed that PKI-587 and sorafenib combination is superior to monotherapy in all HCC and LCSC lines (CD133+, CD44, and CD 24+) that were tested.
We performed Western blot assay to prove inhibition of specific key enzymes in the Ras/Raf/MAPK and PI3 K/AKT/mTOR signaling pathways. Our western blot experiment shows that sorafenib at 10 μM inhibited EGF-stimulated ERK1/2 phosphorylation 42%. Sorafenib, as a single agent, slightly inhibited S6 K phosphorylation (16.7%) but did not inhibit EGF-stimulated AKT phosphorylation in the PI3 K/AKT/mTOR signaling pathway as expected. Instead, sorafenib dramatically stimulated AKT phosphorylation 225.7% compared with EGF treatment alone. PKI-587 at 1 μM, as a single agent, inhibited EGF stimulated S6 K (marker for mTORC1 activity) and AKT (marker for mTORC2 activity) phosphorylation 96% and 55% respectively, thus suggesting that PKI-587 inhibits both mTORC1 and mTORC2 activity. The combination of PKI-587 and sorafenib strongly inhibited both Ras/Raf/MAPK and PI3 K/AKT/mTOR signaling pathways (Fig. 3).
In conclusion, our LCSC demonstrated moderate to significant resistance to therapy compared with other non-LCSC lines (HuH7 and PLC). Combination therapy targeting PI3 K/AKT/mTOR and ras/raf/MAPK pathways using PKI-587 and sorafenib caused additive inhibitory effect on both LCSC and non-stem cell lines highlighting again the importance that could have targeting different signaling pathways in HCC treatment.
Acknowledgment
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- [1].Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- [2].Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lachenmayer A, Hoshida Y, Llovet JM. Hippo tumor supressor pathway: novel implications for the treatment of hepatocellular carcinoma. Gastroenterology. 2010;139:692. doi: 10.1053/j.gastro.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Strumberg D. Preclinical and clinical development of the oral multikinase inhibitor sorafenib in cancer treatment. Drugs Today. 2005;41:773. doi: 10.1358/dot.2005.41.12.937959. [DOI] [PubMed] [Google Scholar]
- [5].Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- [6].Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- [7].Chiba T, Kita K, Zheng Y-W, et al. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240. doi: 10.1002/hep.21227. [DOI] [PubMed] [Google Scholar]
- [8].Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- [9].Tong CM, Ma S, Guan X-Y. Biology of hepatic cancer stem cells. J Gastroenterol Hepatol. 2011;26:1229. doi: 10.1111/j.1440-1746.2011.06762.x. [DOI] [PubMed] [Google Scholar]
- [10].Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Evers BM. PKI-587 and sorafenib targeting PI3K/AKT/mTOR and Ras/Raf/MAPK pathways synergistically inhibit HCC cell proliferation. J Surg Res. 2012;176:542. doi: 10.1016/j.jss.2011.10.045. [DOI] [PubMed] [Google Scholar]
- [11].Gedaly R, Angulo P, Hundley J, et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:495. [PMC free article] [PubMed] [Google Scholar]
- [12].Lobo NA, Shimono Y, Qian D, Clarke MF. The Biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- [13].Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002. [PubMed] [Google Scholar]
- [15].Yin S, Li J, Hu C, et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- [16].Piao LS, Hur W, Kim T-K, et al. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- [17].Ji J, Wang XW. Clinical implications of cancer stem cell biology in hepatocellular carcinoma. Semin Oncol. 2012;39:461. doi: 10.1053/j.seminoncol.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhu Z, Hao X, Yan M, et al. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067. doi: 10.1002/ijc.24868. [DOI] [PubMed] [Google Scholar]
- [19].Lee TKW, Castilho A, Cheung VCH, Tang KH, Ma S, Ng IOL. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- [20].Bellizzi A, Sebastian S, Ceglia P, et al. Co-expression of CD133(þ)/CD44(þ) in human colon cancer and liver metastasis. J Cell Physiol. 2013;228:408. doi: 10.1002/jcp.24145. [DOI] [PubMed] [Google Scholar]
- [21].Ma S. Biology and clinical implications of CD133(+) liver cancer stem cells. Exp Cell Res. 2013;319:126. doi: 10.1016/j.yexcr.2012.09.007. [DOI] [PubMed] [Google Scholar]