Abstract
Mitochondria are the powerhouses of the eukaryotic cell. After billions of years of evolution, mitochondria have adaptively integrated into the symbiont. Such integration is not only evidenced by the consolidation of genetic information, that is, the transfer of most mitochondrial genes into the nucleus, but also manifested by the functional recombination by which mitochondria participate seamlessly in various cellular processes. In the past decade, the field of mitochondria biology has been focused on the dynamic and interactive features of these semiautonomous organelles. Aspects of a complex multilayer quality control system coordinating mitochondrial function and environmental changes are being uncovered and refined. This Forum summarizes the recent progress of these critical topics, with a focus on the dynamic quality control of mitochondrial reticulum, including their biogenesis, dynamic remodeling, and degradation, as well as the homeostasis of the mitochondrial proteome. These diverse but interconnected mechanisms are found to be critical in the maintenance of a functional, efficient, and responsive mitochondrial population and could therefore become therapeutic targets in numerous mitochondrion-implicated disorders. Antioxid. Redox Signal. 22, 961–964.
Introduction
Originated from their bacterial ancestor α-proteobacteria, mitochondria became endosymbionts living inside eukaryotes over a billion years ago. Since then, their unique ability to implement oxidative mechanisms has made them the powerhouses of eukaryotic cells, thus meeting the majority of the cellular energy demands under aerobic conditions and enormously promoting the evolution of eukaryotes to multicellular life. The most prominent metabolic process carried out by mitochondria is oxidative phosphorylation (OXPHOS) to generate ATP, the universal energy currency. Mitochondria also play fundamental roles in multiple cellular processes such as apoptosis, steroid biosynthesis, calcium homeostasis, intermediary metabolism, and cell signaling (2). Mitochondria are also cellular sources of superoxide (O2•−) and hydrogen peroxide (H2O2), which are involved in the redox regulation of cytosolic and nuclear signaling pathways, together with other messengers originating from mitochondria such as ATP/(AMP+ADP) (8) and the fine tuning of the NAD+/NADH redox couple (Fig. 1B).
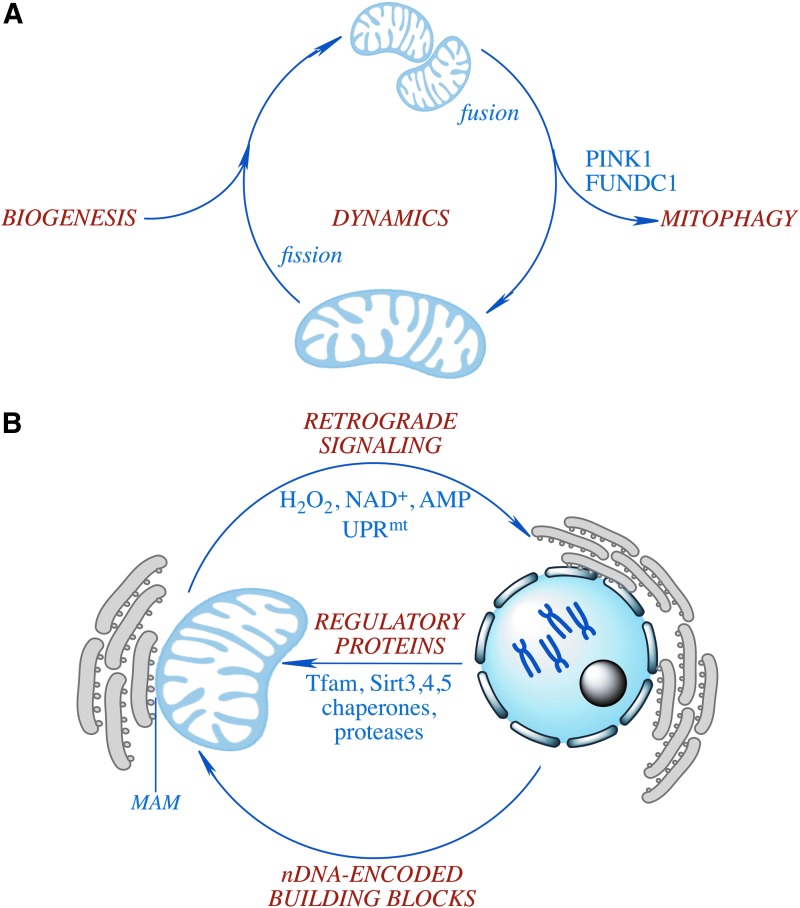
FIG. 1.
Mitochondria: the cellular hub of the dynamic coordinated network. (A) Interrelationship of mitochondrial biogenesis, dynamics, and mitophagy. (B) Coordinated regulation of mitochondrial biogenesis: signals from the nucleus (nDNA-encoded building blocks and regulatory proteins) are regulated (retrograde signaling) by mitochondrion-derived second messengers to the nucleus. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
During the past decades, the classical static and isolated view of the mitochondrion was replaced by an understanding of these organelles within a dynamic network with close interactions with other cellular components. The bioenergetic and redox-modulating capacity of mitochondria not only determines the metabolic rate and redox tone but also interacts with the entire life span and quality control mechanisms of these organelles, including biogenesis, fission and fusion, motility, protein homeostasis, and mitophagy. This complex quality control system grants mitochondria necessary plasticity and protection under various circumstances and allows for adaptation to various specialized and localized metabolic needs in a spatial-temporal manner. Moreover, mitochondria interact with other cellular compartments such as the endoplasmic reticulum (ER), which can be structurally and functionally connected to mitochondria via the mitochondria-associated membranes (MAMs). In this Forum, comprehensive reviews of these critical aspects from leading investigators provide an integrated view of recent advances in mitochondrial research.
Mitochondrial Biogenesis
Mitochondrial biogenesis is a complex process that requires the synthesis, import, and incorporation of proteins and lipids to the existing mitochondrial reticulum, as well as the replication of the mtDNA. The expression of these genes is directly or indirectly regulated by transcription factors, such as nuclear respiratory factor-1 and −2 (NRF-1 and NRF-2) and coactivators such as peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α). As a mechanism to optimize the mitochondrial number and their metabolic capacity, mitochondria biogenesis is activated in response to the imbalance between cellular energy demand and mitochondrial energy transduction induced by a variety of intracellular signals and extracellular stimuli, such as inflammation. The interrelationship of innate inflammation and mitochondrial biogenesis is reviewed in this Forum by Cherry and Piantadosi (3). Acute inflammation such as sepsis induces mitochondrial dysfunction and oxidative stress; conversely, impaired mitochondria lead to further inflammation by releasing various danger-associated molecular patterns and by activating the assembly of inflammasome. Depending on the duration and severity of the inflammatory signal, mitochondrial biogenesis may be activated as a restoration mechanism and correlates significantly with the clinical recovery of sepsis. Signaling pathways (e.g., NFκB, Akt, and MAPKs) and small signaling molecules (e.g., nitric oxide and carbon monoxide) are involved in inflammation-dependent activation of mitochondrial biogenesis by connecting inflammatory signals to the biogenesis machinery, centered on transcriptional factors and coactivators.
Mitochondrial Dynamics
Mitochondria are highly dynamic organelles and undergo fusion and fission continuously in their interconnected network (Fig. 1A). These processes regulate not only mitochondrial morphology but also their biogenesis, transportation and localization, quality control and degradation, and apoptotic cell death. A coordinated balance between fusion and fission serves to maintain the quality of the mitochondria network, while perturbations to this balance are associated with pathologies. Zorzano et al. review the interactions between mitochondrial dynamics and metabolic function, focusing on one of the key GTPases required for mitochondrial fusion, mitofusin-2 (Mfn2) (9). Mfn2 is regulated by metabolic factors such as obesity and type II diabetes, inflammatory factors such as TNFα and interleukin-6, as well as other oxidative and hormonal signals. Conversely, Mfn2 deficiency leads to insulin resistance and metabolic dysfunction through oxidative stress- and ER stress-related signals. Therefore, Mfn2 is proposed as a sensor and regulator of metabolic homeostasis in multiple tissues.
Mitophagy
Through fusion and fission, partially damaged mitochondria can be rescued by diluting (fusion) and/or segregating (fission) deleterious components. When severe mitochondrial damage caused by energy deprivation, oxidative stress, or hypoxia is above a certain threshold, cells activate a mechanism to selectively target and remove damaged mitochondria via the autophagic machinery to invigorate the mitochondrial pool. This process, known as mitochondrial autophagy or mitophagy, includes selective sequestration of damaged mitochondria by the autophagosome and their degradation in the lysosome through various receptor-dependent and -independent mechanisms. Mitophagy is closely linked to mitochondrial fission and fusion processes (Fig. 1A). In this Forum, Wu and Chen summarize the recent advances in mitophagy, with a particular focus on the hypoxia-induced, FUNDC1 (FUN14 domain containing 1)-mediated mitophagy (7). The association between mitophagy and diseases, which suggests the therapeutic potential of novel strategies targeting mitochondria and mitophagy, is also discussed.
PINK1, a Messenger of Mitochondrial Health
PINK1 (PTEN-induced putative kinase 1), the mutation of which causes a form of autosomal recessive Parkinson's disease, is well known for its role in mitophagy by identifying and targeting damaged mitochondria for degradation. The loss of mitochondrial membrane potential prevents the import of PINK1, leading to its accumulation on the outer membrane and the recruitment of the ubiquitin ligase Parkin that initiates the autophagic degradation of these mitochondria. Chu and colleagues review the roles of PINK1 in regulating other mitochondrial function such as respiration, intracellular transport, and oxidant generation, as well as its role in neuron differentiation and neuroprotection is reviewed (6). On the ground of these recent findings, the authors propose a hypothesis to explain the dual role of PINK1 in promoting the degradation of damaged mitochondria and in suppressing the removal of healthy mitochondria; dependent on its subcellular localization, PINK1 is conceptualized as a switch that not only senses damaged mitochondria but also transduces signals from healthy mitochondria to trigger intracellular signaling pathways through its retrotranslocation to the cytosol (Fig. 1A).
Mitochondrial Protein Quality Control
Mitochondrial dynamics and mitophagy maintain mitochondrial homeostasis by repairing or removing damaged mitochondria at the organellar level. In addition, there are proteotoxic damage response mechanisms for mitochondrial quality control at the molecular level (mitochondrial protein quality control). These mechanisms are primarily related to the regulation of protein homeostasis (or proteostasis) within and associated with mitochondria, including the import, folding, and degradation of mitochondrion-targeting proteins (Fig. 1B). The contribution from Khalimonchuk's group to this Forum is a critical review of the current knowledge on several mechanisms governing mitochondrial protein quality control, across all mitochondrial subcompartments, including the matrix, inner and outer membranes, and the intermembrane space (1). These mechanisms, involving a variety of conserved mitochondrial proteases and chaperones, assure the integrity and functionality of the mitochondrial proteome.
Mitochondria-Associated Membrane
Several of the aforementioned mitochondrial functions, such as dynamics and mitophagy, involve a unique interface between mitochondria and the ER, the MAM (Fig. 1B). MAM, which has a complex and highly variable composition, contains hundreds of proteins and may comprise up to 20% of the mitochondrial outer membrane. MAM was found to be enriched in enzymes involved in phospholipid exchange, redox homeostasis, and calcium signaling. Recent findings have expanded the functions of MAM to an array of cellular processes ranging from lipid synthesis and trafficking to calcium signaling to mitochondrial morphology, and to autophagosome formation. These newly recovered roles of MAM render this unique structure a signaling and trafficking hub that integrates mitochondria and ER structurally and functionally. In this Forum, Pinton and colleagues review the knowledge regarding the protein composition of MAM and the connections with its diverse roles in calcium signaling, ER stress, energy metabolism, redox regulation, mitochondrial dynamics, autophagy, and cell death (4). Moreover, this review also discusses recent findings regarding the implications of ER-to-mitochondria signal and MAM in inflammatory response, innate immunity, and the pathogenesis of multiple neurological disorders.
Mitochondrial Sirtuins
The original and most central role of mitochondria is to meet the metabolic needs of the cell, thus maintaining the metabolic homeostasis of the cell. Sirtuins, a family of NAD+-dependent enzymes, have been extensively studied in the past decade for their pivotal role as metabolic regulators involved in stress responses and aging, among others. The mitochondrial sirtuins SIRT3, SIRT4, and SIRT5 regulate several aspects of mitochondrial physiology by controlling the post-translational modifications (deacetylation, demalonylation, or desuccinylation) of mitochondrial protein and the transcription of mitochondrial genes (Fig. 1B). The review by Kumar and Lombard highlights the roles of mitochondrial sirtuins in the maintenance of mitochondrial function and the coordination of cellular metabolic homeostasis (5). Through distinct post-translational modifications, mitochondrial sirtuins target hundreds of key mitochondrial enzymes and are involved in nearly all aspects of mitochondria biology. The implications of mitochondrial sirtuins in aging and the development of various mitochondrion-related pathologies, particularly cancer, are discussed.
Summary
This Forum covers recent advances of critical aspects of mitochondrial biology ranging from the mitochondrial life cycle and quality control mechanisms at the organellar level to the mitochondrial protein homeostasis and post-translational regulation at the molecular level. Via these interconnected repair and self-renewal mechanisms, mitochondria sense the extracellular and intracellular environmental changes and respond adaptively to maximize the overall health and functionality of the mitochondria population and the entire cell. Strategies aiming to maintain or restore the order of these processes are vital for the achievement of optimal metabolic and redox homeostasis in the cell and uncover therapeutic potentials for various pathologies that are correlated with compromised mitochondrial function or dysregulated communication between mitochondria and the rest of the cell.
Abbreviations Used
- ER
endoplasmic reticulum
- MAM
mitochondria-associated membrane
- MAPKs
mitogen-activated protein kinases
- Mfn2
mitofusin-2
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NRF1/2
nuclear respiratory factor 1/2
- PINK1
PTEN-induced putative kinase
- UPRmt
mitochondrial unfolded protein response
References
- 1.Bohovych I, Chan SSL, and Khalimonchuk O. Mitochondrial protein quality control: the mechanisms guarding mitochondrial health. Antioxid Redox Signal 22: 977–994, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadenas E. Mitochondrial free radical production and cell signaling. Mol Aspects Med 25: 17–26, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Cherry AD. and Piantadosi CA. Regulation of mitochondrial biogenesis and its intersection with inflammatory responses. Antioxid Redox Signal 22: 965–976, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, and Pinton P. Mitochondria-associated membranes: composition, molecular mechanisms and physiopathological implications. Antioxid Redox Signal 22: 995–1019, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Kumar S. and Lombard DB. Mitochondrial sirtuins and their relationships with metabolic disease and cancer. Antioxid Redox Signal 22: 1060–1077, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steer EK, Dail MK, and Chu CT. Beyond mitophagy: cytosolic PINK1 as a messenger of mitochondrial health. Antioxid Redox Signal 22: 1047–1059, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H. and Chen Q. Hypoxia activation of mitophagy and its role in disease pathogenesis. Antioxid Redox Signal 22: 1032–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Yin F, Boveris A, and Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid Redox Signal 20: 353–371, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zorzano A, Hernandez-Alvarez MI, Sebastian D, and Muñoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal 22: 1020–1031, 2015 [DOI] [PubMed] [Google Scholar]