Abstract
Autochthonous cases of American cutaneous leishmaniasis (ACL) have been reported since 2001 in the Xakriabá Indigenous Reserve located in the municipality of São João das Missões in northern Minas Gerais state, Brazil. In order to study the presence of Leishmania DNA in phlebotomine sand flies, six entomological collections were carried out from July 2008 through July 2009, using 40 light traps placed in peridomicile areas of 20 randomly selected houses. From October 2011 through August 2012, another six collections were carried out with 20 light traps distributed among four trails (five traps per trail) selected for a previous study of wild and synanthropic hosts of Leishmania. A total of 4,760 phlebotomine specimens were collected belonging to ten genera and twenty-three species. Single female specimens or pools with up to ten specimens of the same locality, species and date, for Leishmania detection by molecular methods. Species identification of parasites was performed with ITS1 PCR-RFLP using HaeIII enzyme and genetic sequencing for SSU rRNA target. The presence of Leishmania DNA was detected in eleven samples from peridomicile areas: Lu. longipalpis (two), Nyssomyia intermedia (four), Lu. renei (two), Lu. ischnacantha, Micropygomyia goiana and Evandromyia lenti (one pool of each specie). The presence of Leishmania DNA was detected in twelve samples from among the trails: Martinsmyia minasensis (six), Ny. intermedia (three), Mi. peresi (two) and Ev. lenti (one). The presence of Leishmania infantum DNA in Lu. longipalpis and Leishmania braziliensis DNA in Ny. intermediasupport the epidemiological importance of these species of sand flies in the cycle of visceral and cutaneous leishmaniasis, respectively. The results also found other species associated with Leishmania DNA, such as Mt. minasensis and Ev. lenti, which may participate in a wild and/or synanthropic cycle of Leishmania transmission in the studied area.
Introduction
Leishmaniases are endemic in many countries where they are considered an important public health problem. The etiological agents for leishmaniases are a variety of protozoan species of the genus Leishmania (Kinetoplastida: Trypanosomatidae) [1,2,3]. Infection occurs in a wide range of vertebrate hosts, including wild and domestic mammals such as rodents, canines, edentates and marsupials, and the vectors are hematophagous insects of the subfamily Phlebotominae (Diptera: Psychodidae) [1,4,5].
Expansion of the geographic distribution of leishmaniasis has been reported in several Brazilian states, including Minas Gerais (MG) where American cutaneous leishmaniasis (ACL) is endemic and widely distributed. Disease-endemic foci occur in the Rio Doce valley [6,7], the Jequitinhonha valley [8], urban centers in the northern region [9,10,11], and on the outskirts of the state capital Belo Horizonte [12,13].
Several species of sand flies (Phlebotaminae) have been associated with species of Leishmania in MG, such as: Ny. intermedia [14]; Lu. longipalpis [10,14], Ny. whitmani [14]; Ny. neivai [15], Pintomyia fischeri, Pi. pessoai, Psychodopygus lloydi and Ps. hirsutus [16,17,18]. In addition to these reports other phlebotomine species, whose epidemiological significance remains unclear, have also been found associated with species of Leishmania, such as: Ev. cortellezzi [19], Ev. sallesi, Ev. termitophila [14,15], Ev. lenti, Pi. christenseni, Pi. monticola, Psathyromyia aragaoi and Ps. lutziana [16].
In a previous study in the Xakriabá Indigenous Reserve (XIR) located in the northern region of MG, wild, synanthropic, and domestic hosts were found to be naturally infected by different species of Leishmania [20]. A recent study on the ecology of the phlebotomine sand fly fauna in the same area reported the presence of Ny. intermedia and Lu. longipalpis mainly in peridomicile areas, and Martinsmyia minasensis and Lutzomyia cavernicola mainly in wild area [21]. The aim of this study was to survey for Leishmania DNA among phlebotomine sand flies collected in a village located in the XIR where autochthonous cases of ACL have been reported since 2001.
Materials and Methods
Study area
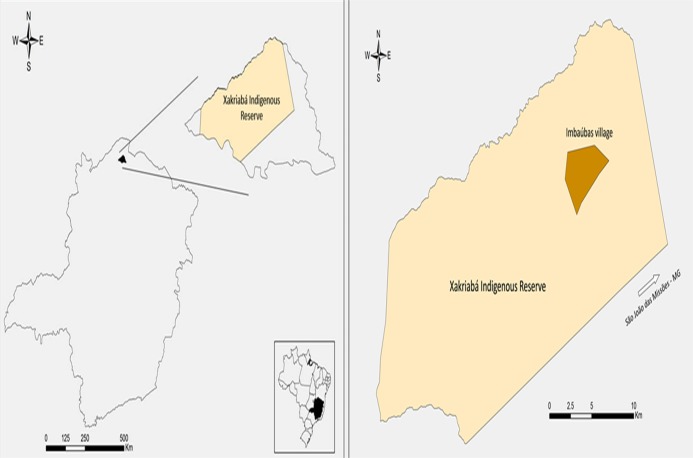
The XIR is located in the municipality of São João das Missões (14°53′4.26“S 44°4′53.19”W) in the northern region of the state of Minas Gerais, Brazil (Fig 1). The indigenous reserve is located in a transition zone between cerrado and caatinga biomes and contains native species of both. This study was conducted in Imbaúbas, an indigenous village which has had both a high prevalence of ACL human cases and numerous wild, synanthropic and domestic Leishmania hosts [20]. Additionally, species incriminated as vectors of Leishmania have been recorded from the village by Rego et al. [21]. This study was conducted with the authorization of FUNAI (National Indian Foundation—Process Number: 2098/08).
Fig 1. Location of study area.
The location of the municipality of São João das Missões in northern Minas Gerais, Brazil. The native village of Imbaúbas located in the Xakriabá Indigenous Reserve, where the study was performed, is indicated.
Sample collection and identification of phlebotomine sand flies
Sand flies used in the present study were originally sampled, collected and identified as published in [21]. The female specimens were pooled and prepared for DNA extraction as shown below.
DNA extraction and Leishmania identification
Sand flies were tested with a minimum of one sand fly female species or pooled to a maximum of ten female specimens of the same species, locality, and date being placed in 1.5 mL tubes containing dimethylsulfoxide 6% (DMSO) and stored at -20°C until DNA extraction. DNA was extracted with Gentra Puregene (QIAGEN, USA) following instructions of the manufacturer. In order to control for potential contamination we included negative control groups (male sand flies) in the DNA extraction step and decontaminated instruments and working areas with DNAZap (Ambion Life Technologies, Inc.).
The extracted DNA from peridomicile areas and trails was screened for Leishmania by the amplification of a 300–350 bp fragment of the intergenic region of the Leishmania DNA (internal transcribed spacer 1—ITS1), using the primers LITSR: 5´ CTGGATCATTTTCCGATG 3´ and L5.8S: 5´ TGATACCACTTATCGCACTT 3 [22,23].
Tests for the presence of Leishmania DNA from peridomicile samples was also performed by nested PCR (LnPCR) with primers that were directed at the small subunit ribosomal ribonucleic acid gene—ssu rDNA [24,25,26]. The first amplification step was performed using R221 and R332 primers and the PCR products were then tested in a subsequent amplification step with R233 and R333 primers [25].
Positive controls for the PCR reactions included DNA extracted from promastigote forms of the following Leishmania strains: Leishmania amazonensis (IFLA/BR/67/PH8), Le. braziliensis (MHOM/BR/75/M2903), Le. infantum chagasi (MHOM/BR/74/PP75) and Le. guyanensis (MHOM/BR/75/M4147). Amplification products were subjected to electrophoresis in 2% agarose gel and stained with ethidium bromide (10mg/mL) with a 100 bp DNA Step Ladder provided as molecular weight size standard.
To identify species of Leishmania, the ITS1 PCR products (10–15 μL) were digested with HaeIII (10U/μL) without prior purification using conditions recommended by the supplier (New England Biolabs, Ipswich, MA, USA). The restriction profiles were analyzed in 4% agarose gel, stained with ethidium bromide (10mg/mL), and compared with the Leishmania reference strains as previously indicated.
Each pool that tested positive by LnPCR and ITS1-PCR were purified using QIAquick PCR Purification kit (QIAGEN, USA) following the instructions of the manufacturer. The purified fragments were then sequenced using Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) in a final volume of 10 μL with 20ng of the purified PCR products and 3.3 pmol of the forward and reverse primers. The products were sequenced in duplicate for each primer (two forward and two reverse). Sequences were then generated by a ABI 3730xl DNA Analyzer, and the software Finch TV (Geospiza, Inc.) and MEGA 5.0 [27] were used to check electropherograms and align sequences with others obtained from GenBank.
Results
A total of 4,760 females of sand flies belonging to ten genera and twenty-three species were tested (Table 1), and arranged in 1,289 pools (263 from peridomicile areas and 1,026 from the trails—Table 2).
Table 1. Females of sand flies collected during the study period in the Xakriabá Indigenous Reserve, Minas Gerais, Brazil.
| Species | Collection sites | Total | |
|---|---|---|---|
| Peridomicile areas | Trails | ||
| Brumptomyia avellari | 3 | 2 | 5 |
| Evandromyia cortelezzii | 7 | 0 | 7 |
| Evandromyia lenti | 17 | 68 | 85 |
| Evandromyia sp. * | 0 | 4 | 4 |
| Evandromyia sallesi | 2 | 0 | 2 |
| Evandromyia spelunca | 5 | 322 | 327 |
| Evandromyia termitophila | 1 | 29 | 30 |
| Lutzomyia cavernicola | 0 | 1026 | 1026 |
| Lutzomyia ischnacantha | 10 | 220 | 230 |
| Lutzomyia longipalpis | 288 | 41 | 329 |
| Lutzomyia sp. * | 0 | 39 | 39 |
| Lutzomyia renei | 6 | 167 | 173 |
| Martinsmyia minasensis | 1 | 1235 | 1236 |
| Micropygomyia capixaba | 1 | 207 | 208 |
| Micropygomyia goiana | 14 | 269 | 283 |
| Micropygomyia longipennis | 0 | 22 | 22 |
| Micropygomyia peresi | 5 | 171 | 176 |
| Micropygomyia sp. * | 0 | 13 | 13 |
| Micropygomyia schreiberi | 0 | 47 | 47 |
| Migonemyia migonei | 4 | 3 | 7 |
| Nyssomyia intermedia | 361 | 139 | 500 |
| Nyssomyia neivai | 0 | 2 | 2 |
| Nyssomyia whitmani | 2 | 0 | 2 |
| Pintomyia misionensis | 0 | 1 | 1 |
| Pintomyia serrana | 2 | 1 | 3 |
| Psathyromyia sp. * | 0 | 1 | 1 |
| Scyopemyia sordellii | 0 | 2 | 2 |
| Total | 729 | 4031 | 4760 |
* Specimens had damaged morphological structures essential for identification.
Table 2. Sand flies collected and grouped in pools of up to ten specimens according to collection site within the Xakriabá Indigenous Reserve, Brazil.
| Species | Sand flies pools from collection sites | Total | |
|---|---|---|---|
| Peridomicile | Trails | ||
| Brumptomyia avellari | 3 | 2 | 5 |
| Evandromyia cortelezzii | 6 | - | 6 |
| Evandromyia lenti | 15 | 40 | 55 |
| Evandromyia sp. * | - | 4 | 4 |
| Evandromyia sallesi | 1 | - | 1 |
| Evandromyia spelunca | 1 | 86 | 87 |
| Evandromyia termitophila | 1 | 20 | 21 |
| Lutzomyia cavernicola | - | 176 | 176 |
| Lutzomyia ischnacantha | 7 | 100 | 107 |
| Lutzomyia longipalpis | 111 | 28 | 139 |
| Lutzomyia sp. * | - | 1 | 1 |
| Lutzomyia renei | 6 | 40 | 46 |
| Martinsmyia minasensis | 1 | 170 | 171 |
| Micropygomyia capixaba | 1 | 45 | 46 |
| Micropygomyia goiana | 11 | 98 | 109 |
| Micropygomyia longipennis | - | 11 | 11 |
| Micropygomyia peresi | 5 | 80 | 85 |
| Micropygomyia sp. * | - | 13 | 13 |
| Micropygomyia schreiberi | - | 16 | 16 |
| Migonemyia migonei | 4 | 3 | 7 |
| Nyssomyia intermedia | 86 | 86 | 172 |
| Nyssomyia neivai | - | 2 | 2 |
| Nyssomyia whitmani | 2 | - | 2 |
| Pintomyia misionensis | - | 1 | 1 |
| Pintomyia serrana | 2 | 1 | 3 |
| Psathyromyia sp. * | - | 1 | 1 |
| Scyopemyia sordellii | - | 2 | 2 |
| Total | 263 | 1026 | 1289 |
* Specimens had damaged morphological structures essential for identification and so pools contained only a single specimen.
The ITS1-PCR did not detect the 300–350 bp fragment that characterizes the sample as positive for Leishmania in peridomicile samples. Thus, amplification using the ssu rDNA primers was performed as suggested by Schonian et al., [23]. The LnPCR detected Leishmania DNA in eleven samples (4.1%) belonging to Ny. intermedia (four), Lu. renei (two), Lu. longipalpis (two) and one sample of each following species: Lu. ischnacantha, Mi. goiana and Ev. lenti. DNA sequencing identified to the species level the Leishmania in nine out of eleven pools (81%) (results summarized in Table 3). The two pools (19%) for which species identification was not possible had hits for Leishmania (Viannia) sp. using the GenBank Blast tool. The most prevalent species of Leishmania in peridomicile areas was Leishmania infantum chagasi (36.3%), however when grouped the other species of Leishmania associated with ACL, the rate of natural infection was 63.6%.
Table 3. Pools of phlebotomine sand flies species naturally infected by Leishmania by collection site within the Xakriabá Indigenous Reserve, Brazil.
| Species | Sand flies pools | Leishmania infection (infection rate) | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Le. braziliensis | Le. guyanensis | Le. (Viannia) sp. | Le. infantum | Le. amazonensis | ||||||||
| Peridomicile | Trails | Peridomicile | Trails | Peridomicile | Trails | Peridomicile | Trails | Peridomicile | Trails | |||
| Evandromyia lenti | 55 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 2 (8.6) |
| Lutzomyia ischnacantha | 107 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (4.3) |
| Lutzomyia longipalpis | 139 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 (8.6) |
| Lutzomyia renei | 46 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 (8.6) |
| Martinsmyia minasensis | 171 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 5 (21.7) |
| Micropygomyia capixaba | 46 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.3) |
| Micropygomyia goiana | 109 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4.3) |
| Micropygomyia peresi | 85 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 (8.6) |
| Nyssomyia intermedia | 172 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 7 (31) |
| Other species | 359 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Total | 1289 | 2 (28.5) | 5 (71.5) | 2 (40) | 3 (60) | 2 (100) | 0 (0) | 4 (57.1) | 3 (42.9) | 1 (50) | 1 (50) | 23 (100) |
| 7 (30.4) | 5 (21.7) | 2 (8.75) | 7 (30.4) | 2 (8.75) | ||||||||
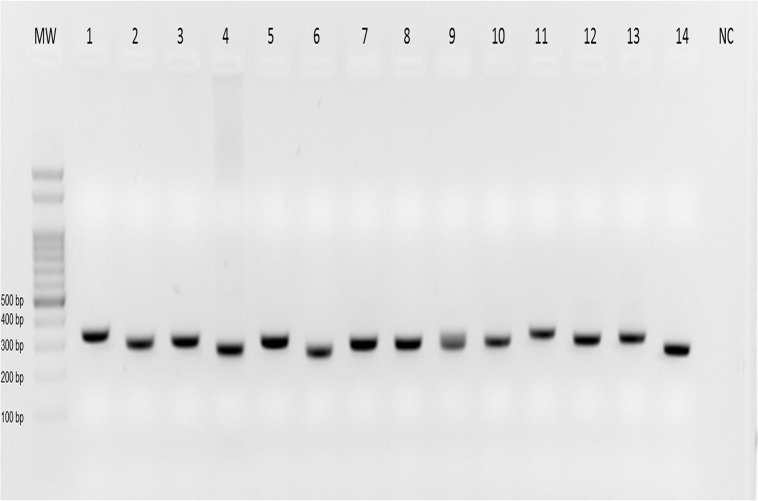
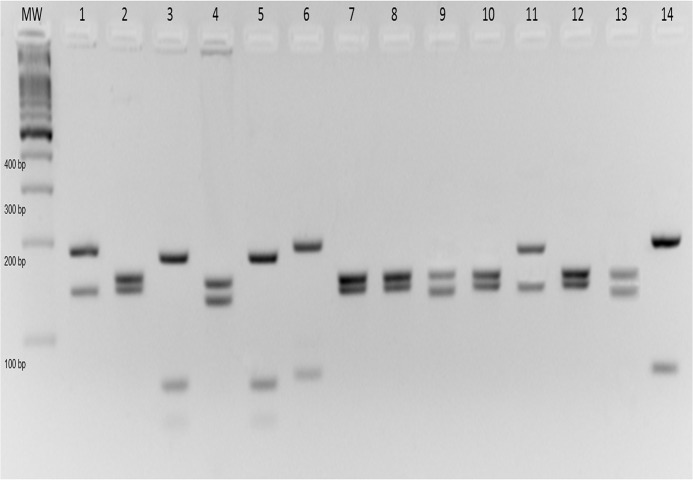
Out of the 1,026 pools from the trails twelve were ITS1-PCR positive (Table 3). The twelve positive pools were Mt. minasensis (five), Ny. intermedia (three), Mi. peresi (two) and one sample of each of the following species: Mi. capixaba and Ev. lenti (Fig 2). The PCR-RFLP technique identified to the species level the Leishmania in all samples (Fig 3). The most prevalent species was Leishmania braziliensis (41.6%), followed by Leishmania guyanensis and Leishmania infantum chagasi (25% of each species).
Fig 2. Detection of Leishmania in sand flies collected from trails in the Xakriabá Indigenous Reserve, Brazil, during the study period.
Sand flies were pooled with up to ten specimens of the same locality, species and date, and ITS1 PCR was performed with total DNA extracted from these pools. The figure represents an ethidium bromide-stained 2% agarose gel in which the amplicons were submitted to electrophoresis. Lanes: MW, molecular weight marker—100 bp; lanes 1–4, positive controls of Le. amazonensis (IFLA/BR/67/PH8), Le. braziliensis (MHOM/BR/75/M2903), Le. infantum (MHOM/BR/74/PP75), Le. guyanensis (MHOM/BR/75/M4147) respectively; 5–14, phlebotomine positive pools; NC, negative control.
Fig 3. Species identification of Leishmania from sand flies collected from trails in the Xakriabá Indigenous Reserve, Brazil, during the study period.
Sand flies were grouped in pools of up to ten specimens of the same species, locality, and date, and ITS1 PCR-RFLP was performed with total DNA extracted from these pools. The figure represents an ethidium bromide-stained 4% agarose gel in which the amplicons were submitted to electrophoresis. Lanes: MW, molecular weight marker—100 bp; lanes 1–4, positive controls of Le. amazonensis (IFLA/BR/67/PH8), Le. braziliensis (MHOM/BR/75/M2903), Le. infantum (MHOM/BR/74/PP75), Le. guyanensis (MHOM/BR/75/M4147) respectively; 5–14, phlebotomine positive pools.
Discussion
One important step towards the incrimination of Leishmania vectors is the occurrence of naturally infected sand flies [28], however, other steps are also necessary for vector incrimination [4,28,29]. Although sand fly digestive tract dissection is the gold-standard method used to study the rate of natural infection in endemic areas, it is laborious and time consuming. Another limiting factor is the difficulty of processing a large number of samples that would be required for epidemiological investigations [30,31]. Moreover, in putative positive cases revealed by sand fly gut dissection, the infection has to be confirmed by in vitro culture of parasites (often susceptible to contamination), or by inoculation into laboratory animals, as other non-identified flagellates are commonly found in the insect midgut [32,33]. Alternatively, molecular techniques allow for DNA detection of a single Leishmania parasite [34] and probably represent a more sensitive tool than manual dissection and microscopic examination [35], which may underestimate natural sand fly infection rates in cases of low parasitaemia.
The use of PCR for detection of Leishmania DNA in wild sand flies is a useful technique for the identification of putative Leishmania vectors in different geographical areas [30,31,34,36,37]. The main advantages of molecular methods are their sensitivity and specificity, independent of the number, stage and location of the parasite in the insect midgut [38].
The present study collected a total of 4,760 specimens from the Xakriabá Indigenous Reserve, where recent ACL human cases and canine visceral leishmaniasis (VL) have been reported [20]. The species most frequently collected from peridomicile areas were Ny. intermedia and Lu. longipalpis, reinforcing the epidemiological role of these species in the transmission of Leishmania braziliensis and Leishmania infantum chagasi, respectively. The species most frequently collected from the trails was Lu. cavernicola, followed by Mt. minasensis; the epidemiological importance of both of these species remains unclear.
The natural infection rate found in peridomicile areas (4.1%) was higher than that observed among the trails (1.1%). This fact can be explained by the use of different molecular targets for PCR, since LnPCR (used only for the peridomicile pools) is more sensitive than ITS1-PCR [23]. Nonetheless, Lu. longipalpis and Ny. intermedia, both incriminated vectors of Leishmania, were found at high rates in peridomestic areas but represented only 1.4% and 3% of the total specimens collected, respectively, from the trails. It should be noted, however, that the peridomicile areas and the trails were sampled at different times, which can also influence detection of Leishmania in phlebotomine sand flies.
The finding of Ev. lenti associated with Le. infantum chagasi in both study areas (peridomicile and trails) is not consistent with that reported by Brazil et al. [39], who demonstrated the resistance of Ev. lenti collected in Minas Gerais to Leishmania. However, Margonari et al. [16] and Paiva et al. [40] have reported finding Leishmania braziliensis in these sand fly species using molecular techniques. In Campo Grande, Mato Grosso do Sul state, this species was closely associated with the peridomestic areas [41] and domestic animal shelters in rural areas [42], as it was observed in our study. Furthermore, Martins et al. [43], reported the significant association between this sand fly species and CL cases in the state of Goiás. Furthermore, Sherlock (1957) [44] and Sherlock & Miranda (1992) [45] reported finding natural infection by promastigotes on this sand fly species. Moreover, according Pinto et al (2012) [46], Ev. lenti and Lu. longipalpis seem to share the same ecological preferences (dry climate areas in different states of Brazil), and the presence of Ev. lenti justifies the establishment of epidemiological surveillance in the area to monitor the appearance of visceral leishmaniasis.
In this study, we observed three species of the genus Lutzomyia associated with Leishmania parasites: Lutzomyia ischnacantha, Lutzomyia longipalpis and Lutzomyia renei. The finding of Leishmania infection in species belonging to this genus is commonly reported in Brazil: Savani et al. [47] reported the infection of Lutzomyia almerioi by Le. infantum chagasi in Mato Grosso do Sul; Pita-Pereira et al. [48] and Missawa et al. [49] reported the infection in Lutzomyia cruzi and Lutzomyia forattinii by Le. infantum chagasi in the same state. However, reports of Leishmania infections in this genus are mostly related to Lutzomyia longipalpis and its association with Leishmania infantum chagasi [10,14,35]. In addition, Paiva et al. [40] reported the association of this sand fly species with Le. braziliensis and Savani et al. [47] with Le. amazonensis.
Lutzomyia longipalpis was found associated with Leishmania infantum chagasi and a parasite belonging to subgenus Leishmania (Viannia) sp.. These findings reinforce the reports about the epidemiological importance of this species mainly in the transmission of Le. infantum chagasi in Brazil [4,50,51,52]. The finding of this sand fly species with a parasite of the subgenus Viannia agrees with the findings of Paiva et al. [40]. However, this is not sufficient to incriminate this sand fly as a vector of species of Leishmania that cause ACL, despite the fact that several studies on experimental infections showed high susceptibility of Lu. longipalpis to different Leishmania species [53,54,55].
Lutzomyia renei and Lutzomyia ischnacantha were never found with Leishmania parasites. It is known that the species of this genus are mostly attracted to a diversity of hosts and use a variety of habitats [56]. The finding of Lu. renei with Leishmania guyanensis and Leishmania (Viannia) sp. DNA, and Lutzomyia ischanacantha with Le. infantum chagasi DNA in XIR may be occasional and with no epidemiological importance or it might be associated with transmission of Leishmania to wild and synanthropic hosts as reported by Quaresma et al. [20] in a study conducted in the same area. To define the role of these sand fly species in the epidemiological context of leishmaniasis, additional studies are necessary.
Martinsmyia minasensis is a phlebotomine species whose feeding habits may be closely related to rodents in the study area [21]. This species was found associated with three species of the genus Leishmania; Le. guyanensis was the most common (3/5 positive pools), followed by Le. braziliensis and Le. amazonensis in the same proportions (1/5 positive pools). The finding of Leishmania guyanensis in the same study area was reported by Quaresma et al. [20] in Thrichomys apereoides (Rodentia: Echimyidae) and Marmosops incanus (Didelphimorphia: Didelphidae). The ecological role of this species should be studied in order to elucidate the epidemiological role in the wild and peridomestic Leishmania transmission cycles. The finding of Mt. minasensis infected by Leishmania braziliensis can also be related to the finding of this parasite in rodents in the same area of study [20], although the finding of Le. amazonensis in the XIR has never been previously reported and human infection by this parasite species is not common, even though it has been identified in some regions of Brazil [57,58,59,60,61,62,63]. The main vector of Le. amazonensis in northern Brazil, Bichromomyia flaviscutellata [64,65,66,67], was not recorded by us during the study period.
The finding of Leishmania in the genus Micropygomyia does not correspond with the behavioral habits of this group, since they have been reported to feed on cold-blooded animals whose participation in the cycle of leishmaniasis is not known in Brazil [56,68,69]. Natural infection with flagellates has been reported in Venezuela in Mi. atroclavata [70,71,72], Mi venezuelensis [72] and Mi cayennensis [71]. Deane et al. [73] also reported infections with flagellates in Mi. cayennensis captured near a bat cave in Venezuela. In Brazil, Micropygomyia ferreirana and Micropygomyia quinquefer have been reported associated with Leishmania braziliensis through molecular methods in the states of Espírito Santo [74] and Mato Grosso [40]. The finding of Leishmania DNA in this genus assumes that these sand flies have fed on hosts susceptible to parasite infection, which is unclear and unknown in cold-blooded animals in Brazil.
Nyssomyia intermedia were positive for Le. braziliensis in four samples (two from each area) reinforcing the epidemiological role of these species in the transmission cycle of ACL given its high abundance in endemic areas in Minas Gerais [75]. It is noteworthy that this species was predominant in peridomestic areas, corroborating the findings of Gontijo et al. [8] in Vale do Jequitinhonha, Minas Gerais and Saraiva et al., [76] in same state, where this species was abundant in environments with anthropic modification.
In this study Ny. intermedia was found associated with Le. infantum chagasi both in peridomicile areas and among the trails, however, the role of this sand fly species in the epidemiological cycle of this parasite is unclear. Oliveira et al. [77] related in a study conducted in VL focus in an indigenous village in Minas Gerais, a high incidence of this species in the absence of Lu. longipalpis, and the same results was reported by Coelho et al. [78] in a VL focus in Goiás state. In addition, Ny. intermedia has been experimentally infected with Le. infantum chagasi [79,80] and recently was found associated with this species of Leishmania in Belo Horizonte, MG [14]. The significance of finding this phlebotomine with Le. amazonensis in the present study is unclear, but in an experimental study Paiva et al., [81] reported this sand fly infected with this parasite. Despite this fact, little is known about the vectorial capacity of this sand fly for this parasite in natural environments.
The entomological data reported in this study may be closely related to the environment found in the XIR: a transition area between cerrado and caatinga biomes, with anthropic modified areas (peridomicile) near forested areas and a variety of wild, synanthropic and domestic animals potentially involved in the transmission cycle of Leishmania [20]. The finding of phlebotomine vectors, as well as species that have no known epidemiological role associated with Leishmania species, reinforces the heterogenous nature of the study area, and calls for additional studies to investigate the vectorial capacity of these species.
Acknowledgments
The authors thank the inhabitants of the XIR for contributing to this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the National Council for Scientific and Technological Development, Minas Gerais Research Foundation, and Oswaldo Cruz Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Leishmaniasis. Fact sheet N° 375. 2014. Available: http://www.who.int/mediacentre/factsheets/fs375/en/.
- 2. Grimaldi G Jr, Tesh RB. Leishmaniasis of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6: 230–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marzochi MC, Marzochi KB. Tegumentary and visceral leishmaniases in Brazil: emerging anthropozoonosis and possibilities for their control. Cad Saúde Pub. 1994;10: 359–375. [DOI] [PubMed] [Google Scholar]
- 4. Killick-Kendrick R. Phlebotomine vectors of leishmaniasis: a review. Med Vet Entomol. 1990;4: 1–24. [DOI] [PubMed] [Google Scholar]
- 5. Galati EAB. Classificação de Phlebotominae In Rangel EF, Lainson R, editors. Flebotomíneos do Brasil: Rio de Janeiro: Fundação Oswaldo Cruz; 2003. pp. 23–51. [Google Scholar]
- 6. Mayrink W, Williams P, Coelho MV, Dias M, Martins AV, Magalhães PA, et al. Epidemiology of dermal leishmaniasis in the Rio Doce valley, State of Minas Gerais, Brazil. Ann Trop Med Parasitol. 1979;73: 123–137. [DOI] [PubMed] [Google Scholar]
- 7. Hermeto MV, Dias DV, Genaro O, Rotondo-Silva A, Costa CA, Toledo VPCP, et al. Outbreak of cutaneous leishmaniasis in the Rio Doce valley, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1994;89: 519–521. [DOI] [PubMed] [Google Scholar]
- 8. Gontijo CMF, Silva ES, Fuccio MB, Sousa MCA, Pacheco RS, Dias ES, et al. Epidemiological studies of an outbreak of cutaneous leishmaniasis in the Rio Jequitinhonha Valley, Minas Gerais, Brazil. Acta Trop. 2002;81: 143–150. [DOI] [PubMed] [Google Scholar]
- 9. Monteiro EM, França da Silva JC, Costa RT, Costa DC, Barata RA, Paula EV, et al. Leishmaniose visceral: estudo de flebotomíneos e infecção canina em Montes Claros, Minas Gerais. Rev Soc Bras Med Trop. 2005;38: 147–152. [DOI] [PubMed] [Google Scholar]
- 10. Michalsky EM, Guedes KS, Silva FOL, França-Silva JC, Fortes-Dias CL, Barata RA, et al. Infecção natural de Lutzomyia (Lutzomyia) longipalpis (Diptera: Psychodidae) por Leishmania infantum chagasi em flebotomíneos capturados no município de Janaúba, Estado de Minas Gerais, Brasil. Rev Soc Bras Med Trop. 2011;44: 58–62. [DOI] [PubMed] [Google Scholar]
- 11. Dias ES, França-Silva JC, Silva JC, Monteiro EM, Paula KM, Gonçalves CM, et al. Flebotomíneos (Diptera: Psychodidae) de um foco de leishmaniose tegumentar no estado de Minas Gerais. Rev Soc Bras Med Trop. 2007;40: 49–52. [DOI] [PubMed] [Google Scholar]
- 12. Passos VM, Falcão AL, Marzochi MC, Gontijo CM, Dias ES, Barbosa-Santos EG, et al. Epidemiological aspects of American cutaneous leishmaniasis in a periurban area of the metropolitan region of Belo Horizonte, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1993;88: 103–110. [DOI] [PubMed] [Google Scholar]
- 13. Luz ZMP, Pimenta DN, Cabral ALLV, Fiúza VOP, Rabello A. A urbanização das leishmanioses e a baixa resolutividade diagnóstica em municípios da Região Metropolitana de Belo Horizonte. Rev Soc Bras Med Trop. 2001;34: 249–254. [PubMed] [Google Scholar]
- 14. Saraiva L, Andrade-Filho JD, Silva SO, Andrade ASR, Melo MN. The molecular detection of different Leishmania species within sand flies from a cutaneous and visceral leishmaniasis sympatric area in Southeastern Brazil. Mem Inst Oswaldo Cruz 2010;105: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 15. Saraiva L, Carvalho GML, Sanguinette CC, Carvalho DAA, Falcão AL, Andrade-Filho AF. Sandflies (Diptera: Psychodidae: Phlebotominae) collected on the banks of the Velhas River in the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2008;103: 843–846. [DOI] [PubMed] [Google Scholar]
- 16. Margonari C, Soares RP, Andrade-Filho JD. Phlebotomine sandflies (Diptera: Psychodidae) and Leishmania infection in Gafanhoto Park, Divinópolis, Brazil. J Med Entomol. 2010;47: 1212–1219. [DOI] [PubMed] [Google Scholar]
- 17. Quaresma PF, de Lima Carvalho GM, Ramos MCNF, Andrade Filho JD. Natural Leishmania spp. reservoirs and phlebotomine sandfly food source identification in Ibitipoca State Park, Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2012;107(4): 480–485. [DOI] [PubMed] [Google Scholar]
- 18. Rangel EF, Ryan L, Lainson R, Shaw JJ. Observations on the sandfly (Diptera: Psychodidae) fauna of Além Paraíba, State of Minas Gerais, Brazil, and the isolation of a parasite of the Leishmania braziliensis complex from Psychodopygus hirsuta hirsuta . Mem Inst Oswaldo Cruz. 1985;80: 373–374. [DOI] [PubMed] [Google Scholar]
- 19. Carvalho GML, Andrade Filho JD, Falcão AL, Rocha ACVM, Gontijo CMF. Naturally infected Lutzomyia sandflies and the transmission of leishmaniasis in an endemic area of Brazil. Vector Borne Zoonotic Dis. 2008;8: 407–414. 10.1089/vbz.2007.0180 [DOI] [PubMed] [Google Scholar]
- 20. Quaresma PF, Rêgo FD, Botelho HA, Silva SR, Junior AJM, Teixeira Neto RG, et al. Wild, synanthropic and domestic hosts of Leishmania in an endemic area of cutaneous leishmaniasis in Minas Gerais State, Brazil. Trans R Soc Trop Med Hyg. 2011;105: 579–585. 10.1016/j.trstmh.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 21. Rêgo FD, Shimabukuro PHF, Quaresma PF, Coelho IR, Tonelli GB, Silva KMS, et al. Ecological aspects of the Phlebotominae fauna (Diptera: Psychodidae) in the Xakriabá Indigenous Reserve, Brazil. Parasites & Vectors. 2014;7(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El Tai NO, Osman OF, El Fari M, Presber W, Schönian G. Genetic heterogeneity of ribosomal internal transcribed spacer (its) in clinical samples of Leishmania donovani spotted on filter paper as revealed by single-strand conformation polymorphisms (sscp) and sequencing. Trans Royal Soc Trop Med Hyg. 2000;94: 1–5. [DOI] [PubMed] [Google Scholar]
- 23. Schonian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47: 349 – 358. [DOI] [PubMed] [Google Scholar]
- 24. Van Eys GJ, Schoone GJ, Kroon NC, Ebeling SB. Sequence analysis of small subunit ribosomal RNA genes and its use for detection and identification of Leishmania parasites. Mol Biochem Parasitol. 1992;51: 133–142. [DOI] [PubMed] [Google Scholar]
- 25. Cruz I, Cañavate C, Rubio JM, Morales MA, Chicharro C, Laguna F, et al. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in coinfected patients with human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2002;96: 185–189. [DOI] [PubMed] [Google Scholar]
- 26. Cruz I, Chicharro C, Nieto J, Bailo B, Cañavate C, Figueras MC, et al. Comparison of new diagnostic tools for management of pediatric Mediterranean visceral leishmaniasis. J Clin Microbiol. 2006;44: 2343–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011. Published online: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Killick-Kendrick R, Ward RD. Ecology of Leishmania. Workshop n°11. Parasitology. 1981;82: 143–152. [Google Scholar]
- 29. Ready PD. Biology of phlebotomine sandflies as vectors of disease agents. Annu Rev Entomol 2013;58: 227–50. 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 30. Aransay AM, Scoulica E, Tselentis Y. Detection and identification of Leishmania DNA within naturally infected sand flies by seminested PCR on minicircle kinetoplastic DNA. Appl Environ Microbiol 2000;66: 1933 – 1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perez JE, Veland N, Espinosa D, Torres K, Ogusuku E, Llanos-Cuentas A, et al. Isolation and molecular identification of Leishmania (Viannia) peruviana from naturally infected Lutzomyia peruensis (Diptera: Psychodidae) in the Peruvian Andes Mem Inst Oswaldo Cruz. 2007;102: 655–658. [DOI] [PubMed] [Google Scholar]
- 32. Ryan L, Brazil RP. Leishmania infections in Lutzomyia longipalpis (Diptera: Psychodidae) on the Island of São Luis, Maranhão State, Brazil. Mem Inst Oswaldo Cruz. 1984;79: 383–384. [DOI] [PubMed] [Google Scholar]
- 33. Freitas RA, Naiff RD, Barrett TV. Species diversity and flagellate infections in the sand fly fauna near Porto Grande, state of Amapá, Brazil (Diptera: Psychodidae. Kinetoplastidae: Trypanosomatidae) Mem Inst Oswaldo Cruz. 2002;97: 53–59 [DOI] [PubMed] [Google Scholar]
- 34. Pita-Pereira D, Alves CR, Souza MB, Brazil RP, Bertho AL, Barbosa AF, et al. Identifications of naturally infected Lutzomyia intermedia and Lutzomyia migonei with Leishmania (Viannia) braziliensis in Rio de Janeiro (Brazil) revealed by a PCR multiplex non-isotopic hybridization assay. Acta Trop. 2005;99: 905–913. [DOI] [PubMed] [Google Scholar]
- 35. Nascimento JC, Paiva BR, Malafronte RS, Fernandes WD, Galati EAB. Natural infection of Phlebotomines (Diptera:Psychodidae) in a visceral-leishmaniasis focus in Mato Grosso do Sul, Brazil. Rev Inst Med Trop. 2007;49: 119–122. [DOI] [PubMed] [Google Scholar]
- 36. Silva OS, Grunewald J. Contribution to the sandfly fauna (Diptera: Phlebotominae) of Rio Grande do Sul, Brazil and Leishmania (Viannia) infections. Mem Inst Oswaldo Cruz 1999;94: 579–582. [DOI] [PubMed] [Google Scholar]
- 37. Miranda JC, Reis E, Schriefer A, Gonçalves M, Reis MG, Carvalho L, et al. Frequency of infection of Lutzomyia Phlebotominae with Leishmania braziliensis in a Brazilian endemic area as assessed by pinpoint capture and polymerase chain reaction. Mem Inst Oswaldo Cruz. 2002;97: 185–188. [DOI] [PubMed] [Google Scholar]
- 38. Perez JE, Ogusuku E, Inga R, Lopez M, Monje J, Paz L, et al. Natural Leishmania infection of Lutzomyia spp. in Peru. Trans R Soc Trop Med Hyg. 1994;88: 161–164. [DOI] [PubMed] [Google Scholar]
- 39. Brazil RP, Carneiro VL, Andrade Filho JD, Alves JCM, Falcão AL. Biology of Lutzomyia lenti (Mangabeira) (Diptera: Psychodidae). An Soc Entomol Brasil. 1997;26: 191–193. [Google Scholar]
- 40. Paiva BR, Oliveira AG, Dorval MEMC, Galati EAB, Malafronte RS. Species-specific identification of Leishmania in naturally infected sand flies captured in Mato Grosso do Sul State, Brazil. Acta Trop. 2010;115: 126–130. 10.1016/j.actatropica.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 41. Oliveira AG, Andrade Filho JD, Falcão AL, Brazil RP. Estudo dos flebotomíneos (Diptera, Psychodidae, Phlebotominae) na zona urbana da cidade de Campo Grande, Mato Grosso do Sul, Brasil, 1999–2000. Cad Saude Pub. 2003;19: 933–944. [DOI] [PubMed] [Google Scholar]
- 42. Galati EAB, Nunes VL, Dorval MEC, Oshiro ET, Cristaldo G, Espíndola MA, et al. Estudo dos flebotomíneos (Diptera, Psychodidae) em área de leishmaniose tegumentar, no Estado de Mato Grosso do Sul, Brasil. Rev Saúde Pub. 1996;30: 115–128. [DOI] [PubMed] [Google Scholar]
- 43. Martins F, Silva IG, Bezerra WA, Maciel JM, Silva HHG, Lima CG, et al. Diversidade e freqüência da fauna flebotomínea (Diptera: Psychodidae) em áreas com transmissão de leishmaniose no Estado de Goiás. Rev Patol Trop. 2002;31: 211–224. [Google Scholar]
- 44. Sherlock IA. Sobre o "Phlebotomus lenti" Mangabeira, 1936 (Diptera: Psychodidae). Rev Bras Biol 1957;17: 77–88. [Google Scholar]
- 45. Sherlock IA, Miranda JC. Observations on the ecology of visceral leishmaniasis in Jacobina, State of Bahia, Brazil (1982–1986) p. 54–80 In Wijeyaratne P, Goodman T, Spinal C, editors. Leishmaniasis Control Strategies: A critical evaluation of IDRC supported research. Mexico City: International Development Research Center; 1992. pp. 54–80. [Google Scholar]
- 46. Pinto IS, Ferreira AL, Valim V, Carvalho FS, Silva GM, Falcão AL, et al. Sand fly vectors (Diptera, Psychodidae) of America visceral leishmaniasis areas in the Atlantic Forest, State of Espiríto Santo, southeastern Brazil. J Vector Ecol. 2012;37: 90–94. 10.1111/j.1948-7134.2012.00204.x [DOI] [PubMed] [Google Scholar]
- 47. Savani ES, Nunes VL, Galati EA, Castilho TM, Zampieri RA, Floeter-Winter LM. The finding of Lutzomyia almerioi and Lutzomyia longipalpis naturally infected by Leishmania spp. in a cutaneous and canine visceral leishmaniases focus in Serra da Bodoquena, Brazil. Vet Parasitol. 2009;160: 18–24. 10.1016/j.vetpar.2008.10.090 [DOI] [PubMed] [Google Scholar]
- 48. Pita-Pereira D, Cardoso MA, Alves CR, Brazil RP, Britto C. Detection of natural infection in Lutzomyia cruzi and Lutzomyia forattinii (Diptera: Psychodidae: Phlebotominae) by Leishmania infantum chagasi in an endemic area of visceral leishmaniasis in Brazil using a PCR multiplex assay. Acta Trop. 2008;107: 66–69. 10.1016/j.actatropica.2008.04.015 [DOI] [PubMed] [Google Scholar]
- 49. Missawa NA, Veloso MAE, Maciel GBML, Michalsky EM, Dias ES. Evidência de transmissão de leishmaniose visceral por Lutzomyia cruzi no município de Jaciara, Estado de Mato, Grosso, Brasil. Rev Soc Bras Med Trop. 2011;44: 76–78. [DOI] [PubMed] [Google Scholar]
- 50.Deane LM. Leishmaniose visceral no Brasil. Estudos sobre reservatórios e transmissores realizados no Estado do Ceará. PhD Thesis, Universidade de São Paulo. 1956.
- 51. Lainson R, Shaw JJ. The role of animals in the epidemiology of South American leishmaniasis In: Lumsden EDA, editor. Biology of the Kinetoplastida. London and New York: Academic Press; 1979;2: 1–116. [Google Scholar]
- 52. Lainson R, Shaw JJ. New World leishmaniasis: The Neotropical Leishmania species. In Collier L, Baeows A, Sussman M, editors. Topley and Wilson’s Microbiology and Microbial Infections. 1998. 5: 241–266. 10.1017/S0950268805004309 [DOI] [PubMed] [Google Scholar]
- 53. da Silva ALFF, Williams P, Melo MN, Mayrink W. Susceptibility of laboratory-reared female Lutzomyia longipalpis (Lutz & Neiva, 1912) to infection by different species and strains of Leishmania Ross, 1903. Mem Inst Oswaldo Cruz. 1990;85: 453–458. [DOI] [PubMed] [Google Scholar]
- 54. Gontijo CMF, Falcão AR, Falcão AL, Coelho MV. The development of species of Leishmania Ross, 1903 in Lutzomyia longipalpis (Lutz & Neiva, 1912). Mem Inst Oswaldo Cruz. 1995;90: 367–373. [DOI] [PubMed] [Google Scholar]
- 55. Barbosa AF, Oliveira SM, Bertho AL, Franco AM, Rangel EF. Single and concomitant experimental infections by Endotrypanum spp. and Leishmania (Viannia) guyanensis (Kinetoplastida: Trypanosomatidae) in the neotropical sand fly Lutzomyia longipalpis (Diptera: Psychodidae). Mem Inst Oswaldo Cruz. 2006;101: 851–856. [DOI] [PubMed] [Google Scholar]
- 56. Deane LM, Deane MP. Observações sobre abrigos e criadouros de flebótomos no noroeste do Estado do Ceará. Rev Bras Malar Doen Trop. 1957;9: 225–246. [Google Scholar]
- 57. Dorval MEC, Oshiro ET, Cupollilo E, Camargo de Castro AC, Alves TP. Ocorrência de leishmaniose tegumentar americana no Estado do Mato Grosso do Sul associada à infecção por Leishmania (Leishmania) amazonensis . Rev Soc Bras Med Trop. 2006;39: 43–46. [DOI] [PubMed] [Google Scholar]
- 58. Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol. 2000;30: 1269–1281. [DOI] [PubMed] [Google Scholar]
- 59. Grimaldi G Jr, Tesh RB, MacMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41: 687–725. [DOI] [PubMed] [Google Scholar]
- 60. Grisard EC, Steindel M, Shaw JJ, Ishikawa EAY, Carvalho-Pinto CJ, Eger-Mangrich I, et al. Characterization of Leishmania sp. strains isolated from autochthonous cases of human cutaneous leishmaniasis in Santa Catarina State, southern Brazil. Acta Trop. 2000;74: 89–93. [DOI] [PubMed] [Google Scholar]
- 61. Lainson R, Shaw JJ, Ryan L, Silveira FT. Leishmaniasis in Brazil XXI. Visceral leishmaniasis in the Amazon Region and further observations on the role of Lutzomyia longipalpis (Lutz & Neiva, 1912) as the vector. Trans R Soc Trop Med Hyg. 1985;79: 223–226. [DOI] [PubMed] [Google Scholar]
- 62. Lainson R, Shaw JJ. Evolution, classification and geographical distribution In: Peters W, Killick-Kendrick R, editors. The Leishmaniases in Biology and Medicine. London: Academic Press; 1987. pp. 1–20. [Google Scholar]
- 63. Passos VM, Fernandes O, Lacerda PA, Volpini AC, Gontijo CMF, Degrave W, et al. Leishmania (Viannia) braziliensis is the predominant species infecting patients with American cutaneous leishmaniasis in the state of Minas Gerais, Southeast Brazil. Acta Trop. 1999; 72, 251–258. [DOI] [PubMed] [Google Scholar]
- 64. Arias JR, Miles MA, Naiff RD, Povoa MM, de Freitas RA, Biancardi CB, et al. Flagellate infections of Brazilian sand flies (Diptera: Psychodidae): isolation in vitro and biochemical identification of Endotrypanum and Leishmania . Am J Trop Med Hyg. 1985;34: 1098–1108. [DOI] [PubMed] [Google Scholar]
- 65. Ryan L, Lainson R, Shaw JJ, Fraiha Neto H. Ecologia de flebotomíneos (Diptera, Psychodidae, Phlebotominae) na região amazônica. Instituto Evandro Chagas “50 anos” 1987;1: 307–320. [Google Scholar]
- 66. Lainson R, Shaw JJ. Leishmaniasis in Brazil: I. Observations on enzootic rodent leishmaniasis-incrimination of Lutzomyia flaviscutellata (Mangabeira) as the vector in the lower amazonian basin. Trans R Soc Trop Med Hyg. 1968;62: 385–395. [DOI] [PubMed] [Google Scholar]
- 67. Ward RD, Shaw JJ, Lainson R, Fraiha H. Leishmaniasis in Brazil: VIII. Observations on the phlebotomine fauna of an area of highly endemic cutaneous leishmaniasis in the Serra dos Carajás, Pará State. Trans R Soc Trop Med Hyg. 1973;67: 174–183. [DOI] [PubMed] [Google Scholar]
- 68. Young DG, Duncan MA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Mem Amer Entomol Inst. 1994;54: 1–881. [Google Scholar]
- 69. Andrade Filho JD, Brazil RP. Phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) of Alagoas State, Northeast of Brazil. Neotrop Entomol. 2009;38: 688–690. [DOI] [PubMed] [Google Scholar]
- 70. Aguilar CM, Fernandez F, de Fernandez R, Deane L. Study of an outbreak of cutaneous leishmaniasis in Venezuela. The role of domestic animals. Mem Inst Oswaldo Cruz. 1984;79: 181–195. [DOI] [PubMed] [Google Scholar]
- 71. Feliciangeli MD. Ecology of sandflies (Diptera: Psychodidae) in a restricted focus of cutaneous leishmaniasis in Northern Venezuela. III—Seasonal fluctuation. Mem Inst Oswaldo Cruz 1987;82: 167–176. [DOI] [PubMed] [Google Scholar]
- 72. Añez N, Nieves E, Carzola D, Oviedo M, Lugo de Yarbuh A, Valera M. Epidemiology of cutaneous leishmaniasis in Merida, Venezuela. III. Altitudinal distribution, age structure, natural infection and feeding behavior of sandflies and their relation to the risk of transmission. Annals of Trop Med and Pasasitol. 1994;88: 279–287. [DOI] [PubMed] [Google Scholar]
- 73. Deane LM, Sargeant S, Fernandez E. Hallazgo de Trypanasoma (Megatrypanum) pessoai Deane & Sugay, 1963, en murcielago de Venezuela, Bol Direc Malar y Saneam Amb 1978;18: 231–237. [Google Scholar]
- 74. Rocha LS, Falqueto A, dos Santos CB, Ferreira AL, da Graça GC, Grimaldi-Jr G, et al. Survey of natural infection by Leishmania in sandfly species collected in southeastern Brazil. Philosophical Trans RS Trop Med Hyg. 2010;104: 461–466. 10.1016/j.trstmh.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 75. Andrade Filho JD, Galati EAB, Falcão AL. Nyssomyia intermedia (Lutz & Neiva, 1912) and Nyssomyia neivai (Pinto, 1926) (Diptera: Psychodidae: Phlebotominae) geographical distribution and epidemiological importance. Mem Inst Oswaldo Cruz. 2007;102: 481–487. [DOI] [PubMed] [Google Scholar]
- 76. Saraiva L, Lopes JS, Oliveira GBM, Batista FA, Falcão AL, Andrade Filho JD. Estudo dos flebotomíneos (Diptera: Psychodidae) em área de leishmaniose tegumentar americana nos municípios de Alto Caparaó e Caparaó, Estado de Minas Gerais, Brasil. Rev Soc Bras Med Trop. 2006;39: 56–63. [DOI] [PubMed] [Google Scholar]
- 77. Oliveira AC, Batista SM, Falcão AL. Calazar em Minas Gerais. Revisão dos dados epidemiológicos obtidos até 1958. Hospital (Rio de Janeiro). 1959;56: 625–643. [PubMed] [Google Scholar]
- 78. Coelho MV, Cunha AS, Falcão AR. Notas sobre um foco de calazar no sudoeste do estado de Goiás. Rev Bras Malar Doen Trop. 1965;17: 143–148. [PubMed] [Google Scholar]
- 79. Chagas AW. Criação de flebótomos e transmissão experimental da leishmaniose visceral americana. Mem Inst Oswaldo Cruz. 1940;35: 327–333. [Google Scholar]
- 80. Paraense WL, Chagas AW. Transmissão experimental da leishmaniose visceral americana pelo "Phlebotomus intermedius". Nota prévia. Brasil-Médico. 1940;54: 179–180. 23005989 [Google Scholar]
- 81. Paiva BR, Secundino NFC, Pimenta PFP, Galati EAB, Andrade-Junior HF, Malafronte RS. Padronização de condições para a detecção de DNA de Leishmania spp. em flebotomíneos (Diptera, Psychodidae) pela reação em cadeia da polimerase. Cadernos de Saúde Pub 2007;23: 87–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.