Abstract
Background: Mouse thyroid side population (SP) cells consist of a minor population of mouse thyroid cells that may have multipotent thyroid stem cell characteristics. However the nature of thyroid SP cells remains elusive, particularly in relation to thyroid cancer. Stanniocalcin (STC) 1 and 2 are secreted glycoproteins known to regulate serum calcium and phosphate homeostasis. In recent years, the relationship of STC1/2 expression to cancer has been described in various tissues.
Method: Microarray analysis was carried out to determine genes up- and down-regulated in thyroid SP cells as compared with non-SP cells. Among genes up-regulated, stanniocalcin 1 (STC1) was chosen for study because of its expression in various thyroid cells by Western blotting and immunohistochemistry.
Results: Gene expression analysis revealed that genes known to be highly expressed in cancer cells and/or involved in cancer invasion/metastasis were markedly up-regulated in SP cells from both intact as well as partial thyroidectomized thyroids. Among these genes, expression of STC1 was found in five human thyroid carcinoma–derived cell lines as revealed by analysis of mRNA and protein, and its expression was inversely correlated with the differentiation status of the cells. Immunohistochemical analysis demonstrated higher expression of STC1 in the thyroid tumor cell line and thyroid tumor tissues from humans and mice.
Conclusion: These results suggest that SP cells contain a population of cells that express genes also highly expressed in cancer cells including Stc1, which warrants further study on the role of SP cells and/or STC1 expression in thyroid cancer.
Introduction
Side population (SP) cells are identified as a small but distinct subset of cells using the dye Hoechst 33342 and dual-wavelength fluorescence–activated cell sorting (FACS) analysis (1). SP cells can efflux Hoechst 33342 dye due to expression of various members of the ATP-binding cassette (ABC) transporter family such as ABCG2 (also called BCRP, MRX) and ABCB1 (also called MDR1, p-gp) (2,3). The activities of these membrane pumps can be specifically blocked by fumitremorgin C and verapamil, respectively (4). SP cells are present in a wide variety of mammalian tissues including hematopoietic and nonhematopoietic tissues such as the liver, skeletal muscle, lung, kidney, and mammary gland (5–12). SP cells appear to contain multipotent stem cells as revealed by various transplantation studies (8,11,12).
The mouse thyroid gland contains a distinct population of Hoechst-effluxing SP cells when examined by the use of verapamil as an inhibitor (13). The thyroid SP cells compose approximately 0.3%–1.4% of total cells that are CD45−/c-kit (CD117)−, with half being Sca1+. They exhibit features characteristic of stem/progenitor cells as judged by the expression of genes specific to stem cells, but not differentiated thyroid cells, and show very few morphological changes during 9 weeks of culture (13). Other studies also suggested the presence of stem/progenitor cells in the human adult thyroid gland (14–16). These results support the long-postulated notion that stem cells are present in the thyroid gland that can replenish the pool of fully differentiated thyrocytes at the frequency of 1 in 1000 cells (17).
There is increasing evidence that cancer cells comprise a small fraction of stem cells that are responsible for constitution of the origin of most, if not all, human tumors and tumor metastases (18–20) although this concept has become controversial in recent years (21). SP cells are characterized by their expression of ABC transporter activity that is associated with multidrug resistance in cancer cells, and a number of studies have shown that SP cells isolated from tumors and tumor cell lines derived from various tissues have tumor initiating potential (22–24). As for the thyroid, Mitsutake et al. (25) demonstrated the presence of SP cells in five different cell lines derived from anaplastic, papillary, and follicular thyroid carcinomas, although two of the cell lines examined later turned out to be originated from nonthyroid cancers (26). Further, it was shown that doxorubicin-resistant anaplastic thyroid cancer cell lines consist of a 70% SP fraction enriched with OCT 4–positive cancer stem cells (27). Epithelial–mesenchymal transition increased the population of SP cells in the thyroid, which highly express stem cell marker genes, and exhibited higher sphere-forming efficiency and higher number of colonies in soft agar assays (28). Despite these studies, very little is known about thyroid SP cells, particularly of the normal mouse thyroid gland, and their relationship to thyroid cancer.
Stanniocalcin (STC) is a secreted glycoprotein known to regulate serum calcium and phosphate homeostasis. Two STCs, STC1 and STC2, are present in fish and mammals and are expressed in a wide variety of tissues, including the heart, lung, liver, adrenal gland, kidney, prostate, and ovary for STC1, and pancreas, spleen, kidney, and skeletal muscle for STC2 (29–31). In recent years, the relationship of STC expression to cancer was described in various tissues including colon, breast, ovary, liver, and esophagus (32–38). The higher expression levels of STC1 and STC2 are generally correlated with poor prognostic outcome of cancers (39,40).
In this study, gene expression profiling of normal mouse thyroid SP cells was carried out to understand the characteristics of mouse thyroid SP cells using Agilent oligonucleotide chips as compared to non-SP cells. The gene profiling results demonstrate that stanniocalcin 1 (Stc1) is among the genes highly up-regulated in thyroid SP fraction of cells as compared to non-SP cells. Higher expression of STC1 was found in thyroid tumor cell lines and/or thyroid tumor tissues of humans and mice.
Materials and Methods
Cell lines
All human thyroid cell lines used in this study were obtained from the European Collection of Cell Cultures (ECACC) through Sigma-Aldrich. FTC133, FTC236, and FTC238 were derived from the same individual, a 42-year-old man with follicular thyroid cancer (FTC); FTC133 was established from the primary tumor, FTC236 from a neck lymph node metastasis, and FTC238 from a lung metastasis (41). RO82-W-1 was established from the metastasis of a follicular carcinoma in a female patient, while 8305C was established from undifferentiated thyroid carcinomas of a 67-year-old female patient (HyperCLDB: cell line database).
Animals and animal procedure
Mice used were C57BL/6NCr (both males and females at approximately a 1:1 ratio). Partial thyroidectomy was carried out by completely removing one thyroid lobe and one third of the other lobe, leaving the proliferative center of the remaining lobe intact as previously described (42). PV/PV mice, a model for thyroid follicular carcinoma were used for histological analysis of thyroid lesions. The details of the mice are described by Suzuki et al. (43). All mouse studies were performed in accordance with the Animals in Intramural Research Guidelines (National Institutes of Health Animal Research Advisory Committee, National Institutes of Health, Bethesda, MD) and after approval by the institutional Animal Care and Use Committee.
Preparation of thyroid cell suspension of mice
To obtain a single cell suspension of mouse thyroid cells, intact thyroids and partial thyroidectomized thyroids 1 week after the surgery were subjected to a two-step enzymatic digestion as previously described (44). The first step was to release solitary thyroid follicles, while the second step was to obtain individual thyroid cells. Briefly, the dissected thyroid lobes were collected in a microtube containing 1 mL of digestion medium, consisting of 100 U/mL type I collagenase (Sigma-Aldrich) and 1.0 U/mL dispase I (Roche Diagnostics) dissolved in Dulbecco's modified Eagle's medium (DMEM)/F12 medium containing 10% fetal bovine serum (FBS). The digestion was performed in a 37°C air incubator with shaking at 250 rpm for 30 minutes or until fragments no larger than thyroid follicles were visible. After digestion, all isolated follicles and individual cells, including interfollicular cells obtained during this process were washed twice with DMEM/F12, seeded in a 60 mm culture dish, and maintained in DMEM/F12 with 10% FBS in a 37°C CO2 incubator for 48 hours. Contaminated nonattached hematopoietic cells were removed by this step. Follicles/cells attached to the dish were carefully washed twice with PBS and then digested with 0.25% trypsin-EDTA for 15 minutes in a 37°C CO2 incubator. This second step of digestion allowed thyroid follicles to dissociate into single follicular cells. To remove undissociated cell clumps and cell debris, the isolated cells were treated with DNaseI (Ambion) for 15 minutes in a 37°C CO2 incubator at a final concentration of 50 μg/mL and filtered through a 40 μm cell strainer (BD Biosciences), followed by two washes with DMEM/F12 containing 10% FBS. Filtered cells were resuspended in DMEM/F12 with 2% FBS and the cell concentration was adjusted to 1×106 cells/mL for the following FACS analysis.
Hoechst 33342 staining for FACS analysis
Isolated thyroid cells were resuspended in prewarmed DMEM/F12 containing 2% FBS and stained for 60 minutes with Hoechst 33342 at a final concentration of 5.0 μg/mL in a 37°C CO2 incubator with occasional shaking to ensure even staining. Control cells were pretreated with verapamil (final concentration of 150 μM, Sigma-Aldrich). At the end of the incubation period, cells were immediately placed on ice to terminate staining and washed twice with ice-cold DMEM/F12 containing 2% FBS.
A FACS Vantage SE with DiVa option (BD Biosciences) was used for cell sorting and analysis in this study. Cells stained with Hoechst 33342 were excited by a krypton laser (multiline ultraviolet [338–356 nm], 50 mW), and the emitted fluorescence was measured at two wavelengths using a 440/40 (Hoechst blue) and 675LP (Hoechst red) optical filter. A beam splitter 610SP was used to separate the emission wavelengths. Seven-amino-actinomycin D (7-AAD) was added to cells immediately before analysis or sorting as a viability dye to exclude dead cells. When the SP1 and SP2 fractions of cells were separately analyzed, the gate of SP1 was set using normal intact thyroid cells based on the complete disappearance of Hoechst 33342–stained cells in the absence of the ABC transporter inhibitor, verapamil as compared with its presence. The SP2 gating was set in transient part from SP1 to non-SP cells by having an equal number of cells to the SP1 cells. Cell sorting was carried out using at least 20 partial thyroidectomized thyroids and 15 intact thyroids three independent times, thus providing three biological replicates for each experimental group. They were subjected to microarray as follows.
Microarray analysis
Total cellular RNA was extracted from a triplicate of SP1, SP2, and non-SP fraction of cells using miRNeasy Mini Kit (Qiagen). Universal Reference Total RNA (Clontech Laboratories, Inc.) was used as reference RNA. Each RNA sample was amplified using Amino Allyl MessageAmp™ II aRNA Amplification Kit (Ambion) following the instructions provided by the manufacturer. Two round amplification was performed. Amplified Universal Reference Total RNA (2 μg) as control and the amplified nine RNA samples (2 μg each) were labeled with Cy3 and Cy5, respectively using CyDye™ Post-Labeling Reactive Dye Pack (GE Healthcare) according to the manufacturer's instruction. The purified dye-coupled amplified RNA samples were hybridized to an Agilent 44 K mouse 60-mer oligo microarrays (Agilent Technologies) and were incubated for 17 hours at 60°C. The slides were washed, dried, and scanned using Agilent G2565AA microarray scanner. The procedures were repeated with independent hybridization and processing. The data were processed and analyzed by Genespring GX software package (Agilent Technologies) and were excluded if their t-test p value was greater than 0.05. The t-test p value gives a confidence measure on how reproducible an expression level measurement is. For the retained confidence signals, they were transformed into the log2 ratio relative to the pooled intact thyroid reference sample. Genes differentially expressed at p≤0.05 in each condition were filtered by Bootstrap t-test with 6000 repetitions (45). Data mining was performed using the Ingenuity Pathway Analysis (IPA) tool (Ingenuity Systems). The significance of each network, function, and pathway was determined by the scoring system provided by the Ingenuity Systems. The raw array files were submitted to the Gene Expression Omnibus (ID GSE34513; www.ncbi.nlm.nih.gov/geo). Hierarchical cluster analysis was performed with Cluster 3.0, and microarray image analysis was performed with TreeView 1.60 (Michael Eisen Laboratory, Lawrence Berkeley National Laboratory and University of California, Berkeley; http://rana.lbl.gov/eisen/).
Quantitative RT-PCR
Approximately 500 ng of amplified RNAs were reverse transcribed into cDNA using SuperScript™ III Reverse Transcriptase (Invitrogen) with random primers. The following PCR amplification was performed with ABI PRISM 7900 thermocycler (Applied Biosystems) using SYBR Premix Taq (Applied Biosystems) and the following primer pairs; human STC1_F: 5′-ATCACATTCCAGCAGGCTTC-3′, human STC1_R: 5′-CCTGAAGCCATCACTGAGGT-3′; mouse Stc1_F: 5′-TGTGCTGACCGTGTCTTCAT-3′, mouse Stc1_R: 5′ GTCATACAGCAGCCCAATCA-3′; Tm4sf1_F: 5′-TTGGGATGGATGAAGAGGAC-3′, Tm4sf1_R: 5′-GCTGGCAAAGGTGTAGTTCC-3′; Vwf_F: 5′-GAAGGAGGCAAAATCGTGAA-3′, Vwf_R: 5′-TGCACATCCTCGATGTCAAT-3′; GAPDH_F: 5′-GGGGTCATTGATGGCAACAATA-3′, GAPDH_R: 5′-AAGGTGAAGGTCGGAGTCAAC-3′; PPIA_F: 5′ TTCTGCTGTCTTTGGGACCT-3′, PPIA_R: 5′-CACCGTGTTCTTCGACATTG-3′. Normal human thyroid RNA was obtained from Clontech. The PCR amplification conditions used were 95°C for 2 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 45 seconds. Relative levels of gene expression were calculated using the ΔΔCt method with normalization to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or peptidylprolyl isomerase A (PPIA, cyclophilin A).
Western blotting
Cells were lysed in a buffer containing 50 mM Tris-HCl (pH 8.0), 0.5% Nonidet P-40, 120 mM NaCl, 100 mM NaF, and 1 mM phenylmethanesulfonyl fluoride at 4°C. After brief sonication, lysates were centrifuged for 10 minutes at 13,000 g at 4°C. Protein concentration was determined using the BCA Protein Assay kit (Thermo Scientific). Two micrograms and 4 μg of STC1 overexpression lysate, and 4 μg of lysate containing empty vector, both purchased from NOVUS (NBL1-16533), were used as positive and negative controls, respectively. Samples (30 μg each) were prepared with 6×sodium dodecyl sulfate loading buffer (125 mM Tris-HCl at pH 6.8, 10% sodium dodecyl sulfate, 30% glycerol, 0.012% bromophenol blue, and 5% beta-mercaptoethanol) and boiled in a water bath at 100°C for 10 minutes. Proteins in lysates were separated in 10% SDS-PAGE gel under denaturing conditions. Proteins were transferred to polyvinylidene fluoride membranes (GE Healthcare Biosciences), and the blot was blocked in 10% nonfat milk (Santa Cruz Biotechnology) overnight at 4°C. Secondary antibody was incubated for 60 minutes at room temperature, and proteins were visualized with SuperSignal West Dura (Thermo Scientific). The primary antibodies used were as follows: STC1 (NBP1-59310; Novus; 1:1000 dilution), and GAPDH (MAB374; Millipore; 1:10,000 dilution). The secondary antibodies were horseradish peroxidase–linked donkey anti-rabbit IgG (NA9340V; GE Healthcare Biosciences). Bands were visualized using electrochemiluminescence kit (GE Healthcare Life Sciences-Amersham Biosciences).
Immunohistochemistry
Immunohistochemistry was carried out using anti-STC1 protein rabbit polyclonal antibody (product NBP1-59310; Novus). The immunogen of this polyclonal antibody is synthetic peptides corresponding to the N-terminal 50 amino acid sequences of STC1 (mouse and human proteins differ by only two amino acids). Tissues used for immunohistochemistry were as follows; two mouse thyroid adenomas obtained from PV/PV mouse (43), and human thyroid tumors including at least three adenomas, and at least five each of follicular and papillary thyroid carcinomas, obtained from the TARP (Tissue Array Research Program) archive (www.cancer.gov/tarp) (46) and from Fukushima Medical University (Fukushima, Japan) pathology archives.
For immunohistochemical staining, 4–5 μm paraffin sections were incubated at 60°C for 10 minutes, deparaffinized in xylene, and hydrated in graded alcohols. For detection of STC1 from mouse samples, sections were subjected for antigen retrieval by boiling in 10 mM sodium citrate buffer (pH 7.0) in a microwave oven for 15 minutes, followed by incubation with 3% hydrogen peroxide in water for 10 minutes to block endogenous peroxidase activity. Sections were then washed in PBS and incubated in 1% bovine serum albumin at room temperature for 1 hour to block nonspecific antibody binding. Specimens were incubated overnight with a primary antibody at 4°C in a humid chamber, and then with peroxidase goat anti-rabbit IgG antibody (Vector Laboratories) for 90 minutes. Immunostaining was visualized with 3,3′-diaminobenzidine as substrate (Sigma-Aldrich). Sections were lightly counterstained with hematoxylin. For immunohistochemical staining of human samples, heat antigen retrieval was performed by submerging slides in pH 6 citrate buffer at 121°C for 20 minutes using a pressure cooker (DAKO) and blocking in 2% skim milk for 20 minutes. Endogenous enzyme activity was inhibited with 3% hydrogen peroxide containing sodium azide for an additional 10 minutes. After rinsing, the sections were incubated at room temperature for 1 hour with antibodies against STC1 (1:400). Detection was performed with DAKO Envision+polymer system and visualized with 3,3′-diaminobenzidine (DAKO), then lightly counterstained with hematoxylin. All slides were dehydrated in graded alcohols, cleared in xylene, mounted, and coverslipped. Immunostaining without primary antibody was used as negative control, and the results were independently evaluated by two pathologists.
Results
Microarray analysis of mouse thyroid SP cells
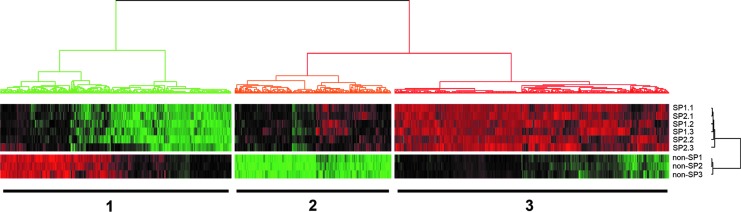
Previously, we demonstrated that mouse thyroid glands possess SP cells that exhibit stem/progenitor cell-like characteristics (13). To further characterize thyroid SP cells, mouse thyroid cells were subjected to cell sorting using verapamil as an inhibitor. RNAs isolated from SP and non-SP fraction of cells right after sorting were used to carry out microarray analysis using the Agilent mouse 44 K oligonucleotides arrays. Initially, SP cells were divided into two regions (SP1 and SP2) by setting a gate where the number of cells in the two regions became equal as described in our previous study (13). This was based on the assumption that the stronger the dye efflux ability is, the higher the stem/progenitor activity might be. Microarray hybridization was carried out using RNAs from three replicate samples of SP1, SP2, and non-SP cells, each using pooled thyroid cells from at least 15 mice. The hierarchical clustering of microarray data demonstrated relative homogenous patterns of gene expression between SP1 and SP2 fraction, which were totally different from those of non-SP cells as shown in Figure 1. Therefore, the merged data of SP1 and SP2 were used for further analysis as an individual SP dataset.
FIG. 1.
Heat map of microarray gene profiling of side population (SP) versus non-SP cells. SP fraction was further divided into two sections, SP1 and SP2, each gated to have the same number of cells. Each SP1, SP2, or non-SP fraction had independent triplicate sample sets, which were subjected to microarray analysis. Genes were clustered in three groups: 1, genes slightly to highly down-regulated in SP cells while highly to slightly up-regulated in non-SP cells; 2, genes slightly up-regulated in SP cells while markedly down-regulated in non-SP cells; and 3, genes highly up-regulated in SP cells while highly to slightly down-regulated in non-SP cells. Red: up-regulated genes; green: down-regulated genes.
Differences in gene expression in SP vs. non-SP cells
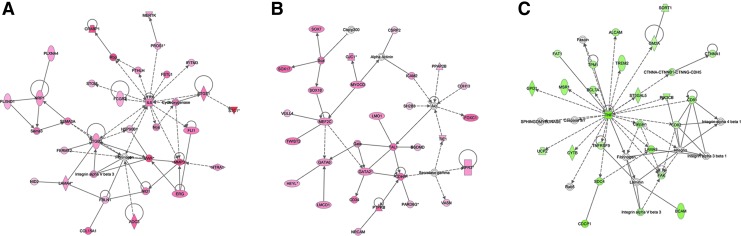
The analysis suggested 3285 up-regulated and 3308 down-regulated genes in SP as compared to non-SP (cut off by more than 1.5-fold changes, and p<0.05 from bootstrap t-test with 6000 random repetitions) and a hierarchical clustering of these expression data showed three distinguishable clusters: 1) genes slightly to highly down-regulated in SP cells while highly to slightly up-regulated in non-SP cells, 2) genes slightly up-regulated in SP cells while markedly down-regulated in non-SP cells, and 3) genes highly up-regulated in SP cells while highly to slightly down-regulated in non-SP cells (Fig. 1). The data were further filtered by threefold difference with p value (p<0.005) from bootstrap t-test and the remaining 1058 probes were subjected to IPA for the functional studies. IPA identified interleukin (IL)-6 (47,48) signaling pathway (Fig. 2A), and GATA (49–51) and SOX (51–54) transcription pathway (Fig. 2B) from the up-regulated genes, and tumor necrosis factor (TNF) signaling pathway (55) (Fig. 2C) from the down-regulated genes in SP relative to non-SP cells. These genes are known to play an important role in either stem cell activity or lineage specifications (see Discussion). Furthermore, genes known to be highly expressed in cancer cells and/or involved in cancer invasion and metastasis, such as stanniocalcin 1 (Stc1), transmembrane 4 superfamily member 1 (Tm4sf1), and von Willebrand factor homolog (Vwf) were drastically up-regulated in SP cells in comparison to non-SP cells (Table 1) (36,56–63). Several multidrug-resistance genes such as Abcg2, Abcb1a, and Abcb1b had 2.99 (2.26 for second probe), 4.13, and 3.05 (2.47 for second probe) fold higher expression in SP cells than non-SP cells, respectively (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy).
FIG. 2.
Ingenuity Pathway Analysis of SP versus non-SP cells. Pathways up-regulated (A, B) and down-regulated (C) in SP cells. (A) Interleukin (IL)-6 signal pathway; (B) GATA and SOX transcription pathway; (C) tumor necrosis factor (TNF) signal pathway.
Table 1.
List of Genes Up-Regulated in Side Population Versus Non–Side Population Cellsa
| GenBank | Gene symbol | Description | Fold changeb | p-value |
|---|---|---|---|---|
| NM_030174 | Mctp1 | Multiple C2 domains, transmembrane 1 | 8.25 | 2.71E-06 |
| AK037205 | Olfml2a | Olfactomedin-like 2A | 7.83 | 2.28E-04 |
| NM_009285 | Stc1 | Stanniocalcin 1 | 7.49 | 5.29E-04 |
| NM_138648 | Olr1 | Oxidized low density lipoprotein (lectin-like) receptor 1 | 7.32 | 1.52E-05 |
| NM_008285 | Hrh1 | Histamine receptor H 1 | 6.99 | 6.49E-04 |
| NM_010045 | Darc | Duffy blood group, chemokine receptor | 6.25 | 2.44E-06 |
| AK034849 | 9430047G12Rik | RIKEN cDNA 9430047G12 gene | 6.20 | 2.20E-05 |
| NM_008536 | Tm4sf1 | Transmembrane 4 superfamily member 1 | 6.19 | 8.71E-07 |
| NM_011127 | Prrx1 | Paired related homeobox 1 | 6.08 | 5.40E-07 |
| NM_013628 | Pcsk1 | Proprotein convertase subtilisin/kexin type 1 | 6.01 | 4.83E-05 |
| NM_011825 | Grem2 | Gremlin 2 homolog, cysteine knot superfamily (Xenopus laevis) | 5.99 | 1.37E-05 |
| AK052718 | Pde3a | Phosphodiesterase 3A, cGMP inhibited | 5.91 | 5.59E-04 |
| X15592 | Ctla2b | Cytotoxic T lymphocyte-associated protein 2 beta | 5.79 | 5.05E-05 |
| NM_008311 | Htr2b | 5-hydroxytryptamine (serotonin) receptor 2B | 5.72 | 3.48E-07 |
| NM_008048 | Igfbp7 | Insulin-like growth factor binding protein 7 | 5.72 | 3.50E-06 |
| NM_019677 | Plcb1 | Phospholipase C, beta 1 | 5.64 | 5.74E-05 |
| NM_011708 | Vwf | von Willebrand factor homolog | 5.64 | 5.88E-06 |
| NM_024406 | Fabp4 | Fatty acid binding protein 4, adipocyte | 5.63 | 1.79E-05 |
| NM_008597 | Mgp | Matrix Gla protein | 5.61 | 4.61E-06 |
| NM_011330 | Ccl11 | Small chemokine (C-C motif) ligand 11 | 5.56 | 3.95E-05 |
| NM_007796 | Ctla2a | Cytotoxic T lymphocyte-associated protein 2 alpha | 5.53 | 8.38E-05 |
| NM_013838 | Trpc6 | Transient receptor potential cation channel, subfamily C, member 6 | 5.44 | 4.62E-05 |
| NM_011701 | Vim | Vimentin | 5.43 | 2.39E-07 |
| AK037771 | Ubr1 | Ubiquitin protein ligase E3 component n-recognin 1 | 5.43 | 1.36E-04 |
| NM_181390 | Mustn1 | Musculoskeletal, embryonic nuclear protein 1 | 5.41 | 9.48E-06 |
| NM_009890 | Ch25h | Cholesterol 25-hydroxylase | 5.37 | 3.99E-05 |
| NM_013496 | Crabp1 | Cellular retinoic acid binding protein I | 5.37 | 1.21E-06 |
| NM_010809 | Mmp3 | Matrix metallopeptidase 3 | 5.34 | 2.06E-05 |
| AK018789 | Ntrk2 | Neurotrophic tyrosine kinase, receptor, type 2 | 5.28 | 9.07E-07 |
| XM_355243 | Prg4 | Proteoglycan 4 (megakaryocyte stimulating factor, articular superficial zone protein) | 5.26 | 1.52E-05 |
| AB031040 | Lhx6 | LIM homeobox protein 6 | 5.23 | 1.62E-05 |
| NM_018782 | Calcrl | Calcitonin receptor-like | 5.22 | 7.26E-06 |
| AK087429 | Mus musculus 0 day neonate eyeball cDNA, RIKEN full-length enriched library, clone:E130107G13 | 5.19 | 2.74E-05 | |
| D16263 | Vcan | Versican | 5.06 | 7.29E-06 |
| NM_145980 | 8430408G22Rik | RIKEN cDNA 8430408G22 gene | 5.06 | 3.05E-06 |
| NM_009364 | Tfpi2 | Tissue factor pathway inhibitor 2 | 5.04 | 4.59E-04 |
| NM_001002927 | Penk1 | Preproenkephalin 1 | 5.04 | 1.01E-06 |
Genes listed are only those up-regulated more than fivefold.
Fold changes are expressed in log2 scale.
Effect of partial thyroidectomy on thyroid SP cells and their gene expression
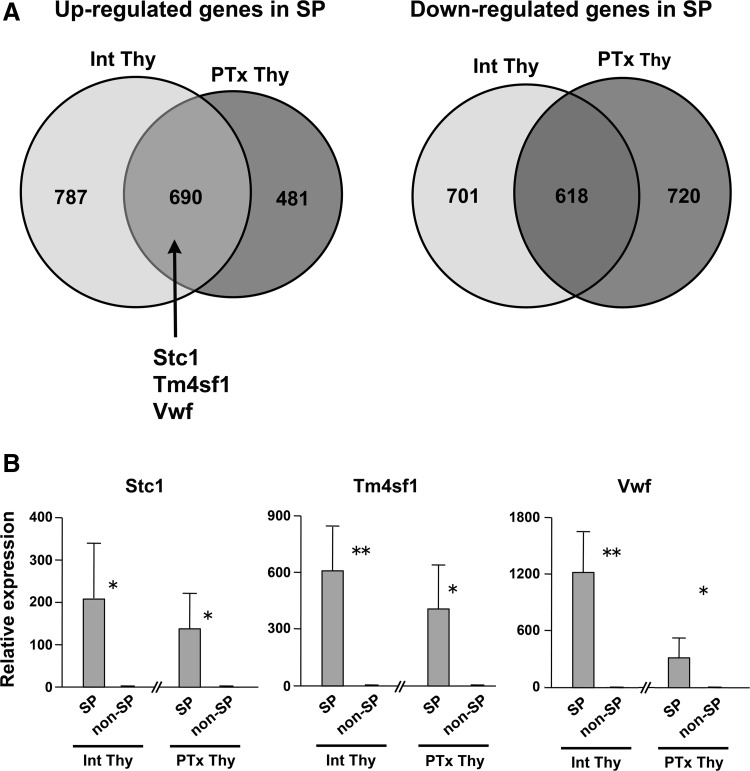
Acute tissue injury may activate stem/progenitor cells to participate in the repair process, which may affect the SP population of cells. Partial thyroidectomy was previously shown to have an effect on thyroid histology, electron microscopic structure, and gene expression patterns, suggestive of repair and/or regeneration of thyroid gland (42). Microarray analysis was carried out using RNAs isolated from SP and non-SP cells that were from pooled thyroid cells of at least 20 partial thyroidectomized mouse thyroids subjected to flow cytometry. Partial thyroidectomized SP versus non-SP cells detected 2509 significant genes with more than 1.5-fold changes and p<0.05 from bootstrap t-test (1171 up-regulated and 1338 down-regulated in SP cells as compared to non-SP cells after partial thyroidectomy). This data set was subjected to comparative analysis with the data set from intact thyroid SP and non-SP cells (1477 up-regulated and 1319 down-regulated genes with more than twofold differences in SP versus non-SP cells in intact thyroid). There were 690 and 618 genes that were commonly up- and down-regulated in both thyroidectomized and nonthyroidectomized SP cells as compared to respective non-SP cells (Fig. 3A, Supplementary Table S2). The aforementioned three genes, Stc1, Tm4sf1, and Vwf were found in the commonly up-regulated group of genes. On the other hand, 481 and 720 genes were up- and down-regulated, respectively only in thyroidectomized SP cells in relative to respective non-SP cells (Fig. 3A, Supplementary Table S3). IPA revealed that Top Bio Functions obtained from these genes are “diseases and disorders,” “molecular and cellular functions,” and “physiological system development and function,” each having subfunctions related to development, cell proliferation, and tissues repair (Table 2).
FIG. 3.
Gene expression analysis with and without partial thyroidectomy. (A) Venn diagram of up- and down-regulated genes in SP versus non-SP cells. Int: intact thyroid, PTx: partial thyroidectomized thyroid. Stc1, Tm4sf1, and Vwf are among genes commonly up-regulated in both intact and partial thyroidectomized thyroids. (B) Quantitative RT-PCR (qRT-PCR) analysis of Stc1, Tm4sf1, and Vwf genes in SP and non-SP cells without (Int Thy) and with (PTx Thy) partial thyroidectomy, using two rounds amplified RNAs. The relative expression levels of SP cells were separately expressed using those of corresponding non-SP cells as 1. Therefore the levels of Int Thy and PTx Thy cannot be directly compared. *p<0.05, **p<0.01.
Table 2.
Pathway Analysis of Up- and Down-Regulated Genes Only in Thyroidectomized Side Population Cells Versus Non–Side Population Cells
| Top biological functions | p-value | No. of molecules |
|---|---|---|
| Diseases and disorders | ||
| Cancer | 8.50E-05 to 2.95E-02 | 281 |
| Reproductive system disease | 8.50E-05 to 2.90E-02 | 95 |
| Hematological disease | 1.94E-04 to 1.86E-02 | 11 |
| Immunological disease | 1.94E-04 to 2.09E-02 | 14 |
| Genetic disorder | 3.10E-04 to 2.94E-02 | 286 |
| Molecular and cellular functions | ||
| Cellular growth and proliferation | 3.87E-05 to 2.95E-02 | 231 |
| Cell death | 4.40E-05 to 2.88E-02 | 203 |
| Cell-to-cell signaling and interaction | 8.50E-05 to 2.62E-02 | 93 |
| Cellular movement | 1.27E-04 to 2.95E-02 | 116 |
| Cell morphology | 2.31E-04 to 2.95E-02 | 113 |
| Physiological system development and function | ||
| Tissue development | 1.95E-04 to 2.88E-02 | 130 |
| Connective tissue development and function | 2.31E-04 to 2.92E-02 | 39 |
| Organismal development | 2.41E-04 to 2.88E-02 | 85 |
| Cardiovascular system development and function | 3.40E-04 to 2.88E-02 | 13 |
| Hepatic system development and function | 3.40E-04 to 1.86E-02 | 6 |
The expression levels of Stc1, Tm4sf1, and Vwf were examined by quantitative RT-PCR (qRT-PCR) using amplified RNAs obtained from SP and non-SP cells of intact as well as partial thyroidectomized thyroids (Fig. 3B). Amplification of RNA was necessary to obtain sufficient amount of RNAs to carry out qRT-PCR for genes of interest. The results demonstrated that all three genes are highly expressed (>100 fold) in SP cells as compared to non-SP cells regardless of partial thyroidectomy. Since STC1 is induced in many cancers and related to thyroid as a thyroid hormone target gene in humans (64), we focused on STC1 for further analysis.
Expression of Stc1 in normal thyroid and thyroid tumor cells
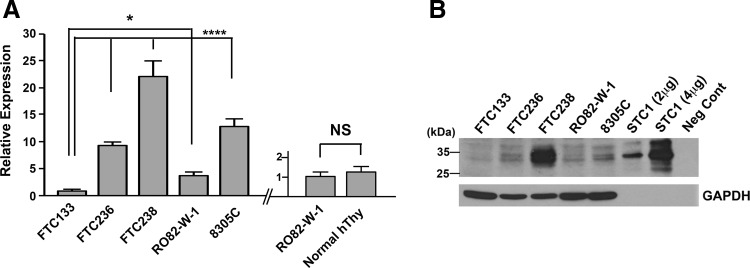
STC1 expression was examined in four human FTC-derived cell lines, FTC133, FTC236, FTC238, and RO82-W-1, and one anaplastic thyroid carcinoma-derived cell line, 8305C, by qRT-PCR (Fig. 4A). No mouse thyroid cancer–derived cell lines were available. The three human FTC lines were derived from the same patient; the higher the number, the more distant the metastasis was, which can be considered less differentiated (65). The expression levels of STC1 mRNA increased as the number became larger in FTC lines of cells, while STC1 expression was lower in RO82-W-1 derived from the metastasis of differentiated follicular carcinoma cells than FTC236, and 8305C obtained from the undifferentiated thyroid carcinoma, had similar STC1 expression level to FTC236. Levels of STC1 mRNA expression in FTC236, FTC238, RO82-W-1, and 8305C were all significantly higher than that of FTC133. STC1 mRNA expression of normal human thyroid was obtained at similar levels to that of RO82-W-1. Western blotting demonstrated the highest protein expression found for FTC238 cells (Fig. 4B). Thus, it appears that both mRNA and protein expression levels for STC1 are roughly correlated with each other and that the expression is roughly inversely correlated with the differentiation status of cells.
FIG. 4.
Analysis of STC1 expression in thyroid cancers. (A) qRT-PCR analysis of STC1 expression in human thyroid carcinoma derived FTC133, FTC236, FTC238, RO82-W-1, and 8305C cells (left). The relative expression was expressed using that of FTC133 as 1. qRT-PCR analysis of STC1 expression in the RO82-W-1 thyroid carcinoma cell line and in normal human thyroid tissue (right). The relative expression was expressed using that of RO82-W-1 as 1. The results are shown as the mean±SD from quadruple analysis. The same experiments were repeated at least two more times, and similar results were obtained. *p<0.05, ****p<0.001 based on the value of FTC133. NS, not significant by One-way ANOVA. (B) A representative Western blotting of STC1 protein expression using a whole cell lysate of the aforementioned cells. The Western blot was done four times. Cell lysates expressing human STC1 by transfection of Stc1 expression plasmid (2 and 4 μg) was used as a positive control, while those carrying a vector only were used as a negative control (lysates purchased from Novus). The multiple bands detected were largely accounted for by glycosylation of STC1 (80). GAPDH was used as a loading control of cell lysate.
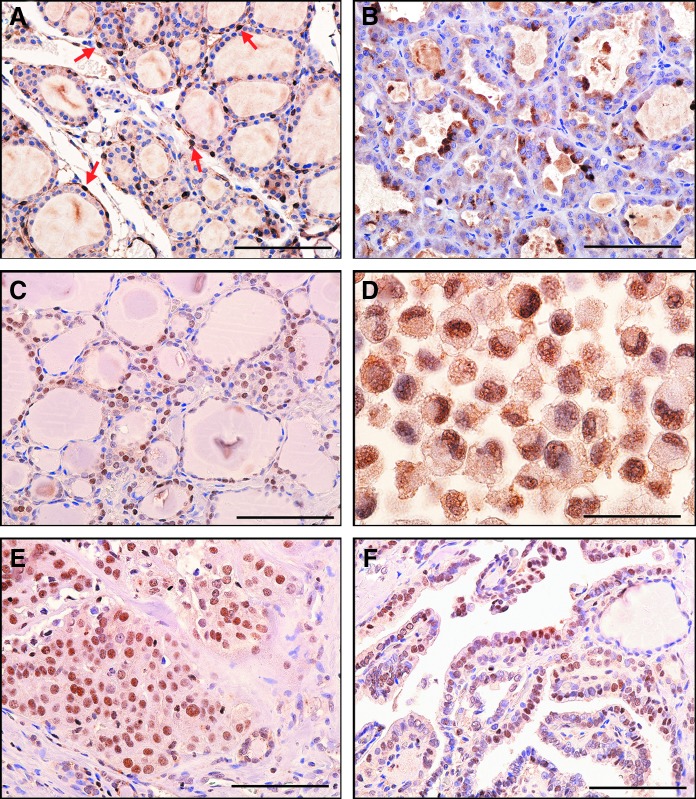
Immunohistochemistry was carried out to determine the pattern of STC1 expression in mouse and human thyroids. The results showed that the STC1 antigen was weakly detected in nuclei of many normal mouse thyroid cells with occasional strong expression (Fig. 5A). Focal strong STC1 staining was clearly observed mainly in the cytoplasm of mouse thyroid adenoma tissues (Fig. 5B). Normal human thyroid follicular epithelium was often, but not always, immunoreactive in the nucleus (Fig. 5C). Some human thyroid carcinomas and a thyroid carcinoma cell line had focal strong STC1 immunoreactivity in the nucleus (Fig. 5D-F).
FIG. 5.
STC1 antigen/protein expression in thyroid tissues. (A) Histology of normal mouse thyroid. Arrows indicate STC1-positive C cells. (B) Mouse thyroid adenoma showing focal cytoplasmic staining. (C) Histology of normal human thyroid. Note nuclear staining. (D) Human RO82-W-1 thyroid carcinoma cell line (cell pellet) showing nuclear staining. (E) Human thyroid follicular carcinoma. (F) Human papillary thyroid carcinoma showing weak nuclear staining. All figures: immunohistochemistry, hematoxylin counterstain,×400. Scale bar: 100 μm.
Discussion
This is the first report of transcriptional profiling of SP and non-SP fraction of cells obtained from normal mouse thyroids. IPA demonstrated that the networks connecting to IL-6, GATA, and SOX are among the top up-regulated networks in thyroid SP cells, while that connected to TNF is among the top down-regulated pathways. The correlation of IL-6 to stem cell activities has been described in glioblastoma (47) and breast carcinomas (48). GATA transcription factors, in particular GATA4/5/6, and SOX transcription factors, in particular SOX17, play a role in definitive endoderm specifications (50–53), while TNF is known to induce neurogenesis in neonatal subventricular zone cell cultures of mice (66) and osteogenic differentiation of human mesenchymal stem cells (67). These results suggest that thyroid SP cells may contain cells in a transient situation where endoderm commitment has been initiated, yet still maintaining stem cell characteristics. Interestingly, SOX2, a stem cell marker, was demonstrated to regulate the Abcg2 gene in anaplastic thyroid carcinoma; this is one of the gene markers that characterize the SP fraction of cells (68). SP cells from partially thyroidectomized thyroids, a condition that is believed to represent repair and/or regeneration of the thyroid (42), have altered expression of many genes that are unique to only partial thyroidectomized thyroid SP cells, but not intact thyroid SP cells. These genes are involved in development, cell proliferation, and tissue repair, suggesting that all thyroid cells including SP cells were in repair after partial thyroidectomy. The ratio of SP cells obtained with verapamil treatment from partial thyroidectomized thyroids increased ∼1.7 fold as compared to that of intact thyroids (Yoshihito Sasaki, personal communication), suggesting that the expression of ABC transporter family genes is also affected by partial thyroidectomy. This may further suggest a correlation between tissue repair/development, SP cells, multidrug-resistance genes, and cancer. However, further studies are required to address this question.
The present results demonstrate that mouse thyroid SP cells highly express genes known to be expressed in cancers and/or those involved in tumor metastasis. They include Tm4sf1, Stc1, and Vwf genes (36,56,59–63), suggesting that the thyroid SP cells may contain cells that could become tumor-initiating cells once they acquire a malignant phenotype. In this study, STC1 expression was elevated in thyroid cancers, particularly those that were less differentiated. It is known that hypoxia is a crucial factor in cancer metabolism (69,70) and the maintenance and/or development of stem cells (71,72), in which hypoxia induces hypoxia-inducible factor-1 (HIF-1). Hypoxia-responsive element is present in the human STC1 gene promoter, and HIF-1 activates STC1 expression in human cancer cells (73,74). Thus, it is possible that STC1 may also be regulated by HIF-1 in stem cells, some of which may be part of the SP cells. Further, the expression of hypoxia-responsive genes including those encoding erythropoietin and endothelin-1, is controlled, in addition to HIF-1, by other transcription factors such as GATA2 (75,76). Therefore GATA transcription factors may be involved in STC1 gene expression in SP cells. Whether this is the case or whether genes in other pathways that are increased in their expression in SP cells, notably those of IL-6 and SOX, are responsible for the increase of STC1 expression in SP cells require further studies.
STC1 is a secreted glycoprotein expressed in a wide variety of tissues to regulate serum calcium and phosphate homeostasis (29). However, the level in serum is generally undetectable, implying that STC1 may act as a local paracrine/autocrine factor (29). In recent years, the relationship of STC1 expression to cancer has been described in various tissues including colon, breast, ovary, and esophagus (32,33–36). Interestingly, the location of STC1 expression varies depending on tissues and/or their physiological conditions. In a virgin rat mammary gland, STC1 expression was found in punctated patterns, highly reminiscent of mitochondrial staining, which was targeted to nuclei as pregnancy progressed into lactation (77). By day 4 post-weaning, nuclear staining was no longer present, and the punctate pattern became evident once more. This nuclear targeting of STC1 during lactation coincided with the fall of gene expression to nearly undetectable levels. In contrast, the normal human mammary gland showed cytoplasmic STC1 expression (36). Mitochondrial localization of STC1 binding was also demonstrated using STC-alkaline phosphatase fusion protein in biochemical analyses and in situ ligand binding in nephron cells and liver hepatocytes (78). On the other hand, cytoplasmic STC1 staining was reported for human gastric cancer, esophageal squamous cell carcinomas, breast carcinomas, and ovarian carcinomas (32,34,36,79). In the current study, the pattern of STC1 expression as demonstrated by immunohistochemistry was highly variable; STC1 expression was low in normal mouse thyroid epithelial cells and was focally found in the cytoplasm of mouse adenomas, while relatively high expression was found in normal human thyroid follicular cells, both mainly found in nuclei. Previously, high expression of STC1 mRNA was demonstrated in the human thyroid (33). In fact, a similar level of STC1 mRNA was found in normal human thyroid compared to that of RO82-W-1 cells. This could be partly due to the fact that RNAs prepared from normal thyroid tissue contain RNAs originated from other cells such as C cells that express STC1, whereas RO82-W-1 cells are originally derived only from a single follicular carcinoma. We could not correlate the levels and patterns of STC1 staining with types of cancers, nor understood the reason why the multiple expression patterns exist even though mRNA and protein levels showed a rough correlation with the differentiation status of cells. Further experiments are required to address these questions.
In conclusion, thyroid SP cells may contain cells in a transient situation in which endoderm commitment has been initiated, yet stem cell properties are still maintained as determined by the transcription profiling. In particular, thyroid SP cells highly express genes such as Tm4sf1, Stc1, and Vwf that are known to be expressed in cancers and/or involved in tumor metastasis. Variable STC1 expression was found in various mouse and human thyroid tumor tissues. These data warrant further studies on SP cells and/or STC1 in relation to thyroid cancer.
Supplementary Material
Acknowledgments
We would like to thank Subhadra Banerjee and Karen M. Wolcott (Flow Cytometry Core Facility, Laboratory of Genome Integrity, CCR, NCI) for their help in FACS analysis. This work was supported by an Intramural Research Program of National Cancer Institute, Center for Cancer Research, ZIABC005522 (SK) and ZICBC010923 (SMH).
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC.1996Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP.2001The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7:1028–1034 [DOI] [PubMed] [Google Scholar]
- 3.Hadnagy A, Gaboury L, Beaulieu R, Balicki D.2006SP analysis may be used to identify cancer stem cell populations. Exp Cell Res 312:3701–3710 [DOI] [PubMed] [Google Scholar]
- 4.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM.2005Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci 118:1715–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asakura A, Rudnicki MA.2002Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol 30:1339–1345 [DOI] [PubMed] [Google Scholar]
- 6.Iwatani H, Ito T, Imai E, Matsuzaki Y, Suzuki A, Yamato M, Okabe M, Hori M.2004Hematopoietic and nonhematopoietic potentials of Hoechst(low)/side population cells isolated from adult rat kidney. Kidney Int 65:1604–1614 [DOI] [PubMed] [Google Scholar]
- 7.Hussain SZ, Strom SC, Kirby MR, Burns S, Langemeijer S, Ueda T, Hsieh M, Tisdale JF.2005Side population cells derived from adult human liver generate hepatocyte-like cells in vitro. Dig Dis Sci 50:1755–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wulf GG, Luo KL, Jackson KA, Brenner MK, Goodell MA.2003Cells of the hepatic side population contribute to liver regeneration and can be replenished with bone marrow stem cells. Haematologica 88:368–378 [PubMed] [Google Scholar]
- 9.Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA.2002Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol 245:42–56 [DOI] [PubMed] [Google Scholar]
- 10.Summer R, Kotton DN, Sun X, Fitzsimmons K, Fine A.2004Translational physiology: origin and phenotype of lung side population cells. Am J Physiol Lung Cell Mol Physiol 287:L477–483 [DOI] [PubMed] [Google Scholar]
- 11.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC.1999Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401:390–394 [DOI] [PubMed] [Google Scholar]
- 12.Challen GA, Little MH.2006. A side order of stem cells: the SP phenotype. Stem Cells 24:3–12 [DOI] [PubMed] [Google Scholar]
- 13.Hoshi N, Kusakabe T, Taylor BJ, Kimura S.2007Side population cells in the mouse thyroid exhibit stem/progenitor cell-like characteristics. Endocrinology 148:4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan L, Cui D, Nowka K, Derwahl M.2007Stem cells derived from goiters in adults form spheres in response to intense growth stimulation and require thyrotropin for differentiation into thyrocytes. J Clin Endocrinol Metab 92:3681–3688 [DOI] [PubMed] [Google Scholar]
- 15.Thomas T, Nowka K, Lan L, Derwahl M.2006Expression of endoderm stem cell markers: evidence for the presence of adult stem cells in human thyroid glands. Thyroid 16:537–544 [DOI] [PubMed] [Google Scholar]
- 16.Fierabracci A, Puglisi MA, Giuliani L, Mattarocci S, Gallinella-Muzi M.2008Identification of an adult stem/progenitor cell-like population in the human thyroid. J Endocrinol 198:471–487 [DOI] [PubMed] [Google Scholar]
- 17.Dumont JE, Lamy F, Roger P, Maenhaut C.1992Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev 72:667–697 [DOI] [PubMed] [Google Scholar]
- 18.Mittal S, Mifflin R, Powell DW.2009Cancer stem cells: the other face of Janus. Am J Med Sci 338:107–112 [DOI] [PubMed] [Google Scholar]
- 19.Pinto CA, Widodo E, Waltham M, Thompson EW.2013Breast cancer stem cells and epithelial mesenchymal plasticity—implications for chemoresistance. Cancer Lett 341:56–62 [DOI] [PubMed] [Google Scholar]
- 20.Shiozawa Y, Nie B, Pienta KJ, Morgan TM, Taichman RS.2013Cancer stem cells and their role in metastasis. Pharmacol Ther 138:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniou A, Hebrant A, Dom G, Dumont JE, Maenhaut C.2013Cancer stem cells, a fuzzy evolving concept: a cell population or a cell property? Cell Cycle 12:3743–3748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda K, Saikawa Y, Ohashi M, Kumagai K, Kitajima M, Okano H, Matsuzaki Y, Kitagawa Y.2009Tumor initiating potential of side population cells in human gastric cancer. Int J Oncol 34:1201–1207 [PubMed] [Google Scholar]
- 23.Wu C, Wei Q, Utomo V, Nadesan P, Whetstone H, Kandel R, Wunder JS, Alman BA.2007Side population cells isolated from mesenchymal neoplasms have tumor initiating potential. Cancer Res 67:8216–8222 [DOI] [PubMed] [Google Scholar]
- 24.Van den Broeck A, Gremeaux L, Topal B, Vankelecom H.2012Human pancreatic adenocarcinoma contains a side population resistant to gemcitabine. BMC Cancer 12:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, Yamashita S.2007Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology 148:1797–1803 [DOI] [PubMed] [Google Scholar]
- 26.Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow LA, Copland JA, Smallridge RC, Haugen BR.2008Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 93:4331–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng X, Cui D, Xu S, Brabant G, Derwahl M.2010Doxorubicin fails to eradicate cancer stem cells derived from anaplastic thyroid carcinoma cells: characterization of resistant cells. Int J Oncol 37:307–315 [DOI] [PubMed] [Google Scholar]
- 28.Lan L, Luo Y, Cui D, Shi BY, Deng W, Huo LL, Chen HL, Zhang GY, Deng LL.2013Epithelial-mesenchymal transition triggers cancer stem cell generation in human thyroid cancer cells. Int J Oncol 43:113–120 [DOI] [PubMed] [Google Scholar]
- 29.Yeung BH, Law AY, Wong CK.2012Evolution and roles of stanniocalcin. Mol Cell Endocrinol 349:272–280 [DOI] [PubMed] [Google Scholar]
- 30.Varghese R, Wong CK, Deol H, Wagner GF, DiMattia GE.1998Comparative analysis of mammalian stanniocalcin genes. Endocrinology 139:4714–4725 [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi K, Miyamoto K, Taketani Y, Morita K, Takeda E, Sasaki S, Imai M.1998Molecular cloning of a second human stanniocalcin homologue (STC2). Biochem Biophys Res Commun 250:252–258 [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Yang G, Chang B, Mercado-Uribe I, Huang M, Zheng J, Bast RC, Lin SH, Liu J.2010Stanniocalcin 1 and ovarian tumorigenesis. J Natl Cancer Inst 102:812–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara Y, Sugita Y, Nakamori S, Miyamoto A, Shiozaki K, Nagano H, Sakon M, Monden M.2000Assessment of Stanniocalcin-1 mRNA as a molecular marker for micrometastases of various human cancers. Int J Oncol 16:799–804 [DOI] [PubMed] [Google Scholar]
- 34.Shirakawa M, Fujiwara Y, Sugita Y, Moon JH, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Mori M, Doki Y.2012Assessment of stanniocalcin-1 as a prognostic marker in human esophageal squamous cell carcinoma. Oncol Rep 27:940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pena C, Cespedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, Hagglof C, Birgisson H, Bojmar L, Jirstrom K, Sandstrom P, Olsson E, Veerla S, Gallardo A, Sjoblom T, Chang AC, Reddel RR, Mangues R, Augsten M, Ostman A.2013STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer Res 73:1287–1297 [DOI] [PubMed] [Google Scholar]
- 36.McCudden CR, Majewski A, Chakrabarti S, Wagner GF.2004Co-localization of stanniocalcin-1 ligand and receptor in human breast carcinomas. Mol Cell Endocrinol 213:167–172 [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Wu K, Sun Y, Li Y, Wu M, Qiao Q, Wei Y, Han ZG, Cai B.2012STC2 is upregulated in hepatocellular carcinoma and promotes cell proliferation and migration in vitro. BMB Rep 45:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kita Y, Mimori K, Iwatsuki M, Yokobori T, Ieta K, Tanaka F, Ishii H, Okumura H, Natsugoe S, Mori M.2011STC2: a predictive marker for lymph node metastasis in esophageal squamous-cell carcinoma. Ann Surg Oncol 18:261–272 [DOI] [PubMed] [Google Scholar]
- 39.Lin S, Guo Q, Wen J, Li C, Lin J, Cui X, Sang N, Pan J.2014Survival analyses correlate stanniocalcin 2 overexpression to poor prognosis of nasopharyngeal carcinomas. J Exp Clin Cancer Res 33:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tohmiya Y, Koide Y, Fujimaki S, Harigae H, Funato T, Kaku M, Ishii T, Munakata Y, Kameoka J, Sasaki T.2004Stanniocalcin-1 as a novel marker to detect minimal residual disease of human leukemia. Tohoku J Exp Med 204:125–133 [DOI] [PubMed] [Google Scholar]
- 41.Havekes B, Schroder van der Elst JP, van der Pluijm G, Goslings BM, Romijn JA, Smit JW.2000Beneficial effects of retinoic acid on extracellular matrix degradation and attachment behaviour in follicular thyroid carcinoma cell lines. J Endocrinol 167:229–238 [DOI] [PubMed] [Google Scholar]
- 42.Ozaki T, Matsubara T, Seo D, Okamoto M, Nagashima K, Sasaki Y, Hayase S, Murata T, Liao XH, Hanson J, Rodriguez-Canales J, Thorgeirsson SS, Kakudo K, Refetoff S, Kimura S.2012Thyroid regeneration: characterization of clear cells after partial thyroidectomy. Endocrinology 153:2514–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suzuki H, Willingham MC, Cheng SY.2002Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid 12:963–969 [DOI] [PubMed] [Google Scholar]
- 44.Kusakabe T, Kawaguchi A, Hoshi N, Kawaguchi R, Hoshi S, Kimura S.2006Thyroid-specific enhancer-binding protein/NKX2.1 is required for the maintenance of ordered architecture and function of the differentiated thyroid. Mol Endocrinol 20:1796–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhauser M, Jockel KH.2006. A bootstrap test for the analysis of microarray experiments with a very small number of replications. Appl Bioinformatics 5:173–179 [DOI] [PubMed] [Google Scholar]
- 46.Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP.1998Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847 [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, Eyler CE, Elderbroom J, Gallagher J, Schuschu J, MacSwords J, Cao Y, McLendon RE, Wang XF, Hjelmeland AB, Rich JN.2009Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells 27:2393–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafe M.2007IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest 117:3988–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsai FY, Orkin SH.1997Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89:3636–3643 [PubMed] [Google Scholar]
- 50.Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H.2002Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev 16:784–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zorn AM, Wells JM.2007Molecular basis of vertebrate endoderm development. Int Rev Cytol 259:49–111 [DOI] [PubMed] [Google Scholar]
- 52.Seguin CA, Draper JS, Nagy A, Rossant J.2008Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell 3:182–195 [DOI] [PubMed] [Google Scholar]
- 53.Qu XB, Pan J, Zhang C, Huang SY.2008Sox17 facilitates the differentiation of mouse embryonic stem cells into primitive and definitive endoderm in vitro. Dev Growth Differ 50:585–593 [DOI] [PubMed] [Google Scholar]
- 54.Pennisi D, Gardner J, Chambers D, Hosking B, Peters J, Muscat G, Abbott C, Koopman P.2000Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat Genet 24:434–437 [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, Park HJ, Hong MH, Kang PM, Morgan JP, Jeong MH, Cho JG, Park JC, Ahn Y.2009TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front Biosci 14:2845–2856 [DOI] [PubMed] [Google Scholar]
- 56.Okabe H, Satoh S, Kato T, Kitahara O, Yanagawa R, Yamaoka Y, Tsunoda T, Furukawa Y, Nakamura Y.2001Genome-wide analysis of gene expression in human hepatocellular carcinomas using cDNA microarray: identification of genes involved in viral carcinogenesis and tumor progression. Cancer Res 61:2129–2137 [PubMed] [Google Scholar]
- 57.Kao YR, Shih JY, Wen WC, Ko YP, Chen BM, Chan YL, Chu YW, Yang PC, Wu CW, Roffler SR.2003Tumor-associated antigen L6 and the invasion of human lung cancer cells. Clin Cancer Res 9:2807–2816 [PubMed] [Google Scholar]
- 58.Shih SC, Zukauskas A, Li D, Liu G, Ang LH, Nagy JA, Brown LF, Dvorak HF.2009The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res 69:3272–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eppert K, Wunder JS, Aneliunas V, Kandel R, Andrulis IL.2005von Willebrand factor expression in osteosarcoma metastasis. Mod Pathol 18:388–397 [DOI] [PubMed] [Google Scholar]
- 60.Smirnov DA, Foulk BW, Doyle GV, Connelly MC, Terstappen LW, O'Hara SM.2006Global gene expression profiling of circulating endothelial cells in patients with metastatic carcinomas. Cancer Res 66:2918–2922 [DOI] [PubMed] [Google Scholar]
- 61.Wang WS, Lin JK, Lin TC, Chiou TJ, Liu JH, Yen CC, Chen PM.2005Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol 11:2166–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terraube V, Marx I, Denis CV.2007Role of von Willebrand factor in tumor metastasis. Thromb Res 120(Suppl 2):S64–S70 [DOI] [PubMed] [Google Scholar]
- 63.Terraube V, Pendu R, Baruch D, Gebbink MF, Meyer D, Lenting PJ, Denis CV.2006Increased metastatic potential of tumor cells in von Willebrand factor-deficient mice. J Thromb Haemost 4:519–526 [DOI] [PubMed] [Google Scholar]
- 64.Moeller LC, Haselhorst NE, Dumitrescu AM, Cao X, Seo H, Refetoff S, Mann K, Janssen OE.2011Stanniocalcin 1 induction by thyroid hormone depends on thyroid hormone receptor beta and phosphatidylinositol 3-kinase activation. Exp Clin Endocrinol Diabetes 119:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rao AS, Goretzki PE, Kohrle J, Brabant G.2005Letter Re: Id1 gene expression in hyperplastic and neoplastic thyroid tissues. J Clin Endocrinol Metab 90:5906. [DOI] [PubMed] [Google Scholar]
- 66.Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO.2008Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells 26:2361–2371 [DOI] [PubMed] [Google Scholar]
- 67.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T.2009TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone 45:367–376 [DOI] [PubMed] [Google Scholar]
- 68.Carina V, Zito G, Pizzolanti G, Richiusa P, Criscimanna A, Rodolico V, Tomasello L, Pitrone M, Arancio W, Giordano C.2013Multiple pluripotent stem cell markers in human anaplastic thyroid cancer: the putative upstream role of SOX2. Thyroid 23:829–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masson N, Ratcliffe PJ.2014Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dang CV.2012Links between metabolism and cancer. Genes Dev 26:877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hawkins KE, Sharp TV, McKay TR.2013The role of hypoxia in stem cell potency and differentiation. Regen Med 8:771–782 [DOI] [PubMed] [Google Scholar]
- 72.Takubo K, Suda T.2012Roles of the hypoxia response system in hematopoietic and leukemic stem cells. Int J Hematol 95:478–483 [DOI] [PubMed] [Google Scholar]
- 73.Yeung HY, Lai KP, Chan HY, Mak NK, Wagner GF, Wong CK.2005Hypoxia-inducible factor-1-mediated activation of stanniocalcin-1 in human cancer cells. Endocrinology 146:4951–4960 [DOI] [PubMed] [Google Scholar]
- 74.Law AY, Ching LY, Lai KP, Wong CK.2010Identification and characterization of the hypoxia-responsive element in human stanniocalcin-1 gene. Mol Cell Endocrinol 314:118–127 [DOI] [PubMed] [Google Scholar]
- 75.Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA.2001Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem 276:12645–12653 [DOI] [PubMed] [Google Scholar]
- 76.Tabata M, Tarumoto T, Ohmine K, Furukawa Y, Hatake K, Ozawa K, Hasegawa Y, Mukai H, Yamamoto M, Imagawa S.2001Stimulation of GATA-2 as a mechanism of hydrogen peroxide suppression in hypoxia-induced erythropoietin gene expression. J Cell Physiol 186:260–267 [DOI] [PubMed] [Google Scholar]
- 77.Hasilo CP, McCudden CR, Gillespie JR, James KA, Hirvi ER, Zaidi D, Wagner GF.2005Nuclear targeting of stanniocalcin to mammary gland alveolar cells during pregnancy and lactation. Am J Physiol Endocrinol Metab 289:E634–642 [DOI] [PubMed] [Google Scholar]
- 78.McCudden CR, James KA, Hasilo C, Wagner GF.2002Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 277:45249–45258 [DOI] [PubMed] [Google Scholar]
- 79.Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Matsushita D, Yanagita S, Nakajo A, Okumura H, Hokita S, Natsugoe S.2012Expression of stanniocalcin 1 as a potential biomarker of gastric cancer. Oncology 83:158–164 [DOI] [PubMed] [Google Scholar]
- 80.Jellinek DA, Chang AC, Larsen MR, Wang X, Robinson PJ, Reddel RR.2000Stanniocalcin 1 and 2 are secreted as phosphoproteins from human fibrosarcoma cells. Biochem J 350(Pt 2):453–461 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.