Abstract
Dengue virus (DENV) of the Flaviviridae family is a single positive-stranded RNA virus that is transmitted by Aedes aegypti and Aedes albopictus mosquitoes. The objective of this study was to investigate the use of chloroquine (CLQ) as an antiviral drug against dengue virus in monkeys. To analyze the action of the drug in vivo, nonhuman primates groups (Aotus azarai infulatus) were inoculated with a subcutaneous injection of a virulent strain of DENV-2, treated and untreated CLQ. Blood hematological, viremia, and serum biochemical values were obtained from 16 DENV-2-inoculated, treated and untreated; four received only CLQ and one mock-infected Aotus monkeys. Monkey serum samples (day 0–10 post-inoculation) were assayed by reverse transcription polymerase chain reaction and Cytometric Bead Array for determination of viremia and inflammatory cytokines, respectively. Additionally, body temperature and activity levels were determined. In the present work, CLQ was effective on replication of DENV-2 in Aotus monkeys; a time viremia reduction was observed compared with the controls. The concentration of tumor necrosis factor alpha and interferon gamma in the serum of the animals had a statistically significant reduction in the groups treated with CLQ after infection compared with the controls. A significant decrease in systemic levels of the liver enzyme aspartate aminotransferase (AST) was also observed in the animals treated with CLQ after infection compared with the controls. These results suggest that CLQ interferes in DENV-2 replication in Aotus monkeys.
Introduction
Dengue fever (DF) is a mosquito-borne viral disease caused by four closely related dengue virus serotypes (DENV-1 to -4) of the genus Flavivirus, of the family Flaviviridae, which includes a number of important human pathogens (6,12). DENV has a positive-sense, single-stranded RNA genome of approximately 11 kb in length that encodes an open reading frame (ORF) of nearly 10,200 nucleotides processed by proteases of viral and cellular origin into three structural proteins—capsid (C), premembrane (PrM), and envelope (E)—and seven nonstructural proteins—NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5—that are required for replication of the virus (3,21). The four serotypes are transmitted to humans and other higher primates by infected Aedes spp. mosquitoes (7). Dengue virus causes the most important arthropod-borne viral disease in the world, characterized by a wide spectrum of clinical manifestations ranging from a flu-like disease (dengue fever) to a life-threatening disease known as dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). The only available way to control dengue is vector control, since no licensed vaccine or effective antiviral therapy is currently available for DENV infections.
The capsid (C) protein, which involves the virus genome, membrane (M), and envelope (E), is embedded in a lipid bilayer of the viral envelope. The E glycoprotein is a major surface protein of the virus and is responsible for attachment and entry. The envelope (E) and precursor membrane (prM) proteins are major structural-protein targets of the antibody response in dengue infection. The lipid composition of the viral envelope is dictated by the composition of the host cell membrane from which the virus emerge. Two types of virions are known: the mature extracellular virions, containing the M protein, and the immature intracellular virions, containing the precursor M (prM), which is proteolitically cleaved during maturation for the production of M glycoprotein (26,31). Dengue viruses enter the target cells via receptor-mediated endocytosis through the direct interaction of viral E glycoprotein with host cell receptors, including DC-specific ICAM-3-grabbing non-integrin (DC-SIGN) and the mannose receptor, followed by acidic pH-dependent fusion with the membrane of the endocytic vesicle (22,24,25,37).
Once exposed to acidic pH inside the endosomal vesicles of the target cell, the virus suffers important structural modifications in the envelope proteins, including trimerization of glycoprotein E and the domain II rearrangement, allowing its projection to the viral envelope surface and exposing its fusion domain (1,35).
Chloroquine (CLQ) is a 9-aminoquinoline known since 1934, a diprotic weak base that increases the pH of acidic organelles such as endosomes, Golgi vesicles, and lysosomes. For viruses, CLQ led to an inhibition of low-pH-dependent entry steps or alteration of post-translational modifications of newly synthesized proteins, especially via inhibition of glycosylation (32,40).
The limited infectivity and replication of DENV in nonhuman species have been hampered by the lack of development of an animal model of DENV infection. Numerous animal models, each with their limitations, have been used as potential dengue models (42). Thus, the present study evaluated the action of CLQ in the replication of DENV-2 in Aotus azarai infulatus monkeys. Nonhuman primates (NHPs) are phylogenetically close to humans, and because of that they were chosen as an experimental model for this study.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the Brazilian Government's ethical and animal experiments regulations. The procedures using animals in this research project were specified in accordance with the ethical principles established by the Brazilian College of Animal Experimentation (COBEA), and the experimental protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Sao Paulo (CETEA/USP, Permit Protocol Number 080/2006) and by the Ministry of the Environment (MMA/SISBIO, Permit Protocol Number 3087896). All surgery was performed under ketamine/xylazine anesthesia, and all efforts were made to minimize animal suffering.
Cell cultures
Vero cells (continuous cell lineage originating from the kidney of African green monkeys) were grown in Leibovitz-15 (L-15) culture medium (Invitrogen, New York) supplemented with 10% fetal bovine serum (FBS), 1% L-glutamine 200 mM, 1% penicillin G (100 IU/mL), streptomycin (100 μg/mL), and 10% triptose phosphate. Vero cells were maintained at 37°C and 5% CO2. C6/36, an Aedes albopictus cell line, was cultured in L-15 medium supplemented with 10% FBS, L-glutamine, 10% triptose phosphate, 1% penicillin G (100 U/mL), and streptomycin (100 μg/mL), at 28°C in the absence of CO2.
Virus stock
Parental DENV-2 New Guinea C strain, recovered from the brains of 1- to 2-day-old suckling Swiss mice, was used in this study. The animals were sacrificed, and the brains were removed in a sterile environment. Each brain was homogenized in 900 μL of bovine skin gelatin 4% (Sigma-Aldrich, St. Louis, MO). The macerate was then harvested and centrifuged at 2,800 g for 20 min at 4°C. After centrifugation, the macerate supernatant fluid was kept in ice to prevent further microbial loss, and then divided into aliquots and stored frozen at −70°C. Virus stock supernatant fluid used in the present study was free of the lipopolysaccharide and mycoplasma.
Dengue virus titration
Virus production was titrated by plaque assay using Vero cells. Vero cells were seeded in a 12-well plate (6×105 cells/well) in L-15 medium with 10% FBS for 48 h at 37°C. The medium was removed, and decimal serial dilutions of virus stock were added (0.1 mL/well) to the cells, which were then incubated for 2 h at 37°C. Subsequently, L-15 medium containing 5% FBS and 3% carboxymethyl-cellulose (1 mL/well; overlay) was added, and the plate was incubated at 37°C for 7 days. The overlay was removed on day 7, and cells were fixed with a solution of 10% formaldehyde in PBS. After 2 h at room temperature, the formaldehyde solution was removed, and cells were washed twice with PBS and stained (15 min) with a 1% crystal violet solution in 20% ethanol. The plaques of cell lysis were counted, and the virus concentration was expressed as plaque-forming units (PFU) per milliliter.
Nonhuman primates
Twenty-one male monkeys of the species Aotus azarai infulatus, 5–10 years old and weighing 0.8–1.2 kg, were used in this study. All animals were kept in the colony of NHPs in the National Primate Center—CENP/SVS/MS, located in a biological reserve with typical vegetation of the Amazon rainforest, with an area of approximately 25 ha in the district of Ananindeua, Pará, Brazil. The institution has currently in its squad primates belonging to the Amazon region, Atlantic rainforest, and one species of the old world. The colony has good reproductive capacity, thus constituting a stable source for the production of animals to be used in experimental studies. The animals infected with DENV-2 were individually housed in stainless steel cages with wire mesh bottoms. The monkeys were fed twice daily with a commercial chow supplemented with fresh fruit, eggs, and vegetables, with water ad libitum. Temperature, humidity, and light/dark cycles were standardized. The animals were identified by “microchip” and a specific tattoo mark on the inner side of the right hind limb. Each animal had a protocol of five letters, according to the records of CENP. The first two letters represented the species, namely, AH, and last three represented the individual.
Before starting the experiment, the biochemical and hematological parameters of the animals were evaluated, such as complete blood count, serum glucose, cholesterol, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (AP), to establish normal values of physiological characteristics, aiming to use this species to establish an experimental model for dengue. Also, the animals were weighed using a digital scale, and the temperature of the animals was measured. Prior to initiation of the study, monkey sera were analyzed for antidengue immunoglobulin G (IgG) antibodies by enzyme-linked immunosorbent assay (ELISA).
IgG indirect ELISA
The indirect ELISA was performed to determine the presence of antidengue IgG in five groups of monkeys before and 20 days after the end of the experiment. DENV-2-infected C6/36 cell supernatant fluid was used as antigen for the dengue IgG ELISA. The DENV-2 antigen was diluted 1:2 with coating buffer (0.05 M carbonate-bicarbonate buffer; pH 9.6). A 96-well EIA flat bottom high binding microplate (Corning Costar®, Corning, NY) was coated with antigen and was then incubated at 4°C overnight. After this period, the plate was washed three times with PBS buffer containing 0.05% Tween 20. The plate was blocked to prevent false positive results by adding 200 μL of PBS buffer with 10% SBF and incubated for 1 h at 37°C. It was then washed three times with PBS buffer containing 0.05% Tween 20. After preparation of the plate, test sera and control serum (positive control) were diluted at 1:1,000 in PBS buffer with 10% SBF, and 100 μL/well of 1:1,000-diluted was added in duplicate to the plate. The plate was incubated at 37°C for 1 h. After this period, the plate was washed six times with PBS buffer containing 0.05% Tween 20, and 100 μL/well of 1:20,000-diluted peroxidase-conjugated antimonkey IgG goat IgG (Sigma-Aldrich in PBS with 10% SBF was added to the plate. The plate was incubated at 37°C for 1 h. After incubation, the plate was washed six times with PBS containing 0.05% Tween 20, followed by initiation of the peroxidase reaction by addition of 100 μL/well of substrate solution (TMB) to the plate. The plate was incubated at room temperature for 15 min in the dark, and then the reaction was stopped by adding 100 μL/well of 1 M phosphoric acid. The optical density readings were performed in an ELISA reader at an excitation wavelength of 490 nm with a reference filter of 620 nm. The threshold (cutoff) was calculated with mean OD of negatives control plus three times the standard deviation (SD). Samples ≥0.537 (cutoff) were considered positives.
Treatment of DENV-2-infected monkeys with CLQ
Different concentrations of CLQ (25; 10; 5 mg/kg) were administered by the oral route to Aotus monkeys, according to its use in humans (38). The drug was also prepared in a laminar flow hood and diluted in saline.
Study groups, inoculation, and sample collection
Dengue naïve Aotus monkeys (n=21) used in the experiment were randomly divided into five groups of four animals each. One control animal (AH-AIR) received control macerate supernatant fluid of the brain of 1- to 2-day-old suckling Swiss mice for the DENV-2 stock (mock infection). Each animal was kept in a separate cage under controlled environmental conditions. The animals were fed as described above. Procedures were performed under anesthesia with 10 mg/kg ketamine chloride (Vetaset, Fort Dodge, IA) intramuscularly. Animals were infected subcutaneously (s.c.) with 0.1 mL of DENV-2 (2×106 PFU/mL) suspension (in the upper arm). Blood was collected daily from the femoral vein for 10 days to detect viremia after infection and analysis of biochemical and hematological parameters, such as complete blood count, ALT, AST, and AP. Group 1 received a dose of CLQ orally (25 mg/kg) on days 1, 2, 3, and 4; a dose of 10 mg/kg on days 5, 6, 7, and 8; and a dose of 5 mg/kg on days 9 and 10. Group 2 (control animals) was infected s.c. with DENV-2. Group 3 received a dose of CLQ (25 mg/kg) and 48 h later was infected s.c. with DENV-2. Then, this group received a dose of CLQ (25 mg/kg) on days 1, 2, 3, and 4; a dose of 10 mg/kg on days 5, 6, 7, and 8; and a dose of 5 mg/kg on days 9 and 10. Group 4 was infected s.c. with DENV-2 and 24 h later received a dose of CLQ (25 mg/kg) on days 1, 2, 3, and 4; a dose of 10 mg/kg on days 5, 6, 7, and 8; and a dose of 5 mg/kg on days 9 and 10. Group 5 was infected s.c. with DENV-2 and 24 h later received a dose of CLQ (25 mg/kg) twice per day on days 1, 2, 3, and 4; a dose of 10 mg/kg twice per day on days 5, 6, 7, and 8; and a dose of 5 mg/kg twice per day on days 9 and 10.
Studies conducted by Styer et al. using WNV showed that mosquitoes inoculate between 104.3 and 106.1 PFU of WNV per bite (36), and similar levels of transmission were observed for DENV. Infection of animals with 104.3–106.1 PFU of DENV is usually considered to mimic the inoculum in a mosquito bite. NHPs can sustain viral replication after s.c. inoculation with a dose of 105 PFU of DENV. However, viral replication is much lower than in humans (23).
Viral RNA extraction
Viral RNA was extracted from 140 μL of each serum sample using the QIAamp Viral RNA kit (QIAGEN, Valencia, CA) according to manufacturer's directions.
Determination of viremia in monkey sera
The amplification of the fragments in each serum of the Aotus monkeys was performed using the reverse transcriptase polymerase chain reaction (RT-PCR) assay. Each RT-PCR contained 5 μL of buffer, 1 μL dNTPs (10 mM), 1 μL of multiscribe (50 U/μL), 0.75 μL of primers (20 nM) DV2U (5′-AAG GTG AGA TGA AGC TGT AGT CTC-3′), and DVL1 (5′-CAT TCC ATT TTC TGG CGT TCT-3′) (14) specifically designed to anneal to the DENV-2 3′ untranslated region (3′-UTR), 6.5 μL of DEPC water, and 10 μL of RNA to a final volume of 25 μL. The amplification protocol consisted of the following steps: 50°C for 30 min, 95°C for 15 min, followed by 45 cycles at 94°C for 1 min, 60°C for 1 min, 72°C for 1 min, and finally 60°C for 1 min. The experiment was carried out in duplicate.
Detection of cytokines by flow Cytometric Bead Array
Blood was drawn daily for the next 10 days. The serum was collected and immediately frozen at −70°C for subsequent determination of cytokine production. The assay was performed by a flow cytometry application that allowed the quantification of multiple cytokines simultaneously. Interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), IL-2, IL-6, IL-4, and IL-5 were measured in the serum using the Cytometric Bead Array (CBA) method (nonhuman primate Th1/Th2 Cytokine Kit II, BD) as recommended by the manufacturer. The experiment was carried out in duplicate, and the results are shown as the mean values obtained from two individual experiments.
Clinical observation
Monkeys were observed for 20 days after the end of the experiment, and rectal temperature, weight, hematological parameters, and biochemical parameters were recorded. Then, the monkeys were released to their colonies.
Statistical analysis
Statistical analysis was used to assess differences of cytokine production and liver enzymes at time-defined intervals from serum monkeys infected in contact with CLQ and control group (without the drug) using two-way analysis of variance (ANOVA) test (multiple comparisons test). The descriptive statistics (mean and standard deviation) was used for hematological parameters and to compare means by group was used two-way ANOVA test. Data were entered into the GraphPad Prism software v6.0, and for all analyses, p-values of <0.01 and <0.05 were considered statistically significant.
Results
IgG indirect ELISA
The results of the ELISA assay on 21 animal serum samples obtained prior to initiation of the study submitted to antidengue-IgG analysis showed negative IgG samples. The animals from group 2, two animals from group 4, and one animal from group 5 were positive IgG when serum samples were obtained at 20 days after the end of the experiment (Table 1).
Table 1.
Serum Titers of Anti1-DENV IgG Determined by ELISA
| Serology IgG anti-DENV | ||
|---|---|---|
| Group 1 | Before infection | After infection |
| 1 | ||
| AH-AKO | Negative | Negative |
| AH-ADA | Negative | Negative |
| AH-BAN | Negative | Negative |
| AH-BBU | Negative | Negative |
| 2 | ||
| AH-BBL | Negative | Positive |
| AH-BAO | Negative | Positive |
| AH-AHE | Negative | Positive |
| AH-BBM | Negative | Positive |
| 3 | ||
| AH-BBF | Negative | Negative |
| AH-BBN | Negative | Negative |
| AH-AKI | Negative | Negative |
| AH-AEV | Negative | Negative |
| 4 | ||
| AH-ADI | Negative | Positive |
| AH-AKU | Negative | Negative |
| AH-BBI | Negative | Negative |
| AH-AIZ | Negative | Positive |
| 5 | ||
| AH-AIL | Negative | Negative |
| AH-BBO | Negative | Negative |
| AH-BBR | Negative | Negative |
| AH-BAF | Negative | Positive |
| * | ||
| AH-AIR | Negative | Negative |
Mock infection with fluid from macerate supernatant of the brain of 1- to 2-day-old suckling Swiss mice for the dengue virus serotype 2 stock. This animal was used as a control.
DENV, dengue virus; IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay.
Viremia
Table 2 presents the results obtained in the viremia evaluation on Aotus monkeys, infected or uninfected with DENV-2, treated and untreated with CLQ, in five experimental groups. In Group 2 (animals infected with DENV-2 and untreated), the DENV-2 induced viremia in all four monkeys with a duration from 5 to 6 days (mean duration of 5.5 days/animal). In Group 4 (animals infected and treated), the CLQ induced a reduction in the time of viremia in all four animals with a duration from 2 to 3 days (mean duration of 2.5 days/animal) compared with controls. In group 5, formed by animals infected and treated twice per day with CLQ, the viremia was detectable in only two animals (AH-BBR and AH-BAF) whereas monkeys presented 1–2 days of viremia (mean duration of 1.5 day/animal) compared with controls. In Group 3 (animals that received a prophylactic dose of CLQ and 48 h later were infected with DENV-2), the viremia was detectable in only one animal (AH-AKI), whereas monkey presented only 1 day of viremia (mean duration of 0.25 day/animal) compared with controls.
Table 2.
Viremia in Aotus azarai infulatus Monkeys Infected or Uninfected with DENV-2, Treated and Untreated Chloroquine
| Days after infection | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group, animal | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Days of viremia |
| 1 | ||||||||||||
| AH-AKO | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-ADA | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-BAN | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-BBU | − | − | − | − | − | − | − | − | − | − | − | 0 |
| 2 | ||||||||||||
| AH-BBL | − | − | + | − | + | − | + | + | − | + | + | 6 |
| AH-BAO | − | + | + | + | − | + | − | + | − | − | − | 5 |
| AH-AHE | − | − | + | + | − | − | + | + | − | + | + | 6 |
| AH-BBM | − | + | + | − | − | − | − | + | + | + | + | 6 |
| 3 | ||||||||||||
| AH-BBF | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-BBN | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-AKI | − | − | − | + | − | − | − | − | − | − | − | 1 |
| AH-AEV | − | − | − | − | − | − | − | − | − | − | − | 0 |
| 4 | ||||||||||||
| AH-ADI | − | − | + | − | − | − | + | − | − | − | − | 2 |
| AH-AKU | − | − | − | + | + | − | − | − | − | − | − | 2 |
| AH-BBI | − | − | + | + | − | − | − | − | − | − | − | 2 |
| AH-AIZ | − | − | − | − | + | − | − | + | + | − | − | 3 |
| 5 | ||||||||||||
| AH-AIL | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-BBO | − | − | − | − | − | − | − | − | − | − | − | 0 |
| AH-BBR | − | − | + | − | + | − | − | − | − | − | − | 2 |
| AH-BAF | − | − | − | − | + | − | − | − | − | − | − | 1 |
| * | ||||||||||||
| AH-AIR | − | − | − | − | − | − | − | − | − | − | − | 0 |
Mock infection with fluid from macerate supernatant of the brain of 1- to 2-day-old suckling Swiss mice for the dengue virus serotype 2 stock. This animal was used as a control.
Detection of cytokines by flow CBA
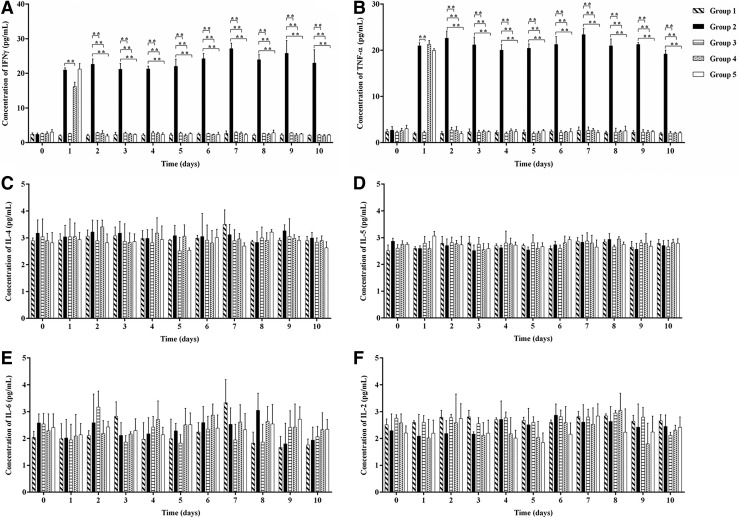
The comparison of serum cytokines (IFN-γ, TNF-α, IL-2, IL-4, IL-5, and IL-6) between the studied groups (180 samples [serum] of 20 animals) showed higher concentration of the cytokines INF-γ and TNF-α in the group of the animals that were only infected with DENV-2 and were untreated (Fig. 1A and B). No statistically significant difference was observed in the production of the cytokines IL-4, IL-5, IL-6, and IL-2 in groups 1, 3, 4, and 5 compared with controls (Fig. 1C–F). The animal (AH-AIR) that was inoculated with mock macerate supernatant fluid of the brain of 1- to 2-day-old suckling Swiss mice for the dengue virus serotype 2 stock used for the present studies failed to produce the cytokines.
FIG. 1.
Detection of cytokines by Cytometric Bead Array (CBA) in the serum of Aotus azarai infulatus monkeys infected or not with the DENV-2 in the presence or absence of chloroquine (CLQ). Interferon gamma (IFN-γ) (A), tumor necrosis factor alpha (B), IL-4 (C), IL-5 (D), IL-6 (E) and IL-2 (F) cytokines production in DENV-2 infected or uninfected animals, treated and untreated CLQ were analyzed by flow cytometry that allowed multiple cytokines to be quantified simultaneously. For all analyses, p-values of <0.01 were considered statistically significant.
Production of IFN-γ and TNF-α was significantly reduced (p<0.01) in the presence of CLQ compared with control, demonstrating that CLQ was able to reduce the viral yield in groups 3, 4, and 5 (Fig. 1A and B).
Clinical observation
Fever and weight
None of the monkeys showed a change in temperature (average temperature 39°C) or weight (average weight 0.9 kg).
Hematological parameters
None of the monkeys showed changes in hematological parameters (complete blood count; Table 3).
Table 3.
Hematological Parameters Expressed as the Mean±Standard Deviation (Data Are Divided by Group)
| Animals | Red blood cells (×106/mm) | Hemoglobin (g/dL) | Hematocrit (%) | MCV (fL) | MCH (pg) | Platelets (103/mm3) | Leukocytes (103/mm3) | Neutrophils (103/mm3) | Lymphocytes (103/mm3) | Eosinophils (103/mm3) | Basophils (103/mm3) | Monocytes (103/mm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | 5.45±0.44 | 16.23±1.13 | 43.58±3.96 | 80.25±2.99 | 26.33±1.41 | 211.3±16.88 | 11.00±1.57 | 2.57±1.89 | 8.15±1.22 | 0.25±0.50 | 0 | 0.02±0.05 |
| Group 2 | 5.12±1.35 | 13.89±3.25 | 42.70±9.07 | 81.50±4.43 | 25.55±1.25 | 232.8±7.63 | 14.60±1.60 | 3.62±1.31 | 10.23±1.65 | 2.10±0.87 | 0 | 0.10±0.20 |
| Group 3 | 5.81±0.52 | 16.13±1.13 | 42.05±3.37 | 77.75±1.70 | 25.20±1.67 | 213.8±23.58 | 11.23±1.18 | 1.87±0.69 | 8.77±1.28 | 0.52±0.45 | 0 | 0.05±0.10 |
| Group 4 | 6.00±0.64 | 16.80±1.53 | 43.98±3.17 | 77.75±2.75 | 25.33±2.13 | 224.5±9.67 | 12.05±2.78 | 3.55±0.78 | 7.47±1.79 | 0.90±0.84 | 0 | 0.07±0.09 |
| Group 5 | 5.89±0.56 | 16.65±1.53 | 47.25±4.38 | 80.00±6.37 | 27.08±0.98 | 224.3±12.69 | 13.15±3.67 | 2.90±1.45 | 8.82±1.09 | 1.35±1.45 | 0 | 0.07±0.15 |
Hematologic values: red blood cells (×106/mm) 5.05–7.06; hemoglobin (g/dL) 14.5–19.4; hematocrit (%) 39.0–53.6; mean corpuscular volume (MCV) (fL) 69.8–83.1; mean corpuscular hemoglobin (MCH) (pg) 22.9–29.9; platelets (103/mm3) 37.0–245.0; leukocytes (103/mm3) 5.67–17.66; neutrophils (103/mm3) 0.91–5.84; lymphocytes (103/mm3) 3.24–10.6; eosinophils (103/mm3) 0.09–4.06; basophils (103/mm3) 0–0.36; monocytes (103/mm3) 0–0.30.
Biochemical parameters
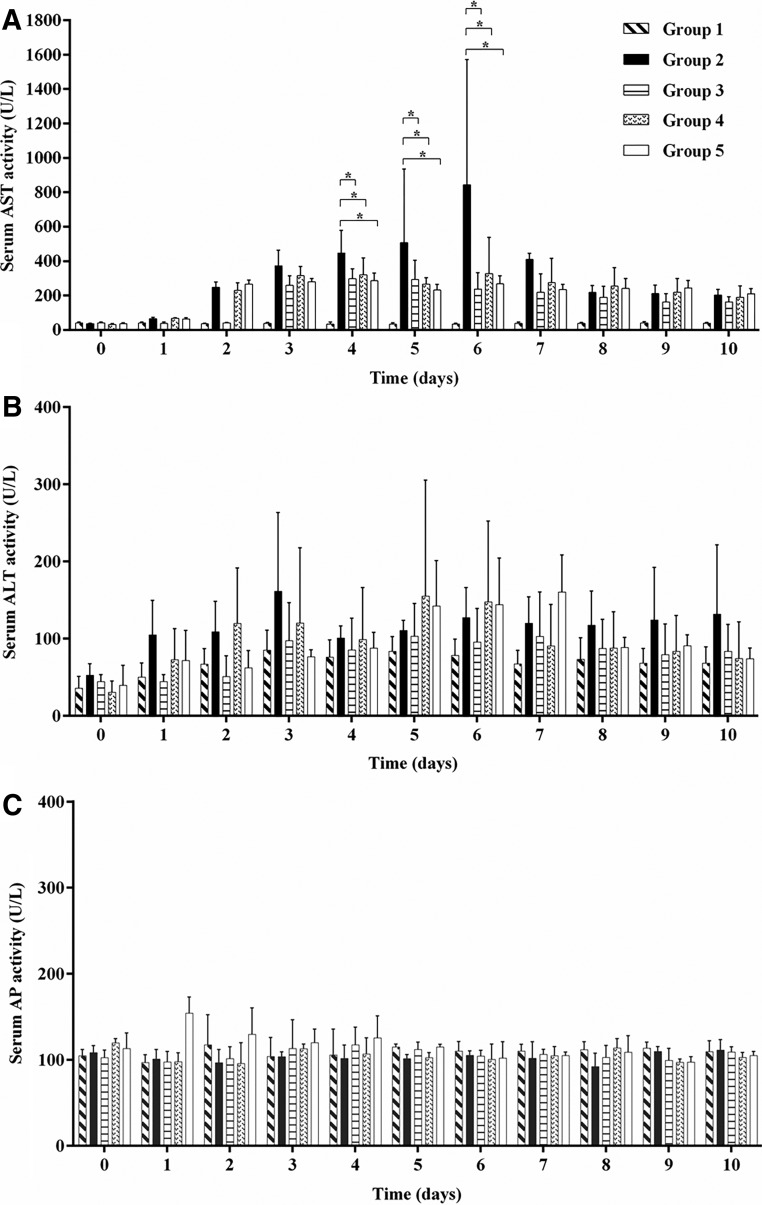
ALT and AST are commonly used biomarkers for liver damage. A significant decrease in serum AST activity was observed on days 4, 5, and 6 for animals treated with CLQ compared with controls (p<0.05; Fig. 2A). Neither serum ALT nor AP activity was altered when compared with controls (Fig. 2B and C).
FIG. 2.
Analysis values of aspartate aminotransferase (AST), (A), alanine aminotransferase (ALT) (B), and alkaline phosphatase (AP) (C), enzymes from Aotus azarai infulatus monkeys infected or not with the DENV-2 in the presence or absence of CLQ. Values of reference: AST, 14–50 IU/L; ALT, 13–61 IU/L; AP, 38–126 IU/L. For all analyses, p-values of <0.05 were considered statistically significant.
Discussion
NHPs have been used to investigate several aspects of DENV. These studies included those involved with the effect of natural and experimental infection, studies of the immune response of animals infected with the virus, and studies evaluating a number of vaccine candidates for DENV (2,17,18,30,33). The generation of specific antibodies to DENV and the kinetics of viremia in these monkeys were shown to be essentially similar to that observed in human infection by DENV (2). Studies with Aotus azarai infulatus also demonstrated the detection of specific antibodies to DENV-2 and the presence of viremia in these animals similar to that observed in humans infected by DENV.
NHPs infected by DENV are considered an acceptable animal model for the study of virological and immunological aspects of experimental infection. The only important exception for the use of monkey as an experimental model has been that these animals infected with DENV do not develop any detectable signs of the disease, including manifestations ranging from classical DF to more severe cases of DHF and DSS, which is characteristic of human infection with this virus (9,10,19,39).
Viremia is one of the major clinical manifestations of dengue virus infection. The present study showed that the animals in group 2 presented viremia for 6 days, while the other groups that were treated with CLQ had a gradual decline to undetectable levels of viremia when compared to the animals in group 2 (Table 2). This suggests that the drug was effective against the infection in the species of the animal studied.
Studies in vivo with NHPs are conducted almost exclusively with HIV. CLQ is currently being evaluated in clinical trials as a potential antiretroviral drug in humans. Chen et al. (5) observed that the drug is safe from HIV infection and improves some immunological parameters in HIV positive patients. Heimlich et al. (11) demonstrated that treating HIV-positive patients with CLQ resulted in an increase in TCD4+ cell counts in these patients. In a study conducted in India with 18 HIV positive patients with TCD4+ cell counts >350/mm3 who received lamivudine, hydroxyurea, and CLQ (250 mg) twice daily for 6 months, there was a statistically significant reduction in viral load, whereas the patients had undetectable levels of viral DNA and an increase in TCD4+ cell count (15). Studies have supported the hypothesis that CLQ may have an application in preventing the transmission of HIV from mother to child through breast milk. These studies reported an accumulation of CLQ 234 times in colostrum cells of HIV-infected African mothers who took CLQ 100 mg per day, suggesting that the drug may be potentially active as an adjunct to antiviral prophylaxis postnatal transmission from mother to child, by decreasing the burden of HIV-1 in breast milk in geographic areas where transmission is higher (20). Onlamoon et al. (28) observed a peak of dengue viremia in infected rhesus monkeys from 3 to 7 days after infection.
Regarding the biochemical parameters, a decrease in the level of liver enzyme AST was observed in serum of animals treated with CLQ compared with the positive control by the fifth and sixth days after infection (Fig. 2A). No change was found in hematological parameters in the animals infected with DENV-2. The animals monitored did not show any other change in weight or temperature during the experiment. Similar results were observed by Onlamoon et al. (28) where the animals did not show any other apparent clinical symptoms, such as fever, inappetence, or lethargy.
DENV-2-infected monkeys (group 2) had significantly higher levels of IFN-γ and TNF-α. Recent studies demonstrated that dengue-infected individuals had significantly higher levels of TNF-α, IFN-γ, and MDA compared to controls (8,29,34). The groups of animals (3, 4, and 5), that were infected and treated with the drug had a statistically significant reduction in the detection of IFN-γ and TNF-α (Fig. 1A and B).
Previous studies demonstrated that the blocking of proinflammatory cytokines by CLQ has been shown to be protective against inflammation and/or sepsis induced by LPS and DNA of Escherichia coli in mice (13). Karres et al. (16) showed that CLQ also inhibited cytokine release in human whole blood, an effect that could be beneficial in diseases that are related to inflammation induced by bacteria. These anti-inflammatory properties of CLQ may have protective effects in the treatment of patients with lupus erythematosus (41). Nishimura et al. (27) analyzed the immunosuppressive effect of CLQ as an effective potential to be exploited to improve the condition of patients with chronic graft-versus-host post-transfusion.
The response induced by the dengue virus may trigger the inflammatory cytokine responses in dengue severity. Preliminary results obtained from this study show that CLQ suppresses TNF-α and IFN-γ production, and it was hypothesized that CLQ may be used to treat patients suspected of having dengue disease, avoiding a severe form of the disease (dengue hemorrhagic fever) and/or shock (dengue shock syndrome). A recent study showed that chloroquine promoted a reduction in the intensity of pain and an improvement in the well-being of patients with dengue infection, but did not alter the duration of the disease or the intensity and days of fever. These results suggest that CLQ should be further tested in dengue disease with a larger number of patients and different CLQ dosages and times of administration to confirm the drug's clinical effect and to assess the side effects of CLQ in dengue patients (4).
The presence of IgG antidengue antibodies in the serum of animals shows that these animals are able to respond to infection of DENV, such as what occurs in humans. The absence of IgG antibodies specific to antidengue serum of animals that received CLQ before infection demonstrates the efficacy of this drug in the prophylactic treatment. In the group of animals that were treated with CLQ after infection, some individual responses were observed, showing that the drug was also effective in the therapeutic treatment of those animals.
Therefore, this model of NHP infection by DENV cannot only provide a valuable tool for the detailed study of this virus infection, but also can provide a comprehensive analysis of virus–host interactions, with the potential to lead to identification of cellular and molecular mechanisms that lead to dengue fever and the replication of this virus. Taken together with the data obtained in these studies, these results suggest that CLQ interferes in DENV-2 virus replication in Aotus monkeys. However, being a relatively safe drug, effective and easy to purchase, used in the treatment of many diseases, including malaria, its therapeutic use is promising because it has been shown that this drug has a significant inhibitory antiviral effect on the replication of DENV-2.
Acknowledgments
This study was supported by FAPESP (São Paulo Foundation for Research; Grant no. 05/04450-4). K.J.S.F. was supported by a FAPESP fellowship (Grant no. 06/55789-4).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Allison SL, Schalich J, Stiasny K, et al. . Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol 1995;69:695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair PJ, Kochel TJ, Raviprakash K, et al. . Evaluation of immunity and protective efficacy of a dengue-3 premembrane and envelope DNA vaccine in Aotus nancymae monkeys. Vaccine 2006;24:1427–1432 [DOI] [PubMed] [Google Scholar]
- 3.Bollati M, Alvarez K, Assenberg R, et al. . Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral Res 2010;87:125–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borges MC, Castro LA, and da Fonseca BAL. Chloroquine use improves dengue-related symptoms. Mem Inst Oswaldo Cruz 2013;108:596–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Xiao B, Xu H, et al. . Procedure and clinical assessments of malariotherapy: recent experience in 20 HIV patients. Chin Med J (Engl) 2003;116:1016–1021 [PubMed] [Google Scholar]
- 6.Christian G, Noble CG, and Shi P. Structural biology of dengue virus enzymes: towards rational design of therapeutics. Antiviral Res 2012;96:115–126 [DOI] [PubMed] [Google Scholar]
- 7.da Fonseca BA, and Fonseca SN. Dengue virus infections. Curr Opin Pediatr 2002;14:67–71 [DOI] [PubMed] [Google Scholar]
- 8.Gandini M, Reis SRNI, Torrentes-Carvalho A, et al. . Dengue-2 and yellow fever 17DD viruses infect human dendritic cells, resulting in an induction of activation markers, cytokines and chemokines and secretion of different TNF-α and IFN-α profiles. Mem Inst Oswaldo Cruz 2011;106:594–605 [DOI] [PubMed] [Google Scholar]
- 9.Guirakhoo F, Pugachev K, Arroyo J, et al. . Viremia and immunogenicity in nonhuman primates of a tetravalent yellow fever-dengue chimeric vaccine: genetic reconstructions, dose adjustment, and antibody responses against wild-type dengue virus isolates. Virology 2002;298:146–159 [DOI] [PubMed] [Google Scholar]
- 10.Guy B, Barban V, Mantel N, et al. . Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg 2009;80:302–311 [PubMed] [Google Scholar]
- 11.Heimlich HJ, Chen XP, Xiao BQ, et al. . Malariotherapy for HIV patients. Mech Ageing Dev 1997;93:79–85 [DOI] [PubMed] [Google Scholar]
- 12.Holmes EC, and Twiddy SS. The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 2003;3:19–28 [DOI] [PubMed] [Google Scholar]
- 13.Hong Z, Jiang Z, Liangxi W, et al. . Chloroquine protects mice from challenge with CpG ODN and LPS by decreasing proinflammatory cytokine release. Int Immunopharmacol 2004;4:223–234 [DOI] [PubMed] [Google Scholar]
- 14.Houng HSH, Chen RC, Vaughn DW, et al. . Development of a fluorogenic RT-PCR system for quantitative identification of dengue virus serotypes 1–4 using conserved and serotype-specific 3′ noncoding sequences. J Virol Methods 2001;95:19–32 [DOI] [PubMed] [Google Scholar]
- 15.Joshi SR, Butala N, Patwardhan MR, et al. . Low cost anti-retroviral options: chloroquine based ARV regimen combined with hydroxyurea and lamivudine: a new economical triple therapy. J Assoc Physicians India 2004;52:597–598 [PubMed] [Google Scholar]
- 16.Karres I, Kremer JP, Dietl I, et al. . Chloroquine inhibits proinflammatory cytokine release into human whole blood. Am J Physiol 1998;274:1058–1064 [DOI] [PubMed] [Google Scholar]
- 17.Kochel TJ, Raviprakash K, Hayes CG, et al. . A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine 2000;18:3166–3173 [DOI] [PubMed] [Google Scholar]
- 18.Kochel TJ, Watts DM, Gozalo AS, et al. . Cross-serotype neutralization of dengue virus in Aotus nancymae monkeys. J Infect Dis 2005;191:1000–1004 [DOI] [PubMed] [Google Scholar]
- 19.Koraka P, Benton S, van Amerongen G, et al. . Characterization of humoral and cellular immune responses in cynomolgus macaques upon primary and subsequent heterologous infections with dengue viruses. Microbes Infect 2007;9:940–946 [DOI] [PubMed] [Google Scholar]
- 20.Kourtis AP, Lee FK, Abrams EJ, et al. . Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis 2006;6:726–732 [DOI] [PubMed] [Google Scholar]
- 21.Kuhn RJ, Zhang W, Rossmann MG, et al. . Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 2002;108:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozach PY, Burleigh L, Staropoli I, et al. . Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DCSIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem 2005;280:23698–23708 [DOI] [PubMed] [Google Scholar]
- 23.Marchette NJ, Halstead SB, Falkler WA Jr, et al. . Nash, D. Studies on the pathogenesis of dengue infection in monkeys. 3. Sequential distribution of virus in primary and heterologous infections. J Infect Dis 1973;128:23–30 [DOI] [PubMed] [Google Scholar]
- 24.Marianneau P, Steffan AM, Royer C, et al. . Differing infection patterns of dengue and yellow fever viruses in a human hepatoma cell line. J Infect Dis 1998;178:1270–1278 [DOI] [PubMed] [Google Scholar]
- 25.Miller JL, Dewet BJ, Martinez-pomares L, et al. . The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog 2008;4:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monath TP, and Burke DS. Flaviviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, and Straus SE, eds. Fields Virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2001:1043–1126 [Google Scholar]
- 27.Nishimura M, Hidaka N, Akaza T, et al. . Immunosuppressive effects of chloroquine: potential effectiveness for treatment of post-transfusion graft-versus-host disease. Transfus Med 1998;8:209–214 [DOI] [PubMed] [Google Scholar]
- 28.Onlamoon N, Noisakran S, Hsiao HM, et al. . Dengue virus-induced hemorrhage in a nonhuman primate model. Blood 2010;115:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto LMO, Oliveira SA, Braga ELA, et al. . Increased pro-inflammatory cytokines (TNF-α and IL-6) and anti-inflammatory compounds (sTNFRp55 and sTNFRp75) in Brazilian patients during exanthematic dengue fever. Mem Inst Oswaldo Cruz 1999;94:387–394 [DOI] [PubMed] [Google Scholar]
- 30.Raviprakash K, Ewing D, Simmons M, et al. . Needle-free biojector injection of a dengue virus type 1 DNA vaccine with human immunostimulatory sequences and the GM-CSF gene increases immunogenicity and protection from virus challenge in Aotus monkeys. Virology 2003;315:345–352 [DOI] [PubMed] [Google Scholar]
- 31.Rey FA. Dengue virus envelope glycoprotein structure: new insight into its interactions during viral entry. Proc Natl Acad Sci U S A 2003;100:6899–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolain J-M, Colson P, and Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents 2007;30:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiavetta AM, Harre JG, Wagner E, et al. . Variable susceptibility of the owl monkey (Aotus nancymae) to four serotypes of dengue virus. Contemp Top Lab Anim Sci 2003;42:12–20 [PubMed] [Google Scholar]
- 34.Soundravally R, Hoti SL, Patil SA, et al. . Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int J Infect Dis 2014;18:68–72 [DOI] [PubMed] [Google Scholar]
- 35.Stiasny K, Allison SL, Mandl CW, et al. . Role of metastability and acidic pH in membrane fusion by tick-borne encephalitis virus. J Virol 2001;75:7392–7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Styer LM, Kent KA, Albright RG, et al. . Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog 2007;3:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tassaneetrithep B, and Burgess TH, Granelli-piperno A, et al. . DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 2003;197:823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valecha N, Joshi H, Eapen A, et al. . Therapeutic efficacy of chloroquine in Plasmodium vivax from areas with different epidemiological patterns in India and their Pvdhfr gene mutation pattern. Trans R Soc Trop Med Hyg 2006;424:1–7 [DOI] [PubMed] [Google Scholar]
- 39.Velzing J, Groen J, Drouet MT, et al. . Induction of protective immunity against dengue virus type 2: comparison of candidate live attenuated and recombinant vaccines. Vaccine 1999;17:1312–1320 [DOI] [PubMed] [Google Scholar]
- 40.Vincent MJ, Bergeron E, Benjannet S, et al. . Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wozniacka A, Lesiak A, Narbutt J, et al. . Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 2006;15:268–275 [DOI] [PubMed] [Google Scholar]
- 42.Zompi S, and Harris E. Animal models of dengue virus infection. Viruses 2012;4:62–82 [DOI] [PMC free article] [PubMed] [Google Scholar]