Abstract
Dietary intervention to prevent inflammation and atherosclerosis has been a major focus in recent years. We previously reported that sesame oil (SO) was effective in inhibiting atherosclerosis in low-density lipoprotein-receptor negative mice. We also noted that the levels of many proinflammatory markers were lower in the SO-treated animals. In this study we tested whether the non-lipid, aqueous components associated with SO would have anti-inflammatory and antioxidant effects. Polymerase chain reaction array data indicated that sesame oil aqueous extract (SOAE) was effective in reducing lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophage cells. Expression of inflammatory cytokines such as interleukin (IL)-1α, IL-6, and tumor necrosis factor α (TNF-α) was also analyzed independently in cells pretreated with SOAE followed by inflammatory assault. Effect of SOAE on TNF-α-induced MCP-1 and VCAM1 expression was also tested in human umbilical vein endothelial cells. We observed that SOAE significantly reduced inflammatory markers in both macrophages and endothelial cells in a concentration-dependent manner. SOAE was also effective in inhibiting LPS-induced TNF-α and IL-6 levels in vivo at different concentrations. We also noted that in the presence of SOAE, transcription and translocation of NF-kappaB was suppressed. SOAE was also effective in inhibiting oxidation of lipoproteins in vitro. These results suggest the presence of potent anti-inflammatory and antioxidant compounds in SOAE. Furthermore, SOAE differentially regulated expression of scavenger receptors and increased ATP-binding cassette A1 (ABCA1) mRNA expression by activating liver X receptors (LXRs), suggesting additional effects on lipid metabolism. Thus, SOAE appears multipotent and may serve as a valuable nonpharmacological agent in atherosclerosis and other inflammatory diseases.
Key Words: : endothelial cells, lipoproteins, macrophages, sesame oil
Introduction
Atherosclerosis is a disease in which chronic inflammation is considered as an independent risk factor.1–3 A number of inflammatory markers such as cytokines (interleukin [IL]-1, IL-6, tumor necrosis factor [TNF], IL-12, and IL-18), adhesion molecules, C-reactive protein (CRP), and enzymes (myeloperoxidase [MPO] and secretory phospholipase A2 [sPLA2]) have been found to be highly expressed in human and animal atherosclerotic lesions.4–7 There is also considerable evidence for the involvement of immune and inflammatory response in atherosclerosis. It is well established that monocyte chemoattractant protein-1 (MCP-1), a chemokine, is produced to recruit monocytes8,9 to the intima and adhesion molecules increase their attachment to activated endothelial cells.10,11 Monocyte-macrophage heterogeneity12,13 adds to the complexity of the process wherein proinflammatory monocytes augment the immune response through the expression of proinflammatory cytokines and other factors such as proteases.14–16 Thus anti-inflammatory therapy as a means to control atherosclerosis has long been in consideration.
Sesame oil (SO) obtained from Sesamum indicum is rich in both monounsaturated and polyunsaturated fatty acids. It contains approximately 47% oleic acid and 39% linoleic acid.17,18 It also contains lignans such as sesamin and sesamolin and several antioxidant compounds such as sesamol and sesaminol19 and other methylenedioxyphenol derivatives. SO has been reported to help reduce high blood pressure and lower the amount of medication needed to control hypertension.20,21 Other beneficial effects include reduction in plasma cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride (TG) levels.22 Our earlier studies in SO-diet fed LDLR−/− mice showed that the plasma levels of total cholesterol, TG, very low-density lipoprotein cholesterol, and LDL cholesterol were decreased while high-density lipoprotein (HDL) was significantly increased in these animals compared with atherosclerotic diet-fed animals. The SO-containing diet effectively prevented atherosclerosis lesion formation in LDLR−/− male mice.23 The fatty acid composition of SO is roughly in between those of olive and sunflower oils (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/jmf), and yet the observed level of inhibition of atherosclerosis was remarkable. This prompted us to wonder whether components in addition to fatty acid unsaturation could be responsible for the observed level of inhibition.
In this study, we tested whether the aqueous extract of SO might be effective in inhibiting inflammation. We also studied its effect on regulating lipid metabolism by studying expression of reverse cholesterol transport (RCT) genes and on macrophage scavenger receptors as a complex interplay of all these factors are involved in atherosclerosis.
Materials and Methods
Detailed description of methods is available in Supplementary Materials and Methods.
Cell culture
RAW 264.7 cells and human umbilical vein endothelial cells (HUVECS) were obtained from ATCC, HepG2-LXR reporter cell line was obtained from System Biosciences and maintained according to supplier's instructions in RPMI 1640, M199, and DMEM mediums respectively.
Isolation and culture of mouse peritoneal macrophages
Eight-week-old Swiss Webster mice were purchased from Charles River (Wilmington, MA, USA) and used for the studies.
Preparation and analysis of aqueous extract
Sesame oil aqueous extract (SOAE) was prepared by using SO and distilled water. Aqueous portion was separated by filtration and lyophilized. Lyophilized sample was reconstituted with pyrogen-free water. Absence of possible endotoxin contamination of SOAE was confirmed using limulus assay.
Isolation and oxidation of lipoproteins
Lipoproteins were isolated from normal plasma by sequential ultracentrifugation.24,25 They were subjected to oxidation with 5 μM copper or 0.2 U MPO with or without 100 μM tyrosine in the presence and absence of SOAE. The formation of conjugated dienes was monitored at an optical density of 234 nm. LDL oxidation was assessed by leucomethylene blue (LMB) assay and thiobarbituric acid reactive substances (TBARS). Minimally modified LDL (mm-LDL) was prepared as described previously.26
Polymerase chain reaction array analysis
RAW 264.7 cells were pretreated with SOAE (500 μg/mL) followed by addition of lipopolysaccharide (LPS, 10 ng/mL). Cells were incubated for 24 h. RNA was extracted and polymerase chain reaction (PCR) array analysis was performed using the atherosclerosis array of Qiagen (Valencia, CA, USA).
Inflammatory cytokine gene expression
Macrophages and HUVECS were pretreated with SOAE followed by addition of LPS or TNF-α for 24 h. Real-time (RT)-PCR analysis was performed for IL-1α, IL-6, and TNF-α gene expressions in macrophages. VCAM1 and MCP-1 expression was analyzed in HUVECs.
Enzyme-linked immunosorbant assay
Medium was collected from RAW 267.4 cells following treatment with SOAE and LPS. Fifty microliters of samples were analyzed using sandwich enzyme-linked immunosorbant assay (ELISA) kit (R & D systems, Minneapolis, MN, USA) following suppliers protocol.
NF-kappaB immunofluorescence assay
Cells were cultured on sterilized glass cover slips in 6-well plates. They were treated with LPS (10 ng/mL) or TNF-α (10 ng/mL)±SOAE (200 μg/mL) for 24 h. Cells were then stained following supplier's protocol. The coverslips were mounted on glass slides and viewed at 100× under oil immersion using Zeiss fluorescence microscope.
Luciferase assay for detection of liver X receptor activity
HepG2-LXR cells were treated with liver X receptor (LXR) agonist T0901317 (50 nM) or SOAE (100 and 300 μg/mL). Cells were incubated overnight and observed for GFP expression. Luciferase activity was determined using the luciferase assay system from Promega (Madison, WI, USA).
In vivo inflammation studies
Forty, six-week-old female C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) weighing approximately 20–22 g were divided into six groups: 12 animals in LPS group (6+6), four groups with 6 animals in each (LPS+SOAE), and 4 animals in control (SOAE 250 μg) group. To evaluate the effect of SOAE on LPS-induced inflammation, LPS (10 μg) and 10 μg LPS plus SOAE at varying concentration (10, 50, 100, and 250 μg/animal) were used. Animals were intraperitoneally injected at two different sites (200 μL/animal total injection volume) first with SOAE followed by LPS after half an hour. Blood and tissue samples were harvested from the animals 2 h after LPS injection. TNF-α and IL-6 cytokines were analyzed in mice serum samples using a sandwich ELISA kit (R & D systems).
Statistical analysis
Values are presented as mean±SD, and statistical analyses were performed by using Student t-test at significance of P<.05.
Experimental Results
PCR array analysis
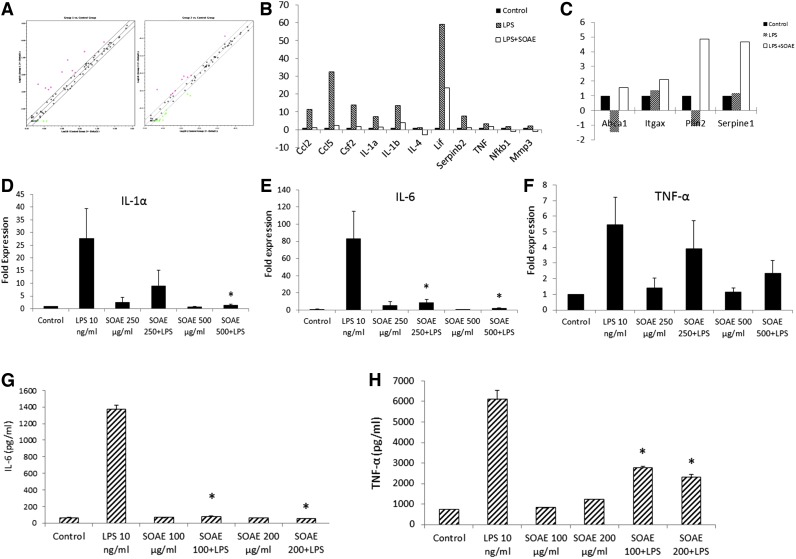
RAW cells were pretreated with 500 μg/mL of SOAE for 2 h followed by addition of LPS (10 ng/mL). RNA was isolated after 24 h and used for atherosclerosis PCR array analysis (Qiagen). PCR array results showed that SOAE inhibited numerous inflammatory markers that were induced by LPS. The scatter plot (Fig. 1A) reveals the difference in expression pattern of numerous genes between LPS and LPS+SOAE groups. Genes such as Ccl2, Ccl5, IL-1α, IL-1β, TNF, Nfkb1, and others were downregulated in the presence of SOAE (Fig. 1B). Genes that were upregulated in the presence of SOAE included ATP-binding cassette A1 (ABCA1), Itgax, Plin2, and Serpine1 (Fig. 1C).
FIG. 1.
SOAE inhibits LPS-induced inflammatory cytokines in RAW cells. RAW cells were pretreated with SOAE followed by treatment with LPS. Following 24 h of incubation, RNA was isolated. The Mouse Atherosclerosis PCR array (Qiagen) was carried out with these samples. Relative expression levels of genes in LPS (A, left panel) and LPS+SOAE (A, right panel) are plotted against control in the scatter plot. Genes that were distinctly downregulated (B) and upregulated (C) are represented as bar diagrams. Real-time PCR analysis was performed for IL-1α (D), IL-6 (E), and TNF-α (F). ELISA to detect mouse IL-6 (G) and TNF-α (H) secretion into the medium was also performed. SOAE significantly attenuated the expression of LPS-induced inflammatory markers. Results are represented as mean±SE from more than three independent experiments. *P<.05. ELISA, enzyme-linked immunosorbant assay; IL, interleukin; LPS, lipopolysaccharide; PCR, polymerase chain reaction; SOAE, sesame oil aqueous extract; TNF-α, tumor necrosis factor α. Color images available online at www.liebertpub.com/jmf
SOAE inhibits LPS-induced inflammatory cytokines in RAW cells
Expression of specific cytokines was tested separately. RAW cells were treated with LPS (10 ng/mL)±SOAE (250 and 500 μg/mL) for 24 h. LPS strongly induced mRNA levels of IL-1α (Fig. 1D), IL-6 (Fig. 1E), and TNF-α (Fig. 1F) in RAW cells. However, in the presence of SOAE, the expression of these inflammatory markers was significantly inhibited in a concentration-dependent manner. The extract alone did not induce any of the inflammatory cytokines.
ELISA for IL-6 and TNF-α
The medium from cells treated with LPS (10 ng/mL)±SOAE (100 and 200 μg/mL) were assayed for IL-6 and TNF-α levels. ELISA for IL-6 (Fig. 1G) and TNF-α (Fig. 1H) also supported mRNA expression results. IL-6 and TNF-α secretion in to the medium was enhanced when cells were treated with LPS alone. However, presence of SOAE significantly inhibited LPS-induced secretion of the cytokines from RAW cells in a concentration-dependent manner.
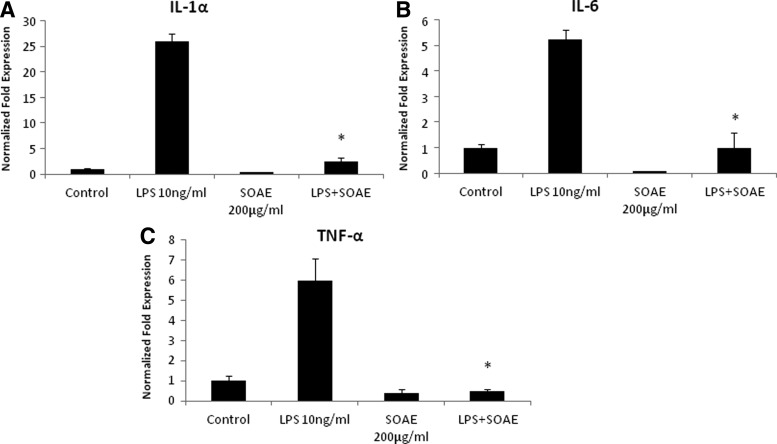
SOAE inhibits LPS-induced inflammatory cytokines in mouse peritoneal macrophages
Peritoneal macrophages were treated with LPS (10 ng/mL)±SOAE (200 μg/mL) for 24 h. RNA was isolated and gene expression of IL-1α, IL-6, and TNF-α was analyzed. Similar results as seen with RAW macrophage cell line were observed. The cells responded well to the positive stimulus of 10 ng/mL of LPS as seen by significant induction of IL-1α, IL-6, and TNF-α. In the presence of SOAE, the mRNA expression of these inflammatory cytokines was significantly inhibited (Fig. 2).
FIG. 2.
SOAE inhibits LPS-induced inflammatory cytokines in mouse peritoneal macrophages. Mouse peritoneal macrophages were pretreated with SOAE (200 μg/mL) followed by addition of 10 ng/mL LPS. Cells were incubated for 24 h following which RNA was isolated and real-time PCR analysis was performed for IL-1α (A), IL-6 (B), and TNF-α (C) gene expression (n=3; *P<.05).
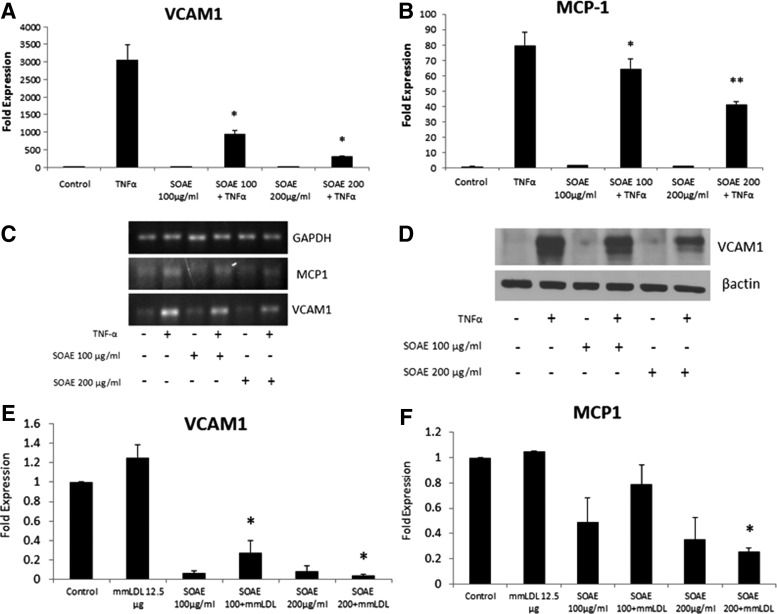
SOAE inhibits MCP-1 and VCAM1 in HUVECs
HUVECs were treated with TNF-α (10 ng/mL)±SOAE (100 and 200 μg/mL). Cells were harvested after 24 h and mRNA expression of VCAM1 and MCP-1 was analyzed. When cells were treated with TNF-α, strong induction of VCAM1 (Fig. 3A) and MCP-1 (Fig. 3B) mRNA expression was observed. However, SOAE was able to inhibit both markers in a concentration-dependent manner. Figure 3C represents PCR products on agarose gel. Western blot for VCAM1 (Fig. 3D) confirmed the results from real-time PCR. As mm-LDL is more physiologically relevant in atherosclerosis, we used it as another inducer of inflammation in HUVECs. SOAE was able to inhibit mm-LDL-induced VCAM1 (Fig. 3E) and MCP-1 (Fig. 3F) mRNA levels in endothelial cells.
FIG. 3.
SOAE inhibits VCAM1 and MCP-1 expression in HUVECs. HUVECs were pretreated with SOAE followed by addition of 10 ng/mL TNF-α. Cells were incubated for 24 h at the end of which total cellular RNA was extracted and reverse transcribed. Real-time PCR analysis was performed to analyze mRNA levels of VCAM1 (A) and MCP-1 (B). PCR products are represented in panel (C). Western blot analysis to determine VCAM1 protein expression was carried out (D). Similar experiments were carried out with HUVECs treated with mm-LDL and SOAE. Gene expression of VCAM1 (E) and MCP-1 (F) were analyzed. Results are representative of three experiments. Error bars represent SEM. *P<.05; **P<.01. HUVECs, human umbilical vein endothelial cells; LDL, low-density lipoprotein; MCP-1, monocyte chemoattractant protein-1; mm-LDL, minimally modified LDL.
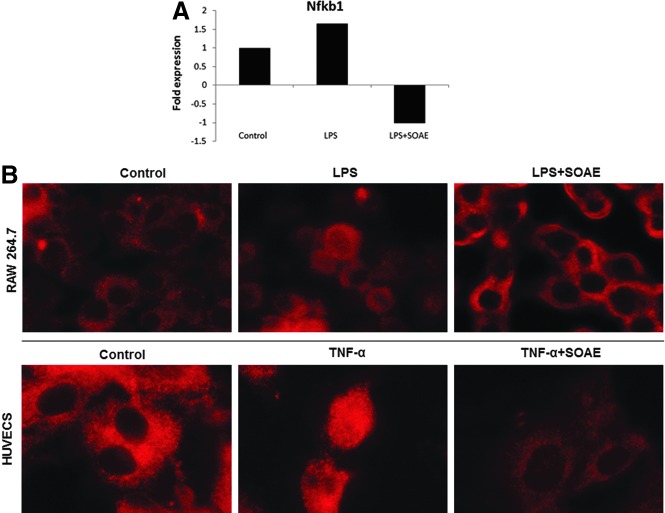
Inhibition of NF-kappaB transcription and translocation in the presence of SOAE
NF-kappaB is involved in LPS-induced expression of many of the inflammatory cytokines. PCR array results indicated suppression of NF-kappaB transcription in the presence of SOAE (Fig. 4A). We also studied the translocation of the transcription factor (p65) in the presence of SOAE. As shown in Figure 4B, immunofluorescence staining of RAW cells and HUVECs showed reduced NF-kappaB translocation into the nucleus thus attenuating LPS and TNF-α-induced inflammatory markers respectively.
FIG. 4.
SOAE inhibits NF-kappaB transcription and translocation. PCR array results showed that SOAE inhibited LPS-induced NF-kappaB mRNA levels in RAW 264.7 cells (A). Immunofluorescence staining revealed that SOAE inhibited NF-kappaB translocation to the nucleus in both macrophages (B, upper panel) and HUVECs (B, lower panel). Images are at 100× and are representative of three independent experiments.
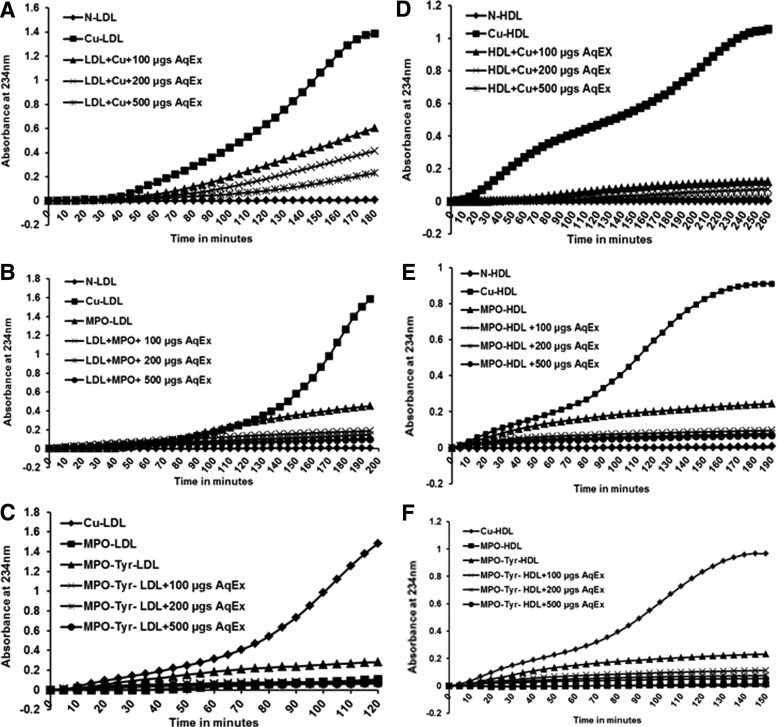
SOAE inhibits oxidation of LDL and HDL by Cu and MPO
Oxidized form of LDL is proatherogenic as it is internalized by macrophages leading to cholesterol accumulation and foam cell formation.27 LDL was subjected to oxidation and the formation of conjugated dienes was measured by following increase in absorption at 234 nm. As shown in Figure 5A, in the presence of increasing amounts of SOAE there was an increase in lag time, suggesting that even low concentrations of SOAE were able to delay the oxidation rate. Similarly, SOAE inhibited the oxidation of LDL by MPO (Fig. 5B) and by MPO and tyrosine (Fig. 5C). LMB, TBARS assay, and electrophoretic mobility results also corroborated with the oxidation curves (data not shown).
FIG. 5.
SOAE inhibits oxidation of lipoproteins. LDL and HDL were isolated from human plasma and oxidation was carried out using copper (A, D); MPO (B, E) and MPO with tyrosine (C, F) in the presence or absence of SOAE. In all cases, SOAE effectively delayed or inhibited oxidation of lipoproteins in a concentration-dependent manner. HDL, high density lipoprotein; MPO, myeloperoxidase.
SOAE was also tested on HDL oxidation in presence and absence of 5 μM copper (Fig. 5D) or 0.2 U MPO (Fig. 5E) or MPO with tyrosine (Fig. 5F). Similar results as with Ox-LDL were observed.
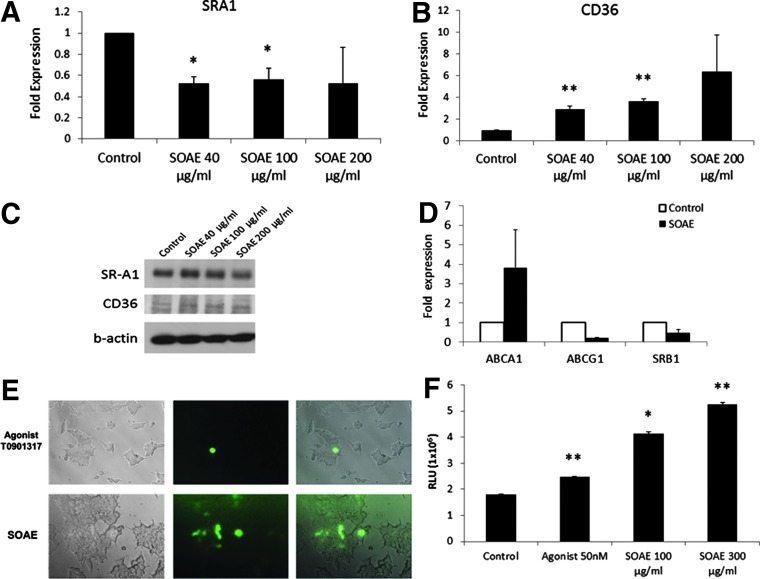
Effect of SOAE on the expression of scavenger receptors
Both class A scavenger receptor (SR-A1) and class B scavenger receptor (CD36) have been recognized for their involvement in atherogenesis.28,29 When RAW cells were treated with SOAE, the mRNA expression of scavenger receptor A1 (SR-A1) was inhibited (Fig. 6A). However, the mRNA levels of CD36 was significantly increased in a concentration-dependent manner (Fig. 6B). The protein expression of the scavenger receptors corroborated with the mRNA expression pattern as confirmed by western blot analysis (Fig. 6C).
FIG. 6.
Differential regulation of expression of scavenger receptors and reverse cholesterol transport genes by SOAE. RAW cells were treated with increasing concentration of SOAE following which expression of SR-A1 and CD36 was analyzed. While SR-A1 mRNA expression was found to decrease with increasing concentration of SOAE (A), CD36 mRNA expression markedly increased (B). The protein expression of SRA1 and CD36 (C) corroborated with the real-time PCR results. The effect of SOAE on reverse cholesterol transport genes was analyzed by real-time PCR (D). Using HepG2-LXR reporter system the effect of SOAE on GFP expression was assayed; the leftmost panel are the bright field images; the middle panel represent fluorescence images; and rightmost panel are the merged images (E). Luciferase activity (F) was also assayed in these cells. Results are average of three independent repeats. *P<.05; **P<.01. LXR, liver X receptor; SR-A1, scavenger receptor A1.
Effect of SOAE on RCT genes
RCT is a mechanism that involves the transport of cholesterol from peripheral tissues to the liver for excretion. Increased RCT is desirable for atherosclerotic plaque regression. ABCA1, ABCG1, and SR-B1 are RCT genes known to be expressed by macrophages.30 Both PCR array analysis and independent gene studies showed that SOAE significantly upregulated ABCA1 in RAW cells (Figs. 1C and 6D). However, there was no significant effect on ABCG1 or SR-B1 (Fig. 6D).
SOAE activates LXRs in HepG2 cells
When HepG2-LXR reporter cell lines were incubated in the presence of SOAE, increased GFP expression (Fig. 6E) was observed, which indicated LXR activation. Increased luciferase activity was seen in the presence of SOAE (Fig. 6F) thus confirming LXR activation by SOAE.
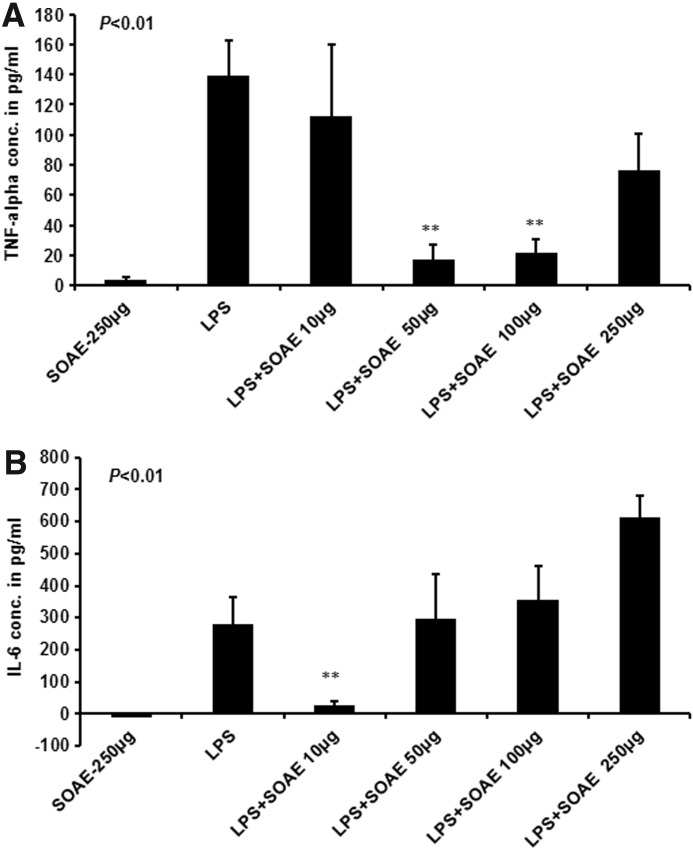
In vivo inflammation studies
To determine whether SOAE would reduce LPS-induced inflammation in mice, animals were injected with LPS alone versus pretreatment with SOAE followed by LPS injection. TNF-α and IL-6 cytokine levels were measured in serum after 2 h. As shown in Figure 7A, pretreatment of mice with 50 and 100 μg SOAE (P<.003) showed a significant reduction in LPS- induced TNFα levels compared with LPS-treatment alone. A similar result was observed with IL-6 at 10 μg SOAE (P<.004). However, a dose-dependent increase in IL-6 (Fig. 7B) levels was observed at concentrations higher than 10 μg SOAE.
FIG. 7.
TNF-α and IL-6 ELISA for mice serum samples. TNF-α and IL-6 levels were measured by ELISA from serum of mice as described in methods. As shown in (A), pretreatment of mice with 50 μg and 100 μg SOAE (**P<.003) showed a significant reduction in LPS-induced TNFα levels compared with LPS-treatment alone. A similar result was observed with IL-6 at 10 μg SOAE (**P<.004). However, a dose-dependent increase in IL-6 (B) levels was observed at concentrations higher than 10 μg SOAE.
Discussion
Vegetable oils have been extensively studied for their wide range of beneficial properties. Our previous studies showed that administration of SO to atherosclerotic mice not only reduced the blood lipids by 50% but also prevented atherosclerosis by over 85%. These effects could be mediated either by the fatty acid components or by the unusual non-saponifiable components. In this study, we primarily tested whether the aqueous components of SO could be anti-inflammatory. We have developed a unique and easy methodology to separate the nonlipid components of SO. The SOAE was characterized by UV spectral analysis along with sesamol and sesamin. After confirming the absence of considerable lipid content (by TLC) or protein content (by SDS PAGE gel), the aqueous extract was used for in vitro studies. This ensured that we eliminated vitamin E or vitamin E-associated effects in the fraction tested. LPS, mm-LDL, and TNF-α were used as inducers of inflammation. Bacterial LPS is an essential component of the membrane of Gram-negative bacteria and is the primary factor associated with the pathogenesis of sepsis.31,32 LPS exerts its toxic effects by potently activating macrophages and endothelial cells, and inducing the expression of inflammatory cytokines such as TNF-α and IL-6.33,34 According to the oxidation hypothesis, LDL in the intima undergoes oxidative modification to different degrees. The modified lipoprotein is considered more proinflammatory and proatherogenic due to the presence of increased peroxides.35
Our current observations show that SOAE has potent anti-inflammatory properties as seen with LPS-induced inflammation in RAW macrophages or mouse peritoneal macrophages. The atherosclerosis PCR array revealed downregulation of a number of inflammatory markers such as Ccl2 or MCP-1, Ccl5 or RANTES, IL-1α, IL-1β, and TNF in the presence of SOAE. Other targets that are thought to play a role in the atherosclerotic disease process such as colony stimulating factor 2 (Csf2), Nfkb1, an important proinflammatory transcription factor, and matrix metalloproteinase 3 (Mmp3) were also suppressed. Independent analysis also revealed significant inhibition of IL-6 expression in the presence of SOAE. An acute phase response is known to be elicited by IL-6 through the release of CRP, which is an inflammatory biomarker of cardiovascular risk.36,37 Thus, components in SOAE might have the potential to regulate many inflammatory pathways. This anti-inflammatory property was unique to SOAE as aqueous extract of sunflower oil did not show any effect on LPS-induced inflammatory markers in macrophages (data not shown). SOAE was also effective in attenuating mm-LDL/TNFα-induced inflammation in HUVECs.
As activation of NF-kappaB transcription factor is primarily involved in directing LPS response,38,39 we wanted to determine whether SOAE was mediating its anti-inflammatory effects through NF-kappaB inhibition. While data from the PCR array already revealed downregulation of NF-kappaB in the presence of SOAE, immunofluorescence assays showed that SOAE inhibited translocation of NF-kappaB from cytoplasm in to the nucleus in both LPS-induced macrophages and TNF-α-induced HUVECs. Thus, it is clear that SOAE has components that inhibit inflammation through blocking NF-kappaB activation. SOAE also effectively inhibited oxidation of LDL and HDL by Cu, MPO, or MPO in the presence of tyrosine. While oxidized LDL promotes foam cell formation,40 oxidation of HDL compromises the function of the latter in RCT and its anti-inflammatory properties.41 Components in SOAE could hence preserve the nature of lipoproteins by preventing oxidation.
Besides its anti-inflammatory and antioxidant effects, we also observed that SOAE affected lipid metabolism by regulating genes involved in RCT. It specifically upregulated ABCA1 as evidenced by mRNA expression in RAW cells. No significant changes were observed in ABCG1 or SR-B1 mRNA levels. LXRs are nuclear receptors that act as transcription factors and have been identified to be involved in the induction of ABCA1 and ABCG1.42,43 Luciferase assays in HepG2-LXR reporter cells confirmed activation of LXRs by SOAE. Thus, components in SOAE have the potential to activate specific RCT mechanisms that could be vital in plaque regression.
Effect of SOAE on scavenger receptors namely SRA1 and CD36 was interesting as it downregulated the former while expression of CD36 was significantly increased. While LPS has been shown to induce macrophage scavenger receptor A44 and the latter is indeed required for LPS-induced activation of NF-kappaB,45 it is highly possible that SOAE could be attenuating LPS-mediated effects by decreasing SR-A expression in macrophages. On the other hand CD36, a multifunctional receptor, is reported to be strongly downregulated by LPS in human peripheral blood monocytes46 while our data show increase in CD36 transcripts and protein expression with SOAE in macrophages. In the event of an infection this could be beneficial as SOAE-mediated upregulation of CD36 could ensure pathogen elimination and suppress inflammation. Hence, compounds acting as LPS-antagonists could be present in SOAE thus providing protection against inflammation. Although one can argue that CD36 is also a receptor for oxidized LDL47 and the uptake of the latter can lead to foam cell formation, we propose that SOAE may restore steady state in macrophages by promoting RCT through ABCA1 via LXR. In addition, CD36 has recently been identified as a regulator of de novo cholesterol synthesis in hepatic tissue, which should limit cholesterol availability and lower plasma cholesterol levels.48
Thus, SOAE appears to contain components that possess a wide variety of beneficial effects. Testing of this fraction in animal models of atherosclerosis and other inflammatory diseases will provide valuable information on the efficacy of components of SOAE in vivo. Our in vivo studies showed that SOAE inhibited LPS-induced proinflammatory markers to varying degrees. The difference in the ability of SOAE to attenuate different inflammatory markers to different extents might depend on numerous factors including, the ability of the inflammatory agent to interact and dissociate from components present in the SOAE. Thus, SOAE is able to inhibit acute inflammation in animals. While identifying the specific components in SOAE will shed light on the nature of compounds, one has to keep in mind that the mixture might be a more potent nonpharmocological agent due to potential synergistic interaction among the components.
Dietary approaches to prevent, treat, or manage atherosclerosis are more desirable than pharmacological control as it is safe and healthy. A better understanding of the nature of the components and their mechanism of action could lead to the development of inexpensive and powerful “adjunct therapy” to existing medications. It would also lead to new class of drug development.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grant 5R01AT004106-05.
The authors thank Dr. Kathryn Young Burge for her assistance during in vivo study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hansson GK, Libby P: The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol 2006;6:508–519 [DOI] [PubMed] [Google Scholar]
- 2.Peter Libby P, Ridker PM, Maseri A: Inflammation and atherosclerosis. Circulation 2002;105:1135–1143 [DOI] [PubMed] [Google Scholar]
- 3.Packard RRS, Libby P: Inflammation in atherosclerosis: From vascular biology to biomarker discovery and risk prediction. Clin Chem 2008;54:24–38 [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Hennekens CH, Buring JE, Rifai N: C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–843 [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V: C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004;350:1387–1397 [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS: Biomarkers of cardiovascular disease: Molecular basis and practical considerations. Circulation 2006;113:2335–2362 [DOI] [PubMed] [Google Scholar]
- 7.Biasucci LM, Biasillo G, Stefanelli A: Inflammatory markers, cholesterol and statins: Pathophysiological role and clinical importance. Clin Chem Lab Med 2010;48:1685–1691 [DOI] [PubMed] [Google Scholar]
- 8.Quinn MT, Parthasarathy S, Fong LG, Steinberg D: Oxidatively modified low density lipoproteins: A potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci USA 1987;84:2995–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM: Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA 1990;87:5134–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Cybulsky MI, Gimbrone MA, Jr., Libby P: An atherogenic diet rapidly induces VCAM-1, a cytokine regulatable mononuclear leukocyte adhesion molecule, in rabbit endothelium. Arterioscler Thromb 1993;13:197–204 [DOI] [PubMed] [Google Scholar]
- 11.Galkina E, Ley K: Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 2007;27:2292–2301 [DOI] [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR: Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964 [DOI] [PubMed] [Google Scholar]
- 13.Selvarajan K, Moldovan L, Chandrakala AN, Litvinov D, Parthasarathy S: Peritoneal macrophages are distinct from monocytes and adherent macrophages. Atherosclerosis 2011;219:475–483 [DOI] [PubMed] [Google Scholar]
- 14.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, et al. : Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest 2007;117:195–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khallou-Laschet J, Varthaman A, Fornasa G, Compain C, Gaston AT, Clement M, et al. : Macrophage plasticity in experimental atherosclerosis. PLoS One 2010;5:e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson HM: Macrophages heterogeneity in atherosclerosis—implications for therapy. J Cell Mol Med 2010;14:2055–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grishina NL, Kuznetsov DI: Fatty acid composition of oils from different varieties of sunflower, peanut and sesame. Vopr Pitan 1970;29:81–88 [PubMed] [Google Scholar]
- 18.Sengupta A, Roychoudhury SK: Triglyceride composition of Sesamum indicum seed oil. J Sci Food Agric 1976;27:165–169 [DOI] [PubMed] [Google Scholar]
- 19.Egbekun MK, Ehieze MU: Proximate composition and functional properties of full fat and defatted beniseed (Sesamum indicum L.) flour. Plant Foods Hum Nutr 1997;51:35–41 [DOI] [PubMed] [Google Scholar]
- 20.Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV: Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta 2005;355:97–104 [DOI] [PubMed] [Google Scholar]
- 21.Sankar D, Rao MR, Sambandam G, Pugalendi KV: Effect of sesame oil on diuretics or Beta-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J Biol Med 2006;79:19–26 [PMC free article] [PubMed] [Google Scholar]
- 22.Satchithanandam S, Chanderbhan R, Kharroubi AT, Calvert RJ, Klurfeld D, Tepper SA, Kritchevsky D: Effect of sesame oil on serum and liver lipid profiles in the rat. Int J Vitam Nutr Res 1996;66:386–392 [PubMed] [Google Scholar]
- 23.Bhaskaran S, Santanam N, Penumetcha M, Parthasarathy S: Inhibition of atherosclerosis in low-density lipoprotein receptor-negative mice by sesame oil. J Med Food 2006;9:487–490 [DOI] [PubMed] [Google Scholar]
- 24.Chung BH, Wilkinson T, Geer JC, Segrest JP: Preparative and quantitative isolation of plasma lipoproteins: Rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J Lipid Res 1980;21:284–291 [PubMed] [Google Scholar]
- 25.Santanam N, Parthasarathy S: Paradoxical actions of antioxidants in the oxidation of low density lipoprotein by peroxidases. J Clin Invest 95:2594–2600, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrakala AN, Sukul D, Selvarajan K, Sai-Sudhakar C, Sun B, Parthasarathy S: Induction of brain natriuretic peptide and monocyte chemotactic protein-1 gene expression by oxidized low-density lipoprotein: Relevance to ischemic heart failure. Am J Physiol Cell Physiol 2012;302:C165–C177 [DOI] [PubMed] [Google Scholar]
- 27.Steinberg D: Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem 1997;272:20963–20966 [DOI] [PubMed] [Google Scholar]
- 28.Babaev VR, Gleaves LA, Carter KJ, Suzuki H, Kodama T, Fazio S, Linton MF: Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler Thromb Vasc Biol 2000;20:2593–2599 [DOI] [PubMed] [Google Scholar]
- 29.Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL: Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 2000;105:1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH: The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 2009;50Suppl:S189–S194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Amersfoort ES, Van Berkel TJC, Kuiper J: Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev 2003;16:379–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danner RL, Elin RJ, Hosseini JM, Wesley RA, Reilly JM, Parillo JE: Endotoxemia in human septic shock. Chest 1991;99:169–175 [DOI] [PubMed] [Google Scholar]
- 33.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S: LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol 2011;31:379–446 [DOI] [PubMed] [Google Scholar]
- 34.Marsh CB, Wewers MD: The pathogenesis of sepsis. Factors that modulate the response to gram-negative bacterial infection. Clin Chest Med 1996;17:183–197 [DOI] [PubMed] [Google Scholar]
- 35.Catapano AL, Maggi FM, Tragni E: Low density lipoprotein oxidation, antioxidants, and atherosclerosis. Curr Opin Cardiol 2000;15:355–363 [DOI] [PubMed] [Google Scholar]
- 36.Majello B, Arcone R, Toniatti C, Ciliberto G: Constitutive and IL-6-induced nuclear factors that interact with the human C-reactive protein promoter. EMBO J 1990;9:457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis: Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol 2009;54:2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayden MS, Ghosh S: Signaling to NF-kappaB. Genes Dev 2004;18:2195–2224 [DOI] [PubMed] [Google Scholar]
- 39.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND: Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol 2007;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witztum JL, Steinberg D: Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 1991;88:1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pirillo A, Uboldi P, Kuhn H, Catapano AL: 15-Lipoxygenase-mediated modification of high-density lipoproteins impairs SR-B1 and ABCA1- dependent cholesterol efflux from macrophages. Biochim Biophys Acta 2006;1761:292–300 [DOI] [PubMed] [Google Scholar]
- 42.Costet P, Luo Y, Wang N, Tall AR: Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 2000;275:28240–28245 [DOI] [PubMed] [Google Scholar]
- 43.Venkateswaran A, Repa JJ, Lobaccaro JM, Bronson A, Mangelsdorf DJ, Edwards PA: Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J Biol Chem 2000;275:14700–14707 [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald ML, Moore KJ, Freeman MW, Reed GL: Lipopolysaccharide induces scavenger receptor A expression in mouse macrophages: A divergent response relative to human THP-1 monocyte/macrophages. J Immunol 2000;164:2692–2700 [DOI] [PubMed] [Google Scholar]
- 45.Yu H, Ha T, Liu L, Wang X, Gao M, Kelley J, Kao R, Williams D, Li C: Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim Biophys Acta 2012;1823:1192–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yesner LM, Huh HY, Pearce SF, Silverstein RL: Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol 1996;16:1019–1025 [DOI] [PubMed] [Google Scholar]
- 47.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 1993;268:11811–11816 [PubMed] [Google Scholar]
- 48.Rodrigue-Way A, Caron V, Bilodeau S, Keil S, Hassan M, Lévy E, Mitchell GA, Tremblay A: Scavenger receptor CD36 mediates inhibition of cholesterol synthesis via activation of the PPARγ/PGC-1α pathway and Insig1/2 expression in hepatocytes. FASEB J 2014;28:1910–1923 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.