Abstract
Porcine epidemic diarrhea virus (PEDV), a coronavirus, can cause acute diarrhea and dehydration in pigs. In the current study, two positive monoclonal cell lines (5D7 and 3H4) specific for PEDV were established, and the immunoreactivity of the monoclonal antibodies was confirmed by immunofluorescence and dot-immunobinding assays. A method, termed antigen capture enzyme-linked immunosorbent assay (AC-ELISA), which used the monoclonal antibody 5D7 as the detecting antibody and rabbit antiserum of PEDV protein S as the capture antibody, was developed. Compared with the reverse transcription polymerase chain reaction method of detecting PEDV in fecal samples, AC-ELISA showed similar sensitivity and specificity. These results suggested that AC-ELISA would be useful for the diagnosis and epidemiological studies of PEDV.
Introduction
Porcine epidemic diarrhea virus (PEDV) is the pathogen of porcine epidemic diarrhea (PED), which is a highly contagious enteric disease of swine, characterized by watery diarrhea, which results in high morbidity in pigs all of ages and mortality in piglets (5). Since the first report in 1978 (13), there have been frequent outbreaks in many swine-raising countries, leading to severe economic losses in Asia, notably in China, Thailand, and Korea in recent years (4,16).
Considering that the clinical symptoms are the similar to transmissible gastroenteritis virus (TGEV), which is also a coronavirus (13), a diagnosis of PED cannot be made on the basis of clinical signs and histopathological lesions unless differential tests in the laboratory are performed (1). Many years of research PED have produced a variety of diagnostic methods, including immunofluorescence (IF) tests, immunohistochemical (IHC) techniques, and enzyme-linked immunosorbent assay (ELISA) (16), or etiological methods, such as direct electron microscopy. The development of molecular biology techniques has led to reverse transcriptase polymerase chain reaction (RT-PCR) and loop-mediated isothermal amplification (LAMP) methods being established, which are rapid and sensitive. However, ELISA is cost-effective and can be used as a rapid screening test for large numbers of samples during epidemics (10,17).
Because of the presence of maternal antibodies and immunization, and the fact that antibodies can be detected at least 1–2 weeks after infection, the antibody detection method is not always correlated and may delay a diagnosis of PED (3). Therefore, the information on a current epizootiological situation in a herd is best obtained by virus detection (15). There would be numerous viruses in the feces when the symptoms of watery diarrhea appear, and the fecal material is easy to collect at the onset of illness rather than taking intestinal contents from dead animals. Therefore, a method of detecting the virus in fecal samples is feasible for PED diagnosis.
In this study, two specific monoclonal antibodies (MAbs) against PEDV were developed and characterized, and an antigen capture ELISA (AC-ELISA) method was established using one of the MAbs to detect PEDV in fecal samples, which could be useful for routine examinations of field samples.
Materials and Methods
Preparation of anti-PEDV MAb
PEDV strain LJB/03(11) was propagated in Vero cells at 37°C in a CO2 incubator and passaged twice a week. Crude PEDV from infective culture fluid, from which cell debris had been removed by low-speed centrifugation at 2,000 g for 15 min, was pelleted by 10% (w/v) PEG-6000 precipitation overnight at 4°C and centrifuged at 50,000 g for 30 min at 4°C. The resulting pellet was resuspended in TE buffer (10 mM Tris and 1 mM EDTA, pH 8.4) and layered on top of a 25%, 40%, 50%, and 65% (v/v) discontinuous sucrose gradient prepared in TE buffer. The gradient was then centrifuged for 2.5 h at 100,000 g and 4°C (1,7). The virus band was collected, followed by detection by electron microscopy (7).
Spleen cells from mice that were immunized via intraperitoneal injection of purified PEDV (50 μg/mouse) were fused to SP2/0 myeloma cells in the presence of polyethyleneglycol (PEG) to produce MAbs according to established techniques (8). To screen the hybridomas antibody produced, the supernatant of the fusion cells was subjected to indirect ELISA, set up using cell culture supernatant from PEDV-infected Vero cells and taintless Vero cells. MAbs were isotyped using the mouse MAb isotyping kit (Sigma) according to the manufacturer's instructions. The mice were handled and maintained under strict ethical conditions according to international recommendations for animal welfare.
Indirect immunofluorescence assays
The MAbs were subjected to indirect immunofluorescence assays according to previously described methods, with modifications (12,18). Vero cells cultured on glass coverslips in 24-well plates were infected with PEDV at 37°C for 24 h. The cells were rinsed in phosphate-buffered saline (PBS) and fixed for 20 min at room temperature with 4% (w/v) paraformaldehyde in PBS, followed by blocking with 1% bovine serum albumin (BSA) in PBS for 1 h at 37°C. The cells were then incubated with hybridoma conditioned supernatants and fluoresceine isothiocyanate (FITC)-labeled goat antimouse IgG (1:100 dilution in 1% BSA) at 37°C for 1 h in succession. After staining the nucleus with propidium iodide, fluorescence microscopy (Leica) was used to detect the fluorescence signals of the sample.
Dot-ELISA assays
The cultured supernatant from Vero cells infected by PEDV or not was loaded onto the nitrocellulose (NC) membrane (Minipore), followed by complete drying at room temperature. After blocking the membrane with 5% nonfat dry milk at 37°C for 2 h, the membrane was sliced into strips and incubated with either the supernatant of the hybridoma culture or that of SP2/0 myeloma cell culture, at 37°C for 1 h. The membrane was further incubated for 1 h at 37°C with anti-HRP-labeled goat antimouse IgG (Zhongshan Company) diluted 1:2,000 and developed using a diaminobenzidine (DAB) substrate buffer at room temperature until an amethyst-colored signal was observed.
AC-ELISA
Purification of the antibody
The positive hybridoma cells were injected into mice intraperitoneally. The ascites fluid was then collected, and ELISA was used to determine its titer. The immunoglobulin fraction of the ascites fluid that showed the highest antibody titer was purified by caprylic saturated ammonium sulfate and affinity chromatography on a protein G column (GE Healthcare).
Optimal antibody dilutions
A system of double antibody sandwich ELISA test was optimized using purified rabbit polyclonal antibodies against the PEDV S protein (9) and the mouse MAb as the capture and detector antibody, respectively. Optimal antibody dilutions were determined by checkerboard titration according to a previous report (6). The wells of the ELISA plates were coated with 100 μL of 25× to 1,600× dilutions of rabbit polyclonal antibodies and incubated at 37°C for 2 h. They were plated and then washed with PBS Tween-20 (PBST) three times and blocked with 200 μL/well of 5% nonfat dry milk for 2 h at 37°C. After washing, PEDV suspensions cultured in Vero cells and the supernatant of Vero cells was added at 100 μL per well, followed by incubation for 1 h at 37°C. The MAb, serially diluted with PBS (from 1:100 to 1:6,400) was added across the plate after the washing steps. Finally, the wells were incubated with HRP-conjugated goat antimouse IgG (1:5,000) at 37°C for 40 min. The reactions were visualized by incubation with orthophenylenediamine (OPD), and absorbance was measured at 490 nm. The optimal dilutions and cutoff values were calculated as described in a previous report (6). The positive–negative threshold value was defined as mean (of 30 PEDV negative samples)±3 standard deviations (SD).
Specificity and sensitivity of AC-ELISA
The cross-reactivity with TGEV, porcine parvovirus (PPV), porcine rotavirus (PRV), and classic swine fever virus (CSFV), which also cause diarrhea, were checked using culture supernatants as antigens in AC-ELISA.
The cell cultures of PEDV (TCID50=103.3/mL) were diluted in serial twofold dilutions from 1:2 to 1:256; 100 μL of each mixture was tested by AC-ELISA, and RT-PCR was performed using primers specific for the membrane protein (M-protein), as previously described (11).
Field samples
One hundred and five fecal specimens of pigs with the diarrheic symptom collected from two farms were diluted in 2 vol of Dulbecco's modified Eagle's medium. After centrifugation (3,000 g for 10 min), the supernatants were collected and examined in parallel using RT-PCR and AC-ELISA.
Statistical analysis
The results of the ELISA are expressed as mean (three repeats of each sample)±SD. The agreement between PCR and ELISA techniques was measured using the kappa statistic value (17).
Results
Preparation of anti-PEDV MAb
Purification of PEDV
Isopycnic ultracentrifugation in a discontinuous sucrose gradient resulted in only one distinct virus band at the junction of the 50% and 65% sucrose gradient, which contained numerous morphologically intact virus particles when examined by electron microscopy. The virus showed classical characteristics of a surface displayed petal-like structure approximately 100 nm in size, which is the spike protein of PEDV (Fig. 1).
FIG. 1.
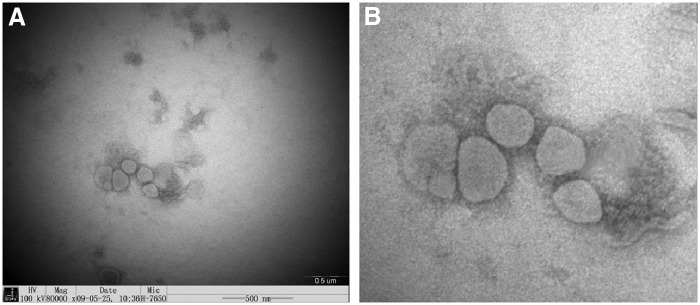
(A) Transmission electron microscope test of purified porcine epidemic diarrhea virus (PEDV; 80,000×). (B) Magnification of virions in (A) showing the spike of the virus particle clearly.
Identification of hybridoma cell lines
The result of indirect ELISA confirmed that two hybridoma cell lines, 5D7 and 3H4, were able to secrete stably MAbs specific only for PEDV. These two MAbs were immunotyped using ELISA, revealing that 3H4 was IgG1 and 5D7 was IgG2a. The titers of ascites fluid from 3H4 and 5D7 were 5×105 and 1×107, respectively; ascites fluid 5D7 was purified for use as a detector antibody in AC-ELISA.
Biological activity of the MAbs
The immunoreactivity of the MAbs to PEDV was analyzed by dot-ELISA and immunofluorescence. PED viral antigen suspensions and Vero cells cultures were spotted onto NC membranes. Incubation with 3H4 and 5D7 hybridoma suspensions showed strong positive signals for PEDV; Vero cells produced a negative reaction. By contrast, a negative signal for the presence of PEDV and Vero was observed with SP2/0 incubation (Fig. 2).
FIG. 2.
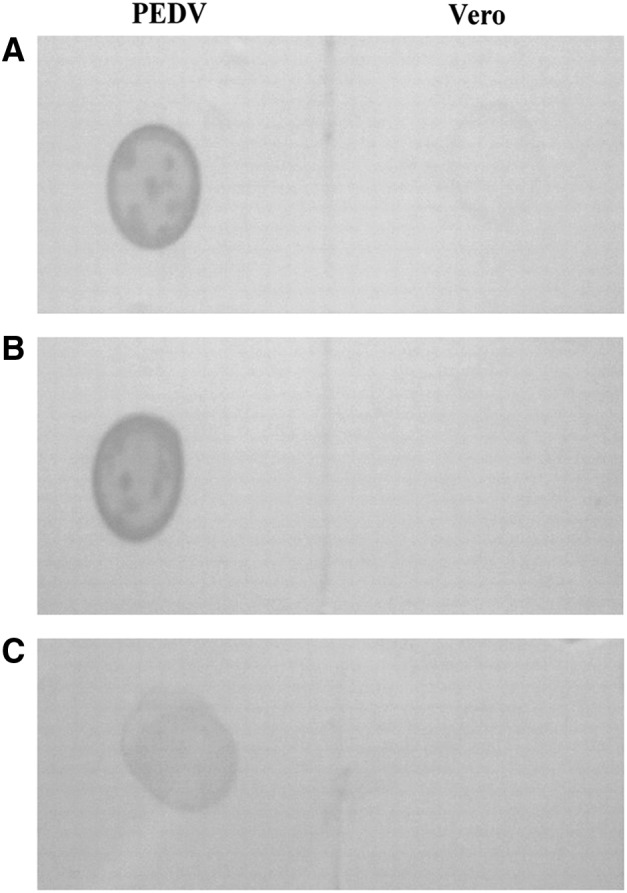
Dot-ELISA assay of monoclonal antibodies (MAbs). PEDV-containing cell culture fluid and cell cultures were spotted onto nitrocellulose membranes. The supernatants from hybridoma 3H4 (A), 5D7 (B), or SP2/0 (C) myeloma cells were used as primary antibodies. Distinct spots with (A) and (B) indicated a positive result, while fuzzy spots with (C) and the lack of a spot with Vero as the antigen indicated a negative result.
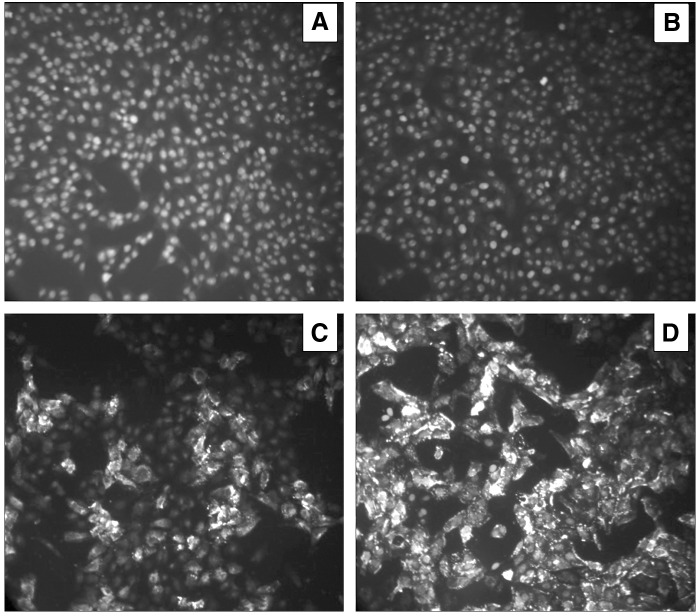
Vero cells infected by PEDV were subjected to immunofluorescence analysis, using normal cells with no infection as a negative control. Vero cells infected with PEDV showed strong green fluorescence in the cytoplasm and cell membranes after interacting with MAbs; the noninfected cells interacted with two MAbs did not show any fluorescence (Fig. 3), indicating that the MAbs against PEDV were highly specific to detect pathogenicity.
FIG. 3.
Indirect immunofluorescence assay of MAbs. MAbs 3H4 (C) and 5D7 (D), which recognized PEDV in the affected Vero cells, shown in white. In contrast, no fluorescence signals were detected on the mock-infected cells reacting with 3H4 (A) or 5D7 (B).
AC-ELISA
Optimal antibody dilutions
AC-ELISA by checkerboard titration was performed to select the optimal antibody dilution for test samples. Dilutions of 1:800 (4.0 ng/mL) for polyclonal antibodies and 1:800 (2.4 ng/mL) for the MAb were selected, respectively, which provided the widest window between the positive and negative antiserums. The negative cutoff value according to the mean value of negative fecal samples±3 SD was determined as 0.1046.
Specificity and sensitivity of AC-ELISA
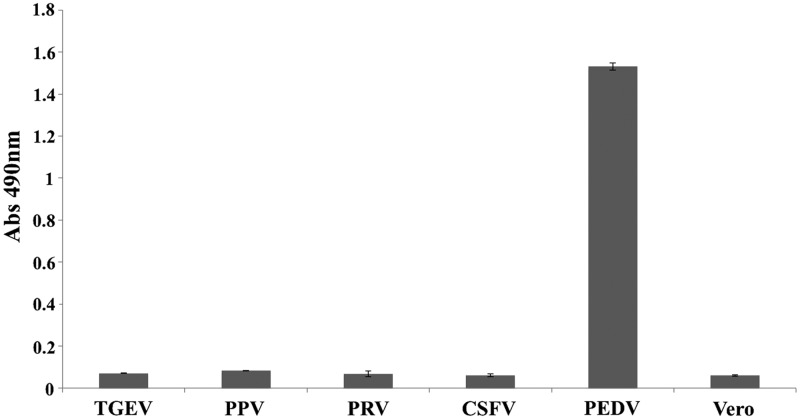
To check the specificity of AC-ELISA, the cell cultures of TGEV, PPV, PRV, and CSFV were detected, and no cross-reactivity was observed in the ELISA (Fig. 4). The results of RT-PCR and AC-ELISA were positive when the samples were diluted from 1:2 to 1:128 (TCID50=103.3/mL). Thus, the sensitivity of the AC-ELISA was determined to be 15.6 TCID50 (Table 1).
FIG. 4.
Specificity testing of antigen capture enzyme-linked immunosorbent assay (AC-ELISA). Several kinds of virus related to diarrhea disease—TGEV, PPV, PRV, CSFV, and PEDV—were tested by AC-ELISA; Vero cultures were used as the control. Data are the geometric means from duplicate samples. The error bars represent standard deviations.
Table 1.
Sensitivity of Antigen Capture-Enzyme-Linked Immunosorbent Assay
| Dilution of feces | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Method variant | 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | 1:256 | Negative control |
| A490 of AC-ELISA* | 0.533 | 0.199 | 0.208 | 0.179 | 0.141 | 0.139 | 0.110 | 0.090 | 0.087 |
| RT-PCR | + | + | + | + | + | + | + | − | − |
The OD of ELISA≥0.105 was considered as positive.
AC-ELISA, antigen capture-enzyme-linked immunosorbent assay; RT-PCR, reverse transcriptase polymerase chain reaction.
Field samples of feces
Among the 105 samples from two swine farms, 96 were positive by RT-PCR, and 93 were positive by AC-ELISA. The kappa value was 0.839, representing a moderate agreement, as shown in Table 2.
Table 2.
Comparison of RT-PCR and AC-ELISA for the Detection of PEDV in Fecal Samples
| RT-PCR | ||||
|---|---|---|---|---|
| ELISA | + | − | Total tested | Kappa |
| + | 93 | 0 | 93 | |
| − | 3 | 9 | 12 | |
| Total tested | 96 | 9 | 105 | 0.839 |
PEDV, porcine epidemic diarrhea virus.
Discussion
PEDV infection is an acute disease that poses a serious threat to the pig industry in China. It causes enteritis, with clinical signs similar to those caused by other viruses, leading to serious and continuous losses to the swine industry (14,19). Thus, more effective, sensitive, and quick diagnostic methods are required for the surveillance of PED. Considering its characteristics of mucosal infection and shedding, and the feasibility of clinical samples collection, the AC-ELISA method for virion detection in feces was chosen. In a previous study of ELISA for PEDV, comparing the effect of polyclonals and monoclonals as binding antibodies, the authors found lower sensitivity of ELISA with conjugated MAbs, despite their high specificity (15). In this study, to improve the sensitivity and specificity, high titer, purified polyclonal serum was used as capture antibodies, and the MAb of PEDV was used as the detecting antibody. There was no cross-reactivity in AC-ELISA when detecting TGEV, PRV, PPV, and CSFV. The sensitivity of AC-ELISA was 15.6 TCID50 (TCID50=103.3/mL), which was same as that of RT-PCR.
It is worth mentioning that although the MAbs obtained showed positive signals for PEDV by immunofluorescence assays, they showed negative signals by Western blotting. Western blotting was performed using the purified PEDV as antigen on the 12% SDS-PAGE, and using the supernatant of hybridomas as primary antibody and antimouse HRP conjugate as secondary antibody, there was no band shown on the NC membrane. The MAbs were then subjected to dot-ELISA assays, showing positive signals with PEDV, suggesting that the MAbs were probably novel and specific for spatial epitopes of PEDV, explaining the high specificity of this system.
On testing clinical samples from two farms, there was a substantial, but not complete, agreement (K=0.839) between PCR and ELISA (three samples were negative by AC-ELISA, but positive by RT-PCR). The three discordant samples were collected from the same farm, and the results might be explained by the presence in feces of specific antibodies that form immunocomplexes, or an excessive delay between collection and examination, resulting in sample degradation and loss of antigenic sites. The high fragility of coronaviruses in the contents of the intestines (2) has frequently been cited as an explanation of the lower sensitivity of ELISA in the clinical setting compared with RT-PCR. Additionally, more field samples need to be assessed to achieve statistical validity.
In conclusion, MAbs 5D7 and 3H4 against PEDV were produced, which are highly specificity and stable, as shown in Table 3. These MAbs might represent a useful molecular reagent for accurate diagnosis of PED. Using 5D7, a sensitive and specific AC-ELISA system was established, which is suitable for screening large numbers of specimens. The PEDV AC-ELISA system might be reliable for the diagnosis and collection of the epidemiological characteristics of PEDV.
Table 3.
Stability of Antigen Capture-Enzyme-Linked Immunosorbent Assay
| OD490 nm | Generation 1 | Generation 10 | Generation 20 | Generation 35 | Generation 50 | After frozen |
|---|---|---|---|---|---|---|
| 3H4 | 0.240±0.007 | 0.335±0.013 | 0.293±0.005 | 0.401±0.005 | 0.347±0.006 | 0.394±0.014 |
| 5D7 | 0.204±0.002 | 0.352±0.009 | 0.428±0.011 | 0.333±0.010 | 0.419±0.001 | 0.451±0.008 |
Value are mean±standard deviation (by three repeats).
Acknowledgments
This work was supported by Grant No. 2012AA101304-3 from the 863 Program and No. 30671574 from the National Natural Science Funds of China.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ashley CR, and Caul EO. Potassium tartrate-glycerol as a density gradient substrate for separation of small, round viruses from human feces. J Clin Microbiol 1982;16:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aynaud JM, and Bottreau E. Transmissible gastroenteritis of swine: stability of coronavirus in gastric and intestinal contents. Ann Rech Vet 1984;15:359–364 [PubMed] [Google Scholar]
- 3.Carvajal A, Lanza I, Diego R, et al. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J Vet Diagn Invest 1995;7:60–64 [DOI] [PubMed] [Google Scholar]
- 4.Chae C, Kim O, Choi C, et al. Prevalence of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus infection in Korean pigs. Vet Rec 2000;147:606–608 [DOI] [PubMed] [Google Scholar]
- 5.Ducatelle R, Coussement W, Pensaert MB, et al. In vivo morphogenesis of a new porcine enteric coronavirus, CV 777. Arch Virol 1981;68:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas B, Hinz KH, and Glunder G. Biotin-streptavidin enzyme-linked immunosorbent assay for the detection of antibodies to Campylobacter jejuni and C. coli in chickens. Zentralbl Veterinarmed B 1999;46:163–171 [DOI] [PubMed] [Google Scholar]
- 7.Hofmann M, and Wyler R. Quantitation, biological and physicochemical properties of cell culture-adapted porcine epidemic diarrhea coronavirus (PEDV). Vet Microbiol 1989;20:131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt R, Bragina O, Drews M, et al. Generation and characterization of mouse monoclonal antibody 5E1 against human transcription factor GLI3. Hybridoma (Larchmt) 2007;26:231–240 [DOI] [PubMed] [Google Scholar]
- 9.Jiang YP, Ge JW, Li YJ, et al. Prokaryotic expression of four S gene segments of porcine epidemic diarrhea virus and reactivity analysis of their expressed products. Chinese Vet Sci 2009;39:602–607 [Google Scholar]
- 10.Kumar J, Khan M, Gupta G, et al. Production, characterization, and application of monoclonal antibodies specific to recombinant (E2) structural protein in antigen-capture ELISA for clinical diagnosis of Chikungunya virus. Viral Immunol 2012;25:153–160 [DOI] [PubMed] [Google Scholar]
- 11.Mao YY, Zhang GH, Ge JW, et al. [Isolation and characteristics of virus culture of porcine epidemic diarrhea virus LJB/03]. Bing Du Xue Bao 2010;26:483–489 [PubMed] [Google Scholar]
- 12.Meng F, Yin J, Li X, et al. Production and characterization of a monoclonal antibody against spike protein of transmissible gastroenteritis virus. Hybridoma (Larchmt) 2010;29:345–350 [DOI] [PubMed] [Google Scholar]
- 13.Pensaert MB, and de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol 1978;58:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pijpers A, van Nieuwstadt AP, Terpstra C, et al. Porcine epidemic diarrhoea virus as a cause of persistent diarrhoea in a herd of breeding and finishing pigs. Vet Rec 1993;132:129–131 [DOI] [PubMed] [Google Scholar]
- 15.Rodák L, Valícek L, Smid B, et al. An ELISA optimized for porcine epidemic diarrhoea virus detection in faeces. Vet Microbiol 2005;105:9–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song D, and Park B. Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 2012;44:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sozzi E, Luppi A, Lelli D, et al. Comparison of enzyme-linked immunosorbent assay and RT-PCR for the detection of porcine epidemic diarrhoea virus. Res Vet Sci 2010;88:166–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sui X, Yin J, and Ren X. Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res 2010;85:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun D, Feng L, Shi H, et al. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet Microbiol 2008;131:73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]