Abstract
In this article, we identify and discuss a timeline of historical events and scientific breakthroughs that shaped the principles of tissue engineering and regenerative medicine (TERM). We explore the origins of TERM concepts in myths, their application in the ancient era, their resurgence during Enlightenment, and, finally, their systematic codification into an emerging scientific and technological framework in recent past. The development of computational/mathematical approaches in TERM is also briefly discussed.
Introduction
It is in the timbres of past that the future is echoed. This is particularly applicable to the realm of technology, which clearly has a cumulative character. The fact that a lot of technologies find their precedent in myths should therefore come as little surprise, for myths shape ideas, and ideas technologies. Tissue engineering and regenerative medicine (collectively TERM, henceforth), therapeutic techniques that rely on, both in vivo and ex vivo, reactivation of developmental processes to form products that can help alleviate healthcare problems related to the diseases of cellular deficiency,1 including loss of tissue/organ functionality due to trauma, share this template too. Where the objective of tissue engineering is to produce functional, preferably autologous, substitutes that can improve or replace a failing tissue2–5; regenerative medicine, as per Daar and Greenwood6 and Mason and Dunnill,7 aims to “repair, replace or regenerate cells, tissues, or organs to restore impaired function.”6–8 Already, despite the fields being in their nascence, examples abound of successful clinical implementation9: corneal surfaces,10 replacement of bronchial segment,11 reconstitution of bone,12 as well as cartilage13 defects and diseased bladder.14 On the same note, external support devices such as extracorporeal liver15 or engineered tissues that can serve as in vitro models to investigate pathogenesis, stem cell behavior, and developmental processes, in addition to developing new molecular therapeutics,16–18 further underpin the capacity for therapy and knowledge on offer.
The toolkit employed to achieve this includes, but is not limited to: (stem) cells, controlled release matrices (synthetic and natural), scaffolds, soluble molecules (nucleic acids, proteins, hormones, even viruses) directing cell function, and bioreactors. The applicability of each of these components is discussed in detail in The Principles of Tissue Engineering.19 There are of course slight differences distinguishing the two fields, which, according to Daar and Greenwood,6 include a broader range of methodological alternatives available to regenerative medicine, such as genetic engineering of cells followed by their transplantation and pharmaceutical targeting of developmental pathways. Nevertheless, they are treated collectively in this article since the underlying goals (repair, regeneration, replacement, and restoration of impaired function),6 tools (involving either isolated cells or substances that induce tissue growth, or cells placed on/encapsulated within a biocompatible scaffold),3 and, more importantly, principles (as we discuss in this article) are almost identical in both cases.
In this article, we explore the origins and historical development of the principles that are responsible for the availability of, possibly, the most promising healthcare technology ever put forward. Given space constraints, the events catalogued herein were chosen based on their relevance to the aforementioned toolkit. Additionally, the development of theoretical and computational concepts is discussed, albeit very briefly. We conclude with a concise review of the current status of TERM followed by a glance at some immediate and long-term frontiers of the future.
A Timeline of the Development of TERM
TERM in myth and art
While the principles of TERM acquired their current form in the late 20th century, conceptually—and even practically—these principles are as old as humanity. The concept of totipotencya first finds expression in the Hindu myth of Raktabeej (Rakta=blood; beej=seed); an Asurab whose blood drops could form his clones ex vivo.c Quite apt then that stem cells, some of which possess a totipotent character, were first reported in 1963 by the hematopoietic community.20 We first encounter, in Hesiod's Theogony (lines 507–616) circa 700 BC, in vivo regeneration in the Greek myth of Prometheus; a Titan who stole fire from Zeus to offer it to humanity and was sentenced to a lifelong cycle of misery where his liver, pecked out by an eagle, would regenerate each day to prolong his suffering. The application of a bioreactor to support and stimulate development ex vivo is first documented in the great Indian epic, Mahabharata, which tells the story of the birth of the Kaurava brothers. After 2 years of pregnancy, their mother produced a mass of flesh, which a sage divided into a hundred parts that were kept in pots treated with herbs and ghee (a form of butter)—the combination of both, presumably, serving as culture media—for 2 additional years.21 At the end of the second year, each pot produced a viable human, and thus, a hundred Kaurava brothers were born. The oldest preserved parts of the text are considered to be written circa 400 BC. This, quite possibly, also constitutes the first reference in human history to perfusion culture. In modern fiction, a bioreactor appeared in the laboratory scene of Faust Part II, published in 1832, as the phial employed to create the homunculus.22 Both the pots in Mahabharata and the phial in Faust were clearly meant to be used as devices that could provide controlled environments to support (ex vivo) development, much like a uterus would in vivo.
Rig Veda, considered to be compiled between 3500 and 1800 BC, contains the first mention of a biomaterial. Queen Vishpala, who was amputated in a battle, was fitted with an iron leg once her wound healed enabling her to return to the battlefield.d Another example of the use of a prosthesis occurs in the Greek story of Hegesistratus, who in order to escape his Spartan captors cuts off his own foot and later replaces it with a wooden one, as narrated in the ninth Book, Chapter 37, of the Histories by Herodotus. The article was published circa 400 BC.
Ancient applications of TERM principles
From the practical perspective, wound closure through the application of sutures has been known to exist since the Neolithic period (10,000 BC).23 The range of materials varied from synthetic (linen by Egyptians), to natural (catgut by Europeans), and biological (heads of large biting ants in the Indian subcontinent and South Africa).23 Skin grafts were first employed in the Indian subcontinent as early as 2500 BC,24 with Susruta, the father of Surgery, at the forefront of this technology. Susruta employed free gluteal fat and skin grafts to treat the mutilations of the ear, nose, and lip.24 Variations of the procedure, among others, included “slapping the skin of the buttock with a wooden paddle until it was quite congested and then, with a leaf cut to proper shape as a pattern, cutting out a piece of skin with its subcutaneous fat, transplanting it and sewing it into place, uniting it to the freshened edges of the defect.”25 The Papyrus of Ebers presented us with the most classical example of TERM; cell-supportive scaffold guiding regeneration: Egyptians, around 1500 BC, treated skin wounds using lint, grease, and honey,26 where honey served as the antibiotic—presumably due to its hypertonicity—and grease provided a barrier to the entry of pathogens26; lint acted as the fibrous scaffold that would promote wound regeneration.26 Metallic sutures have been documented to be in application since the second century AD in Greece and were described by the physician, surgeon, philosopher Galen of Pergamon (129–216).23
One of the earliest evidence of the use of biomaterials comes from a mummified body discovered in Egypt. Paleopathological examination of this mummified woman showed that the big toe of her right foot was amputated. The missing toe, however, was replaced by a “delicately manufactured” wooden one.27 Additional evidence of Iron being used as a biomaterial stretches as far back as the Gallo-Roman period. Crubezy et al.28 reported a skull from that era containing an osseointegrated dental implant of a right second upper premolar. Pieces of blue nacre shell, which achieved successful osseointegration,29 were employed as dental implants by the Mayans as early as 600 AD.23,30
Enlightenment and the growth of TERM principles
While a lot of practical information from the medieval era was lost, TERM concepts managed to survive as art forms and myths, refer to Figure 1, and with the advent of the age of Enlightenment started to regain lost momentum. The Enlightenment period (late 17th century to the end of 18th century) was the first significant milestone in the history of TERM; in many ways, it sparked the revolution that provided impetus for the birth of this technology. The Enlightenment era brought with it an emphasis on the empirical sciences, as well as a push toward objectivity and a reasoned approach to study nature.31 Experiments became an integral part of this reasoned approach.31 It was during this era that philosophers started investigating the phenomena of reproduction and regeneration to understand the origins and governing dynamics of life.
FIG. 1.
Tissue engineering and regenerative medicine in literature and art. (Left) The figure depicts transplantation of a leg by Saints Cosmas and Damian. The twin Christian martyrs were physicians and lived during the third century. The painting shown in the figure was created in the late 15th century. (Right) The figure shows the use of a phial, representing a bioreactor, in the engraving of Homunculus from Goethe's Faust, Part II.22 The play was published in 1832. Both figures belong to the public domain.
The mechanistic thought gained prominence with Rene Descartes, who concluded that “all natural objects were caused by inert particles of matter in motion,”31 followed by Sir Isaac Newton's understanding of man as a complex physicochemical machine and disease as a fault in that machine.32 The mechanical philosophy, thus, emerged as the medium to explore physiological phenomena. Giovanni Alfonso Borelli (1608–1678), for example (Fig. 2), explained muscular contraction as a hydraulic inflation.31 The mechanical philosophy was, however, unable to account for truly complex phenomena, such as regeneration, reproduction, or even growth. We need to be reminded though that even today, current computational and theoretical methodologies still struggle to account properly and capture some of this complexity. As a result, 18th century physiology, influenced by experimental physics and chemistry, adopted a more phenomenological approach.31
FIG. 2.
Mechanistic physiology. G.A. Borelli's De Motu Animalium (on the motion of animals) (1680) looks at the human body as a machine functioning as a system of levers and pulleys demonstrated in the figure along with leg joints and the action of muscles in balancing the body. The subfigures show Borelli's analysis of various joints in humans (adapted from Pope153). (1) Conjunction of two levers (IFS and HDR in the figure) at pivot point C. (2) Role of elastic bands (muscles) attached to the levers (at D and F) and the pivot (B) in bringing the levers closer together. (3) The role of the elastic bands in “expanding” the levers. (4) A system composed of multiple levers of unequal length. (5, 6) Muscle and bone configurations in humans carrying different loads. (7, 8) Investigations in pulley arrangements. (9, 10) Muscular action that enables a human to hold a weight with an extended arm. While the mechanistic approach was able to explain the motions of bones, muscles, and, even, the heart, it was not able to capture the vital functions of the human body. The figure was derived from the digital copy of Borelli's De Motu Animalium (1680) and reprinted under the Creative Commons Attribution License.
In 1663, 2 years before Robert Hooke discovered the cell and two centuries before Rudolf Virchow's (1858) “omnis cellula e cellula,”e Benedict de Spinoza (1632–1677) in a letterf to Henry Oldenburg, the first secretary of the Royal Society, opined the existence of homeostasis and dynamic cross talk between various elements within the body. He wrote, “All natural bodies can and ought to be considered in the same way as we have here considered the blood, for all bodies are surrounded by others, and are mutually determined to exist and operate in a fixed and definite proportion, while the relations between motion and rest in the sum total of them, that is, in the whole universe, remain unchanged.”33 Benedict de Spinoza, in his Newtonian articulation, thus laid, what perhaps can be called the foundation of modern TERM.
One of the most remarkable sets of experiments in this period was conducted 80 years later by Abraham Trembley (1710–1784) who was investigating the regenerative capability of hydra (Fig. 3). Each time he cut a hydra, irrespective of the body axes or number of pieces, he observed regeneration of the entire organism from each piece.31,34 The results, published in 1744, provided the first glimpse into the regenerative potential of the cell. The conclusions were revolutionary, for regeneration, until then, as observed in salamanders and crabs, was considered a property of the organism,31 and not the cell.
FIG. 3.
Trembley's polyps. The ability to regenerate an entirely new polyp from a severed part made polyps closer to plants. But their ability to move and hunt to feed themselves, as shown in this illustration from Trembley's “Memoires pour server a l'histoire d'un genre de polypes d'eau douce, a bras en forme de cornes” (Memoir on the natural history of a species of fresh water polyps) (1744), suggested they were animals. Their regeneration, however, contradicted the Preformation theory. (1–9) Planche 3 (top) display two different styles of locomotion adopted by a polyp. In Planche 13 (bottom), Trembley's attempts to unite one polyp inside another are displayed. (1) State of two polyps after one had been passed through another. The external polyp is represented as “a b” and the internal polyp as “c d.” (2) Internal polyp splitting the lower part of the external polyp at i. (3) State of the two polyps following a meal ingested by the internal polyp. They are both dilated, including the foot of the external polyp, which indicated communication between the two polyps. (4) The internal polyp (c d) splits the upper region of the external polyp (a b) and emerges at o. The inner polyp is now represented by c o i d. (5) Dilation is again observed following a meal ingested by the external polyp, indicating that the two polyps share the same interior.154 (6) After maintaining that state for more than a month, and following multiple cycles of nourishment, the two polyps display growth and division. Here, the inner polyp is represented by c o i d; the external by a i b; and the progenies by e. (7) A constriction appears at n on the neck of the grafted polyp (c d in frame [1]) two months after transplantation. n acts as the point of separation for the head of the internal polyp (c d), which detaches itself (not shown here) a month after the formation of this constriction. The authors gratefully acknowledge Polly Akhurst (BA Hons, Oxon) for translation of relevant text from French to English. Planche 3 (top) was derived from the digital copy of Memoires Pour Server A L'histoire D'un Genre De Polypes D'eau Douce, A Bras En Forme De Cornes, and reprinted under the Creative Commons Attribution License. Planche 13 (bottom), which originally appeared in Trembley's “Memoires..,” was reprinted from British Journal of Plastic Surgery, 19, Tom Gibson, “The first homografts: Trembley and the polyps,” 301–307, © (1966), with permission from Elsevier.
Making the assumption that humans had some appreciation, however rudimentary, of the concept of bioreactivity is probably justified, because of, one would presume, everyday life experience on the ill-effects of using a nonbiocompatible material. However, the first-documented instance of evaluating in vivo bioreactivity of implant materials only occurred in the first half of the 19th century. Henry S. Levert, an American doctor, in 1829, began testing the efficacy and safety of various metallic sutures on dogs. Levert reported superior performance of platinum sutures over those made of gold, silver, and lead.35 The next few decades witnessed a departure from the use of splints and braces to fix bones, with application of metal screws and plates garnering considerable medical interest23 toward this end. About a century later, in 1924, Arthur Zierold, building on the investigations conducted by Levert, reported on the ill-effects of using materials, such as iron and steel (rapid corrosion); copper, aluminum, and zinc (tissue discoloration); and gold, silver, lead, and aluminum (lack of mechanical robustness).36 It was not until the discovery of stainless steel technology, according to Buddy Ratner,23 that the routine use of metals in the body, at reasonable cost, became the mainstream.
During the first half of the 19th century, Christian H. Pander (1817) hypothesized the dependence of tissue development on a dynamic interplay between cells and their surrounding microenvironment37,38 (Fig. 4). Forty years later, in 1858, Rudolf Virchow proposed that tissue regeneration depended on cellular proliferation.39 This resulted in pioneering efforts to grow cells ex vivo, first suggested by Leo Loeb in 1897.40 It was also toward the end of the 19th century that the phrase stem cell,g owing to research on the development and regeneration of the hematopoietic system, first made its appearance in the scientific literature.41 It would take investigators working on the hematopoietic system another 60 years to actually prove the existence of these versatile precursor cells.
FIG. 4.

The development of the embryo as a result of interactions between primordial tissue. Christian Heinric Pander (1794–1865)38 is attributed to have played a major role in our understanding of the development of germ layers that are responsible for the generation of the organ systems of organisms. Pander, in collaboration with Döllinger and d'Alton, observed chicken embryos from several thousand incubated eggs using a methodology155 that enabled them to describe early development with unprecedented accuracy. Pander reported, in the surface of the yolk, a developing thin and delicate membrane, that is, the embryo, which he coined the “blastoderm.”156 The drawing, by d'Alton, demonstrates the earliest observation of circulation in the chicken embryo—a later stage in embryonic development.155 His findings were linked to induction and interactions between the primordial tissue that help devise the body plan without intervention from an external principle. Pander's work, validating the theory of Epigenesis, was a strong blow to the now annulled preformation theory, quite popular at the time. The authors gratefully acknowledge Elisabeth Forster (DPhil Candidate, Oxon) for translation of relevant text from German to English. The figure was derived from the digital copy of Pander's Beitrage zur Entwickelungs-Geschichte des Huhnchens im Eye and reprinted under the Creative Commons Attribution License.
Ex vivo cell growth was finally achieved42 in 1910 in a seminal piece of work by Ross G. Harrison (1870–1959) who was primarily motivated to determine whether nerve fibers originated from nerve cells of the central nervous system or were secreted by cells of the tissues through which the nerve fibers eventually passed. Harrison investigated this by forming a hanging-drop culture.43 He explanted a fragment from the nerve cord of a frog tadpole into a drop of frog lymph on a coverslip that was inverted and sealed to a hollow-ground slide and observed the growth of nerve fibers pushing their way through the lymph.18,40,44 The article42 reporting the findings is considered as probably “the most important paper ever published in the Journal of Experimental Zoology.”45 The discovery of contact inhibition,46 the observation that cells cease their movement upon “collision” rather than sliding past each other, is attributed to the hanging-drop culture44 study.
Alexis Carrel (1873–1944), recipient of the 1912 Nobel Prize in Medicine, extended this work by growing cells of endothermal animals ex vivo, and, in his efforts toward developing a methodology to grow cells in large numbers, invented the technique of cell culture. Carrel's culture techniques involved embedding tissue fragments in a thin layer of clotted plasma (presumably analogous to the modern scaffold), which was maintained under a fluid phase (usually of embryo extract) that had to be replaced every few weeks. Once confluent, several pieces of the clot would be used to seed new flasks.44 An editorial published in the Journal of the American Medical Association in 1911 opined that Carrel's—along with Montrose T. Burrows' (Carrel's colleague at the Rockefeller Institute)—efforts have laid bare “practically a whole new field for experimental attack on many of the most fundamental problems in biology and the medical sciences.”47 By 1912, the technique of tissue culture (Fig. 547) had been firmly established.48
FIG. 5.

The beginnings of tissue culture. The figure shows two technicians working in the Carrel laboratory at the Rockefeller Institute. They are wearing full-length black, hooded gowns that were adopted by Carrell.47 The image first appeared as Figure 23 in R.C. Parker's “Methods of Tissue Culture,” New York, Paul B. Hoeber (erstwhile Medical Book Department of Harper & Brothers), 1938. Reproduced with kind permission of HarperCollins.
Carrel's observation that fragments of embryonic chick heart explanted into blood plasma supplemented with saline extract of embryonic tissues could maintain a state of active growth for long periods49 was important, for it supported Pander's hypothesis of the interdependence of cells and their environment toward tissue development. But, in terms of the development of culture media, an essential tissue engineering material, this observation constituted a significant advance47 and a push to move away from using saline culture media, which had dominated the scene until then. While the development of the culture technique and advances in the composition of culture media solved, to an extent, the problem of growing cells in vitro, the cultured cells failed to demonstrate much beyond survival and proliferation.44 This was partly due to the fact that to develop into tissues, cells require spatial as well as mechanical cues that primitive culturing scaffolds were unable to provide, and, second, an increased metabolic demand that static cultures often failed to meet. The need for a perfusion device had arisen.
In 1930, Carrel, interested in ex vivo perfusion of organs, was joined in his investigations by the great American aviator Charles A. Lindbergh48,50 (1902–1974). Lindbergh was motivated by the death of his sister-in-law who had rheumatic valvular disease48 and could not undergo surgery on her mitral valve due to the absence of any means to perform temporary heart bypass.50 Lindbergh met Carrel on the recommendation of one of his sister-in-law's physicians,48 and the two together created a continuous perfusion flask that can provide a tightly controlled environment to sustain organs ex vivo. The perfusion chamber could additionally provide pulsatile circulation under sterile conditions and had the means of controlling the composition of the gas in the circulating “perfusion fluid” as well as the exterior of the organ.48 While the brewing industry had employed industrial scale bioreactors aimed at controlled fermentation for centuries until then, this was the first application of the bioreactor as it is known in the TERM sector. Carrel was able to keep a human thyroid “alive and in good condition” in the Lindbergh apparatus, shown in Figure 6, for at least 3 weeks and in a usable condition for 1–2 months.51
FIG. 6.
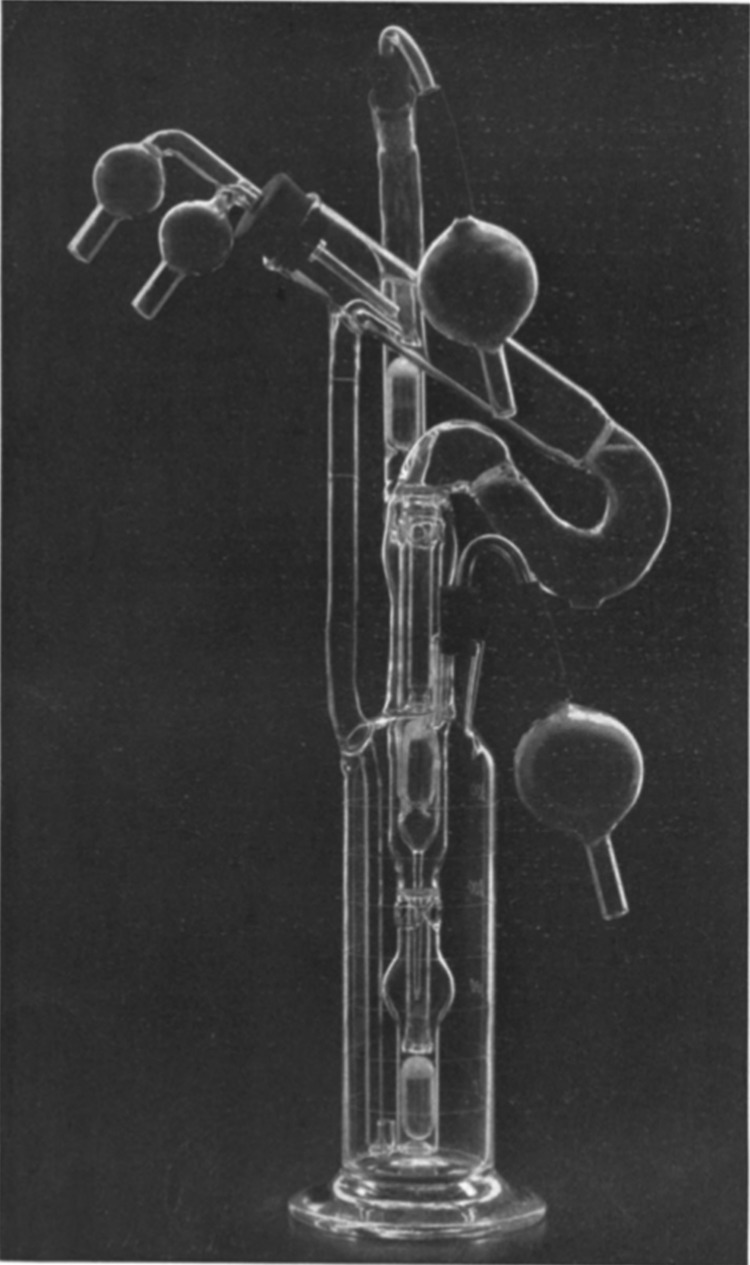
The Lindbergh pump. The figure shows the Pyrex® glass-based pulsating perfusion pump developed by Charles Lindbergh and Alexis Carrel. The perfusion pump, along with its inventors, appeared on the cover of Time in 1935. The apparatus consisted of two portions: the perfusion pump containing the organ and perfusion fluid, and the other for “creating and transmitting a pulsating gas pressure to the perfusion fluid contained in the first.”138 The perfusion pump involved the use of three chambers, one above another. The lowest chamber served as fluid reservoir, which was maintained under a pulsating pressure and passed fluid to the organ chamber (highest). After passing through the organ, the fluid returned to the reservoir through the (central) equalization chamber. The perfusion pump shown in the figure was photographed by Louis Schmidt and Joseph B. Haulenbeek, and was reprinted from © (1935) Lindbergh, Journal of Experimental Medicine, 62:409–431, with permission from Rockefeller University Press.
The dark and sullen backdrop of the two world wars, exacerbated and prolonged by the immeasurably superior (compared with previous years) technological advances made during the Industrial revolution, which enabled the mass production of weapons with enhanced accuracy, power, and range, had profound (social, economic, material, ethical, and philosophical)52 consequences. However, scientific advancements during the war years, which witnessed peacetime availability of newly developed high-performance materials (metals, ceramics, and polymers) and their application by surgeons to address a multitude of medical problems,23 constituted a few instances shining brightly against that background. The brightest of them being the serendipitous observations of Sir Harold Ridley (1906–2001), which even to this day23 form the touchstone for clinical acceptability and efficacy of implants.
Following the Second World War (1939–1945), Sir Harold observed53 in aviators who had retained in their eyes splinters of plastic from shattered canopies in Spitfire and Hurricane fighter planes stable, noninflammatory, and nonirritable healing of these unintentionally implanted shards.23 The widely held view23 at the time was that human body could not tolerate foreign objects. Sir Harold's observation, therefore, constituted a paradigmatic advance, for it was “perhaps the first”23 observation of biocompatibility in humans, as per the currently held definition of the concept. Sir Harold went onto use poly(methyl methacrylate) to fabricate intraocular lenses, which were employed as substitutes for natural lenses clouded by cataracts.53 Ignoring the—extremely important in its own right—matter of revolutionizing the treatment of individuals suffering from cataract, Sir Harold's observation that foreign objects can indeed be compatible with the human body proved catalytic to the development and availability of other implants.23
The concept of osseointegration shares similar fortuitous origins as that of biocompatibility. Per Ingvar Brånemark, an orthopedic surgeon based in Sweden, was involved, since 1952, in investigations pertaining to tissue-integrated prostheses and healing reactions.54 Following the conclusion of a study, in early 1960s, in which a titanium cylinder had been screwed into the bone of a rabbit, Brånemark observed the integration of the implant into the bone.55 He coined the phenomenon “osseointegration.”54,55 Gösta Larsson, who had a cleft palate defect, was the first human patient to receive a dental implant, which remained functional until the subject's death in 2006.56 To further put the implant's functionality and shelf-life into perspective: Brånemark had conducted the procedure in 1965.
Currently, titanium metal and alloys are the premier choice of materials for dental and orthopedic implants.23 By 1954, the first prosthetic vascular graft developed from a silk handkerchief and Vinyon “Y” (the then parachute fabric) had been implanted in a human patient.57 The innovation was inspired by Arthur Voorhees' observation that an implanted silk suture traversing the chamber of the right ventricle of a dog's heart “became coated in a few months throughout its length by a glistening film, free of macroscopic thrombi.”58 For a detailed history of biomechanical implants and grafts, we recommend perusing the historical review on Biomaterials by Buddy Ratner.23
With the advent of digital computing machines, the second quarter of the 20th century also witnessed several efforts to enhance our quantitative understanding of development and regeneration. In 1930, Nicolas Rashevsky coupled chemical reactions with mechanical processes in cells to make sense of morphogenesis,59 and in doing so attempted to unite the phenomenological aspect of physiology with the mechanistic philosophy. Rashevsky assumed cells to be spherical entities and considered only abstract patterns of chemical reactions occurring across and within their boundaries.60 It was, however, an article published in 1952 by the British mathematician and cryptanalyst, Alan Turing (1912–1954) that marked a seismic event that contributed, albeit indirectly, to further advances of TERM. In his seminal article, The Chemical Basis of Morphogenesis,61 Turing investigated coherent patterns of permanence (in a statistical sense) that would emerge out of instabilities disturbing the homogeneous equilibrium of reaction–diffusion systems and presented, arguably, the first computational model of a biological phenomenon, founded on first principles.
Turing was particularly interested in the onset of these instabilities that force a system out of its equilibrium, toward a new stable state. Turing demonstrated that coupling the chemical reactions occurring within cells with the diffusion of these chemicals could give rise to complex differentiation patterns. His findings heralded a new era of computational models where his reaction–diffusion equations, complemented with nonlinear coupling between the chemical reactants and kinetic data, became the “universal mathematical language for describing the biochemistry of spatiotemporal pattern formation in cells and developing organisms.”60 His conclusion, “[…] principles which have been discussed, should be of some help in interpreting real biological forms,” now forms the basis of the computational TERM/biology sector.
John von Neumann (1903–1957), Turing's eminent peer, was the scientific computing pioneer who recognized the dependence of an “automaton” on the “milieu” it constitutes a part of as well as responds to. In a series of lectures, von Neumann stated, “[…] it's meaningless to say that an automaton is good or bad, fast or slow, reliable or unreliable, without telling in what milieu it operates,”62 thus contextualizing the hypotheses proposed by Spinoza and Pander as well as the observations of his contemporary experimentalists using computational parlance. His investigations on self-replication and biological pattern formation led to the birth of automata theory,60,62 which provides arguably the most suitable ontology to represent biological systems. Von Neumann, furthermore, viewed organisms as composed of “analog” (or continuous, in the mathematical sense) and “discrete” elements.62 The increasingly popular hybrid models find at their heart Von Neumann's attempt to unify63 the two.
The modern history
The discovery of stem cells was beyond doubt a significant event in the history of medicine. Along with experimental observations regarding cell growth and histogenesis, many of which came from investigations conducted by Judah Folkman (1933–2008), it kick-started the early modern history of TERM. Coming to the fore in the early 1960s, stem cell research fueled the remarkable advances in TERM. While it was not until the 1990s that the use of stem cell and tissue engineering concepts merged to produce elegant solutions to healthcare problems arising due to cellular deficiency and/or physical trauma, transplantation of stem cells had started as early as 1968.
Stem cells
Although the concept of stem cells, as the kind of cells capable of forming cells of a different, more specialized, phenotype, emerged in the late 19th century, it was not until 1960s that the existence of these multipotent cells was first validated. Becker et al.20 reported, in murine spleen, colonies of dividing hematopoietic cells, some of which were differentiating “into cells of erythrocytic, granulocytic and megakaryocytic series, respectively.”20 Altman and Das, in 1965, published evidence of postnatal neurogenesis,64 but their report was largely ignored as neurogenesis was, and is still,65 by many, considered a prenatal phenomenon. In 1978, hematopoietic stem cells were discovered in human cord blood,h and it was in 1981 that the term Embryonic Stem Cell66 entered the medical lexicon, after pluripotent stem cells were derived from the inner cell massi of a mouse embryo.66,67 Finally, in 1998, human embryonic stem cells (hESCs) were derived from blastocyst-stage human embryos.68
Governing principles
The discovery of stem cells was paralleled by investigations on the nature of cell growth. Judah Folkman, a distinguished medical scientist, was at the forefront of these inquiries during the 1970s. Folkman reported the dependence of histogenesis on mass transport requirements of the growing tissue structure69; the essence of a substrate to cell shape, which in turn governed growth and proliferation70; and the ability of dissociated cells to create structures if presented with cues from their native environment.71,72 These observations necessitated the use of scaffolds, analogous to cellular matrices, and morphogens (growth factors, hormones, etc.) to supplement growth of cells and their development into tissues. Furthermore, Folkman's conclusions on the importance of vascularization to the growth of solid tumors, which could easily be extended to most developing tissues, must have acted as the precursor to construction of more sophisticated bioreactors capable of supporting cellular growth in the third dimension. Several other investigators, including Joseph Vacanti, had reported similar results.73–76
Extending these principles to practice, William T. Green, an orthopedic surgeon, in the late 1970s, used chondrocytes to grow cartilage. Green77 seeded these progenitor cells onto spicules of bone (the scaffold), which he implanted into nude mice. His attempts were not particularly successful, but his efforts spawned a theoretical approach that led to a new methodology of experimental techniques. In 1985, Paul S. Russell wrote a review on selective cell transplantation78; an alternative approach to organ transplantation whereby only a selective part of an organ or tissue would be transplanted. The concept had merit but was rife with logistical challenges.79 Isolated cells implanted into tissues as cell suspension would neither integrate with the tissue nor initiate regeneration due to lack of a template guiding those processes.79 Metabolic needs of the cells constituted an additional problem.79 The following years witnessed classical tissue engineering experiments, in the laboratory as well as on human subjects, such as the one where a collagen matrix seeded with fibroblasts was employed to create neodermis.80 The basic platform had been laid.
However, it was only in the mid-1980s, when Joseph Vacanti approached Robert Langer to design scaffolds appropriate for cell delivery as opposed to seeding them on a matrix, that TERM made its first serious impression as an emerging technology.3,40,79,80 Vacanti and Langer's efforts resulted in the publication of Tissue Engineering,3 the most heavily cited article in the field to date. Realizing the benefits, this new field had to offer, a lot of centers, aiming to explore its potential, had sprung up around the world by mid-1990s.80 TERM entered public consciousness with the BBC broadcast that featured auriculosaurus, the mouse with the human ear80 (Fig. 7). During the 1990s, Balkrishan G. Matapurkar successfully employed adult stem cells found in the peritoneum—the membrane that forms the lining of the abdominal cavity—to aid organ regeneration.81–84 Matapurkar and his team harnessed the metaplastic capacity of these autogenic, pluripotent mesodermal stem cells to aid the regeneration of certain urogenital tissues, also mesodermal in origin.82 Finally, in 2007, stem cell researchers were able to induce pluripotency in adult stem cells.85,86 As they are not derived from embryos, the induced pluripotent stem cells may finally put an end to the ethical debates that have impeded the use of hESCs. While the discovery of stem cells was one of the most significant achievements of the 20th century, and although techniques have emerged to get around the ethical, legal, and logistical issues that have hindered the progress in stem cell research, we have merely witnessed, as evident in Figure 8, the tip of this iceberg.
FIG. 7.

The auriculosaurus. The “human-ear-bearing” mouse developed by the Vacanti laboratory that went onto epitomize Tissue Engineering. License to reproduce the image was obtained from the © (2002) BBC Photo Library. Color images available online at www.liebertpub.com/teb
FIG. 8.
Ear growth on the arm. Figure 7 presented the auriculosaurus, the mouse with a human ear. Similar principles were employed by a team of surgeons to grow an external ear for this patient on her own arm. The set of images show the compromised organ, perfusion to meet the transport demands of the synthetically growing new organ, the fully developed organ, and, finally, its attachment to the patient. Cartilage for the new ear was derived from the patient's ribs, matched with her right ear, and implanted under the arm for growth. The work was carried on by surgeons led by Dr. Patric Byrne at the Johns Hopkins University in 2012. The photographs issued along a media release belong to the public domain. The set of images was retrieved from an online article published by CBS Baltimore.157 Color images available online at www.liebertpub.com/teb
The State of the Art
The early years of the 21st century have witnessed TERM take major strides as a therapeutic rehabilitator, not only building on certain ancient techniques but also making tangible the stuff of legends and fiction. Availability of autologous or allogenic epidermal (Epicel®, Epidex™, Myskin™, ReCell®), dermal (AlloDerm®, Integra®, Matriderm®), and dermoepidermal (Apligraf®, Orcel®) substitutes represent the current advance87 made on the earliest form of TERM technique practiced as early as 2500 BC in the Indian subcontinent. Regeneration of dentine-pulp complex using cells encapsulated within scaffolds; treatment of periodontal defects through guided bone regeneration membrane, growth factors and cytokines; and replacement of lost teeth by transplantation of the bioengineered tooth germ constitute state of the art of the rudimentary practice of employing blue nacre shells as functional dental substitutes (by the Mayans circa 600 AD). These advancements have been reviewed by Abou Neel et al.88 Transplantation of bone marrow stem cells (encapsulated within a suitable scaffold),89 to repair articular cartilage (patellar, patellofemoral, and femoral)90 and osteochondral defects90 as well as local bone defects91 in humans forms another significant achievement of a TERM therapy89 practiced first by the Egyptians. The use of TERM to treat skeletal defects has been reviewed elsewhere.89,91,92
While TERM is still decades away from replicating the feat mentioned in the Mahabharata and Faust, of creating human forms within a phial, the existing technology has made possible development and availability of tissues and, very recently, organs for clinical applications. A bioengineered vessel implanted to replace the right intermediate pulmonary artery in a child suffering from single right ventricle and pulmonary atresia epitomizes the state of the art.93 The methodology employed consisted of harvesting cells from a peripheral vein and seeding them in a biodegradable polymer, which was maintained in a bioreactor for 10 days. Imaging the patient 9 years postimplantation revealed a patent graft.94 The same technology has been exploited to bioengineer bladders14 and urethras.95 Postimplantation follow-ups showed improved functionality and compliance.94
Using the same technology, with the exception that a human scaffold was employed, a trachea was bioengineered ex vivo and implanted in a woman with end-stage bronchomalacia.12 A deceased donor trachea, which was decellularized following retrieval, served as the scaffold within which autologous epithelial cells (derived from bronchoscopy samples) and chondrocytes (differentiated from mesenchymal cells obtained from patient's bone marrow), were seeded.12 Before implantation, the construct was maintained for 4 days in a bioreactor designed to address seeding requirements, provide biomechanical cues, and achieve good laboratory practice.94 This technology of employing decellularized organ scaffolds is advantageous as the native three-dimensional (3D) tissue architecture, vascular tree, and ECM-related cues, all with high developmental significance, seem to be well preserved94,96 in the organ scaffold. A variety of complex modular organs have now been bioengineered, some of which, such as the larynx97,98 and vagina,99 have found clinical use. We highly recommend reviews87,98 including those by Orlando et al.94 and Badylak et al.96 on this topic.
The bioengineered tissue constructs thus developed recapitulate the native architecture, physiology, dynamic conditions, intercellular, and cell–matrix interactions more accurately than two-dimensional culture monolayers, cadaveric tissues, as well as animal models.100–103 As such, these ex vivo constructs can be employed as preclinical models for high-throughput drug screening,100,102 pharmacokinetic and pharmacodynamics analyses of drugs,102 and device testing.100 The advantages offered by these “synthetic” tissues include decreased costs, increased reproducibility, precise control over culture conditions, incorporation of human cells, and systematic evaluation of the product being tested.100 The platforms utilized include spheroids and cell sheet stacking, matrix-embedded, lithography,103 microfluidic cell culture, hollow-fiber, and multicellular layer models.102 Specific examples, which have been reviewed extensively here,100,102,103 include blood vessels for device (stents)104,105 and drug testing106; bioengineered blood–brain barriers to test drug permeability107 and disease modelling108–110; musculoskeletal tissue to optimize drugs aimed at improving muscular growth and function,111 as well as to evaluate the impact of prosthetic devices on muscular tissues.112 Corneal tissue to conduct tests for toxicology and eye irritancy113; skin to test for cytotoxicity,114 phototoxicity,115,116 and wound healing117; and reproductive tissues to study sexually transmitted infections, product efficacy,118–120 and, as models of the blood–testis barrier, to predict toxicity and permeability in vivo,121,122 form prime examples of in vitro models (reviewed here in Refs.100,102,103).
The most significant advantage, though, remains their availability as a superior and more accurate alternative to animal testing. After all, “∼92% of all drugs that enter clinical trials following extensive animal testing fail to achieve FDA approval because they are not safe or not effective in humans,”101 and about half of the remaining 8% are either withdrawn or relabeled due to adverse effects that go undetected during animal testing.101 This endeavor constitutes a quantum leap in making TERM a more humane academic, research, and technological enterprise.
Within the TERM paradigm, the “product is the process.”123 The phrase implies that efficacy and functionality of TERM products is intimately linked with product design and manufacturing. This is evident by the fact that manufacturing processes responsible for the aforementioned constructs has itself undergone transition from being manual, inherently variable, static, and noncompliant to automated, dynamic, reproducible, and increasingly compliant with current good manufacturing practices (GMP).123,124 The state of the art in TERM manufacturing constitutes robotic systems, such as the CompacT SelecT,125 to conduct automated cell culture124; computer-assisted bioprinting to manufacture two- and three-dimensional biological patterns to recapitulate the microenvironmental niche more precisely2,126,127; and bioreactors that can perform multiple roles such as nutrient and waste transport, mechanical conditioning, cell seeding, supporting cell viability, and promoting their 3D organization.128 These accomplishments by no means represent the peak of this systematic evolution. Quite the contrary, the automated procedures and manufacturing processes are being continuously scrutinized and optimized, using a variety of quality control tools, such as six-sigma and, the data-driven, Define, Measure, Analyze, Improve and Control (DMAIC) tool.123,124 Of significant value has been the development of mathematical techniques60,129 in synergy with the availability of increasingly powerful and sophisticated semi-conductor chips and customized way of packaging digital computing services.60 Mathematical formulations, thus reinforced, have not only guided the design and development of devices, such as the bioreactor, employed to synthesize TERM products, they have also made available previously inaccessible data, such as flow profiles and concentration gradients within a physical construct and the cells' response to them.128,130–136
New Frontiers and Future Directions
As is the nature of scientific revolutions, the information sourced by the scientific community until the late 1980s answered certain fundamental questions and thereby provided the impetus behind TERM acquiring a formal framework to operate with. And, although the holy grail137 of TERM—that of developing vascularized, fully functional, complex bioengineered tissues and organs—still lies out of reach, the foregoing efforts have brought the community ever so closer to achieving that coveted eventuality. However, a lot of work is still required to overcome grass root challenges that stymie further advancement. These include the need for: increased cell expansion,137 more sophisticated in vitro systems that can expose cells to physiologically relevant developmental cues,101 resistance of implantable scaffolds against stricture,93 better drug screening models, ensuring product bioneutrality rather than inducing product tolerance in the patient, and developing more sophisticated bioreactors capable of capturing the niche characteristics of the targeted tissue/organ.101,128
Scientific investigators of the 20th century, armed with verified principles and sophisticated apparatuses that can recreate tissue microenvironments with great fidelity, made major breakthroughs that shaped the course of future investigations. These included the ability to sustain organ and cellular growth ex vivo,47,138,139 determining the impact of the microenvironment on cellular behaviour,71,72 understanding the nature of interactions between cells (e.g., contact inhibition),46 and introducing the classical tissue engineering technique of transplanting into the human body cells seeded within polymeric scaffolds.77 In doing so, these investigators exposed certain other questions, which required a new paradigm of exploration. One of these, still relevant questions, pertains the induction of ex vivo histogenesis in a growing population of cells, for which decellularized scaffolds have been recently utilized. The most immediate of these include quantifying the complexity inherent in biological systems129 as well as gaining understanding of how much complexity is actually required to create a functional tissue.101 This is a multipronged problem that calls for integration of developmental biology methodologies with those of TERM, but more importantly, the application of computational approaches in developmental biology to quantify the cellular niche. The latter, however, is not an attempt to impose a way of investigation on another. In fact, the computational TERM community must also rise to the occasion and advance their techniques and approaches to address the challenges of capturing biocomplexity. An example of this collective endeavor is the principle of Dynamic Assimilation, proposed by Kaul in his doctoral thesis,140 which serves as the touchstone of performance for computational platforms developed to analyze biocomplexity, especially in the TERM context. The underlying idea being that once a system is quantified, the information can then be applied to create scaffolds, conditioning tools, and bioreactors that are based on the niche characteristics of the targeted tissue/organ—a concept that has gained wide attention.17,96,101,128,137
A significant challenge, however, remains nontechnological. As technologies often do, TERM, in the wake of its advancements, has already established itself as a significant player on the economic landscape. Despite, however, its vast clinical and commercial potential, regenerative medicine has thus far underperformed,141 with the industry in aggregate yet to make a profit. A multitude of nonquantifiable reasons have contributed to this modest performance of TERM industry. These include extraneous factors such as misconceptions regarding the genuine achievements of the sector,142 lack of strong political leadership,142 the historical lack of an industry voice,143,144 among others.145,146 Regulatory challenges,9,147 such as lack of quality control biomarkers and potency markers, process and product variability, risk factors associated with operator handling, continue to impede the marketability of TERM-based products. Similarly, a complex Intellectual Property situation, parallel to the biotech industry,148 owing to uncertain outcomes with poorly defined and overlapping patents constitutes another reason behind TERM's modest industrial and clinical translation. This is perhaps the most challenging and unique hurdle that TERM faces, overcoming which necessitates collaboration between scientists, innovators, entrepreneurs, and policy makers. Progress, however, is being continuously made, although at a glacial pace, in this regard with the identification of business models (“integrated,” “fully integrated,” and “royalty model”),87 appreciation of the distinctions between TERM and nonliving medical products,87,137 shift from the conventional pharmaceutical manufacturing ethos based on economies of scale,87 and introduction of quality control and GMP toward the development of TERM products.9,123,149
Conclusion
TERM has completed its initial iteration. Initial because little conceptual advance has been made since the early years of the 20th century when efforts to grow cellular structure ex vivo were first realized. The push back then was to create better media, more sophisticated 3D perfusion systems, scaffolds capable of architectural and mechanical conditioning, and making cell culture more of a science than art. In realizing the technological advances, which now constitute state of the art (decellularized scaffolds, a multitude of bioreactors, and robotic culture systems), humanity was acquainted with innovative therapies, which had until recently belonged in the realm of fiction. These exciting accomplishments left in their wake some more heretofore unconsidered questions, not all scientific, exposing newer horizons, which promise, in the most stunning manner, to usher a new dawn of scientific, technological, industrial, and regulatory endeavors, as discussed in the last section. Initial also because the signature of the next iteration can be distinctly identified from the first and is embodied in the collective effort to recapitulate the microenvironmental niche through the use of bioprinters, condensing laboratory into a computer and experiments into a code as part of the third approach,150 employing the emergent151,152 paradigm for understanding cellular behavior, and moving to an integrated approach to better understand, develop, and test TERM products. The TERM community stands at the edge of another transition with its horizon irrevocably broadened.
Acknowledgments
The authors gratefully acknowledge Neelam Kaul, Polly Akhurst (BA Hons, Oxon), and Elisabeth Forster (DPhil Candidate, Oxon) for translation of relevant texts from Sanskrit, French, and German (respectively) into English.
Disclosure Statement
No competing financial interests exist for either author.
The ability of a cell to divide and produce all the differentiated cells of an organism.
According to Rig Veda, Asuras are a group of power-seeking deities that preside over moral and social phenomena.
The legend of Raktabeej appears in the eighth chapter of Devi Mahatmya, which forms a part of Markandeya Purana. The Purana is supposed to have been composed around 400–500 CE, although establishing the origin is difficult, for a lot of Hindu literature existed in oral form before it was printed.
The authors gratefully acknowledge the translation of the relevant Sanskrit hymns found in the Rig Veda (10th Mandala, 39th Sukta) into Hindi by Neelam Kaul.
Cells arise from preexisting cells.
Letter XV. (XXXII.): Spinoza to Oldenburg.
A precursor cell with limitless proliferative potential capable of forming more specialized cells.
Cord Blood is the blood that remains in the placenta and in the attached umbilical cord after childbirth.
Pluripotent mass of cells inside the early embryo, which form the source of embryonic stem cells.
References
- 1.Murry C.E., and Keller G.Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132,661, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Khademhosseini A., and Langer R.Microengineered hydrogels for tissue engineering. Biomaterials 28,5087, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Langer R., and Vacanti J.P.Tissue engineering. Science 260,920, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Berthiaume F., Maguire T.J., and Yarmush M.L.Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng 2,403, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Science and Technology Committee, House of Lords. Regenerative Medicine [Report]. Authority of the House of Lords, London, 2013. Available at: http://www.publications.parliament.uk/pa/Id201314/ldselect/Idsctech/23/23.pdf (Last accessed November25, 2014) [Google Scholar]
- 6.Daar A.S., and Greenwood H.L.A proposed definition of regenerative medicine. J Tissue Eng Regen Med 1,179, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Mason C., and Dunnill P.A brief definition of regenerative medicine. Regen Med 3,1, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Gurtner G.C., Callaghan M.J., and Longaker M.T.Progress and potential for regenerative medicine. Annu Rev Med 58,299, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Martin I., Smith T., and Wendt D.Bioreactor-based roadmap for the translation of tissue engineering strategies into clinical products. Trends Biotechnol 27,495, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini G., Traverso C., Franzi A., Zingirian M., Cancedda R., and DeLuca M.Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349,990, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Tsai R., Li L., and Chen J.Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med 343,86, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Macchiarini P., Jungebluth P., Go T., Asnaghi M., Rees L., Cogan T., Dodson A., Martorell J., Bellini S., Parnigotto P., Dickinson S., Hollander A., Mantero S., Conconi M., and Birchall M.Clinical transplantation of a tissue-engineered airway (vol 372, pg 2023, 2008). Lancet 373,462, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Quarto R., Mastrogiacomo M., Cancedda R., Kutepov S., Mukhachev V., Lavroukov A., Kon E., and Marcacci M.Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 344,385, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Atala A., Bauer S., Soker S., Yoo J., and Retik A.Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367,1241, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Chamuleau R.Artificial liver support in the third millennium. Artif Cells Blood Substit Immobil Biotechnol 31,117, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Griffith L., and Naughton G.Tissue engineering—current challenges and expanding opportunities. Science 295,1009, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Martin I., Wendt D., and Heberer M.The role of bioreactors in tissue engineering. Trends Biotechnol 22,80, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Burdick J.A., and Vunjak-Novakovic G.Engineered microenvironments for controlled stem cell differentiation. Tissue Eng Part A 15,205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanza R.P., Langer R.S., and Vacanti J.Principles of Tissue Engineering. London: Academic Press, 2007 [Google Scholar]
- 20.Becker A.J., McCulloch E.A., and Till J.E.Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 197,452, 1963 [DOI] [PubMed] [Google Scholar]
- 21.Mishra P.How India Reconciles Hindu Values and Biotech [Online Newspaper Article]. New York City: The New York Times, 2005 [Google Scholar]
- 22.Goethe J.W.V., and Greenberg M.F.Faust. Part Two. New Haven: Yale University Press, 1998 [Google Scholar]
- 23.Ratner B.D.History of biomaterials. In: Ratner B.D., Hoffman A.S., Schoen F.J., and Lemons J.E., eds. Biomaterials Science—An Introduction to Materials in Medicine. 3rd ed. London: Academic Press, 2013, pp. xli–liii [Google Scholar]
- 24.Chick L.R.Brief history and biology of skin grafting. Ann Plast Surg 21,358, 1988 [DOI] [PubMed] [Google Scholar]
- 25.Ehrenfried A.Reverdin and other methods of skin-grafting—historical. Boston Med Surg J 161,911, 1909 [Google Scholar]
- 26.Jaklenec A., Stamp A., Deweerd E., Sherwin A., and Langer R.Progress in the tissue engineering and stem cell industry “Are we there yet?”. Tissue Eng Part B Rev 18,155, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Nerlich A.G., Zink A., Szeimies U., and Hagedorn H.G.Ancient Egyptian prosthesis of the big toe. Lancet 356,2176, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Crubezy E., Murail P., Girard L., and Bernadou J.P.False teeth of the Roman world. Nature 391,29, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Westbroek P., and Marin F.A marriage of bone and nacre. Nature 392,861, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Bobbio A.The first endosseous alloplastic implant in the history of man. Bull Hist Dent 20,1, 1972 [PubMed] [Google Scholar]
- 31.Hankins T.L.Science and the Enlightenment. Cambridge: Cambridge University Press, 1985 [Google Scholar]
- 32.Wulff H.R.The concept of disease: from Newton back to Aristotle. Lancet 354,50, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Spinoza B.D., and Elwes R.H.M.The Chief Works of Benedict de Spinoza. London: G. Bell and Sons, 1883 [Google Scholar]
- 34.Lenhoff S.G., Lenhoff H.M., and Trembley A.Hydra and the Birth of Experimental Biology, 1744: Abraham Trembley's Mémoires Concerning the Polyps. Pacific Grove, CA: Boxwood Press, 1986 [Google Scholar]
- 35.Levert H.S.Experiments on the use of metallic ligatures, as applied to arteries. Am J Med Sci 4,17, 1829 [Google Scholar]
- 36.Zierold A.A.Reaction of bone to various metals. Arch Surg 9,365, 1924 [Google Scholar]
- 37.Pander C.H.Beitraege zur Entwickelungsgeschichte des Hühnchens im Eye. Würzburg: Nitribitt, 1817 [Google Scholar]
- 38.Wessel G.M.Christian Heinrich Pander (1794–1865). Mol Reprod Dev 77,i, 2010 [Google Scholar]
- 39.Virchow R., and Chance F.Cellular Pathology, as Based upon Physiological and Pathological Histology. Twenty Lectures Delivered in the Pathological Institute of Berlin During the Months of February, March and April, 1858. New York: R. M. De Witt, 1860 [Google Scholar]
- 40.Schultheiss D., Bloom D.A., Wefer J., and Jonas U.Tissue engineering from Adam to the zygote: historical reflections. World J Urol 18,84, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Ramalho-Santos M., and Willenbring H.On the origin of the term “stem cell”. Cell Stem Cell 1,35, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Harrison R.G.The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool 9,787, 1910 [DOI] [PubMed] [Google Scholar]
- 43.Harrison R.G., Greenman M.J., Mall F.P., and Jackson C.M.Observations of the living developing nerve fiber. Anat Rec 1,116, 1907 [Google Scholar]
- 44.Fell H.B.Tissue culture and its contribution to biology and medicine. J Exp Biol 57,1, 1972 [DOI] [PubMed] [Google Scholar]
- 45.Keshishian H.Ross Harrison's “The outgrowth of the nerve fiber as a mode of protoplasmie movement”. J Exp Zool A Comp Exp Biol 301A,201, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Abercrombie M., and Heaysman J.E.Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res 6,293, 1954 [DOI] [PubMed] [Google Scholar]
- 47.Witkowski J.A.Alexis Carrel and the mysticism of tissue culture. Med Hist 23,279, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malinin T.I.Remembering Alexis Carrel and Charles A. Lindbergh. Tex Heart Inst J 23,28, 1996 [PMC free article] [PubMed] [Google Scholar]
- 49.Carrel A.Artificial activation of the growth in vitro of connective tissue. J Exp Med 17,14, 1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dente C.J., and Feliciano D.V.Alexis Carrel (1873–1944): Nobel Laureate, 1912. Arch Surg 140,609, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Foot N.C., Baker L.E., and Carrel A.The behavior of abnormal human thyroid tissue cultivated in the Lindbergh apparatus. J Exp Med 70,39, 1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zapotoczny W.S.The Impact of the Industrial Revolution on Warfare [Essay]. 2006. Available at: http://www.wzaponline.com/yahoo_site_admin/assets/docs/InductrialRevolution.292125935.pdf (Last accessed November25, 2014)
- 53.Ridley H.Intra-ocular acrylic lenses; a recent development in the surgery of cataract. Br J Ophthalmol 36,113, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brånemark P.I.Osseointegration and its experimental background. J Prosthet Dent 50,399, 1983 [DOI] [PubMed] [Google Scholar]
- 55.Breine U., Johansson B., Roylance P.J., Roeckert H., and Yoffey J.M.Regeneration of bone marrow—a clinical and experimental study following removal of bone marrow by curettage. Acta Anat (Basel) 59,1, 1964 [PubMed] [Google Scholar]
- 56.McClarence E.Close to the Edge—Brånemark and the Development of Osseointegration. London: Quintessence, 2003 [Google Scholar]
- 57.Blakemore A.H., and Voorhees A.B.The use of tubes constructed from vinyon N cloth in bridging arterial defects; experimental and clinical. Ann Surg 140,324, 1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voorhees A.B., Jaretzki A., and Blakemore A.H.The use of tubes constructed from vinyon N cloth in bridging arterial defects. Ann Surg 135,332, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rashevsky N.Mathematical Biophysics; Physico-Mathematical Foundations of Biology. New York: Dover Publications, 1960 [Google Scholar]
- 60.Semple J.L., Woolridge N., and Lumsden C.J.In vitro, in vivo, in silico: computational systems in tissue engineering and regenerative medicine. Tissue Eng 11,341, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Turing A.M.The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci 237,37, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Von Neumann J., and Burks A.W. Theory of Self-Reproducing Automata. Urbana: University of Illinois Press, 1966 [Google Scholar]
- 63.Istrail S., and Marcus S.Alan Turing and John von Neumann—their brains and their computers. In: Csuhaj-Varju E., Gheorghe M., Rozenberg G., Salomaa A., and Vaszil G., eds. Membrane Computing Lecture Notes in Computer Science. Berlin Heidelberg: Springer, 2013, pp. 26–35 [Google Scholar]
- 64.Altman J., and Das G.D.Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124,319, 1965 [DOI] [PubMed] [Google Scholar]
- 65.Nowakowski R.S., and Hayes N.L.New neurons: extraordinary evidence or extraordinary conclusion? Science 288,771, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Martin G.R.Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 78,7634, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans M.J., and Kaufman M.H.Establishment in culture of pluripotential cells from mouse embryos. Nature 292,154, 1981 [DOI] [PubMed] [Google Scholar]
- 68.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., and Jones J.M.Embryonic stem cell lines derived from human blastocysts. Science 282,1145, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Folkman J., and Hochberg M.Self-regulation of growth in three dimensions. J Exp Med 138,745, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Folkman J., and Moscona A.Role of cell shape in growth control. Nature 273,345, 1978 [DOI] [PubMed] [Google Scholar]
- 71.Folkman J., and Haudenschild C.Angiogenesis in vitro. Nature 288,551, 1980 [DOI] [PubMed] [Google Scholar]
- 72.Folkman J., and Haudenschild C.Angiogenesis by capillary endothelial cells in culture. Trans Ophthalmol Soc U K 100,346, 1980 [PubMed] [Google Scholar]
- 73.Morse M.A., and Vacanti J.P.Time-lapse photography: a novel tool to study normal morphogenesis and the diseased hepatobiliary system in children. J Pediatr Surg 23,69, 1988 [DOI] [PubMed] [Google Scholar]
- 74.Benzeev A., Farmer S.R., and Penman S.Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell 21,365, 1980 [DOI] [PubMed] [Google Scholar]
- 75.Vacanti J.P., Morse M.A., Saltzman W.M., Domb A.J., Perezatayde A., and Langer R.Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg 23,3, 1988 [DOI] [PubMed] [Google Scholar]
- 76.Vacanti J.P.Beyond transplantation. Third annual Samuel Jason Mixter lecture. Arch Surg 123,545, 1988 [DOI] [PubMed] [Google Scholar]
- 77.Green W.T.Articular-cartilage repair. Behavior of rabbit chondrocytes during tissue culture and subsequent allografting. Clin Orthop Relat Res 124,237, 1977 [PubMed] [Google Scholar]
- 78.Russell P.S.Selective transplantation. An emerging concept. Ann Surg 201,255, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaignaud B.E., Langer R.S., and Vacanti J.P.The history of tissue engineering using synthetic biodegradable polymer scaffold and cells. In: Atala A., Mooney D., Vacanti J.P., and Langer R.S., eds. Synthetic Biodegradable Polymer Scaffolds. Boston: Birkhauser, 1997, pp. 1–14 [Google Scholar]
- 80.Vacanti C.A.The history of tissue engineering. J Cell Mol Med 10,569, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matapurkar B.G., Rehan H.M.S., Agarwal A.K., and Bhatia M.Large recurrent incisional hernia: ultrasonographic mapping of abdominal wall defects and repair by Marlex peritoneal sandwich technique. Indian J Surg 15,321, 1995 [Google Scholar]
- 82.Matapurkar B.G., Bhargave A., Dawson L., and Sonal B.Organogenesis by desired metaplasia of autogenous stem cells. Ann N Y Acad Sci 857,263, 1998 [DOI] [PubMed] [Google Scholar]
- 83.Matapurkar B.G.Method of organogenesis and tissue regeneration/repair using surgical techniques [Patent No.: US 6,227,202 B1]. Official Gazette of the United States Patent and Trademark Office, 2001 [Google Scholar]
- 84.Matapurkar B.G., Gupta A.K., and Agarwal A.K.A new technique of marlex-peritoneal sandwich in the repair of large incisional hernias. World J Surg 15,768, 1991 [DOI] [PubMed] [Google Scholar]
- 85.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S.Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131,861, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., Slukvin I.I., and Thomson J.A.Induced pluripotent stem cell lines derived from human somatic cells. Science 318,1917, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Kemp P.History of regenerative medicine: looking backwards to move forwards. Regen Med 1,653, 2006 [DOI] [PubMed] [Google Scholar]
- 88.Abou Neel E.A., Chrzanowski W., Salih V.M., Kim H.W., and Knowles J.C.Tissue engineering in dentistry. J Dent 42,915, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Evans C.H.Advances in regenerative orthopedics. Mayo Clin Proc 88,1323, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamasaki S., Mera H., Itokazu M., Hashimoto Y., and Wakitani S.Cartilage repair with autologous bone marrow mesenchymal stem cell transplantation: review of preclinical and clinical studies. Cartilage 5,196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gomez-Barrena E., Rosset P., Mueller I., Giordano R., Bunu C., Layrolle P., Konttinen Y.T., and Luyten F.P.Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med 15,1266, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amini A.R., Laurencin C.T., and Nukavarapu S.P.Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40,363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin'oka T., Imai Y., and Ikada Y.Transplantation of a tissue-engineered pulmonary artery. N Engl J Med 344,532, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Orlando G., Wood K.J., Stratta R.J., Yoo J.J., Atala A., and Soker S.Regenerative medicine and organ transplantation: past, present, and future. Transplantation 91,1310, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Raya-Rivera A., Esquiliano D.R., Yoo J.J., Lopez-Bayghen E., Soker S., and Atala A.Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet 377,1175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Badylak S.F., Weiss D.J., Caplan A., and Macchiarini P.Engineered whole organs and complex tissues. Lancet 379,943, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Omori K., Tada Y., Suzuki T., Nomoto Y., Matsuzuka T., Kobayashi K., Nakamura T., Kanemaru S., Yamashita M., and Asato R.Clinical application of in situ tissue engineering using a scaffolding technique for reconstruction of the larynx and trachea. Ann Otol Rhinol Laryngol 117,673, 2008 [DOI] [PubMed] [Google Scholar]
- 98.Gugatschka M., Ohno S., Saxena A., and Hirano S.Regenerative medicine of the larynx. Where are we today? A review. J Voice 26,e7, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Raya-Rivera A.M., Esquiliano D., Fierro-Pastrana R., López-Bayghen E., Valencia P., Ordorica-Flores R., Soker S., Yoo J.J., and Atala A.Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet 384,329, 2014 [DOI] [PubMed] [Google Scholar]
- 100.Gibbons M.C., Foley M.A., and Cardinal K.O.H.Thinking inside the box: keeping tissue-engineered constructs in vitro for use as preclinical models. Tissue Eng Part B Rev 19,14, 2013 [DOI] [PubMed] [Google Scholar]
- 101.Rouwkema J., Gibbs S., Lutolf M.P., Martin I., Vunjak-Novakovic G., and Malda J.In vitro platforms for tissue engineering: implications for basic research and clinical translation. J Tissue Eng Regen Med 5,e164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Elliott N.T., and Yuan F.A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci 100,59, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Kimlin L., Kassis J., and Virador V.3D in vitro tissue models and their potential for drug screening. Expert Opin Drug Discov 8,1455, 2013 [DOI] [PubMed] [Google Scholar]
- 104.Cardinal K.O.H., Bonnema G.T., Hofer H., Barton J.K., and Williams S.K.Tissue-engineered vascular grafts as in vitro blood vessel mimics for the evaluation of endothelialization of intravascular devices. Tissue Eng 12,3431, 2006 [DOI] [PubMed] [Google Scholar]
- 105.Cardinal K.O.H., and Williams S.K.Assessment of the intimal response to a protein-modified stent in a tissue-engineered blood vessel mimic. Tissue Eng Part A 15,3869, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Laflamme K., Roberge C.J., Pouliot S., D'Orléans-Juste P., Auger F.A., and Germain L.Tissue-engineered human vascular media produced in vitro by the self-assembly approach present functional properties similar to those of their native blood vessels. Tissue Eng 12,2275, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Neuhaus W., Lauer R., Oelzant S., Fringeli U.P., Ecker G.F., and Noe C.R.A novel flow based hollow-fiber blood-brain barrier in vitro model with immortalised cell line PBMEC/C1-2. J Biotechnol 125,127, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Cucullo L., Aumayr B., Rapp E., and Janigro D.Drug delivery and in vitro models of the blood-brain barrier. Curr Opin Drug Discov Devel 8,89, 2005 [PubMed] [Google Scholar]
- 109.Grant G.A., Abbott N.J., and Janigro D.Understanding the physiology of the blood-brain barrier: In vitro models. News Physiol Sci 13,287, 1998 [DOI] [PubMed] [Google Scholar]
- 110.Janigro D., Leaman S.M., and Stanness K.A.Dynamic in vitro modeling of the blood-brain barrier: a novel tool for studies of drug delivery to the brain. Pharm Sci Technolo Today 2,7, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Vandenburgh H., Shansky J., Benesch-Lee F., Barbata V., Reid J., Thorrez L., Valentini R., and Crawford G.Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37,438, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Breuls R.G.M., Bouten C.V.C., Oomens C.W.J., Bader D.L., and Baaijens F.P.T.Compression induced cell damage in engineered muscle tissue: an in vitro model to study pressure ulcer aetiology. Ann Biomed Eng 31,1357, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Tegtmeyer S., Papantoniou I., and Muller-Goymann C.C.Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur J Pharm Biopharm 51,119, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Roguet R., Regnier M., Cohen C., Dossou K.G., and Rougier A.The use of in vitro reconstituted human skin in dermotoxicity testing. Toxicol In Vitro 8,635, 1994 [DOI] [PubMed] [Google Scholar]
- 115.Cohen C., Dossou K.G., Rougier A., and Roguet R.Episkin: An in vitro model for the evaluation of the phototoxicity and sunscreen photoprotective properties. Toxicol In Vitro 8,669, 1994 [DOI] [PubMed] [Google Scholar]
- 116.Lelievre D., Justine P., Christiaens F., Bonaventure N., Coutet J., Marrot L., and Cotovio J.The EpiSkin phototoxicity assay (EPA): development of an in vitro tiered strategy using 17 reference chemicals to predict phototoxic potency. Toxicol. In Vitro 21,977, 2007 [DOI] [PubMed] [Google Scholar]
- 117.Xie Y., Rizzi S.C., Dawson R., Lynam E., Richards S., Leavesley D.I., and Upton Z.Development of a three-dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng Part C Methods 16,1111, 2010 [DOI] [PubMed] [Google Scholar]
- 118.Ayehunie S., Cannon C., Lamore S., Kubilus J., Anderson D.J., Pudney J., and Klausner M.Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol. In Vitro 20,689, 2006 [DOI] [PubMed] [Google Scholar]
- 119.Ayehunie S., Cannon C., LaRosa K., Pudney J., Anderson D.J., and Klausner M.Development of an in vitro alternative assay method for vaginal irritation. Toxicology 279,130, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schaller M., Korting H.C., Borelli C., Hamm G., and Hube B.Candida albicans-secreted aspartic proteinases modify the epithelial cytokine response in an in vitro model of vaginal candidiasis. Infect Immun 73,2758, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Legendre A., Froment P., Desmots S., Lecomte A., Habert R., and Lemazurier E.An engineered 3D blood-testis barrier model for the assessment of reproductive toxicity potential. Biomaterials 31,4492, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Li M.W.M., Mruk D.D., Lee W.M., and Cheng C.Y.Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol 41,2302, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Williams D.J., Thomas R.J., Hourd P.C., Chandra A., Ratcliffe E., Liu Y., Rayment E.A., and Archer J.R.Precision manufacturing for clinical-quality regenerative medicines. Philos Trans A Math Phys Eng Sci 370,3924, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Thomas R.J., Chandra A., Hourd P.C., and Williams D.J.Cell culture automation and quality engineering: a necessary partnership to develop optimized manufacturing processes for cell-based therapies. JALA Charlottesv Va 13,152, 2008 [Google Scholar]
- 125.Thomas R.J., Anderson D., Chandra A., Smith N.M., Young L.E., Williams D., and Denning C.Automated, scalable culture of human embryonic stem cells in feeder-free conditions. Biotechnol Bioeng 102,1636, 2009 [DOI] [PubMed] [Google Scholar]
- 126.Campbell P.G., and Weiss L.E.Tissue engineering with the aid of inkjet printers. Expert Opin Biol Ther 7,1123, 2007 [DOI] [PubMed] [Google Scholar]
- 127.Mironov V., Reis N., and Derby B.Review: bioprinting: a beginning. Tissue Eng 12,631, 2006 [DOI] [PubMed] [Google Scholar]
- 128.Kaul H., Cui Z., and Ventikos Y.A multi-paradigm modeling framework to simulate dynamic reciprocity in a bioreactor. PLoS One 8,e59671, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaul H., and Ventikos Y. Investigating biocomplexity through the agent-based paradigm. Brief Bioinform 2013. [Epub ahead of print]; doi: 10.1093/bib/bbto77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Garza-Garcia L.D., Carrillo-Cocom L.M., Araiz-Hernandez D., Soto-Vazquez P., Lopez-Meza J., Tapia-Mejia E.J., Camacho-Leon S., Garcia-Lopez E., Rodriguez-Gonzalez C.A., and Alvarez M.M.A biopharmaceutical plant on a chip: continuous micro-devices for the production of monoclonal antibodies. Lab Chip 13,1243, 2013 [DOI] [PubMed] [Google Scholar]
- 131.Song M.J., Dean D., and Knothe Tate M.L.In situ spatiotemporal mapping of flow fields around seeded stem cells at the subcellular length scale. PLoS One 5,7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lapin A., Schmid J., and Reuss M.Modeling the dynamics of E. coli populations in the three-dimensional turbulent field of a stirred-tank bioreactor—a structured-segregated approach. Chem Eng Sci 61,4783, 2006 [Google Scholar]
- 133.Storck T., Picioreanu C., Virdis B., and Batstone D.J.Variable cell morphology approach for individual-based modeling of microbial communities. Biophys J 106,2037, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bailey A.M., Thorne B.C., and Peirce S.M.Multi-cell agent-based simulation of the microvasculature to study the dynamics of circulating inflammatory cell trafficking. Ann Biomed Eng 35,916, 2007 [DOI] [PubMed] [Google Scholar]
- 135.Picioreanu C., van Loosdrecht M.C.M., and Heijnen J.J.Mathematical modeling of biofilm structure with a hybrid differential-discrete cellular automaton approach. Biotechnol Bioeng 58,101, 1998 [DOI] [PubMed] [Google Scholar]
- 136.Picioreanu C., Vrouwenvelder J.S., and van Loosdrecht M.C.M.Three-dimensional modeling of biofouling and fluid dynamics in feed spacer channels of membrane devices. J Memb Sci 345,340, 2009 [Google Scholar]
- 137.Atala A., Kasper F.K., and Mikos A.G.Engineering complex tissues. Sci Transl Med 4,160rv12, 2012 [DOI] [PubMed] [Google Scholar]
- 138.Lindbergh C.A.An apparatus for the culture of whole organs. J Exp Med 62,409, 1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Carrel A., and Lindbergh C.A.The culture of whole organs. Science 81,621, 1935 [DOI] [PubMed] [Google Scholar]
- 140.Kaul H.A multi-paradigm modelling framework for simulating biocomplexity [D.Phil. thesis]. Department of Engineering Science, University of Oxford, Oxford, 2014 [Google Scholar]
- 141.Parson A.B.Stem cell biotech: seeking a piece of the action. Cell 132,511, 2008 [DOI] [PubMed] [Google Scholar]
- 142.Mason C., and Manzotti E.Regenerative medicine cell therapies: numbers of units manufactured and patients treated between 1988 and 2010. Regen Med 5,307, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Mason C., and Dunnill P.The need for a regen industry voice. Regen Med 3,621, 2008 [DOI] [PubMed] [Google Scholar]
- 144.Mason C.ISSCR 2009 Industry panel session: promoting translation and commercialization. Cell Stem Cell 5,379, 2009 [DOI] [PubMed] [Google Scholar]
- 145.Martin P.A., Coveney C., Kraft A., Brown N., and Bath P.Commerical development of stem cell technology: lessons from the past, strategies for the future. Regen Med 1,801, 2006 [DOI] [PubMed] [Google Scholar]
- 146.Lysaght M.J., Jaklenec A., and Deweerd E.Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A 14,305, 2008 [DOI] [PubMed] [Google Scholar]
- 147.Naughton G.K.From lab bench to market—critical issues in tissue engineering. Ann N Y Acad Sci 961,372, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Mason C., and Dunnill P.Lessons for the nascent regenerative medicine industry from the biotech sector. Regen Med 2,753, 2007 [DOI] [PubMed] [Google Scholar]
- 149.Ratcliffe E., Thomas R.J., and Williams D.J.Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br Med Bull 100,137, 2011 [DOI] [PubMed] [Google Scholar]
- 150.Anderson J.Computational Fluid Dynamics. New York: McGraw-Hill, 1995 [Google Scholar]
- 151.Morowitz H.J.The Emergence of Everything: How the World Became Complex. Oxford: Oxford University Press, 2002 [Google Scholar]
- 152.Hazen R.M., Griffin P.L., Carothers J.M., and Szostak J.W.Functional information and the emergence of biocomplexity. Proc Natl Acad Sci U S A 104 (Suppl 1), 8574, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pope M.H.Giovanni Alfonso Borelli—the father of biomechanics. Spine 30,2350, 2005 [DOI] [PubMed] [Google Scholar]
- 154.Gibson T.The first homografts: trembley and the polyps. Br J Plast Surg 19,301, 1966 [DOI] [PubMed] [Google Scholar]
- 155.Schmitt S.From eggs to fossils: epigenesis and transformation of species in Pander's biology. Int J Dev Biol 49,1, 2005 [DOI] [PubMed] [Google Scholar]
- 156.Pander C.H.Dissertatio inauguralis sistens historiam metamorphoseos quam ovum incubatum prioribus quinque diebus subit. Würzburg: Nitribitt, 1817 [Google Scholar]
- 157.Valcourt D.Hopkins doctors grow new ear on woman's arm [Electronic News Article]. CBS Baltimore, Baltimore, MD, 2012. Available at: http://baltimore.cbslocal.com/2012/09/26/hopkins-doctors-give-woman-a-new-ear/ (Last Accessed: December11, 2014) [Google Scholar]






