Abstract
A role for type A Clostridium perfringens in acute hemorrhagic and necrotizing gastroenteritis in dogs and in necrotizing enterocolitis of neonatal foals has long been suspected but incompletely characterized. The supernatants of an isolate made from a dog and from a foal that died from these diseases were both found to be highly cytotoxic for an equine ovarian (EO) cell line. Partial genome sequencing of the canine isolate revealed three novel putative toxin genes encoding proteins related to the pore-forming Leukocidin/Hemolysin Superfamily; these were designated netE, netF, and netG. netE and netF were located on one large conjugative plasmid, and netG was located with a cpe enterotoxin gene on a second large conjugative plasmid. Mutation and complementation showed that only netF was associated with the cytotoxicity. Although netE and netG were not associated with cytotoxicity, immunoblotting with specific antisera showed these proteins to be expressed in vitro. There was a highly significant association between the presence of netF with type A strains isolated from cases of canine acute hemorrhagic gastroenteritis and foal necrotizing enterocolitis. netE and netF were found in all cytotoxic isolates, as was cpe, but netG was less consistently present. Pulsed-field gel electrophoresis showed that netF-positive isolates belonged to a clonal population; some canine and equine netF-positive isolates were genetically indistinguishable. Equine antisera to recombinant Net proteins showed that only antiserum to rNetF had high supernatant cytotoxin neutralizing activity. The identifica-tion of this novel necrotizing toxin is an important advance in understanding the virulence of type A C. perfringens in specific enteric disease of animals.
Introduction
C. perfringens is an important Gram-positive anaerobic pathogen of humans and animals that is found ubiquitously in soil and the gastrointestinal tract of vertebrates. It causes a number of histotoxic infections, enteritis and enterotoxemias. The species produces an array of extracellular toxins, four of which (alpha, beta, epsilon and iota) form the basis for a toxin-typing scheme, which identifies five toxin types (types A, B, C, D or E) [1]. In recent years, a novel toxin, NetB, was shown to be produced by the majority of type A isolates recovered from chickens with necrotic enteritis (NE), an important disease in broiler chicken production, and to play a critical role in NE pathogenesis [2]. This important advance raises the possibility that type A strains in a number of other poorly understood but clinically and pathologically distinct enteric diseases of different animal species [1] might contain other as-yet-undescribed necrotizing toxin genes [3].
A number of important toxins, C. perfringens enterotoxin (CPE, in non-food-poisoning strains and in a minority of food poisoning strains), and all the typing toxins except for alpha-toxin (CPA), are encoded on a conserved family of large plasmids related to the pCW3 tetracycline-resistance plasmid. These plasmids share a conserved core region that includes the transfer of clostridial plasmid (tcp) locus required for conjugation [4,5]. The transmissible nature of key C. perfringens toxin and related virulence genes suggests that virulence of different toxin types can change through plasmid acquisition or loss. However, phylogenetic studies of C. perfringens disease strains also suggest a contribution of the chromosomal background to virulence that varies with the source of the strain. For example, clonality has been described for the majority of bovine type E isolates, for porcine type C isolates, and for isolates from chickens with NE [6–9].
C. perfringens type A-associated diarrhea and enteric disease in dogs is not well characterized, but its association with disease may range in severity from mild and self-limiting to fatal acute hemorrhagic diarrhea [10]. The acute hemorrhagic gastroenteritis form of disease is marked by severe necrotizing inflammation of the intestinal tract, especially of the small intestine, by hemorrhage and in some cases by rapid death [11,12]. The presence of large numbers of C. perfringens adhering to the necrotic intestinal mucosa is a striking and common feature [11–14]. Morbidity may be more common than mortality. Because the infection is not well characterized, no gold standard for diagnosis exists [10]. An association of cpe-positive C. perfringens with a case of fatal canine hemorrhagic enteritis has been described [13]. Although not well characterized, acute hemorrhagic gastroenteritis associated with C. perfringens occurs particularly in small breed dogs [15].
The role of type A C. perfringens in enteric diseases of horses is also not well understood. There is evidence that CPB2 toxin-producing C. perfringens play a role in the fatal progression of colitis in horses [16,17]. Vilei and others [18] demonstrated that some out-of-frame cpb2-positive equine disease isolates produced the CPB2 toxin when grown in sub-inhibitory concentrations of gentamicin. They proposed a feasible direct role of these isolates in antibiotic-associated diarrhea in horses, since treatment with aminoglycoside antibiotics allowed translation of the cpb2 mRNA through induction of a ribosomal frame-shift. Anecdotally, there was an association between the isolation of cpb2-positive C. perfringens from cases of equine colitis and the use of gentamicin in hospitalized diarrheic horses, which ended when the antibiotic was stopped [18]. The correlation between CPB2 with severe and sometimes fatal colitis in horses is intriguing but the association remains unproven [19]. An apparent association has also been noted between the presence of the C. perfringens enterotoxin (CPE) and diarrheal illness in adult horses and in foals, including severe enteric disease [20–23]. Type A C. perfringens with cbp2 and (rarely) cpe genes are commonly found in the feces of healthy foals, whereas type C is seldom found in healthy horses [24]. Considerable work has been done on the important role of type C C. perfringens in foal neonatal enterocolitis [25,26]. The role of type A in fatal enterocolitis in foals is less clear, but necrosis of the small intestine and colon in 1-14-day-old foals caused by type A C. perfringens has been described in Kentucky [27]. These isolates possessed both cpb2 and cpe [28]. Other sporadic case reports in neonatal foals associate type A C. perfringens with fatal enterocolitis [29,30].
The present study describes a novel pore-forming toxin toxigenic C. perfringens associated with fatal hemorrhagic gastroenteritis of dogs and fatal necrotizing enterocolitis of neonatal foals.
Results
Identification and Analysis of Novel Putative Necrotizing Toxins
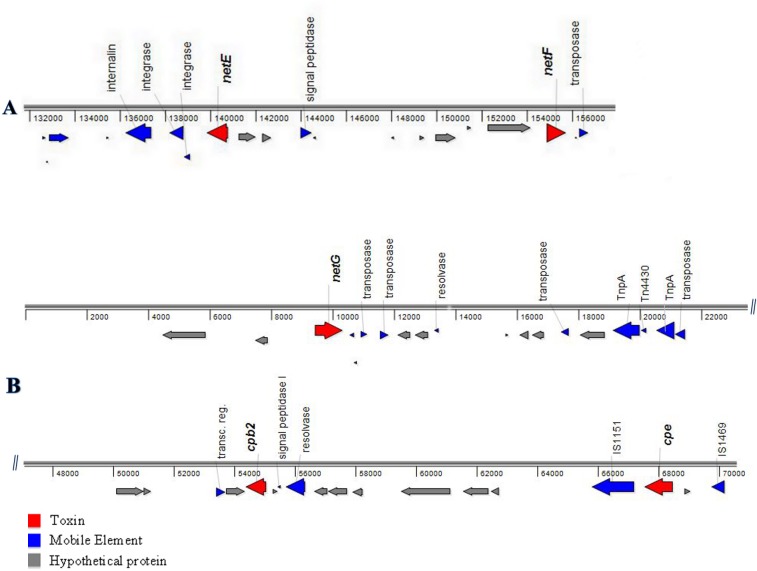
C. perfringens strain JFP718 was isolated from a case of fatal canine hemorrhagic gastroenteritis diagnosed in a 2-year-old female Pomeranian dog that was found dead the day after attending a dog show [13]. The strain was sequenced because of both the classic appearance of the disease in this dog and the highly cytotoxic effect of its supernatant. The automated annotation of the draft genome sequences of C. perfringens canine strain JFP718 were essential to the discovery of the three putative toxin genes, initially denominated as Panton-Valentine leukocidins (PVLs). Two PVL open reading frames (ORF) were located in scaffold00006 (nucleotides 139809–140777 [–] and 154804–155721[+]) (GenBank KP739975), while the third was in scaffold00012 (nucleotides 9399–10319 [+]) (GenBank KP739976), both surrounded by transposon-related sequences. Interestingly, both scaffolds (00006 and 00012) contained mainly plasmid genes, suggesting that these sequences were associated with a transposable element and/or plasmid. The newly identified toxin genes were named netE, netF and netG. The predicted proteins (NetE, NetF and NetG) encoded by these three ORFs showed 79%, 48% and 52% identity, respectively, with the pore-forming toxin NetB from C. perfringens (EU143239) (Table 1 and S1 Fig). Scaffold 00006 harbors netE and netF toxin genes among 140 ORFs. Immediately upstream of the netE and netF genes, which are 14,027 nt apart, are two mobile element proteins classified as an integrase (C. botulinum BKT015925) and a transposase (C. perfringens), whereas downstream of the netF gene there is another integrase (C. botulinum BKT015925) (Fig 1A). Scaffold 00012 harbors the netG gene as well as the cpb2 (CPB2 toxin) (54351–55052) and cpe (67515–68474) genes (Fig 1B).
Table 1. Characterization of Net toxins.
| Protein 1 | Length (aa) | Molecular size (mature protein) in kDa | Predicted Product | E-Value | % of Identity 2 | Localization 3 | Conserved Domain | Accession | PSSM-ID | SignalP 4 |
|---|---|---|---|---|---|---|---|---|---|---|
| NetE | 322 | 36.1 (32.9) | Putative beta-pore-forming toxin | 3.14E-71 | 254/322 (79%) | Extracellular | Leukocidin/Hemolysin toxin family | cl08468 | 244969 | 30–31 |
| NetF | 305 | 34.3 (31.7) | Putative beta-pore-forming toxin | 4.41E-57 | 143/299 (48%) | Extracellular | Leukocidin/Hemolysin toxin family | cl08468 | 244969 | 24–25 |
| NetG | 306 | 34.3 (31.7) | Putative beta-pore-forming toxin | 2.89E-70 | 143/276 (52%) | Extracellular | Leukocidin/Hemolysin toxin family | cl08468 | 244969 | 24–25 |
1Based on strain JFP718 genome
2Percent amino acid identity with NetB (query length/total length of the subject protein)
3Subcellular location as predicted by pSortb
4Signal peptide was predicted by SignalP cleavage position
Fig 1. Genetic organization of JFP718 scaffolds.
The genetic organization of (A) partial view of scaffold00006 (GenBank KP739975), (B) partial view of scaffold00012 (GenBank KP739976) is shown, each arrow representing a predicted gene and its orientation. Predicted functional annotations and respective positions are shown above each gene, respectively. Genes are color-coded by their putative role based upon sequence analyses.
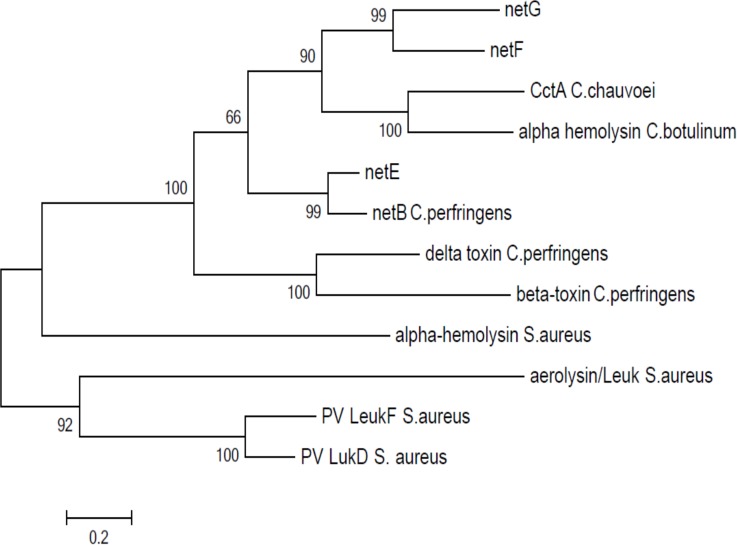
Sequence analyses of these ORFs were performed using BLAST, BLASTP, the Conserved Domains Database (CDD) [31], SignalP [32], pSortB [33], and InterProScan [34] (Table 1). Analysis against the CDD conserved domain database showed that these newly described net genes encode proteins belonging to the Leukocidin/Hemolysin superfamily. InterProScan analysis and classification also confirmed the presence of the Leukocidin/Hemolysin domain (IPR001340) and classified them as members of the alpha-hemolysin branch of the beta-pore-forming toxin family. The new genes are predicted by pSortB and SignalP to encode extracellular proteins. netE encodes a putative 322 amino acid (aa) protein with a signal peptide region of 30 residues, netF encodes a 305 aa protein, and netG encodes a 306 aa protein, both with a signal peptide region of 24 residues. The predicted respective molecular size of the mature NetE toxin is 32.9 kDa, and of NetF and NetG are 31.7 kDa. The close relationship of the novel Net toxins to other members of the Leukocidin/Hemolysin pore-forming toxin family is shown through the Clustal W alignment (S1 Fig) and phylogenetic tree (Fig 2) [35]. For NetE, the closest ortholog (79% identity) is NetB from C. perfringens, whereas NetF and NetG were placed in their own branch (Fig 2).
Fig 2. Phylogenetic analysis of representative members of the Leukocidin/Hemolysin superfamily.
The phylogenetic tree was built by the Neighbor-joining algorithm using (1000 interactions) MEGA5 software (35). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Toxins that were used included: alpha-hemolysin of C. botulinum (YP_004394739.1), hemolysin II of B. cereus (YP_002447023.1), alpha-hemolysin of S. aureus (WP_001788633), putative CctA of C. chauvoei (WP_021874975) and beta-toxin of C. perfringens (CAA58246.1).
The VirR/VirS two-component signal transduction system regulates several virulence genes in C. perfringens, including cpa (alpha-toxin or phospholipase C), cpb (beta-toxin), pfoA (perfringolysin O or theta-toxin), colA (kappa-toxin or collagenase), and netB [36,37]. Analysis of the upstream region of net genes revealed the presence of potential VirR boxes [38] in the promoter region of netE and netG, but not of netF S1 Table.
Cytotoxicity of Clostridium perfringens Type A Isolates
To determine whether C. perfringens type A strains isolated from fatal cases of canine hemorrhagic enteritis and foal necrotizing enteritis produced a distinct secreted toxin, sterile culture supernatants of strains JFP718 (canine origin) and JFP728 (equine origin) were tested for cytotoxicity against 10 cell lines derived from 9 animal species (Table 2). The culture supernatants of the two strains showed potent cytotoxic effects for an equine ovarian cell line (EO) at a dilution of 1:128, with far less or no toxicity against other cell lines tested (Table 2). By contrast, supernatants derived from NCTC3110 (cpa, cpb positive), JFP564 (cpa, cpb2 and cpe positive), SM101 (cpa, cpe positive) and CW504 (cpa positive) displayed mild or no cytotoxic effects on EO cells, suggesting that toxicity of JFP718 and JFP728 strains might be caused by a secreted component(s) distinct from other known toxins (Table 3).
Table 2. Cytotoxicity of different cell lines with bacterial culture supernatant of netF-positive (JFP726, JFP728) and cpb-positive (NCTC3110) C. perfringens strains.
| Cytotoxicity titer 2 | ||||
|---|---|---|---|---|
| Cell lines 1 | Origin of cell lines | NCTC3110 | JFP728 (Equine) | JFP726 (Canine) |
| EO | Equine | 16 | 128 | 128 |
| MDCK | Canine | N 3 | 4 | 4 |
| A72 | Canine | 4 | 8 | 8 |
| MDBK | Bovine | N | 2 | 2 |
| PK15 | Pig | N | 4 | 4 |
| 208F | Rat | 2 | 2 | 2 |
| NIH 3T3 | Mouse | N | N | N |
| CaCo2 | Human | N | 2 | 2 |
| LMH | Chicken | 2 | N | N |
| Vero | Monkey | N | N | N |
1EO: Equine ovarian cell line, MDCK: Madin Darby canine kidney cell line, A72: Canine fibroblasts cell line, MDBK: Madin Darby bovine kidney cell line, PK15: Porcine kidney cell line, 208F: Rat Fischer fibroblast cell line, N1H 3T3: Mouse embryo fibroblast cell line, CaCo2: Human colon epithelial cell line, LMH: Primary chicken hepatocellular carcinoma epithelial cell line, Vero: African green monkey kidney cell line.
2 Cytotoxicity was evident after 8 h of exposure of 2-fold dilution series of the filter-sterilized culture supernatants up to 1:1024 to different cells. The titer is the final dilution showing > 2+ cell toxicity.
3 No cytotoxicity.
Table 3. Cytotoxicity of equine ovarian cell line (EO) with different bacterial culture supernatants.
| Strains (toxin genes) 1 | Cytotoxin titer 2 |
|---|---|
| NCTC3110 (cpb+) | 16 |
| JFP564 (cpe+/cpb2+) | 8 |
| SM101 (cpe+) | 4 |
| JFP728 (netE/F/G+) | 128 |
| JFP718 (netE/F/G+) | 128 |
| CW504 (cpa+) | N 3 |
| TPG broth control | N |
1NCTC3110: type B, JFP564: type A, SM101: Derivative of NCTC 8798, JFP728: equine necrotizing enteritis, JFP718: canine hemorrhagic enteritis, CW504: lab strain. All strains possessed the cpa gene. Toxin titers were assessed over 10 times.
2Dilution of filter-sterilized bacterial supernatants from TPG broth cultures with an OD600 of 0.6–0.8 that showed end-point > 2+ cytotoxicity
3No cytotoxicity
Characterization of Large Plasmids by PFGE Analysis and Southern Blots (SB)
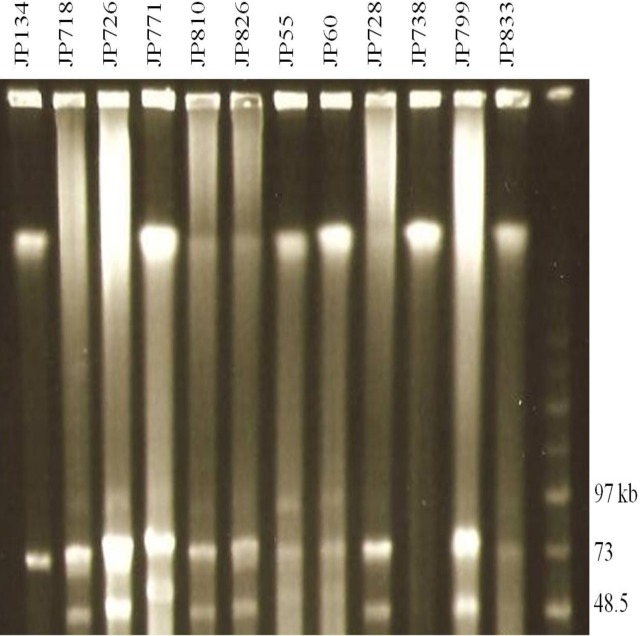
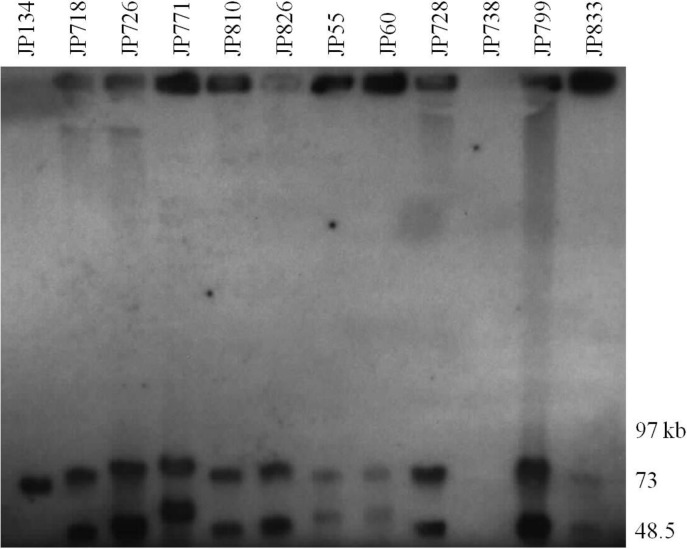
To determine the presence of large plasmids in equine and canine type A C. perfringens isolates, genomic DNA from six canine and six equine isolates (Table 4) were subjected to PFGE. The PFGE profiles of the canine and equine C. perfringens strains digested with NotI revealed the presence of two to three large plasmids ranging in size from 45–97 kb in all strains (Fig 3 and Table 5). PFGE analysis (Fig 3) shows the diversity of plasmids among these type A isolates and their marked size variations, which were confirmed by PFGE/SB studies (Fig 4). Specifically, the partially sequenced canine type A C. perfringens JFP718 strain carried three large plasmids (Fig 3) in which the netE and cpe genes were on a distinct plasmid (Fig 4). The SBs showed the presence of tcpF in all the large plasmids (data not shown). In addition, cpb2 was identified by SB in the netG plasmid as described in the scaffold 00012 (data not shown) confirming the hypothesis that these two scaffolds represent two distinct plasmids. Interestingly, the non-cytotoxic strains JFP134 (canine) and JFP738 (equine) which showed one and no large plasmids, respectively, were also SB negative for netE (Fig 4).
Table 4. Bacterial strains used in this study.
| Strain | Name | Relevant characteristics | Source |
|---|---|---|---|
| C. perfringens | NCTC3110 | Type B | NCTC, UK |
| NCTC7368 | Type B | NCTC, UK | |
| NCTC3181 | Type C | NCTC, UK | |
| ATCC3628 | Type C | ATCC, USA | |
| SM101 | Derivative of NCTC 8798 | J.Gong, AAFC | |
| CW504 | RifR2 NalR; conjugation recipient | J.I.Rood, Monash University | |
| JIR325 | Strain 13 Rif RNalR; electroporation recipient | J.I.Rood, Monash University | |
| Strain 33 | C. perfringens strain 33 was used to normalize the gel and allow for gel-to-gel comparisons | This study | |
| CP1 | netB+ C. perfringens/Chicken necrotic enteritis | [39] | |
| CP4 | netB+ C. perfringens/Chicken necrotic enteritis | [39] | |
| JFP55 | Equine strain/Undifferentiated diarrheal disease | This study | |
| JFP60 | Equine strain/Undifferentiated diarrheal disease | This study | |
| JFP134 | Canine strain/Undifferentiated diarrheal disease | This study | |
| JFP564 | Human strain/ Type A | This study | |
| JFP718 | Canine strain/Hemorrhagic gastroenteritis | This study | |
| JFP726 | Canine strain/Hemorrhagic gastroenteritis | This study | |
| JFP728 | Equine strain/Necrotizing enteritis | This study | |
| JFP738 | Canine strain/Undifferentiated diarrheal disease | This study | |
| JFP771 | Canine strain/ Hemorrhagic gastroenteritis | This study | |
| JFP799 | Equine strain/Necrotizing enteritis | This study | |
| JFP810 | Canine strain/Hemorrhagic gastroenteritis | This study | |
| JFP826 | Canine strain/Hemorrhagic gastroenteritis | This study | |
| JFP833 | Equine strain/Necrotizing enteritis | This study | |
| JFP838 | Equine strain/Hemorrhagic enterocolitis | This study | |
| JFP838E-05 | JFP838::netE::ErmRAM—ClosTron insertion in netE gene | This study | |
| JFP838F-05 | JFP838::netF::ErmRAM—ClosTron insertion in netF gene | This study | |
| JFP838G-07 | JFP838::netG::ErmRAM-ClosTron insertion in netG gene | This study | |
| T504-05::netE | CW504 derived transconjugant RifRNalRErmRwith plasmid pNetE/NetF from JFP838E-05 | This study | |
| JFPnetF | JIR325 derived transconjugant RifRNalRCmR with plasmid pNetF07 | This study | |
| VN-22C | JFP838F-05 ErmRCmR complemented with pNetF07 | This study | |
| E. Coli | DH5α | F - Φ80 lacZΔM15Δ (lacZYA-argF) U169 endA1 recA1 hsdr17 (r K - m K- ) deoR thi-1 supE44 gyrA96 relA1 | Stratagene, La Jolla, CA |
| BL21-Star (DE3) pLysS | E. coli B F — dcm ompT hsdS (r B — m B – ) gal λ(DE3) [pLysS CamR] | Invitrogen | |
| CA434 | E. coli HB101 carrying the Incβ conjugative plasmid R702 | [40]; G. Vedantam, University of Arizona | |
| CA434-netE | E. coli CA434 carrying plasmid pMTL::netE | This study | |
| CA434-netF | E. coli CA434 carrying plasmid pMTL::netF | This study | |
| CA434-netG | E. coli CA434 carrying plasmid pMTL::netG | This study |
Fig 3. PFGE analyses of plasmids from canine and equine C. perfringens strains.
Agarose plugs containing DNA from each specified isolate were digested with NotI and subjected to PFGE and staining with ethidium bromide. Line numbers indicate isolate numbers; M: Mid-Range II PFG molecular DNA ladder (Kb).
Table 5. Features of type A canine and equine Clostridium perfringens strains.
| PCR 1 | Southern blot 2 | |||||
|---|---|---|---|---|---|---|
| Strains | Plasmid number | netF/netF | netG | cpe | netE | cpe |
| JFP134 | 1 | - | - | + | - | 70 |
| JFP718 | 3 | + | + | + | 75 | 45 |
| JFP726 | 3 | + | + | + | 75 | 48 |
| JFP771 | 3 | + | - | + | 75 | 50 |
| JFP810 | 2 | + | + | + | 75 | 48 |
| JFP826 | 2 | + | + | + | 75 | 48 |
| JFP55 | 3 | + | - | + | 75 | 50 |
| JFP60 | 3 | + | - | + | 75 | 50 |
| JFP728 | 2 | + | + | + | 75 | 48 |
| JFP738 | - | - | - | - | - | - |
| JFP799 | 2 | + | + | + | 75 | 48 |
| JFP833 | 2 | + | - | + | 75 | 48 |
1Genes detected by PCR amplification (-) negative and (+) positive
2Approximate size of plasmids in kb
Fig 4. PFGE-Southern blot of plasmids from canine and equine C. perfringens strains.
Southern blotting of PFGE was performed with DIG-labelled probes for netE and cpe genes. Results from both netE and cpe probes are shown overlayed. In all lanes with two bands, the upper band represents netE and the lower band cpe. M: Mid-Range IIPFG molecular DNA ladder (Kb).
PFGE Analysis of netF-Positive and Negative in Canine and Equine Type A C. perfringens
There is increasing evidence that C. perfringens associated with different enteric diseases may belong to clonal populations. We used PFGE to examine the relatedness of canine and equine netF+ and netF- isolates from our strain collection. The PFGE analysis and the dendogram are shown in Fig 5. Out of the 70 isolates (35 equine and 35 canine) typed by PFGE, 56 (80%) individual pulse types were identified based on an identical banding pattern (genetic similarity of 100%). Eighteen of these 56 were positive for netF, 37 were negative for netF, and one type contained both netF+ and netF- isolates that were equivalent. There was no pattern distinguishable between equine and canine types (Fig 5).
Fig 5. Dendogram of Clostridium perfringens isolates.
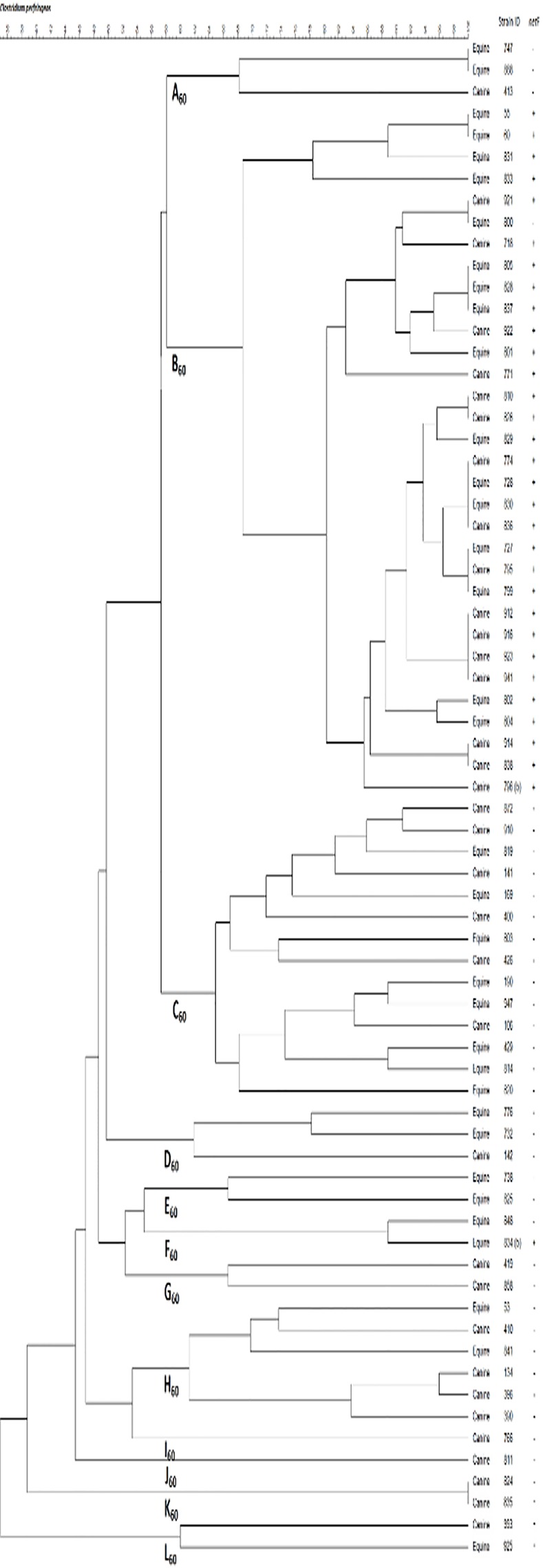
Dendogram of C.perfringens isolates typed by pulsed-field gel electrophoresis and analysed using BioNumerics software. The BioNumerics software used was version 7.1 from Applied Maths, Austin, TX.
Using the criterion of 60% genetic similarity, 11 clades (A to L) were distinguished (Fig 5). All but one of the netF+ strains belonged to one clade when using this relatedness criterion (Fig 5). The netF- isolates comprised a wider diversity of clades (Fig 5). Only one netF+ isolate, JFP834, was included in one of these netF- clades. Using a less stringent genetic similarity cut-off of 70%, all except one of the netF+ strains belonged to two clades whereas the netF- strains belonged to 21 clades.
Mutation of netE, netF and netG and Conjugation
In order to confirm which one of the Net proteins was responsible for cytotoxicity on EO cells, several assays were performed.
Firstly, the netE, netF and netG genes were insertionally inactivated in strain JFP838 by the ClosTron method, resulting in the mutant strains JFP838E-05, JFP838F-05 and JFP838G-07, respectively. The insertion of ErmB-carrying introns into each of the three target genes was confirmed by Southern blot and PCRs. Southern blot assay using specific intron probe (S2 Table and S2 Fig) showed the insertion of the intron in the target sites as well in other sites in the case of the netF and netG mutants (S2 Fig). The insertion of ErmB-carrying introns into each of the three target genes was confirmed by two PCRs, one using specific primers flanking the insertion site (netE, netF and netG primers) and another using a combination of specific primers with intron primer (EBS-universal) (S2 Table and S3A and S3B Fig). The mutant strains JFP838E-05 (::netE) and JFP838G-07(::netG) retained wild-type cytoxicity on EO cells, whereas the JFP838F-05 (::netF) mutant was not cytotoxic.
Secondly, the non-cytotoxic mutant strain JFP838F-05 was complemented in trans with pNetF-07, which contains the entire netF gene. The netF gene in pNetF-07 restored the cytotoxicity of the JFP838::netF mutant (JFP838F-05) similar to that of the wild-type JFP838 (Fig 6). Western blot attested to the expression of NetF in the complemented but not the mutant strain (S4 Fig).
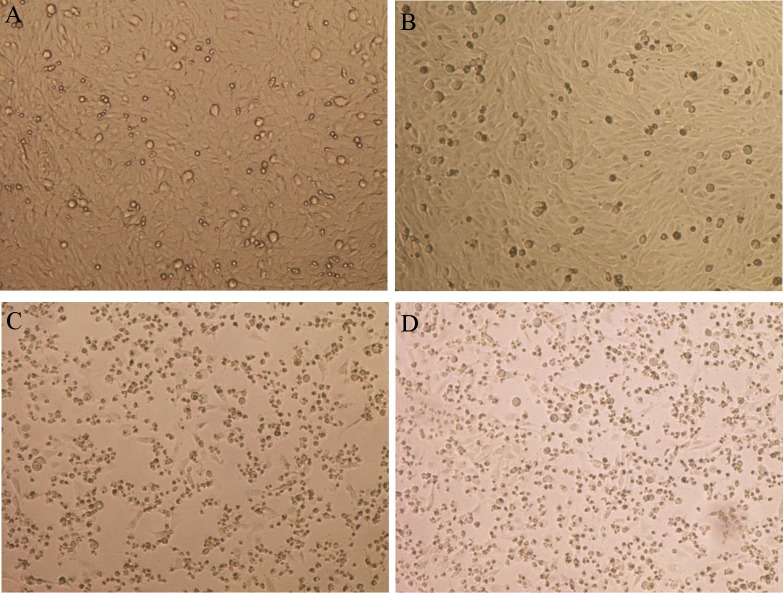
Fig 6. Infection and cytotoxic effects on equine ovarian (EO) cells by supernatant from JFP838 and its isogenic derivatives.
Confluent EO cell cultures were infected for 8 h at 37°C/5%CO2. Filter-sterile broth culture supernatants were used for these infections: (A) Typical morphology of EO cells, (B) JFP838-F05 (netF null mutant), (C) Wild-type JFP838, (D) Complementing strain VN-22C. Cytotoxic effects to EO cells in these conditions were cell rounding, detachment and death of cell in the cell plate, seen in Fig 6C and 6D. Magnification×100.
Thirdly, conjugation assays were performed using mutant strains (JFP838E-05 and JFP838F-05) as host and the laboratory strain CW504 as recipient, where plasmids (pCP718netF and pCP718cpe) were transferred to the recipient strain. The erythromycin-resistant transconjugants were confirmed by specific PCR amplifications of netE, netF and netG genes; the ermB gene was amplified from the transconjugants but not from the wild-type or recipient strain (Table 4). The transconjugant T504-05::netE, which harbored only the pCP718netF::netE plasmid, and not the pCP718cpe plasmid containing netG as confirmed by PCR (data not shown), showed cytotoxicity similar to that of the wild-type JFP838 strain. This evidence also suggested that the NetF toxin was producing the cytotoxic effect.
Finally, to confirm that the cytotoxicity was related entirely to NetF and not to any other protein expressed by the pCP718netF plasmid containing netE and netF, the pNetF-07 plasmid, which harbors the entire netF gene, was electroporated into laboratory strain C. perfringens JIR325. This recombinant strain, named JFPnetF, which expressed the NetF toxin but not the other Net toxins, was found to have cytotoxicity for EO cells similar to that of wild-type strain JFP838, whereas the laboratory strain JIR325 used as control was not cytotoxic.
Taken together, the results described above are clear evidence that the cytotoxic effect observed is produced by netF and that, of the three net genes found in these cytotoxic strains, netF alone is responsible for the cytotoxic activity.
Prevalence of net Genes and their Association with Foal Necrotizing Enteritis and Canine Hemorrhagic Enteritis, and with Cytotoxicity
The netF gene was identified in 11 of 15 isolates (74%) from different foals with fatal necrotizing enteritis whereas it was not found in 11 foals with undifferentiated diarrheal disease (p<0.00019; MUE = 31.45, CI = 5.35-∞). The netF gene was only identified in 4 of 58 (6.8%) isolates from adult horses with undifferentiated diarrheal disease (p<0.0000004 compared to foals with fatal necrotizing enteritis; CMLE≃33.63, CI = 6.58–170.95). In canine fatal hemorrhagic gastroenteritis, the netF gene was found in 8 of 11 isolates (73%) compared to 8 of 81 (10%) undifferentiated canine diarrheal isolates (p<0.000000081; CMLE≃40.44, CI = 8.01–204.72).
PCR analysis of equine and canine netF-positive isolates (n = 31) showed that the netF gene was always (100%) found in association with both the netE gene and the cpe enterotoxin gene, but in fewer (55%) isolates with the netG gene. netG was found in 5 of 11 (46%) isolates from canine hemorrhagic gastroenteritis compared to 7 of 81 (9%) isolates from dogs with undifferentiated diarrheal disease (p<0.0049; CMLE≃8.46, CI = 2.05–36.36). netG was only identified in 7 of 15 (47%) of foal necrotizing enteritis isolates compared to none of 11 foals with undifferentiated diarrheal disease (p<0.01; MUE = 11.15, CI = 1.9-∞) and none of 58 adult horses with undifferentiated diarrheal disease (p<0.000004, MUE = 59.95, CI = 10.75-∞). None of 24 caprine, 28 ovine and 47 bovine different source isolates tested was netE, netF or netG positive. Subsequent PCR amplifications of the netE, netF and netG genes from three canine hemorrhagic enteritis isolates and three other foal necrotizing enteritis isolates and DNA sequencing showed these genes to be fully conserved at the nucleotide level.
The supernatant of 29 of 31 canine and equine netF-positive isolates was as toxic as that of JFP728 for the EO cell line. Of 176 different C. perfringens type A isolates tested, 29 of 31 netF-positive strains were both cytotoxic and immunoblot positive using NetF antiserum compared to none of 145 netF-negative enteric strains of avian, bovine, canine, caprine, equine or human origin tested from our strain collection (details not given); immunoblots of the two non-toxic netF-positive strains revealed that NetF was not produced in these two strains (data not shown).
Recombinant Net Toxins
Recombinant Net toxins (rNetE, rNetF and rNetG) were engineered without the signal peptide (SP) sequence and, because of solubility problems, were purified under denaturing conditions using 8 M urea. To obtain soluble recombinant Net toxins, rNet proteins were constructed with the E. coli fusion protein NusA (45 kDa). SDS-PAGE and immunoblot results showed that the fusion proteins were expressed at a high level in a soluble form (data not shown).
Cytotoxin Neutralization by Equine Polyclonal Antibodies
Immunoblotting of SDS-PAGE separated rNetE-NusA, rNetF-NusA or rNetG-NusA showed cross-reactivity of sera prepared against each of the recombinant proteins (data not shown). Cross-reactivity similar to that noted in immunoblotting was also apparent in ELISA when these sera were tested against individual rNet-NusA proteins (Table 6), with the titer (and immunoblot cross-reactivity) being highest against the homologous protein. Following immunization, only horses immunized with rNetF-NusA showed a high degree of cytotoxin neutralizing activity when tested against supernatant from wild-type (netE/F/G) positive strains (Table 6).
Table 6. ELISA cross-reactivity and cytotoxin neutralizing titers to recombinant toxins rNetE, rNetF and rNetG in horses immunized with different rNet-NusA proteins.
| ELISA | ||||||
|---|---|---|---|---|---|---|
| Antigens | Horses 1 | Neutralizing anti-serum titers 2 | rNetE | rNetF | rNetG | rNetB |
| rNetE | 1 | 643 | 102400 | 6400 | 12800 | 51200 |
| 2 | 128 | 25600 | 1600 | 1600 | 12800 | |
| rNetF | 3 | 25600 | 12800 | 204800 | 12800 | 6400 |
| 4 | 6400 | 6400 | 102400 | 12800 | 12800 | |
| 5 | 3200 | 12800 | 204800 | 12800 | 6400 | |
| rNetG | 6 | 64 | 6400 | 12800 | 51200 | 12800 |
| 7 | 128 | 51200 | 51200 | 102400 | 51200 | |
1Immunization: Horses 1, 2, rNetE-NusA; horses 3–5, rNetF-NusA; horses 6, 7, rNetG-NusA
2The neutralization of cytotoxicity by polyclonal antibody was performed with the EO cell line
3The neutralizing antibody titer was that showing an inhibition of 2+ or greater
To determine the specificity of antibodies produced in horses to the individual rNet-NusA proteins, immunoblot of culture supernatants of two type B (cpb-positive), two type C (cpb-positive) and two type A netB-positive strains was performed using horse sera prepared against rNet-NusA proteins. Antisera prepared against each of the recombinant proteins interacted weakly with NetB but not with the CPB toxin in type B or C strains (data not shown).
Discussion
This paper advances understanding of type A C. perfringens as an enteric pathogen of animals by identifying a novel pore-forming toxin within the Leukocidin/Hemolysin superfamily family, NetF, and the association of a clonal population expressing this toxin with two distinct severe enteric diseases, canine hemorrhagic gastroenteritis and foal necrotizing enteritis.
A partial genome sequence identified three distinct genes (named netE, netF and netG) within the Leukocidin/Hemolysin pore-forming superfamily to which the α-toxin of Staphylococcus aureus and the CPB and NetB toxins of C. perfringens belong. Pore-forming toxins (PFTs) to which these new Net proteins belong, are potent cytolytic agents secreted by pathogenic bacteria that protect microbes against the cell-mediated immune system, disrupt epithelial barriers, and liberate nutrients necessary for growth and colonization. C. perfringens type A strains isolated from fatal cases of canine hemorrhagic enteritis and foal necrotizing enteritis produced secreted cytotoxin(s) toxic for an equine ovarian cell line (EO). The challenge was to determine whether one or all of the Net toxins was responsible, since immunoblotting showed that all three proteins were expressed (S4 Fig).
A series of mutations and conjugation assays proved that NetF was responsible for the marked cytotoxicity of EO cells. Mutations in the netE and netG genes did not cause any reduction in the cytotoxicity activity; only mutation of netF was able to eliminate the cytotoxic effect. Despite the fact that another two sites had intron insertions in the netF mutant, the complementation of the netF mutant by the netF gene completely restored the cytotoxic activity for EO cells (Fig 6). Two other approaches were also used to confirm NetF as the toxin responsible for cytotoxicity, firstly by transferring the pCP718netF plasmid containing netE and netF from JFP838E-05 (mutated netE) to C. perfringens CW504 by conjugation and secondly by electroporating the shuttle vector with the entire netF gene (pNetF-07) to C. perfringens JIR325. In both cases, bacterial supernatants produced cytotoxicity on EO cells equivalent to wild-type (JFP838). In addition, only antiserum against recombinant NetF protein neutralized the cytotoxicity of supernatants at high titer (Table 6).
The particular susceptibility of the EO cells compared to other cell lines (Table 2) may relate to specific receptors or factors other than those relating simply to the animal host of origin of the cell line. Others have found marked differences between chicken cell lines in susceptibility to NetB, a related pore-forming toxin important in the pathogenesis of necrotic enteritis of chickens [2]. Further work is required to identify the receptor for NetF.
Although netE and netG are expressed (S4 Fig), we found no evidence that they had cytotoxic activity. It is possible that they might contribute to the ability to cause disease in hosts other than dogs and horses. An alternate possibility is that NetE and NetG might have cytotonic effects, such as proinflammatory effects, as has been shown with pore-forming Leucocidins of Staphylococccus aureus [41]. There may also be synergism with other toxin molecules [41]. The consistent presence of cpe on a separate large plasmid in the netF-positive strains is an intriguing finding. A recent study of a type C human enteritis necroticans strain, and of purified CPB and CPE, showed the marked synergistic interaction of small amounts of these two toxins in causing fluid accumulation and histologic damage the intestine of rabbits [42].
The consistent presence of cpe in netF-positive strains is indirect evidence that CPE may contribute to the disease process in dogs and foals. CPE was not produced under the growth conditions used in this study (S4 Fig) but speculatively may have an important synergistic interaction with NetF in promoting disease in vivo. It is also possible that NetE and NetG might synergize with CPE in producing intestinal toxicity in vivo. netG was however present in just over half of the strains carrying netE, netF, and cpe, suggesting that its role in canine hemorrhagic enteritis and foal necrotizing enteritis is relatively unimportant.
Pore-forming toxins are released as water-soluble monomeric or dimeric species, bind specifically to a variety of receptors in membranes, and assemble transmembrane channels leading to cell damage and commonly to lysis [43]. De and Olsen [43] have shown that conservation of many of the key amino acids seems to be essential for their pore-forming function S1 Fig. Savva et al. [44] have identified conserved and non-conserved amino acid positions that affect binding and toxicity of NetB. Since the tyrosine at position 202 (NetB:Y202) is only conserved in the clostridial pore-forming toxins, Savva et al. [44] suggested that its proximity to Arg-200 may contribute to an interaction with the glycerol backbone, which reduces the hemolytic activity of NetB Y202A. In NetF, the tyrosine is not conserved, but rather histidine is present at the equivalent position S1 Fig. Yan et al. [45] proposed that the serine at position 254 (S254) of NetB is essential for the formation of an oligomer on the surface of the target cell. Notably, there is a substitution of S245T in NetF which could also impact the oligomerization of this toxin. The clostridial and staphylococcal members of the β-PFTs family may bind to host membranes using different mechanisms involving interactions with different lipids or proteins. The structure of the β-PFTs shows a ring-shaped complex which resembles a mushroom; on the rim domain, Trp-257 and Trp-262 have been shown to be involved in cell binding of NetB [44, 45]. Substitution to alanine at either position (W257A and W262A) reduced NetB cytotoxicity and hemolysis on LMH cells and on RBCs, respectively. Da Costa et al. [46] showed the importance of lysine at position 41 (K41) for oligomerisation and pore-formation of NetB. These residues are conserved in β-barrel PFTs of S. aureus and C. perfringens, as well as in NetE, NetF and NetG S1 Fig. The identification of three new members of the β-barrel PFT family may provide additional proteins with which to explore the receptor-based selectivity and specificity of members of this family [45,47].
Clonal expansion and niche specialization is well recognized in particular types of C. perfringens [48], such as cpe-bearing type A food poisoning isolates [9], porcine cna and cpb2-containing isolates [49], and cpb2 and netB-containing poultry isolates [7,50]. The clonality of the canine and equine netF-positive strains identified here, and the lack of any immediately obvious common infectious source relationship between dogs and neonatal foals, might suggest that they are infected by strains adapted to a common environmental source rather than to dogs or horses. The close relatedness, or in some cases the identity, of canine and equine isolates identified by PFGE was mirrored in the complete nucleotide conservation of the net genes, though size differences were noted in the plasmids encoding these genes (Fig 3). Additional studies will determine which are the fully conserved regions of these plasmids, and whether these plasmids have common pathogenicity loci [4,51].
The virulence of C. perfringens is dependent on its remarkable ability to produce a variety of toxins, of which some of the most toxic are found on the large family of conjugative tcp plasmids [4,52,53]. Although strains may carry up to three different large toxin plasmids [5,51], we found only two in the majority of pCP718netF-positive isolates, one carrying netE and netF, and the other consistently carrying the cpe enterotoxin gene and often the netG gene. C. perfringens virulence plasmids share a conserved backbone sequence which contains among other genes the tcp conjugation locus [4]. The tcp locus is present on all known conjugative plasmids from C. perfringens and consists of 11 genes (tcpA to tcpJ), of which two (tcpF, tcpH) are essential for conjugative transfer [54]. pCP718netF and pCP718cpe plasmids carried tcpF genes as shown by SB, but further work is required to show whether these are both conjugative. This study has added to understanding of the role of plasmids in the adaptability and flexibility of virulence in C. perfringens [53]. The likely basis of the presence of independently conjugative plasmids with large common genetic regions has been suggested [52] and identified [4,8]. Although toxin gene-bearing large plasmids are critical in the virulence of C. perfringens, the chromosomal background in which these plasmids exist may enhance both the host specificity and the virulence of the strain [7,8,9]. The netF-positive strains were almost entirely clonal (Fig 5), suggesting that the chromosomal background is important for maintenance of these plasmids and/or for virulence. A feature of the netF-positive strains identified here was that they always contained one plasmid with netE and netF and another with cpe and usually, but not always, with netG. The relatedness of strains carrying a plasmid-encoded cpe has been noted previously [55] but not at the degree of conservation identified here.
Many toxin genes in C. perfringens are positively regulated at exponential phase by the two-component VirR/VirS that is a major regulator of virulence in C. perfringens [36], including production of NetB system toxin by chicken necrotic enteritis strains [37] and CPB in type C strains [36]. We found that netE and netG genes have a putative VirR box upstream of their start site, and it is therefore possible that these toxin genes are regulated by the VirR/VirS system as are many other C. perfringens toxins. Obana and Nakamura [56] have shown that CPE1447 and CPE1446 controls target genes as novel transcriptional regulators. This might also be the case in the regulation of NetF toxin, or alternatively it may be regulated by VirR-regulated RNA [57].
Canine hemorrhagic gastroenteritis has long been a poorly understood disease of dogs, known to be often associated with C. perfringens and characterized by its dramatic and sometimes fatal nature [14,15]. The pathology of fatal cases of C. perfringens-associated canine hemorrhagic gastroenteritis is characterized by coagulative necrosis in the small intestine, a disease process typically associated with pore-forming toxins in C. perfringens. This study associates netF-producing strains with this disease, and will contribute to improved diagnosis, treatment and control. In the light of the current advance in understanding the basis of canine hemorrhagic gastroenteritis, the well-recognized association of this disease with small breed dogs [14,15,58] may be explained by the increased incidence of pancreatitis in small rather than in large breed dogs [59], since pancreatitis will disrupt pancreatic trypsin production. By analogy, fatal type C C. perfringens enteritis has commonly been associated with trypsin inhibition by foods such as cassava, or by trypsin inhibitory factors in colostrum, with the consequence that CPB toxin produced in the small intestine is not destroyed by the proteolytic action of trypsin but rather initiates intestinal necrosis and cascading clostridial disease [1]. Although there was a highly significant association between the presence of netF and canine hemorrhagic gastroenteritis compared to undifferentiated canine enteritis, we only obtained netF-positive isolates from about 75% of canine hemorrhagic gastroenteritis cases; failure to isolate netF from all cases might relate to loss of the pCP718netF plasmid, to isolation of non-toxigenic C. perfringens, to the presence of other etiologic agents, or for other reasons.
Enterocolitis in neonatal foals associated with types A and C C. perfringens is associated with high mortality [60], but the role of type A isolates has not been defined. This study identified the highly significant association of type A strains producing the novel pore-forming toxin NetF with this disease, and opens the way for control based on immunoprophylaxis or for measures based on future understanding of the epidemiologic basis of the disease [24,60]. Most foals with C. perfringens-associated enterocolitis have been younger than three days of age [25], also thus supporting a role for the trypsin-inhibitory action of colostrum [61] in interfering with the breakdown of C. perfringens toxins as an important feature of its pathogenesis. Failure to identify netF in all cases of type A C. perfringens-associated neonatal foal necrotizing enteritis might have explanations similar to those suggested for failure to isolate these bacteria from all cases of canine hemorrhagic gastroenteritis.
Materials and Methods
Bacterial Strains, Plasmids and Growth Media
Bacterial strains and plasmids used are described in Tables 4 and 7, respectively. C. perfringens strains were grown overnight at 37°C under anaerobic conditions (80% N2, 10% H2, 10% CO2) on either TPG medium (5% Tryptone [Becton, Dickinson and Company, Sparks, MD], 0.5% proteose peptone [Fisher Scientific, ON], 0.4% glucose [Fisher Scientific], and 0.1% thioglycolic acid [Sigma-Aldrich, St. Louis, MO]) or Brain Heart Infusion (BHI) agar (Becton, Dickinson and Company). All C. perfringens isolates were also cultivated in blood agar (Trypticase Soy Agar [Fisher Scientific] with 5% sheep blood) plates aerobically to confirm purity. E. coli strains DH5α (Stratagene, La Jolla, CA) was used as the host for plasmid construction, E. coli BL21-Star (DE3) pLysS (Invitrogen, Carlsbad, CA) was used for the overexpression of histidine-tagged fusion proteins, and E. coli CA434 for conjugation assays (Table 4). E. coli strains were grown on Luria-Bertani (LB) broth or agar plates (Becton, Dickinson and Company) overnight at 37°C. All strains were stored at -70°C in lyophilizing medium (25 g powdered skim milk [Fisher Scientific], 18.75 g glucose, 25 g sucrose [Sigma-Aldrich], 2.5 g bovine albumin [Sigma-Aldrich] in 250 ml distilled water).
Table 7. Plasmids used in this study.
| Plasmid | Relevant characteristics | Source |
|---|---|---|
| pJIR750 | E. coli—C. perfringens shuttle vector, CmR | J.I.Rood, Monash University |
| pNetF07 | pJIR750CmR containing netF gene and 250 bp of its upstream region | This study |
| pMTL007 | Inducible clostridial expression vector for expression of ClosTron, containing Erm RAM, ColE1, pCB102, CmR | [62] |
| pMTLnetE | pMTL007 containing intron retargeted to C. perfringens netE (sense insertion at 883–884 bp) | This study |
| pMTLnetF | pMTL007 containing intron retargeted to C. perfringens netF (antisense insertion at 152–153 bp) | This study |
| pMTLnetG | pMTL007 containing intron retargeted to C. perfringens netG (antisense insertion at 583–584 bp) | This study |
| pET-28a | E. coli expression vector. PT7, KanR, ori pBR322, ori f1, lacI, T7, Tag N-terminal 6xHis, C-terminal 6xHis | (Novagen, Gibbstown, NJ) |
| pET28::netE | pET28a containing the DNA fragment amplified by PCR using primers netE-F-EcoRI and netE-R- HindIII | This study |
| pET28::netF | pET28a containing the DNA fragment amplified by PCR using primers netF-F- EcoRI and netF-R-XhoI | This study |
| pET28::netG | pET28a containing the DNA fragment amplified by PCR using primers netG-F-BamHI and netG-R- XhoI | This study |
| pET43.1a | E. coli expression vector. PT7, AmpR, ori pBR322, ori f1, lacI, T7, Tag N-terminal Nus, C-terminal 6xHis | (Novagen, Gibbstown, NJ) |
| pET43::netE | pET43.1a containing the DNA fragment amplified by PCR using primers netE-F- EcoRI and netE-R- HindIII | This study |
| pET43::netF | pET43.1a containing the DNA fragment amplified by PCR using primers netF-F- EcoRI and netF-R-XhoI | This study |
| pET43::netG | pET43.1a containing the DNA fragment amplified by PCR using primers netG-F-BamHI and netG-R- XhoI | This study |
Identification of Necrotizing Toxin Genes
The identification of necrotizing toxin genes netE, netF and netG was made possible by the sequencing of a canine strain of C. perfringens (JFP718) carried out by the McGill University and Genome Quebec Innovation Centre (Montreal, QC) (data not shown). The pseudochromosome, plasmid fragments, and unplaced contigs were automatically annotated by Rapid Annotation using Subsystem Technology (RAST). BLASTN and BLASTX analyzes were performed to compare the established sequences to known C. perfringens sequences in the NCBI database.
Genomic and Plasmid DNA Isolation
A 1 ml aliquot of TPG medium cultured at 37°C overnight was spun down at 13,500 x g and DNA of the samples were extracted using InstaGene Matrix (Bio-Rad Laboratories, Mississauga, ON) following the manufacturer’s directions. Plasmid DNA was purified using midi-Qiagen columns (Qiagen, Mississauga, ON) following the manufacturer’s instructions.
PCR Amplifications for Toxin Genes
Detection of various toxin genes was carried out by PCR amplification of each C. perfringens isolate. The presence of the netE, netF and netG toxin genes was detected by PCR amplification, using primers designed from the C. perfringens JFP718 sequences. PCR-based toxin gene identification of cpa, cpb2 and cpe was also done. Primers used are described in S2 Table. Amplifications were performed in a 25 μl total volume containing the following: 5 μl of template DNA; 1X PCR buffer with Mg2+ (New England BioLabs, Pickering, ON); 0.2 mM deoxynucleoside triphosphate mixture; 2.5 units of TaqDNA polymerase (New England BioLabs); and 200 nM of each primer. The PCR program for netF was: 94°C for 3 min, 30 cycles of 94°C for 30 sec, 45°C for 30 sec, extension at 72°C for 1 min, and finally, 72°C for 5 min. The PCR programs for netE and netG were similar with the exception of the annealing temperature, 48°C for 30 sec/cycle. PCR product sizes were determined by agarose gel electrophoresis and visualized by ethidium bromide staining and photographed under UV light.
Prevalence of netE, netF and netG Genes in C. perfringens Isolates from Different Animal Species
C. perfringens type A isolates examined were from a collection of equine (n = 84), canine (n = 92), bovine (n = 47), caprine (n = 24) and ovine (n = 28) origin. Some isolates were from cases of diarrheal disease in animals presented to the Animal Health Laboratory, University of Guelph, some of which had detailed pathological diagnostic descriptions that included cases of fatal enteritis. Other isolates were from diarrheic horses (Dr. J.S. Weese, University of Guelph) or calves (Dr. K. Leslie, University of Guelph). In addition, some isolates were from fatal necrotizing enteritis in foals or fatal canine hemorrhagic enteritis (Dr. T. Besser, Washington State University, Pullman, WA) or originated from undifferentiated diarrheal illness in dogs (Dr. R. J. Carman, TechLabs, Blacksburg, VA; Dr. V. Perreten, Institute of Veterinary Bacteriology, University of Bern, Switzerland). Positive identifications of C. perfringens were based on the presence of the characteristic double zone of hemolysis and colonial morphology as well as the presence of the alpha-toxin gene cpa by PCR.
Cytotoxicity Assay and Cell lines
Culture supernatants of equine and canine netE, netF, and netG positive C. perfringens isolates were evaluated for cytotoxicity on several cell lines. Equine Ovarian cell line, EO (Dr. E. Nagy, University of Guelph; [63]); Madin Darby Bovine Kidney cell line, MDBK (ATCC, CCL-22); Madin Darby Canine Kidney cell line, MDCK (ATCC, CCL-34); Porcine Kidney cell line, PK15 (ATCC, CCL-33); Rat Fischer Fibroblast cell line, 208F (Life Technologies, ON); Mouse Embryo Fibroblast cell line, NIH 3T3 (ATCC, CRL- 1658); African green monkey kidney cell line, Vero (ATCC, CCL-81); and human colon epithelial cell line, CaCo-2 (ATCC, HTB-37) were grown in EMEM complete media (EMEM 450 ml, 10% fetal calf serum [VWR International, Radnor, PA], 2 mM L-glutamine [Sigma-Aldrich]). The A72, a cell line derived from canine fibroblasts, was maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum [VWR International], 2 mM L-glutamine [Sigma-Aldrich]. The primary chicken hepatocellular carcinoma epithelial cell line, LMH (ATCC, CRL-2117) was grown in Waymouth's complete media (90% Waymouth's MB 752/1 [Invitrogen, ON], 10% fetal calf serum). All cells were incubated at 37°C in an atmosphere of humidified 5% CO2. To test for cytotoxicity, cell lines were cultured to almost 100% confluence in 96-well plates (Corning Inc., Corning, NY) grown in their respective growth medium in 5% CO2 at 37°C.
For bacterial cytotoxin testing, bacterial supernatants from TPG broth cultures with an OD600 of 0.6–0.8 were filtered through a 0.22 μm filter (Fisher Scientific). Subsequently, 100 μl of sterile bacterial culture supernatant was added to the wells, in a 2-fold dilution series up to 1:1024 (v/v) in duplicate for each strain tested. Cytotoxicity was evaluated microscopically over 8 h as described in detail in Mehdizadeh Gohari et al. [64]. C. perfringens strains NCTC3110 (cpb-positive) and CW504 (cpa-positive) were used as positive and negative controls, respectively. The titer was the final dilution that showed >2+ cytotoxicity.
Mutation of netE, netF, and netG Toxin Genes and Conjugation
The generation of C. perfringens mutants was conducted as described in Heap et al. [62]. The ClosTron intron targeting and design tool (http://clostron.com) identified possible intron target sites. The insertion sites at the positions 883/884bp in the netE open-reading frame (ORF), at positions 152/153bp in the netF ORF, and positions 583/584 bp in the netG ORF were preferentially chosen to generate ClosTron-intron modifications, which were obtained by PCR from primers (IBS, EBS2, EBS1 and EBS universal) designed by the ClosTron website (S2 Table). The 350 bp PCR products and pMTL007 ClosTron-shuttle vector were digested with HindIII and BsrGI, ligated and then transformed by heat shock into E. coli DH5α. Recombinant plasmids were isolated and sequenced in order to verify sequences of the retargeted intron specific for netE, netF and netG insertions. The recombinants pMTLnetF, pMTLnetE and pMTLnetG containing the modified netE, netF and netG intron were then electroporated into electrocompetent E. coli CA434 for conjugation experiments. Conjugation was conducted as described in Heap et al. [65]. Plasmid transfer experiments were carried out with E. coli CA434 carrying recombinant plasmids pMTLnetE, pMTLnetF, and pMTLnetG, respectively. Overnight BHI cultures of the resultant E. coli CA434 were used as donor strains and the equine-source C. perfringens JFP838 strain was used as recipient. Donor and recipient cells were mixed at a ratio of 3:1 and a total of 200 μl of both cultures were spread onto BHI agar without antibiotics and incubated anaerobically at 37°C overnight. Subsequently, the bacterial growth was removed and resuspended in 1 ml of PBS. Transconjugants were selected on BHI agar plates containing cycloserine (250 μg/ml) and thiamphenicol (15 μg/ml). Transconjugants screening were performed as described in Parreira et al. [4]. Transconjugants were confirmed by PCR amplifications of specific genes (netE, netF and netG) (S2 Table).
Complementation of C. perfringens JFP838::netF
For complementation assays, the plasmid pJIR750 was used to construct the recombinant pNetF07 which contains the entire netF gene together with the 250 bp upstream region. C. perfringens JFP838::netF mutant was complemented with pNetF1A by the same conjugation method described above with E. coli CA434 as donor strain, using erythromycin and thiamphenicol to select transconjugants. The recombinant plasmid pNetF was also introduced into C. perfringens JIR325in order to analyze NetF expression and cytotoxicity.
Construction and Purification of Recombinant Toxins NetE, NetF and NetG using pET-28a
The chromosomal DNA of C. perfringens strain JFP728 was used as a template in PCR reactions. PCR reactions were performed with a Platinum PCR SuperMix high-fidelity kit (Invitrogen) and specific primers are described in S2 Table. The PCR amplified netE, netF and netG genes were cloned into the EcoRI—HindIII, EcoRI—XhoI, and BamHI—XhoI sites of vector pET-28a vector (Novagen, Gibbstown, NJ) to generate proteins fused with histidine residues (6-His), then transformed into E. coli DH5α. The nucleotide sequences of the cloned PCR products were verified by sequencing. The resulting plasmids (pET28::netE, pET28::netF, pET28::netG) were introduced into E. coli BL21-Star (DE3) pLysS. The recombinant E. coli BL21 strains harboring recombinant plasmids were grown in LB medium with kanamycin (50 μg/ml) and chloramphenicol (34 μg/ml) at 37°C until the absorbance at 600 nm reached 0.5, and then induced by adding 1 mM isopropyl-b-D-thiogalactopyranoside (IPTG) at 37°C for 4 h. Purification of recombinant His-tagged NetE, NetF and NetG proteins from E. coli BL21 was performed under denaturing conditions according to the manufacturer’s instructions (Qiagen). Briefly, for purification, the cell pellet was resuspended in 5 ml of lysis buffer (100 mM NaH2PO4, 10 mM Tris-Cl, 8M Urea, pH 8.0). The mixture was stirred for 60 min at room temperature. The lysate was centrifuged at 10,000xg for 30 min at room temperature to harvest the cellular debris. One ml of the 50% affinity chromatography on nickel-nitrilotriacetic acid agarose (Ni-NTA) was added to cleared lysate and mixed gently on a shaker for 60 min at 4°C. The column was washed twice with 4 ml of lysis buffer- pH 6.3, followed by 4 times with 1 ml lysis buffer- pH 5.9, and rNetE, rNetF and rNetG eluted with 4 times lysis buffer- pH 4.5. The final products were characterized by SDS-PAGE analysis.
Construction and Purification of Recombinant Toxins NetE, NetF and NetG using pET43.1a
The PCR amplified netF gene was cloned into the EcoRI and XhoI sites of vector pET43.1a (Novagen), whereas PCR products of netE and netG genes were cloned into the EcoRI—HindIII and BamHI—XhoI sites of vector pET43.1a. Transformation into expression strain (E. coli BL21) and induction was done as described above. Purification of rNetE-NusA, rNetF-NusA and rNetG-NusA was done by Ni-NTA agarose under native conditions following the manufacturer’s instructions (Qiagen). The resulting protein expressions and solubility levels were evaluated by SDS gel electrophoresis.
Horse Immunization using rNet-NusA Proteins
The recombinant fusion proteins were used for polyclonal antibody production in 7 adult horses (3 horses for rNetF-NusA and 2 horses each for rNetE-NusA or rNetG-NusA). A 1:4 ratio of aluminium hydroxide gel and sterile antigen was mixed together using 2.0 mg of antigen in 2 ml PBS, for a total of 2.5 ml per horse. For the primary immunization 1.0 mg of recombinant fusion protein and for the 2 subsequent immunizations 2.0 mg of fusion protein were used. Horses were immunized intramuscularly at day 0, 14, and 28, and bled at these times as well as on day 35 and 42 after initial immunization. Horses were examined for 4 days after each immunization and body reactions to injected antigen were recorded.
Cytotoxin Neutralization by Antibodies to rNetF, rNetE and rNetG
The neutralization of cytotoxicity by equine polyclonal antibody produced against each of the rNet-NusA proteins was performed with the EO cell line. An overnight culture supernatant of a netE, netF and netG-positive strain (JFP728) was prepared as described above. The supernatant was diluted in EMEM medium; the dilution of supernatant used for determination of neutralization titers was 128. Subsequently, serial 2-fold dilutions of sera up to 1:102,400 were made in a new 96-well plate (100 μl/well). Diluted antibodies were transferred into the diluted toxin plate and manually homogenized for 30 sec, then incubated for 2 h at 37°C. After incubation, 100 μl of the toxin-antibody dilution series was added into a plate with confluent EO cells which was incubated in a humidified environment of 5% CO2 at 37°C for 8 h. The neutralizing antibody titer was that showing an inhibition of 2+ or greater [66].
Enzyme-Linked Immunosorbent Assay (ELISA)
rNetE, rNetF and rNetG separated by SDS-PAGE were recovered by electro-elution using the Bio-Rad model 422 electro-eluter according to manufacturer’s protocol. Briefly, recombinant proteins were electrophoresed on a 12% polyacrylamide gel and the zone with rNet protein was cut from the gel, macerated into small pieces and then placed in electro-elution tubes containing electrophoretic buffer (25 mM Tris base, 192 mM Glycine and 0.1% SDS). Transferred recombinant proteins underwent dialysis to remove SDS.
For ELISA, 96-well plates (MaxiSorp, Nunc, Roskilde, Denmark) were coated with 0.5 μg /well of individual electro-eluted rNet proteins in carbonate-bicarbonate buffer pH 9.6 for 1 h at 37°C followed by overnight incubation at 4°C [67]. Briefly, plates were washed twice with buffer (phosphate-buffered saline pH 7.4 (PBS), 0.05% Tween 20) and once with PBS, and the coated plates were blocked using blocking buffer (PBS, 0.05% Tween 20, 0.5% fish skin gelatin [Norland HiPure Liquid Gelatin, Norland Products Inc, Cranbury, NJ]) for 2 h at 37°C. After washing 3 times with wash buffer, 100 μl/well of horse polyclonal serum (2-fold serial dilutions up to 1:409600) were added to the plate in duplicate. Washing buffer with no polyclonal antibodies was used as a negative control. After incubation at room temperature for 2 h, followed by 3 times washings with washing buffer, 100 μl/well of enzyme-labeled detecting goat anti-horse antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) (diluted 1:5000 in wash buffer) was applied, and the plate was incubated for another 1 h at room temperature. The plate was then washed 3 times with wash buffer and 100 μl/well of the chromogenic substrate 2,2'-azino-di-[3-ethylbenzthiazoline sulfonate] diammonium salt (ABTS) (Roche Applied Science) was added. The reaction was stopped after 30 min to 1 h of further incubation at room temperature using 0.5% SDS (50 μl/well) and the OD measured at 405 nm in an ELISA reader (BioTek Instruments Inc., Power Wave XS, Winooski, VT).
Western Blot Analysis
Immunoblotting was used for three main reasons. Firstly, to assess the specificity of horse polyclonal serum prepared against rNet proteins. For this purpose, 2 type B C. perfringens (NCTC3110, NCTC7368), 2 type C C. perfringens (ATCC3628, NCTC3181), and 2 netB-positive C. perfringens strains (CP1, CP4) were tested. Secondly, to confirm the mutation of three net genes and complementation of netF gene, as well as expression of all three native Net proteins in wild-type strain. For this, 3 mutant strains (JFP838E-05, JFP838F-05 and JFP838G-07), complemented netF strain (VN-22C), and wild-type strain (JFP838) were tested using horse polyclonal serum prepared against rNet proteins. Thirdly, to indicate the absence of expression of the cpe gene under the growth conditions used in this study. For this purpose, wild-type strain (JFP383) was tested using sheep polyclonal rCPE antibody (Dr. Michel Popoff, Institut Pasteur, France, and recombinant CPE toxin (TechLab) was used as a positive control.
Broth culture supernatants were mixed with a 1:1 ratio of Laemmli Sample Buffer (Bio-Rad) and separated by SDS-PAGE in 12% acrylamide gel. Subsequently, proteins were transferred onto a nitrocellulose 0.45 μm membranes (BioTrace NT, Gelman Laboratory, Laurent, QC) for 60 min at constant power supply of 95 V. Membranes were then placed in blocking buffer (PBS, 0.05% Tween 20, 0.5% fish skin gelatin) at 4°C overnight, followed by incubation with serum from horses immunized with 1 of the 3 rNet-NusA proteins at 1:2000 dilution, for 90 min at room temperature. After washing 3 times, the membranes were incubated with alkaline phosphatase-conjugated goat anti-horse IgG (Jackson ImmunoResearch Laboratories) at 1:5000 dilution. Specific protein bands were visualized using the alkaline phosphatase conjugate substrate kit (Bio-Rad) [68]. BLUeye Prestained Protein Ladder (FroggaBio, ON) was used in Western blot analysis. For the third experiment, the same condition was employed. The only difference was that sheep polyclonal serum against rCPE and alkaline phosphatase-conjugated goat anti-sheep IgG (Jackson ImmunoResearch Laboratories) were used in this experiment.
Pulse Field Gel Electrophoresis (PFGE)
PFGE was performed to analyze the presence of plasmids in 12 C. perfringens isolates (6 canine isolates and 6 equine isolates), as described by Parreira et al. [4], and on chromosomal DNA to examine the clonality of 35 equine (16 netF+, 19 netF-) and 35 canine (16 netF+, 19 netF-) C. perfringens isolates, using the method described by Chalmers et al. [50]. NotI digestion was used to linearize plasmids since this cuts large Tcp-positive conjugative C. perfringens plasmids only once [4, 51]. Thirty-two isolates were selected on the basis of the presence of the netF gene, and thirty-eight isolates were randomly selected from fecal isolates from diarrheal or healthy animals that were negative for the netF gene. Electrophoresis was performed in a 1% PFGE-certified gel and separated with the CHEF-III PFGE system (Bio-Rad) in 0.56 Tris-borate-EDTA buffer supplemented with 200 μM thiourea (Fisher Scientific) at 14°C at 6 V for 19 h with a ramped pulsed time of 1 to 12 sec (plasmid protocol) and 4 to 38 sec (clonality protocol). As a size guide, C. perfringens strain 33 was electrophoresed on each gel, with markers being used in lanes 1, 2, 7 and 15. Gels were stained in ethidium bromide and visualized by UV light. Mid-Range II PFG markers (New England Biolabs) were used as the molecular DNA ladder.
Band matching was performed using a 0.8% position tolerance; cluster analysis was completed utilizing the Dice similarity coefficient and unweighted pair group method with arithmetic mean. The isolate host species (equine or canine), laboratory numeric code, and presence of netF (+ or-) were included. Genotypes were designated through application of the Tenover criteria [69] with 60% band pattern similarity equating to a 7-band difference. These genotypes were labelled alphabetically from top to bottom, with a subscript representing the percent relatedness of the banding pattern. Analysis of the PFGE gels was completed utilizing BioNumerics software version 7.1 (Applied Maths, Austin, TX).
Preparation of DIG Probes and Plasmid PFGE for Southern Blotting
DNA probes for all plasmid PFGE Southern blot steps were labelled by PCR amplification in the presence of digoxigenin-11-dUTP (DIG; Roche Applied Science) according to the manufacturer’s recommendation. DNA probes were amplified from C. perfringens strain JFP718. DNA probes for netE and cpe genes were prepared with specific primers (S2 Table). DNA from PFGE gels was transferred to nylon membranes (Roche Applied Science, Mannheim, Germany). DNA hybridizations and detection were performed by using the DIG labelling and CSPD substrate according to the manufacturer’s recommendation (Roche Applied Science). For Southern blot hybridizations, nylon membranes were prehybridized for at least 2 h at 42°C in hybridization solution without labelled probe and then hybridized separately at 42°C with specific DNA probes for 16 h. The membranes were washed at 68°C under high-stringency conditions. For each different DIG labelled probe, the membrane was first stripped with 0.2 N NaOH and 0.1% sodium dodecyl sulfate, incubated with prehybridization solution, and then reprobed.
Nucleotide Sequence Accession Numbers
The net toxin sequences were assigned GenBank accession numbers KJ606985 for netE, KJ606986 for netF and KJ606987 for netG. The incomplete plasmid sequences are available in GenBank as KP739975 (pCP718netF [scaffold 00006]) and KP739976 (pCP718cpe [scaffold 00012]).
Statistical Analyses
Fisher’s exact test was used to make Odds Ratio estimates about the association of netF with canine hemorrhagic gastroenteritis or foal necrotizing enteritis, since the test uses the hypergeometric distribution that allows us to make exact statements. Conditional means likelihood estimates (CMLE) of Odd Ratios were obtained, with 95% confidence intervals, except where one of the cell counts was 0, in which case mean unbiased estimates (MUE) were determined [70].
Ethics Statement
Horse immunization studies were approved by the University of Guelph Animal Care Committee (Animal Utilization Protocol approval number 1818) in accordance with the guidelines of the Canadian Council on Animal Care.
Supporting Information
Residue numbers for individual proteins are given on the top of the sequences. Filled- in boxes represent identical residues, whereas outlined boxes represent similar residues. The secondary structure is shown on top based on their respective crystallographic structures (β = beta-strand, η = 310 helix, α = alpha-helix, strict β-turns = TT and strict α-turns = TTT). The alignment was performed using ClustalW of the toxins Panton-Valentine leukocidin S of Staphylococcus aureus (AHC29058), putative CctA of C. chauvoei (WP_021874975), alpha-hemolysin of C. botulinum (YP_004394739.1), NetE (KJ606985), NetF (KJ606986), NetG (KJ606987) of C. perfringens, NetB of C. perfringens (EU143239) and Delta toxin of C. perfringens (EU652406) and Beta-toxin of C. perfringens (CAA58246.1). The figure was created using the ESPript 2.2.program.
(TIFF)
SB was performed using DIG system (Roche Applied Science) for labelling and detection according to the manufacturer’s recommendation. PCR probe was amplified using probe Intron primer pairs (S2 Table) designed based on the group II intron sequence, Lane 1: JFP838E-05, Lane 2:JFP838F-05, Lane 3:JFP838G-07, Lane 4: wild-type JFP838, Lane 5: DNA DIG-ladder VII (Roche).
(TIF)
PCR amplifications of genomic DNA of mutants netE, netF and netG (A) using specific primer pairs (netE-F/R, netF-F/R, netG-F/R described on S2 Table) Lane M: 1 kb ladder (NEB), Lane 1: JFP838E-05, Lane 3: JFP838F-05, Lane 5: JFP838G-07 and Lanes 2,4,6: wild-type JFP838 and (B) using target primers (netE-F, netF-R and netG-R) in combination with EBS-Universal primer. Lane M: 100 bp ladder (NEB), Lane 1: JFP838E-05, Lane 3: JFP838F-05, Lane 5: JFP838G-07 and Lanes 2,4,6: wild-type JFP838.
(TIFF)
Fig. A: Western blot using horse polyclonal Ab against rNetF. Lane 1: Culture supernatant of wild-type netF-positive strain (JFP838); Lane 2: Culture supernatant of mutant strain, showing absence of NetF; Lane 3: Culture supernatant of complementing strain, showing production of NetF. Fig. B: Western blot using horse polyclonal Ab against rNetG. Lane 1: Culture supernatant of netG mutant strain, showing absence of NetG; Lane 2: Culture supernatant of wild-type of netG; positive strain. Fig. C: Western blot using horse polyclonal Ab against rNetE. Lane 1: Culture supernatants of netE mutant strain, showing absence of NetE; Lane 2: Culture supernatant of wild-type netE positive strain. Fig. D: Immunoblot using sheep polyclonal Ab against CPE showing the lack of expression of CPE under the growth condition used in this study. Lane 1: Purified rCPE (positive control); Lane 2: Culture supernatant of a canine netF- and cpe-positive strain (JFP718).
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Tom Besser, Department of Veterinary Microbiology and Pathology, Washington State University, Pullman, WA, USA, Dr. Bob Carman, TechLab, Virginia, Dr. Vincent Perreten, Institute for Veterinary Bacteriology, University of Bern, Switzerland, Dr. Durda Slavic, Animal Health Laboratory, University of Guelph and Dr. Scott Weese, Department of Pathobiology, University of Guelph for provision of strains of C. perfringens from dogs and horses, and Dr. Michel Popoff, Institut Pasteur for antiserum to CPE. We thank Dr. Davor Ojkic, Animal Health Laboratory, University of Guelph, and Dr. Eva Nagy, Department of Pathobiology, University of Guelph for provision of cell lines. We also thank Dr. David Hobson, Catalyst Centre, University of Guelph, for discussion of this work, and Dr. Gayatri Vedantam, University of Arizona, Tucson, for advice relating to net gene mutation and for careful review of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided from Natural Sciences and Engineering Discovery Grant, by the Ontario Ministry of Agriculture, Food and Rural Affairs (Proof of Principle grant program), and by the Ontario Centres of Excellence (C4 Proof of Principle grant program). Equine Guelph - University of Guelph funds supported the initial steps of this study.
References
- 1. Songer JG (1996) Clostridial enteric diseases of domestic animals. Clin Microbiol Rev 9: 216–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, et al. (2008) NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens . PLoS Pathog 4: e26 10.1371/journal.ppat.0040026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nowell VJ, Kropinski AM, Songer JG, MacInnes JI, Parreira VR, Prescott JF (2012) Genome sequencing and analysis of a Type A Clostridium perfringens isolate from a case of bovine clostridial abomasitis. PLoS One 7: e32271 10.1371/journal.pone.0032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parreira VR, Costa M, Eikmeyer F, Blom J, Prescott JF (2012) Sequence of two plasmids from Clostridium perfringens chicken necrotic enteritis isolates and comparison with C. perfringens conjugative plasmids. PLoS One 7: e49753 10.1371/journal.pone.0049753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Rood JI, et al. (2013) Toxin plasmids of Clostridium perfringens . Microbiol Mol Biol Rev 77: 208–233. 10.1128/MMBR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jost BH, Trinh HT, Songer JG (2006) Clonal relationships among Clostridium perfringens of porcine origin as determined by multilocus sequence typing. Vet Microbiol 116: 158–165. [DOI] [PubMed] [Google Scholar]
- 7. Hibberd MC, Neumann AP, Rehberger TG, Siragusa GR (2011) Multilocus sequence typing subtypes of poultry Clostridium perfringens isolates demonstrate disease niche partitioning. J Clin Microbiol 49: 1556–1567. 10.1128/JCM.01884-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lepp D, Gong J, Boerlin P, Parreira VR, Songer JG, Prescott JF (2013) Identification of accessory genome regions in poultry Clostridium perfringens isolates carrying the NetB plasmid. J Bact 195: 1152–1166. 10.1128/JB.01032-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deguchi A, Miyamato K, Kuwahara K, Miki Y, Kaneko I, Li J, et al. (2009) Genetic characterization of type A enterotoxigenic Clostridium perfringens strains. PLoS One 4: e5598, 10.1371/journal.pone.0005598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marks SL (2012) Clostridium perfringens- and Clostridium difficile- associated diarrhea In: Greene CE, editors. Infectious diseases of the dog and cat, 4th ed. St. Louis: Saunders Elsevier; pp. 393–398. [Google Scholar]
- 11. Prescott JF, Johnson JA, Patterson JM, Bulmer WS (1978) Haemorrhagic gastroenteritis in the dog associated with Clostridium welchii . Vet Record 103: 116–117. [DOI] [PubMed] [Google Scholar]
- 12. Sasaki J, Goryo M, Asahina M, Makara M, Shisido S, Okada K (1999) Hemorrhagic enteritis associated with Clostridium perfringens Type A in a dog. J Vet Med Sci 61: 175–177. [DOI] [PubMed] [Google Scholar]
- 13. Schlegel BJ, Van Dreumel T, Slavic D, Prescott JF (2012) Clostridium perfringens type A fatal acute hemorrhagic gastroenteritis in a dog. Can Vet J 53: 549–553. [PMC free article] [PubMed] [Google Scholar]
- 14. Unterer S, Busch K, Leipig M, Hermanns W, Wolf G, Straubinger RK, et al. (2014) Endoscopically visualized lesions, histologic findings, and bacterial invasion in the gastrointestinal mucosa of dogs with acute hemorrhagic diarrhea syndromes. J Vet Intern Med 28: 52–58. 10.1111/jvim.12236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burrows CF (1977) Canine hemorrhagic gastroenteritis. J Am Anim Hosp Assoc 13: 451–458. [Google Scholar]
- 16. Herholz C, Miserez R, Nicolet J, Frey J, Popoff M, Gibert M, et al. (1999) Prevalence of beta2-toxigenic Clostridium perfringens in horses with intestinal disorders. J Clin Microbiol 37: 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacciarini LN, Boerlin P, Straub R, Frey J, Grone A (2003) Immunohistochemical localization of Clostridium perfringens beta2-toxin in the gastrointestinal tract of horses. Vet Pathol 40: 376–381. [DOI] [PubMed] [Google Scholar]
- 18. Vilei EM, Schlatter Y, Perreten V, Straub R, Popoff MR, Gibert M, et al. (2005) Antibiotic-induced expression of a cryptic cpb2 gene in equine beta2-toxigenic Clostridium perfringens . Mol Microbiol 57: 1570–1581. [DOI] [PubMed] [Google Scholar]
- 19. Waters M, Raju D, Garmory HS, Popoff MR, Sarker MR (2005) Regulated expression of the beta2-toxin gene (cpb2) in Clostridium perfringens type A isolates from horses with gastrointestinal diseases. J Clin Microbiol 43: 4002–4009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Kanoe M, Inoue S, Abe T, Anzai T, Kamada M, Imagawa H, et al. (1990) Isolation of Clostridium perfringens from foals. Microbios 64: 153–158. [PubMed] [Google Scholar]
- 21. Netherwood T, Wood JL, Mumford JA, Chanter N (1998) Molecular analysis of the virulence determinants of Clostridium perfringens associated with foal diarrhea. Vet J 155: 289–294. [DOI] [PubMed] [Google Scholar]
- 22. Donaldson MT, Palmer JE (1999) Prevalence of Clostridium perfringens enterotoxin and Clostridium difficile toxin A in feces of horses with diarrhea and colic. J Am Vet Med Assoc 215: 358–361. [PubMed] [Google Scholar]
- 23. Weese JS, Staempfli HR, Prescott JF, Kruth SA, Greenwood SJ, Weese HE. (2001) The roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in diarrhea in dogs. J Vet Intern Med 15: 374–378. [PubMed] [Google Scholar]
- 24. Tillotson K, Traub-Dargatz JL, Dickinson CE, Ellis RP, Morley PS, Hyatt DR, et al. (2002) Population-based study of fecal shedding of Clostridium perfringens in broodmares and foals. J Am Vet Med Assoc 220: 342–348. [DOI] [PubMed] [Google Scholar]
- 25. Traub-Dargatz JL, Jones RL (1993) Clostridia-associated enterocolitis in adult horses and foals. Vet Clin North Am Equine Pract 9: 411–421. [DOI] [PubMed] [Google Scholar]
- 26. East LM, Savage CJ, Traub-Dargatz JL, Dickinson CE, Ellis RP (1998) Enterocolitis associated with Clostridium perfringens infection in neonatal foals: 54 cases (1988–1997). J Am Vet Med Assoc 212: 1751–1756. [PubMed] [Google Scholar]
- 27. Donahue M, Williams N (2002) Clostridial enterocolitis in horses. Equine Dis Quart 10: 4–5. [Google Scholar]
- 28. Timoney JF, Hartmann M, Fallon L, Fallon E, Walker J (2005) Antibody responses of mares to prepartum vaccination with Clostridium perfringens bacterin and beta2 toxin. Vet Rec 157: 810–812. [DOI] [PubMed] [Google Scholar]
- 29. Diab SS, Kinde H, Moore J, Shahriar MF, Odani J, Anthenill L, et al. (2011) Pathology of Clostridium perfringens Type C enterotoxemia in horses. Vet Pathol 49: 255–263. 10.1177/0300985811404710 [DOI] [PubMed] [Google Scholar]
- 30. Hazlett MJ, Kircanski J, Slavic D, Prescott JF (2011) Beta2 toxigenic Clostridium perfringens type A colitis in a three-day-old foal. J Vet Diagn Invest 23: 373–376. [DOI] [PubMed] [Google Scholar]
- 31. Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, et al. (2009) CDD: specific functional annotation with the Conserved Domain Database. Nucl Acids Res 37: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dyrløv Bendtsen J, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795. [DOI] [PubMed] [Google Scholar]
- 33. Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Brinkman FS (2005) PSORTb v.2.0: Expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21: 617–623. [DOI] [PubMed] [Google Scholar]
- 34. Zdobnov EM, Apweiler R (2001) InterProScan–an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17: 847–848. [DOI] [PubMed] [Google Scholar]
- 35. Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 36. Ohtani K, Hirakawa H, Tashiro K, Yoshizawa S, Kuhara S, Shimizu T (2010) Identification of a two-component VirR/VirS regulon in Clostridium perfringens . Anaerobe 16: 258–264. 10.1016/j.anaerobe.2009.10.003 [DOI] [PubMed] [Google Scholar]
- 37. Cheung JK, Keyburn AL, Carter GP, Lanckriet AL, Van Immerseel F, Moore RJ, Rood JI (2010) The VirSR two-component signal transduction system regulates NetB toxin production in Clostridium perfringens . Infect Immun 78: 3064–3072. 10.1128/IAI.00123-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheung JK, Dupuy B, Deveson DS, Rood JI (2004) The spatial organization of the VirR boxes is critical for VirR-mediated expression of the perfringolysin O gene, pfoA, from Clostridium perfringens . J Bacteriol 186: 3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson DR, Parreira VR, Kulkarni RR, Prescott JF (2006) Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet Microbiol 113: 25–34. [DOI] [PubMed] [Google Scholar]
- 40. Purdy D, O’Keeffe TAT, Elmore M, Herbert M, McLeod A, Ostrowski A, et al. (2002) Conjugative transfer of clostridial shuttle vectors from Escherichia coli to Clostridium difficile through circumvention of the restriction barrier. Mol Microbiol 46: 439–452. [DOI] [PubMed] [Google Scholar]
- 41. Alonzo F 3rd, Torres VJ (2014) The bicomponent pore-forming leucocidins of Staphylococcus aureus . Microbiol Mol Biol Rev 78: 199–230. 10.1128/MMBR.00055-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma M, Gurjar A, Theoret JR, Garcia JP, Beingesser J, Fisher DJ, et al. (2014) Synergistic effects of Clostridium perfringens enterotoxin and beta toxin in rabbit small intestinal loops. Infect Immun 82: 2958–2970. 10.1128/IAI.01848-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De S, Olson R (2011) Crystal structure of the Vibrio cholerae cytolysin heptamer reveals common features among disparate pore-forming toxins. Proc Natl Acad Sci USA 108: 7385–7390. 10.1073/pnas.1017442108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Savva CG, Fernandes da Costa SP, Bokori-Brown M, Naylor CE, Cole AR, Titball RW, et al. (2013) Molecular architecture and functional analysis of NetB, a pore-forming toxin from Clostridium perfringens . J Biol Chem 288: 3512–3522. 10.1074/jbc.M112.430223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yan X, Porter CJ, Hardy SP, Steer D, Smith AI, Hughes V, et al. (2013) Structural and functional analysis of pore-forming toxin NetB from Clostridium perfringens . MBio 4: e00019–13. 10.1128/mBio.00019-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fernandes da Costa SP, Savva CG, Bokori-Brown M, Naylor CE, Moss DS, Titball RW (2014) Identification of a key residue for oligomerisation and pore-formation of Clostridium perfringens NetB. Toxins 12: 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huyet J, Naylor CE, Savva CG, Gibert M, Popoff MR, Basak AK (2013) Structural insights into Clostridium perfringens delta toxin pore formation. PLoS One 8: e66673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Popoff MR, Bouvet P (2013) Genetic characteristics of toxigenic Clostridia and toxin gene evolution. Toxicon 75: 63–89. 10.1016/j.toxicon.2013.05.003 [DOI] [PubMed] [Google Scholar]
- 49. Jost BH, Trinh HT, Songer JG (2006) Clonal relationships among Clostridium perfringens of porcine origin as determined by multilocus sequence typing. Vet Microbiol 116: 158–165. [DOI] [PubMed] [Google Scholar]
- 50. Chalmers G, Martin SW, Hunter DB, Prescott JF, Weber LJ, Boerlin P (2008) Genetic diversity of Clostridium perfringens isolated from healthy broiler chicken at a commercial farm. Net Microbiol 127: 116–127. [DOI] [PubMed] [Google Scholar]
- 51. Lepp D, Roxas B, Parreira VR, Marri PR, Rosey EL, Gong J, et al. (2010) Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS ONE 5: e10795 10.1371/journal.pone.0010795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bannam TL, Yan XX, Harrison PF, Seemann T, Keyburn AL, Stubenrauch C, et al. (2011) Necrotic enteritis-derived Clostridium perfringens strain with three closely related independently conjugative toxin and antibiotic resistance plasmids. MBio 2: e00190–11. 10.1128/mBio.00190-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Rood JI, et al. (2013) Toxin plasmids of Clostridium perfringens . Microbiol Mol Biol Rev 77: 208–233. 10.1128/MMBR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bannam TL, Teng WL, Bulach D, Lyras D, Rood JI (2006) Functional identification of conjugation and replication regions of the tetracycline resistance plasmid pCW3 from Clostridium perfringens . J Bacteriol 188: 4942–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Deguchi A, Miyamoto K, Kuwahara T, Miki Y, Kaneko I, Li J, et al. (2009) Genetic characterization of type A enterotoxigenic Clostridium perfringens strains. PLoS One 4: e5598 10.1371/journal.pone.0005598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Obana N, Nakamura K (2011) A novel toxin regulator, the CPE1446-CPE1447 protein heteromeric complex, controls toxin genes in Clostridium perfringens . J Bacteriol 193: 4417–4424. 10.1128/JB.00262-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shimizu T, Yaguchi H, Ohtani K, Banu S, Hayashi H (2002) Clostridial VirR/VirS regulon involves a regulatory RNA molecule for expression of toxins. Mol Microbiol 43: 257–265. [DOI] [PubMed] [Google Scholar]
- 58. McGavin MD, Zachary JF (2007) Pathologic basis of veterinary disease, 4th edition Mosby. 1476 p. [Google Scholar]
- 59. Chase K, Jones P, Martin A, Ostrander EA, Lark KG (2009) Genetic mapping of fixed phenotypes: disease frequency as a breed characteristic. J Hered 1: 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. East LM, Dargatz DA, Traub-Dargatz JL, Savage CJ (2000) Foaling-management practices associated with the occurrence of enterocolitis attributed to Clostridium perfringens infection in the equine neonate. Prev Vet Med 46: 61–74. [DOI] [PubMed] [Google Scholar]
- 61. Quigley JD, Martin KR, Dowlen HH (1995) Concentrations of trypsin inhibitor and immunoglobulins in colostrum of Jersey cows. J Dairy Sci 78: 1573–1577. [DOI] [PubMed] [Google Scholar]
- 62. Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods 70: 452–464. [DOI] [PubMed] [Google Scholar]
- 63. Glaser AL, de Vries AA, Dubovi EJ (1995) Comparison of equine arteritis virus isolates using neutralizing monoclonal antibodies and identification of sequence changes in GL associated with neutralization resistance. J Gen Virol 76: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 64. Mehdizadeh Gohari I, Arroyo L, MacInnes JI, Timoney JF, Parreira VR, Prescott JF (2014) Characterization of Clostridium perfringens in the feces of adult horses and foals with acute diarrhea. Can J Vet Res 78: 1–7. [PMC free article] [PubMed] [Google Scholar]
- 65. Heap JT, Pennington OJ, Cartman ST, Minton NP (2009) A modular system for Clostridium shuttle plasmids. J Microbiol Meth 78: 79–85. [DOI] [PubMed] [Google Scholar]
- 66. Roth F, Jansen K, Petzke S (1999) Detection of neutralizing antibodies against alpha-toxin of different Clostridium septicum strains in cell culture. FEMS Immunol Med Microbiol 24: 353–359. [DOI] [PubMed] [Google Scholar]
- 67. Kircanski J, Parreira VR, Whiteside S, Pei Y, Prescott JF (2012) The majority of atypical cpb2 genes in Clostridium perfringens isolates of different domestic animal origin are expressed. Vet Microbiol 159: 371–374. 10.1016/j.vetmic.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 68. Kircanski J, Hodgins D, Soltes G, Pei Y, Parreira VR, et al. (2012) Development of an antigen-capture enzyme-linked immunosorbent assay for Clostridium perfringens beta2-toxin in porcine feces and the neonatal piglet intestine. J Vet Diagn Invest 24: 895–902. 10.1177/1040638712453584 [DOI] [PubMed] [Google Scholar]
- 69. Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, et al. (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33: 2223–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hirji KF, Tsiatis AA, Mehta CR (1989) Median unbiased estimation for binary data. Am Stat 43: 7–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Residue numbers for individual proteins are given on the top of the sequences. Filled- in boxes represent identical residues, whereas outlined boxes represent similar residues. The secondary structure is shown on top based on their respective crystallographic structures (β = beta-strand, η = 310 helix, α = alpha-helix, strict β-turns = TT and strict α-turns = TTT). The alignment was performed using ClustalW of the toxins Panton-Valentine leukocidin S of Staphylococcus aureus (AHC29058), putative CctA of C. chauvoei (WP_021874975), alpha-hemolysin of C. botulinum (YP_004394739.1), NetE (KJ606985), NetF (KJ606986), NetG (KJ606987) of C. perfringens, NetB of C. perfringens (EU143239) and Delta toxin of C. perfringens (EU652406) and Beta-toxin of C. perfringens (CAA58246.1). The figure was created using the ESPript 2.2.program.
(TIFF)
SB was performed using DIG system (Roche Applied Science) for labelling and detection according to the manufacturer’s recommendation. PCR probe was amplified using probe Intron primer pairs (S2 Table) designed based on the group II intron sequence, Lane 1: JFP838E-05, Lane 2:JFP838F-05, Lane 3:JFP838G-07, Lane 4: wild-type JFP838, Lane 5: DNA DIG-ladder VII (Roche).
(TIF)
PCR amplifications of genomic DNA of mutants netE, netF and netG (A) using specific primer pairs (netE-F/R, netF-F/R, netG-F/R described on S2 Table) Lane M: 1 kb ladder (NEB), Lane 1: JFP838E-05, Lane 3: JFP838F-05, Lane 5: JFP838G-07 and Lanes 2,4,6: wild-type JFP838 and (B) using target primers (netE-F, netF-R and netG-R) in combination with EBS-Universal primer. Lane M: 100 bp ladder (NEB), Lane 1: JFP838E-05, Lane 3: JFP838F-05, Lane 5: JFP838G-07 and Lanes 2,4,6: wild-type JFP838.
(TIFF)
Fig. A: Western blot using horse polyclonal Ab against rNetF. Lane 1: Culture supernatant of wild-type netF-positive strain (JFP838); Lane 2: Culture supernatant of mutant strain, showing absence of NetF; Lane 3: Culture supernatant of complementing strain, showing production of NetF. Fig. B: Western blot using horse polyclonal Ab against rNetG. Lane 1: Culture supernatant of netG mutant strain, showing absence of NetG; Lane 2: Culture supernatant of wild-type of netG; positive strain. Fig. C: Western blot using horse polyclonal Ab against rNetE. Lane 1: Culture supernatants of netE mutant strain, showing absence of NetE; Lane 2: Culture supernatant of wild-type netE positive strain. Fig. D: Immunoblot using sheep polyclonal Ab against CPE showing the lack of expression of CPE under the growth condition used in this study. Lane 1: Purified rCPE (positive control); Lane 2: Culture supernatant of a canine netF- and cpe-positive strain (JFP718).
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.