Abstract
Objective
There is evidence of altered neural taste response in female adolescents who are obese, and in adolescents who are at risk for obesity. To further understand risk factors for the development of overeating and obesity, we investigated response to tastes of sucrose and water in 23 obese and healthy weight children.
Methods and design
Thirteen healthy weight (HW) and 10 obese (OB) 8–12 year old children underwent functional magnetic resonance imaging while tasting sucrose and water. Additionally, children completed an eating in the absence of hunger paradigm and a sucrose liking task.
Results
A region of interest analysis revealed an elevated BOLD response to taste (sucrose and water) within the bilateral insula and amygdala in OB children relative to HW children. Whole brain analyses revealed a group by condition interaction within the paracingulate, medial frontal, middle frontal gyri, and right amygdala: post hoc analyses suggested an increased response to sucrose for OB relative to HW children, whereas HW children responded more strongly to water relative to sucrose. In addition, OB children, relative to HW, tended to recruit the right putamen as well as medial and lateral frontal and temporal regions bilaterally.
Conclusion
This study showed increased reactivity in the amygdala and insula in the OB compared to HW children, but no functional differentiation in the striatum, despite differences in the striatum previously seen in older samples. These findings support the concept of the association between increased neural processing of food reward in the development of obesity, and raise the possibility that emotional and interoceptive sensitivity could be an early vulnerability in obesity.
Keywords: obesity, sucrose, fMRI, brain imaging, eating in the absence of hunger
Introduction
Approximately one-third of children in the United States are overweight or obese,1 affecting 4 to 5 million children. Children who are obese are at an increased risk for negative health sequelae in childhood and adulthood, including orthopedic and endocrine conditions, cardiovascular disease, cancer, and all-cause mortality.2–5 Unfortunately, obesity tracks well from childhood to adolescence and adulthood, with over 80% of obese youth remaining obese in adulthood.6
Although several factors contribute to weight gain, eating above and beyond daily caloric requirements plays a primary etiological role in obesity across the lifespan.7 Appetite regulation as a whole is a complex process that involves the integration of multiple physiological signals, including energy needs in the body, neural processes and cognitive processes. Neural mechanisms, in particular motivation and reward, are considered central components in regulating eating behavior.8 Recent studies of obesity suggest that top-down neural processes, which encode the rewarding, emotional, and cognitive aspects of food ingestion, may lead to important understandings of why some individuals over-consume food, even in the presence of satiety and replete energy stores.9,10
A number of models regarding the neural processes of obesity and overeating have been proposed. One model suggests that obese compared to normal weight people have decreased neural processing of food reward,11 while another suggests an increase in neural processing of food reward.12 Others have proposed that those at risk for obesity have an initial hyper-reactivity in the striatum and somatosensory and gustatory regions, which drives overeating. Over time, there is a decrease in striatal response and increases in regions that encode incentive salience of food cues, which leads to further overeating and weight gain.13,14 All of these models implicate neural regions associated with dopamine functioning, including the striatum, mesolimbic pathway and mesocortical pathways.15,16 The primary gustatory pathway, which partially overlaps with the above circuits but extends to the insula, is responsible for the integration of sensory information and taste from the mouth. Additionally, the insula has been implicated in the anticipation of reward and outcomes.17,18 Together with the anterior cingulate cortex and orbitofrontal cortex, the insula codes the sensory-hedonic response to taste, is implicated in the anticipation of outcomes, and innervates a broad region of the rostral ventral-central striatum.19–22 This network is thought to play a crucial role in linking sensory-hedonic experiences to the motivational components of reward19 that may govern eating behavior.
The functional development of these regions is not well understood, and very little is known about the development of the reward pathways in childhood, let alone about the reward pathways related to taste and food consumption. Studying taste reward in children earlier in life will allow for a greater understanding of the conflicting reports of the hypo- vs hyper- reward sensitivity seen in obesity. Understanding the development of the taste reward pathways in childhood is crucial for understanding the development of reward over the lifespan and will assist in the development of interventions to address overeating.
To date, there are relatively few studies evaluating the neural responsivity to food in obese and healthy weight youth, and these studies have used paradigms that include both pictures of food 23,24 and food delivery 25,26 to assess reward, motivation and interoceptive circuitry. The studies that have evaluated the delivery of reward have compared the taste of chocolate milkshake and tasteless solution in obese and healthy weight female adolescents. Results indicated that obese compared to healthy weight teens showed increased activation in the gustatory cortex (anterior and mid insula, frontal operculum) and in the somatosensory regions (parietal operculum and rolandic operculum) in anticipation of consumption of a chocolate milkshake compared to actual consumption. However, obese compared to healthy weight females showed decreased activation in the caudate nucleus in response to consumption of chocolate milkshake compared to a tasteless solution.25 Another study by the same group compared normal weight female adolescents determined to be at high or low risk of obesity (based on parent’s obesity status) and found that adolescents at high risk for obesity showed activation in the caudate, parietal operculum and frontal operculum in response to chocolate milkshake compared to a tasteless solution,26 suggesting that many of these neural changes may exist prior to adolescence and prior to obesity onset. To date, there is no data on food reward delivery in younger children, which is crucial in understanding the development of food reward, overeating and obesity development.
To address these gaps in the literature, we chose to study neural processing of taste in preadolescent children, as there are developmental changes in dopamine receptors,27 reward seeking behaviors 28,29 and reactivity to sweet substances 30 as children transition to puberty. Due to the significant developments in dopamine receptors throughout childhood, it is important to carefully stratify children and use tightly controlled age ranges when examining factors that activate the reward system.27 Additionally, we are specifically interested in neural responsivity to taste when sated, as eating past nutritional needs is a key contributor to the obesity epidemic. Thus, the purpose of this study was to compare neural taste responsivity to sucrose and water in obese and healthy weight preadolescent children when sated. We hypothesized that obese children would show increased activation to sucrose in the insula, amygdala, and anterior striatum consistent with the hyper-reactivity model and prior studies.25 As an exploratory aim, we evaluated correlations between BOLD responses and behavioral task performance evaluating sucrose liking and overeating.
Methods
Subjects
Ten obese (OB) children (5 boys, 5 girls) aged 8–12 (BMI percentile >95th for age) were recruited from the community. Thirteen age-matched healthy weight (HW; BMI<85th%) children (8 boys, 5 girls) were recruited through local advertisements and pediatric clinics. Inclusion criteria included willingness to participate in the study, meeting weight criteria for the two groups (OB or HW), right-handedness, fluent in English (for speaking, reading and writing) and liking of cheese pizza (necessary to complete the Eating in the Absence of Hunger assessment). Exclusion criteria included any eating disorder (diagnosed by chEDE-C)31 or other significant psychiatric disorder (MINI-kid),32 or other medical or neurologic concerns and conditions contraindicative to MRI. Child height was measured using a portable Schorr height board (Schorr Inc, Olney, MD) in duplicate. Height was recorded to the nearest 0.1 cm for both trials. Body weight in kilograms was measured in duplicate on a Tanita Digital Scale (model WB-110A). Body weight was recorded to the nearest 0.1 kg. The average of the 2 values for height and weight was converted to body mass index (BMI=[kg/m2]). Because children are growing, BMI was translated to BMI for age percentile score using the CDC growth charts.33
The study was conducted according to the Institutional Review Board regulations of the University of California, San Diego. Participants gave written informed assent, and their parents gave written informed consent. Participants completed an Eating in the Absence of Hunger evaluation (EAH) and a Monell forced-choice paired-comparison task for assessing sweet taste preference (i.e., sucrose liking). Between group comparisons of assessment scores were performed using Student’s t-tests and assumed unequal variance when indicated (Table 1).
Table 1.
Clinical and demographic characteristics.
| Characteristic | HW (N=13) | OB (N=10) | T or Chi Sq. value | DF | P value | Hedge’s g |
|---|---|---|---|---|---|---|
| Age at time of scanning (years) | 10.4 ± 0.3 [8.0–12.0] | 9.9 ± 0.3 [8.0–11.0] | 1.03 | 20.97 | 0.31 | 0.4 |
| Child BMIa | 17.7 ± 0.5 [14.8–20.0] | 26.1 ± 1.0 [22.3–30.7] | −7.60 | 14.27 | <0.001 | −3.1 |
| Child % BMIa | 53.9 ± 6.9 [7.0–83.0] | 96.8 ± 0.5 [94.0–98.0] | −6.18 | 12.13 | <0.001 | −2.5 |
| Child BMI-za | 0.1 ± 0.2 [−1.5–1.0] | 2.0 ± 0.1 [1.6–2.3] | −8.68 | 16.54 | <0.001 | −3.5 |
| Sucrose Liking | 0.6 ± 0.1 [0.2–1.1] | 0.6 ± 0.1 [0.1–1.1] | −0.23 | 18.10 | 0.82 | −0.1 |
| EAH %b | 10.4 ± 3.1 [0.5–33.6] | 16.6 ± 2.5 [6.0–30.2] | −1.54 | 19.81 | 0.14 | −0.6 |
Note: Entries are of the form mean ± SD [min – max]. Statistical comparisons were by means of Welsh’s t-tests. BMI: body mass index; DF: degrees of freedom; EAH: Eating in the Absence of Hunger percent calories consumed; HW: healthy weight; OB: obese;
Equal variances not assumed;
one HW was missing responses on this measure.
Experimental Design
In the first visit, the children completed psychiatric interviews, sucrose liking and the EAH paradigm. Experienced interviewers (graduate students trained to competency by a licensed psychologist) used the MINI-KID32 to rule out significant anxiety, depression and attentional disorders in children. The EAH paradigm has been described in publications with children from preschool to age 12 and yields a measure of percent of daily caloric needs consumed when sated in a free access session (EAH%).34,35 Additionally, based on methods described by Small, we also calculated percent of daily calories consumed in the EAH free access procedure using only the sweet foods (to correlate with BOLD response to the sweet taste of sucrose).36 Children also completed a Monell forced-choice paired-comparison task, which yields a measure of aqueous sucrose solution preference (sucrose liking).37 On a separate day, following a standardized breakfast, functional magnetic resonance imaging (fMRI) images were acquired using the taste paradigm task developed in our laboratory.38 One mL fluid sample of 10% sucrose or ionic water was delivered to children with a semi-automatic programmable customized syringe pump (J-Kem Scientific, St. Louis, MO) in 4 blocks of 20 pseudorandom trials each using a manifold. Four runs were acquired, each lasting 6 minutes 40 seconds, and fluid was delivered every 20 seconds. Children were trained to perform one tongue motion (swishing the solutions across the tongue) after each application of taste stimulant, in order to wash the taste stimulus around the mouth and stimulate taste buds. Children were instructed to remain still with eyes closed during the paradigm.
Apparatus
The macronutrient solutions were contained in two 25 ml syringes, which were attached to a semiautomatic and programmable customized syringe pump (J-Kem Scientific, St Louis, MO), positioned in the scanner control room.39 Tastes were delivered to the subjects via two separate approximately 10-m long FDA approved food grade Teflon tubes (Cole-Parmer Vernon Hills, IL). The syringes were also attached to a computer-controlled valve system, which enabled the two solutions to be delivered independently along the tubing. Taste delivery was controlled by E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA) software operating on a PC positioned in the control room. The stimuli were also synchronized with MR scanning.
fMRI
Imaging data were collected with a 3T Signa Excite scanner (GE Medical Systems). FMRI was performed with gradient-recalled echoplanar imaging (TR=2000 ms, TE=30 ms, flip angle=80°, 64 x 64 matrix, ASSET factor=2, 40 2.6-mm ascending interleaved axial slices with a 0.4-mm gap, 200 volumes).40,41 The first four volumes of each run were discarded so as to discount T1 saturation. EPI-based field maps were acquired for correcting susceptibility-induced geometric distortions. A high resolution T1-weighted image (SPGR, TI=600 ms, TE=min full, flip angle=8°, 256 x 192 matrix, 170 1.2 mm contiguous slices) was obtained for subsequent spatial normalization.
Definition of Anatomical Regions of Interest
Regions of interest (ROI) were chosen based on the neurocircuitry of reward to include both the sensory aspects of taste (e.g., insula), and limbic areas that modulate pleasure, motivation, and the cognitive aspects of taste, including the amygdala and anterior ventral striatum.15,42,43 Insula and amygdala ROIs were derived from the Harvard-Oxford Atlas.44 We attempted to include the orbitofrontal cortex in our ROI analysis for its involvement in the gustatory circuit, but due to signal drop-out and artifact we do not report these results. The anterior striatum ROI was defined a priori based upon known functional distinctions to include the nucleus accumbens, and segments of the dorsal caudate and dorsal putamen residing anterior to the anterior commissure. Using the anterior commissure in the coronal plane, the caudate nucleus and putamen were sliced into anterior and posterior subdivisions, and only anterior subdivisions were retained for analysis.
Statistical Analysis
Functional images were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software45 and R statistical packages (http://www.r-project.org). EPI images were motion-corrected and aligned to high-resolution anatomical images.46 Time points with isolated head movements not corrected by coregistration were censored from the statistical analysis. Outliers were generated using AFNI’s 3dToutcount. Volumes with more than 10% of the voxels marked as outliers were censored from subsequent analyses. Approximately 5.2% of all volumes were censored overall (for all subjects M = 36.4 volumes; SD = 8.1; range = 18–55). Statistical analyses were performed using a general linear model (GLM), whereby taste blocks were modeled using AFNI’s SPMG function. Six motion parameters (3 rotations and 3 translations) were used as nuisance regressors to account for motion artifact. Within-voxel auto-correlations of time-series data were controlled with AFNI’s 3dREMLfit. Registration to the MNI-152 atlas was performed using FMRIB’s Non-linear Image Registration Tool (FNIRT), part of FSL (http://fsl.fmrib.ox.ac.uk/fsl/), resulting in 2x2x2 mm voxels. Functional data were scaled to percent signal change (PSC) and smoothed with a 4 mm FWHM Gaussian kernel. The PSC map for each individual was visually inspected for outliers before inclusion in the group analyses.
In order to better account for morphometric variation and constrain extracted fMRI signal to gray matter tissue, we segmented each individual’s anatomical image into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes using FMRIB’s Automated Segmentation Tool (FAST).47 ROIs applied to the fMRI data were constrained to exclude CSF or WM but retain GM
A linear mixed effects (LMEs) analysis in R was performed for each voxel within each of the limbic ROIs.48 Two separate models were constructed: in both models, subject was treated as a random effect. In one model, diagnosis (OB, HW) was treated as the between subjects factor and condition (sucrose, water) as the within subjects factor. As no group x condition interaction was observed in any of the ROIs, we reduced the model to include diagnosis (OB, HW) and condition (sucrose, water) as the between subjects factors. Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives. To achieve an a posteriori ROI-wise probability of p < 0.05, an a priori voxel-wise probability of p < 0.05 in a cluster of 112 mm3 comprised of 14 connected voxels in the amygdala, a cluster of 178 mm3 comprised of 22 connected voxels in the insula, a cluster of 160 mm3 comprised of 20 connected voxels in the left striatum, and a cluster of 152 mm3 comprised of 19 connected voxels in the right striatum was required. Post hoc analyses were conducted using Tukey’s HSD. Effect size was computed with Hedge’s g, which provides a better estimate for smaller sample sizes.
Pearson product-moment correlation coefficients using the mean PSC of each ROI and raw scores of behavioral measures of interest (EAH%, sugar liking) were computed to explore potential correlations, using FDR correction to control for multiple comparisons (p<0.05).49
We also performed an exploratory voxelwise analysis using a 2 group (OB, HW) by 2 condition (sucrose, water) LME model as performed with the ROIs. A threshold adjustment based upon Monte-Carlo simulations (via AFNI’s 3dClustSim) was used to guard against false positives. Based on the whole brain analysis, an a priori voxel-wise probability of p < 0.05 in a cluster of 488 μL with 61 connected voxels (faces touching) resulted in an a posteriori cluster-wise probability of p < 0.05. The results of the main effect of group were more robust than the main effect of condition or the interaction of group by condition. Thus, a more stringent a priori voxel-wise probability of p < 0.01 in a cluster of 168 μL with 21 connected voxels (faces touching) which resulted in an a posteriori cluster-wise probability of p < 0.01 was implemented to discern distinct regions of activation for the group contrast.
Results
Demographics and clinical assessments
OB and HW were of similar age (Table 1), but as expected OB had higher BMI. Groups did not differ on percent calories consumed during the Eating in the Absence of Hunger free access protocol (EAH%).
ROI analyses
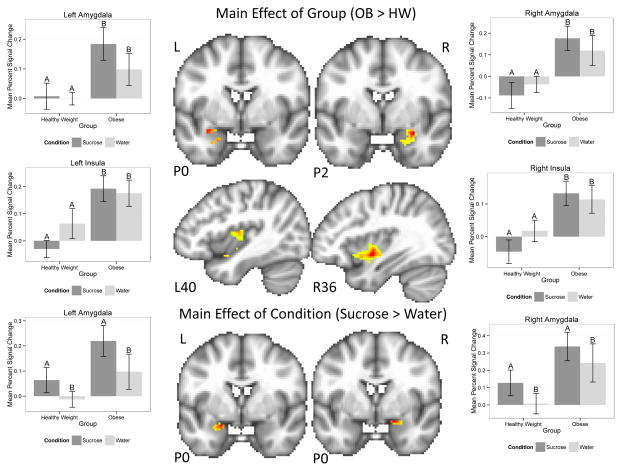
There were main effects of group and condition detected in the left and right amygdala and insula ROIs, but no main effects were seen in the anterior striatum ROIs (Figure 1, Table 2). Post hoc analyses revealed that, relative to HW, OB children had a stronger response to taste (e.g., sugar and water collapsed across condition) within regions of the bilateral amygdala, left anterior insula, and bilateral posterior insula. In addition, regions of the bilateral amygdala demonstrated a main effect of condition (Figure 1, Table 2). Post hoc analyses revealed this was driven by a stronger response to sucrose relative to water.
Figure 1.
Plots demonstrating main effects of group (upper) and condition (lower) within regions of interest for the sucrose task. Upper: Within the bilateral amygdala and insula, overweight children had an elevated response to sucrose and water. Lower: Within the bilateral amygdala, brain response was elevated for sucrose relative to water across all participants. Error bars represent the standard error for each group. Bars with different letters (A vs B) are significantly different from one another. Hot colors indicate voxels reflecting a greater response to sucrose or water within the regions of interest, all voxels p<0.05. L=left; R=right; P=posterior; OB: obese children; HW: healthy weight children
Table 2.
Anatomically constrained limbic regions of interest analysis of variance results.
| Group (OB > HW) | Condition (Sucrose > Water) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | L/R | Volume (μL) | x | y | z | F | t | g | Volume (μL) | x | y | z | F | t | g |
| Amygdala | L | 184 | −30 | 0 | −16 | 7.2 | 2.8 | 1.1 | -- | -- | -- | -- | -- | -- | -- |
| L | 160 | −20 | −2 | −26 | 6.3 | 2.5 | 1.0 | 208 | −20 | 0 | −18 | 10.0 | 3.2 | 1.3 | |
| L | 144 | −28 | −12 | −14 | 7.6 | 2.8 | 1.1 | -- | -- | -- | -- | -- | -- | -- | |
| R | 1064 | 30 | −2 | −18 | 10.1 | 3.2 | 1.3 | 184 | 20 | 0 | −16 | 8.8 | 3.0 | 1.2 | |
| Insula (anterior) | L | 488 | −38 | 0 | −16 | 8.3 | 2.9 | 1.2 | -- | -- | -- | -- | -- | -- | -- |
| (posterior) | L | 472 | −42 | −12 | 6 | 13.6 | 3.7 | 1.5 | -- | -- | -- | -- | -- | -- | -- |
| (posterior) | R | 1984 | 36 | −4 | −6 | 12.2 | 3.5 | 1.4 | -- | -- | -- | -- | -- | -- | -- |
| Anterior striatum | L | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| R | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
Small volume correction was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives resulting in an a posteriori ROI-wise probability of p < 0.05; minimum cluster size for amygdala = 112 μL, insula = 178 μL, left anterior striatum = 160 μL, right anterior striatum = 152 μL. Coordinates given in MNI space. L: left; R: right; HW: healthy weight; OB: obese.
Whole brain analysis
Multiple areas showed a significant group by condition (Table 3) interaction in the whole brain voxel-wise analysis. Post hoc analyses suggested that OB children demonstrated elevated brain response compared to HW children for sucrose in nearly all significant clusters, and that for HW children, brain response was elevated for water compared to sucrose. These areas included the left and right paracingulate gyrus, right medial frontal gyrus, left and right lingual gyrus, left and right middle frontal gyrus, right amygdala, and left posterior middle temporal gyrus.
Table 3.
Voxelwise analysis of variance reporting significant clusters for an interaction of group x condition and significant main effects of group and of condition.
| Region | BA | L/R | Volume (μL) | x | y | z | F | g | Contrast | t |
|---|---|---|---|---|---|---|---|---|---|---|
| Group X Conditiona | ||||||||||
| Medial frontal gyrus/superior frontal gyrus, paracingulate gyrus | 10/9/32 | R | 7256 | 20 | 56 | 10 | 11.4 | 1.4 | Sucrose: OB>HW | 2.9 |
| Water: OB>HW | 1.1 | |||||||||
| OB: Sucrose>Water | 2.2 | |||||||||
| HW: Water>Sucrose | 2.6 | |||||||||
| Anterior cingulate/paracingulate gyrus, superior frontal gyrus | 9/32 | L | 3624 | −20 | 54 | 16 | 17.5 | 1.7 | Sucrose: OB>HW | 3.3 |
| Water: OB>HW | 1.5 | |||||||||
| OB: Sucrose>Water | 1.3 | |||||||||
| HW: Water>Sucrose | 4.0 | |||||||||
| Cuneus, lingual gyrus/calcarine gyrus | 17/18 | L/R | 3456 | 8 | −84 | −6 | 13.4 | 1.5 | Sucrose: OB>HW | 3.0 |
| Water: OB>HW | 1.1 | |||||||||
| OB: Sucrose>Water | 1.3 | |||||||||
| HW: Water>Sucrose | 4.0 | |||||||||
| White matter/middle frontal gyrus | 9 | L | 1840 | −22 | 20 | 28 | 14.9 | 1.6 | Sucrose: OB>HW | 2.2 |
| Sucrose: OB>HW | Water: OB>HW | 1.1 | ||||||||
| Water: OB>HW | OB: Sucrose>Water | 4.1 | ||||||||
| OB: Sucrose>Water | HW: Water>Sucrose | 1.1 | ||||||||
| Amygdala/parahippocampal gyrus/anterior fusiform gyrus | 37/36 | R | 1168 | 18 | −2 | −28 | 7.1 | 1.1 | Sucrose: OB>HW | 3.2 |
| Water: OB>HW | 0.9 | |||||||||
| OB: Sucrose>Water | 0.7 | |||||||||
| HW: Water>Sucrose | 3.3 | |||||||||
| Paracingulate gyrus | 32/9 | R | 824 | 10 | 38 | 32 | 7.3 | 1.1 | Sucrose: OB>HW | 3.2 |
| Water: OB>HW | 1.0 | |||||||||
| OB: Sucrose>Water | 0.3 | |||||||||
| HW: Water>Sucrose | 3.7 | |||||||||
| Inferior frontal gyrus/middle frontal gyrus | 45/9 | R | 800 | 40 | 18 | 18 | 9.6 | 1.3 | Sucrose: OB>HW | 2.6 |
| Water: OB>HW | 0.9 | |||||||||
| OB: Sucrose>Water | 1.6 | |||||||||
| HW: Water>Sucrose | 2.8 | |||||||||
| Middle temporal gyrus/inferior temporal gyrus | 21/20 | L | 728 | −62 | −18 | −20 | 24.3 | 2.0 | Sucrose: OB>HW | 1.4 |
| Water: OB>HW | 0.2 | |||||||||
| OB: Sucrose>Water | 4.5 | |||||||||
| HW: Water>Sucrose | 2.3 | |||||||||
| Cingulate gyrus/precentral gyrus | 31/4 | L | 576 | −16 | −28 | 48 | 11.2 | 1.4 | Sucrose: OB>HW | 2.1 |
| Water: OB>HW | 0.5 | |||||||||
| OB: Sucrose>Water | 3.4 | |||||||||
| HW: Water>Sucrose | 1.2 | |||||||||
| Anterior parahippocampal gyrus | 36 | L | 512 | −32 | −16 | −30 | 12.7 | 1.4 | Sucrose: OB>HW | 1.9 |
| Water: OB>HW | 1.5 | |||||||||
| OB: Sucrose>Water | 2.8 | |||||||||
| HW: Water>Sucrose | 2.2 | |||||||||
| Main effect of group (OB vs. HW)b | ||||||||||
| Calcarine sulcus/occipital pole | 19/18/17 | L/R | 3336 | 0 | −90 | 2 | 13.2 | 1.5 | OB>HW | 3.6 |
| Brain stem | L/R | 3264 | −12 | −16 | −26 | 16.0 | 1.6 | OB>HW | 4.0 | |
| Cerebellum (culmen) | L | 1384 | −4 | −42 | −14 | 13.6 | 1.5 | OB>HW | 3.7 | |
| Superior frontal gyrus | 6 | L | 1376 | −14 | 14 | 62 | 13.5 | 1.5 | OB>HW | 3.7 |
| Superior temporal gyrus/insula | 22/13 | R | 1280 | 60 | −10 | −2 | 15.6 | 1.6 | OB>HW | 3.9 |
| Posterior cingulate gyrus | 29/30 | L/R | 1152 | −8 | −50 | 20 | 13.8 | 1.5 | OB>HW | 3.7 |
| Calcarine sulcus/occipital pole | 17/18 | R | 1000 | 14 | −98 | 0 | 28.9 | 2.2 | OB>HW | 5.4 |
| Superior frontal gyrus/frontal pole | 10 | L | 768 | −10 | 66 | 18 | 12.9 | 1.5 | OB>HW | 3.6 |
| Postcentral gyrus/precentral gyrus | 3/2/1/4 | R | 752 | 12 | −34 | 70 | 15.7 | 1.6 | OB>HW | 4.0 |
| Anterior cingulate gyrus (dorsal)/paracingulate gyrus | 32/24 | R | 744 | 6 | 30 | 26 | 14.0 | 1.5 | OB>HW | 3.7 |
| Middle temporal gyrus (posterior) | 21 | L | 712 | −58 | −30 | −14 | 14.3 | 1.5 | OB>HW | 3.8 |
| Inferior frontal gyrus (pars triangularis) | 45 | L | 616 | −34 | 34 | 14 | 13.6 | 1.5 | OB>HW | 3.7 |
| Hippocampus/parahippocampal gyrus | 27/36/35 | R | 608 | 28 | −36 | −2 | 18.3 | 1.7 | OB>HW | 4.3 |
| Insula/putamen | 13/14 | R | 472 | 36 | −4 | −6 | 12.2 | 1.4 | OB>HW | 3.5 |
| Insula/superior temporal gyrus | 13/38 | L | 456 | −44 | 16 | −12 | 14.3 | 1.5 | OB>HW | 3.8 |
| Middle temporal gyrus (posterior) | 21 | R | 432 | 56 | −38 | −4 | 11.8 | 1.4 | OB>HW | 2.8 |
| Middle temporal gyrus (posterior) | 37/19 | L | 424 | −48 | −58 | 0 | 14.1 | 1.5 | OB>HW | 3.8 |
| Middle frontal gyrus/superior frontal gyrus | 8 | L | 392 | −28 | 32 | 52 | 10.8 | 1.3 | OB>HW | 3.3 |
| Parahippocampal gyrus | 36 | L | 336 | −30 | −42 | -10 | 11.7 | 1.4 | OB>HW | 3.4 |
| Posterior cingulate gyrus | 23/31 | L | 320 | −14 | −40 | 40 | 10.8 | 1.3 | OB>HW | 3.3 |
| Posterior insula | 13 | L | 272 | −42 | −12 | 6 | 13.6 | 1.5 | OB>HW | 3.7 |
| Thalamus (ventral lateral nucleus, ventral anterior nucleus) | L | 248 | −6 | −8 | −2 | 11.5 | 1.4 | OB>HW | 3.4 | |
| Middle frontal gyrus | 8 | L | 248 | −48 | 20 | 44 | 10.9 | 1.3 | OB>HW | 3.3 |
| Parahippocampal gyrus/fusiform gyrus | 36/37 | L | 208 | −40 | −30 | −16 | 11.6 | 1.4 | OB>HW | 3.4 |
| Cerebellum (lobules I–IV) | L/R | 208 | 0 | −54 | 2 | 10.6 | 1.3 | OB>HW | 3.3 | |
| Posterior cingulate | 30/29 | R | 208 | 14 | −52 | 24 | 11.8 | 1.4 | OB>HW | 3.4 |
| Middle temporal gyrus | 21 | L | 200 | −56 | −16 | −22 | 12.4 | 1.4 | OB>HW | 3.5 |
| Inferior occipital gyrus/fusiform gyrus | 37/19 | L | 200 | −38 | −68 | −8 | 11.4 | 1.4 | OB>HW | 3.4 |
| Middle temporal gyrus/angular gyrus | 39 | L | 200 | −44 | −68 | 32 | 9.7 | 1.3 | OB>HW | 3.1 |
| Main Effect of Taste (Sucrose vs. Water)a | ||||||||||
| Cerebellum | R | 31088 | 24 | −34 | −26 | 15.7 | 1.6 | Sucrose > Water | 3.8 | |
| Precentral gyrus/postcentral gyrus | 3/4 | L | 7928 | −46 | −16 | 24 | 22.9 | 1.9 | Sucrose > Water | 5.0 |
| Precentral gyrus/postcentral gyrus | 40/43/4 | R | 3352 | 44 | −14 | 26 | 15.1 | 1.6 | Sucrose > Water | 4.1 |
| Superior temporal gyrus | 38 | R | 2200 | 42 | 16 | −30 | 12.5 | 1.4 | Sucrose > Water | 3.4 |
| Anterior cingulate | 32 | R | 1728 | 12 | 42 | −20 | 19.4 | 1.8 | Sucrose > Water | 4.1 |
| Lingual gyrus/cuneus | 30 | L | 1392 | −26 | −72 | 6 | 29.3 | 2.2 | Sucrose > Water | 5.3 |
| Inferior parietal lobe | 40 | R | 1392 | 26 | −34 | 26 | 10.7 | 1.3 | Sucrose > Water | 3.2 |
| Cingulate gyrus | 24 | L | 1160 | −22 | 10 | 24 | 15.0 | 1.6 | Sucrose > Water | 4.0 |
| Caudate tail/posterior cingulate | 23 | L | 904 | −20 | −40 | 22 | 13.6 | 1.5 | Sucrose > Water | 3.6 |
| Anterior cingulate/medial frontal gyrus/subcallosal cortex | 32/11/12 | L | 752 | −12 | 32 | −18 | 8.7 | 1.2 | Sucrose > Water | 3.0 |
| Superior temporal gyrus | 41/42 | R | 712 | 62 | −22 | 10 | 16.1 | 1.6 | Sucrose > Water | 4.1 |
| Middle temporal gyrus | 21/36 | L | 592 | −38 | 0 | −28 | 10.4 | 1.3 | Sucrose > Water | 3.2 |
| Cingulate gyrus | 24 | R | 512 | 20 | 2 | 28 | 8.3 | 1.2 | Sucrose > Water | 2.9 |
| Medial frontal gyrus | 10 | L | 6912 | −16 | 68 | 4 | 16.6 | 1.7 | Water > Sucrose | 4.0 |
| Middle frontal gyrus/medial frontal gyrus | 8/9 | R | 5104 | 24 | 30 | 40 | 17.0 | 1.7 | Water > Sucrose | 4.0 |
| Middle frontal gyrus/superior frontal gyrus | 8/9 | L | 4392 | −22 | 30 | 52 | 16.2 | 1.6 | Water > Sucrose | 3.8 |
| Parahippocampal gyrus/middle temporal gyrus | 36/21 | L | 2216 | −40 | −22 | −18 | 15.8 | 1.6 | Water > Sucrose | 3.8 |
| Middle occipital gyrus/lingual gyrus | 17/18 | R | 1784 | 24 | −100 | 12 | 11.2 | 1.4 | Water > Sucrose | 3.1 |
| Superior occipital gyrus | 19 | L | 1448 | −38 | −78 | 32 | 12.1 | 1.4 | Water > Sucrose | 3.3 |
| Inferior frontal gyrus | 44 | L | 1128 | −52 | 14 | 20 | 8.3 | 1.2 | Water > Sucrose | 2.8 |
| Middle occipital gyrus/lingual gyrus | 17/18 | L | 1048 | −14 | −94 | 12 | 7.2 | 1.1 | Water > Sucrose | 2.3 |
| Precentral gyrus/postcentral gyrus | 4/3 | L/R | 976 | 4 | −36 | 72 | 10.1 | 1.3 | Water > Sucrose | 3.1 |
| Superior parietal lobule | 7 | L | 840 | −26 | −52 | 52 | 9.0 | 1.2 | Water > Sucrose | 2.9 |
| Middle temporal gyrus/temporal pole | 38/21 | L | 808 | −52 | 2 | −38 | 9.5 | 1.3 | Water > Sucrose | 2.8 |
| Middle frontal gyrus | 46/9 | R | 800 | 32 | 32 | 20 | 7.8 | 1.1 | Water > Sucrose | 2.5 |
| Middle temporal gyrus | 21 | R | 648 | 58 | −14 | −10 | 9.6 | 1.3 | Water > Sucrose | 2.9 |
| Lateral occipital cortex | 19 | R | 616 | 46 | −74 | 30 | 9.8 | 1.3 | Water > Sucrose | 3.1 |
| Precentral gyrus/Postcentral gyrus | 4/3 | R | 552 | 36 | −18 | 64 | 7.8 | 1.1 | Water > Sucrose | 2.5 |
| Superior frontal gyrus | 6 | L | 536 | −2 | 16 | 68 | 12.3 | 1.4 | Water > Sucrose | 3.2 |
| Parahippocampal gyus/uncus | 34/36 | R | 496 | 28 | −6 | −34 | 10.2 | 1.3 | Water > Sucrose | 2.9 |
Statistics relate to the peak voxel within each functional cluster. Coordinates given in MNI space. BA: Brodmann area; L: left; R: right; OB: Obese; HW: healthy weight.
Statistical threshold was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives resulting in an a posteriori ROI-wise probability of p < 0.05; minimum cluster size = 488 mm3
Statistical thresholding was determined with Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives resulting in an a posteriori ROI-wise probability of p < 0.01; minimum cluster size = 168 mm3.
Multiple areas showed a main effect of group (Table 3, Figure 1), even at a more conservative statistical threshold (p <.01). Overall, OB children tended to recruit medial and lateral frontal and temporal regions bilaterally, including the anterior cingulate, inferior and middle frontal gyri, insula, and middle temporal gyrus more than HW children, whereas HW children did not recruit any regions more than OB children. There was also a main effect of condition (Table 3); a greater response to sucrose relative to water was reported for several clusters, including the inferior temporal lobe, operculum, caudate and insula. Regions more responsive to water relative to sucrose included the middle frontal gyrus, inferior frontal gyrus, and occipital cortex.
Relationships to other measures
We conducted separate exploratory correlations between behavioral measures (EAH%, sucrose liking) for each group (OB or HW) and BOLD response to sucrose and water averaged across the entire ROI. When evaluating eating in the absence of hunger, we also evaluated sweet food consumption and found comparable results to the full EAH paradigm so results are not presented. Several comparisons were significant when uncorrected, but did not survive after correcting for multiple comparisons (Table 4). Overall, an interesting but non-significant pattern emerged in the obese and healthy weight children’s response to sucrose. In OB children, there was a positive correlation between EAH% and bilateral striatum and bilateral insula, while the same relationships were negative in HW children. Similarly, there was a positive correlation in OB children between sucrose liking and bilateral amygdala, while the same relationships were negative in HW children.
Table 4.
Correlations between behavioral measures (EAH%, Sucrose liking) and sucrose and water in obese and healthy weight children.
| Sucrose | Water | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obese | Healthy weight | Obese | Healthy weight | |||||||||
| r | p | p(FDR-corr) | r | p | p(FDR-corr) | r | p | p(FDR-corr) | r | p | p(FDR-corr) | |
| EAH% | ||||||||||||
| L Anterior striatum | 0.61 | 0.057 | 0.26 | −0.53 | 0.079 | 0.26 | 0.75 | 0.012 | 0.13 | 0.44 | 0.15 | 0.34 |
| R Anterior striatum | 0.65 | 0.044 | 0.23 | −0.64 | 0.025 | 0.18 | 0.75 | 0.013 | 0.13 | 0.33 | 0.29 | 0.45 |
| L Insula | 0.69 | 0.026 | 0.18 | −0.35 | 0.26 | 0.43 | 0.76 | 0.01 | 0.13 | 0.56 | 0.06 | 0.26 |
| R Insula | 0.74 | 0.014 | 0.13 | −0.31 | 0.328 | 0.48 | 0.84 | 0.002 | 0.10 | 0.52 | 0.08 | 0.26 |
| L Amygdala | 0.55 | 0.1 | 0.30 | 0.13 | 0.69 | 0.71 | 0.57 | 0.08 | 0.26 | 0.36 | 0.26 | 0.43 |
| R Amygdala | 0.51 | 0.14 | 0.34 | 0.53 | 0.2 | 0.40 | 0.65 | 0.042 | 0.23 | 0.46 | 0.13 | 0.33 |
| Sucrose liking | ||||||||||||
| L Anterior striatum | 0.38 | 0.28 | 0.45 | 0.35 | 0.24 | 0.43 | 0.29 | 0.42 | 0.54 | 0.24 | 0.43 | 0.54 |
| R Anterior striatum | 0.4 | 0.25 | 0.43 | 0.26 | 0.4 | 0.53 | 0.36 | 0.3 | 0.45 | 0.23 | 0.46 | 0.57 |
| L Insula | 0.14 | 0.7 | 0.71 | 0.38 | 0.21 | 0.40 | 0.15 | 0.69 | 0.71 | 0.14 | 0.65 | 0.71 |
| R Insula | 0.32 | 0.38 | 0.52 | 0.46 | 0.12 | 0.32 | 0.22 | 0.54 | 0.63 | 0.18 | 0.55 | 0.63 |
| L Amygdala | 0.44 | 0.21 | 0.40 | −0.51 | 0.07 | 0.26 | 0.14 | 0.7 | 0.71 | −0.2 | 0.51 | 0.61 |
| R Amygdala | 0.54 | 0.11 | 0.31 | −0.41 | 0.17 | 0.37 | 0.32 | 0.37 | 0.52 | −0.08 | 0.81 | 0.81 |
FDR: False discovery rate; EAH: eating in the absence of hunger; L: left; R: right
Discussion
To ultimately develop interventions to address overeating, it is crucial to understand the development of the taste reward pathways in childhood and the development of reward over the lifespan. To our knowledge, this is the first study to compare brain activation to sucrose and water administration in preadolescent (8–12 year old) OB and HW children. Our ROI results showed that OB children had a significantly greater neural response to taste (e.g., sugar and water collapsed across condition) within regions of the bilateral amygdala, left anterior insula, and bilateral posterior insula compared to HW children. Surprisingly, there were no significant differences seen between OB and HW children in the ventral striatum. Our whole brain analyses showed a main effect of group in that OB children tended to recruit medial and lateral frontal and temporal regions bilaterally, whereas HW children did not recruit any regions more than OB. We also found that OB children had an elevated neural response to sucrose compared to water in the paracingulate gyrus, medial frontal gyrus, middle frontal gyrus, and amygdala, compared to HW children. Taken together, our data support the hypothesis that OB children relative to HW children show a neural hyper-reactivity to the taste of sucrose and suggest that increased emotional and interoceptive susceptibility could be a vulnerability marker of obesity in youth.
Surprisingly, we did not find differences between OB and HW youth in response to sucrose/water administration in the anterior striatum. However, differences did emerge between OB and HW youth in the putamen in response to the task in the whole brain analyses. Studies have suggested that the striatum is less responsive to food reward in OB compared to HW controls.50 It is possible that our sample was too small to detect differences in this study, or alternatively, there is wider variation in the neural taste responsivity of children in this age range. It is also possible that sucrose alone was not as stimulating as administration of chocolate milkshake or that water was more rewarding than artificial saliva, resulting in smaller differences than in other studies.25;26Studies evaluating the development of food reward in children may need to target children younger than the pre-adolescent age range, to understand developmental issues related to higher levels of food reward.
Children had enhanced neural processing not only to sucrose but also to water in this study, although the activation was higher for sucrose compared to water in most of the regions studied. This is not surprising, as attempting to taste water produces large, bilateral activations in the insula, opercular, rolandic and cerebellar cortices relative to resting or smelling and it is not considered a neutral substrate.51 For this reason, we collapsed sucrose and water activation to analyze group differences, which were found in the amygdala and insula ROIs. Our whole brain analysis suggests that the differences were driven by increased neural processing of sucrose, but our sample size may have been too small to allow us to detect this potential difference. These results suggest that OB children have higher activation to sucrose and water solutions, which may be a result of a focus on the liquid in the mouth, expectation of reward, or the attempt to taste it.
This is also the first study to evaluate the associations between these regions associated with reward and a laboratory measure of overeating and sucrose liking in youth. Of note, the two groups (OB and HW) did not differ on overeating or sucrose liking at baseline. Although our results suggest an interesting differential pattern between OB and HW youth, after adjusting for multiple comparisons, these patterns were no longer significant. Larger studies are needed to explore these patterns further, and to determine whether neural activation can be associated with behavioral data.
The age of the children should be considered when interpreting these results. One study demonstrated that adult levels of dopaminergic neuromodulation of the subcortical structures are reached by the age of 9,52 while another observed adult levels of striatal D1 and D2 receptors at the age of 10.53 Published studies comparing OB to HW youth using food delivery 25 were conducted with adolescents, while those using pictures of food 23,24 were conducted with youth in late childhood and adolescence (10–17 years of age). Although the subcortical dopiminergic system matures early in life, some of the children in our study may not have reached full maturity in the striatum at the time of the study, impacting our ability to detect differences between groups. Clearly, more information is needed about the development of higher levels of food reward in youth.
There are several strengths and limitations that should be noted. This is the first study to interrogate the reward regions using sucrose in a tight age range (8–12 years of age) of preadolescent children. The restricted age range in this study should provide some reduced concern regarding developmental variability. Additionally, we studied delivery of a taste in children when sated, which is important to elucidate the mechanisms that drive eating past nutritional needs. Furthermore, we used laboratory paradigms to assess overeating and sucrose liking. Limitations include the relatively small sample size; further studies with larger samples are necessary to support its validity. There are potential concerns about using transformation to adult stereotaxic space in children, due to differences in brain size and morphometry (e.g., due to synaptic pruning and myelination). Other evidence, however, suggests the developing brain achieves 95% of its peak size by age six, and aligning the brains of children over the age of seven to an adult template does no worse than when aligning typical adults.54–56 To address this limitation, we used nonlinear registration to the template atlas so as to improve accuracy. We did not report results for the ventromedial regions of the PFC due to susceptibility artifact. This signal drop out in the OFC limits our ability to make comparisons to frontal opercular findings in other studies. Additionally, this study utilized sucrose and water, which did not differentiate in the manner expected. Overall, OB children responded more to both sucrose and water compared to HW children. Other studies have used artificial saliva, which could be considered an aversive stimulus, potentially enhancing differentiation. Finally, this is a cross sectional study, and causal relationships cannot be inferred. There may be unmeasured variables that could contribute to these finding, such as socioeconomic status among others. Future studies should replicate these results in larger samples, and extend them longitudinally to evaluate developmental differences between OB and HW children over time, and how changes relate to overeating.
While these data from this study join a very small base of research on the development of food reward and overeating and obesity in children, they do provide some intriguing hypotheses regarding overeating. Our data support the hypothesis that obese children are hyper-responsive to the taste of sucrose and water compared to healthy weight children during the preadolescent years. Although non-significant in this small sample, the overeating paradigm and sucrose liking tasks could be used to evaluate the differences in neural substrates of liking and wanting. As this research is in its infancy, it is an important first step toward the goal of understanding neural development that may contribute to overeating, and to the development of tailored obesity interventions and prevention for youth in the future.
Acknowledgments
Supported by grants to KB (R01DK094475; R01 DK075861; K02HL112042; Department of Pediatrics, UCSD) and WK (MH046001, MH042984, MH066122; MH001894; MH092793), the Price Foundation, and the Davis/Wismer Foundation.
Footnotes
Disclaimer statement: Authors have no conflicts to declare
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biro F, Wien M. Childhood obesity and adult morbidities. Am J Clin Nut May. 2010;91(5):1499S–505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franks P, Hanson R, Knowler W, Sievers M, Bennett H, Looker H. Childhood obesity, other cardiovascular risk factors, and premature death. New England J of Med. 2010;362(6):485–93. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobstein T, Bauer L, Uauy R. Obesity in children and young people: a crisis in public health. Obesity Reviews. 2004;5 (Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 5.Reilly J, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int J Obes. 2011;35(7):891–98. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 6.Herman K, Craig C, Gauvin L, Katzmarzyk P. Tracking of obesity and physical activity from childhood to adulthood: The Physical Activity Longitudinal Study. Int J Obes. 2009;4(4):281–88. doi: 10.3109/17477160802596171. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen S, Siega-Riz A, Popkin B. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obesity Research. 2002;10(5):370–78. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- 8.Tataranni P, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obesity Reviews. 2003;4(4):229–38. doi: 10.1046/j.1467-789x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 9.Berthoud H. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity. 2006;14(Suppl 5):197S–200S. doi: 10.1038/oby.2006.308. [DOI] [PubMed] [Google Scholar]
- 10.Volkow N, Wang G, Baler R. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15(1):37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang G, Volkow N, Logan J, Pappas N, Wong C, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–57. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 12.Dawe S, Loxton N. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev May. 2004;28(3):343–51. doi: 10.1016/j.neubiorev.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. Current drug abuse reviews. 2011;4(3):182–9. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol Psychiatry. 2013;73(9):804–10. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Volkow N, Wang G, Tomasi D, Baler R. Obesity and addiction: neurobiological overlaps. Obesity reviews. 2012;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wise R. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends in Neurosciences. 2009;32(10):517–24. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 18.Volz KG, Schubotz RI, von Cramon DY. Predicting events of varying probability: uncertainty investigated by fMRI. Neuroimage. 2003;19(2 Pt 1):271–80. doi: 10.1016/s1053-8119(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 19.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 20.Haber SN, Kim K, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26(32):8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaye W, Fudge J, Paulus M. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10(8):573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 22.Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85(1):45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes. 2010;34(1):94–104. doi: 10.1038/ijo.2009.193. [DOI] [PubMed] [Google Scholar]
- 24.Bruce A, Holsen L, Chambers R, Martin L, Brooks W, Zarcone J, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34:1494–500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31(12):4360–6. doi: 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Development. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- 29.Chambers R, Potenza M. Neurodevelopment, impulsivity, and adolescent gambling. Journal of gambling studies Journal of Gambling Studies. 2003;19(1):53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- 30.Post G, Kemper H. Nutrient intake and biological maturation during adolescence. The Amsterdam growth and health longitudinal study. Europ J of Clin Nutr. 1993;47(6):400–08. [PubMed] [Google Scholar]
- 31.Watkins B, Frampton I, Lask B, Bryant-Waugh R. Reliability and validity of the child version of the eating disorder examination: A preliminary investigation. Int J of Eat Dis. 2005;38(2):183–87. doi: 10.1002/eat.20165. [DOI] [PubMed] [Google Scholar]
- 32.Sheehan D, Sheehan K, Shytle R, Janavs J, Bannon Y, Rogers J, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) J Clin Psychiatry. 2010;71(3):313–26. doi: 10.4088/JCP.09m05305whi. [DOI] [PubMed] [Google Scholar]
- 33.Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;324:1–27. [PubMed] [Google Scholar]
- 34.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J of Clin Nutr. 2002;76(1):226–31. doi: 10.1093/ajcn/76.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutelle KN, Zucker NL, Peterson CB, Rydell SA, Cafri G, Harnack L. Two novel treatments to reduce overeating in overweight children: A randomized controlled trial. J Consult Clin Psychol. 2011;79(6):759–71. doi: 10.1037/a0025713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nolan-Poupart S, Veldhuizen M, Geha P, Small D. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite. 2013;60(1):168–74. doi: 10.1016/j.appet.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mennella J, Lukasewycz L, Griffith J, Beauchamp G. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chemical Senses. 2011;36(4):345–55. doi: 10.1093/chemse/bjq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner A, Aizenstein H, Frank GK, Figurski J, May JC, Putnam K, et al. Altered insula response to a taste stimulus in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33(3):513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 39.Frank G, Kaye W, Carter C, Brooks S, May C, Fissel K, et al. The evaluation of brain activity in response to taste stimuli--a pilot study and method for central taste activation as assessed by event related fMRI. J Neurosci Methods. 2003;131(1–2):99–105. doi: 10.1016/s0165-0270(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 40.Kwong K, Belliveau J, Chesler D, Goldberg I, Weisskoff R, Poncelet B, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci USA. 1992;89(12):5675–79. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa S, Tank D, Menon R, Ellermann J, Kim S, Merkle H, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89(13):5951–55. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rolls E. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1123–36. doi: 10.1098/rstb.2006.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Small D. Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes. 2009;33(Suppl 2):S44–48. doi: 10.1038/ijo.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desikan R, Segonne F, Fischl B, Quinn B, Dickerson B, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 46.Saad Z, Glen D, Chen G, Beauchamp M, Desai R, Cox R. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44(3):839–48. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 48.Pinheiro J, Bates D, DebRoy S, Sarkar D The R Development Core Team. R package version 3.1–109. 2013. nlme: Linear and Nonlinear Mixed Effects Models. [Google Scholar]
- 49.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc B-Methodological. 1995;57:289–300. [Google Scholar]
- 50.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zald D, Pardo J. Cortical activation induced by intraoral stimulation with water in humans. Chem Senses. 2000;25(3):267–75. doi: 10.1093/chemse/25.3.267. [DOI] [PubMed] [Google Scholar]
- 52.Haycock J, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish S. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry. 2003;87(3):574–85. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- 53.Seeman P, Bzowej N, Guan H, Bergeron C, Becker L, Reynolds G, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1(5):399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- 54.Giedd J, Rapoport J. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67(5):728–34. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muzik O, Chugani D, Juhasz C, shen C, Chugani H. Statistical parametric mapping: assessment of application in children. NeuroImage. 2000;12(5):538–49. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- 56.Burgund E, Kang H, Kelly J, Buckner R, Snyder A, Petersen S, et al. Feasibility of a common stereotactic space for children and adults in fMRI studies of development. NeuroImage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]