Abstract
Background/Objectives
We have reported that maternal overnutrition/obesity (OB) in sheep resulting from feeding 150% of National Research Council (NRC) requirements throughout gestation, leads to maternal hyperglycemia and hyperinsulinemia. Further, newborn lambs born to OB vs. control-fed (CON, 100% of NRC) ewes exhibited greater adiposity, increased blood cortisol, insulin and glucose and the elimination of the postnatal leptin spike seen in lambs born to CON ewes. This early postnatal leptin peak is necessary for development of hypothalamic circuits which program appetite in later life. This study evaluated the multigenerational impact of OB on insulin:glucose dynamics of mature female F1 offspring fed only to requirements throughout gestation, and on their lambs (F2 generation).
Design and Methods
Adult F1 female offspring born to OB (n=10) or CON (n=7) ewes were utilized. All F1 ewes were subjected to a glucose tolerance test at midgestation and late gestation. Jugular blood was obtained from F2 lambs at birth (day 1) through postnatal day 11, and plasma glucose, insulin, cortisol and leptin concentrations determined. Dual Energy X-ray Absorptiometry (DEXA) was utilized to determine bone mineral density (BMD), bone mineral content (BMC), lean tissue mass, and fat tissue mass.
Results
Fasted blood glucose and insulin concentrations were greater (P < 0.05) in OBF1 than CONF1 ewes at mid- and late gestation. Further, after glucose infusion, both glucose and insulin concentrations remained higher in OBF1 ewes (P < 0.05) than CONF1 ewes demonstrating greater insulin resistance. Blood concentrations of glucose, insulin, and cortisol, and adiposity were higher (P < 0.01) in OBF2 lambs than CONF2 lambs at birth. Importantly, OBF2 lambs failed to exhibit the early postnatal leptin peak exhibited by CONF2 lambs.
Conclusions
These data suggest that these OBF2 lambs are predisposed to exhibit the same metabolic alterations as their mothers, suggesting a multi-generational programming effect.
Keywords: sheep, fetal programming, neonatal leptin spike, multi-generational effect, obesity
INTRODUCTION
Obesity is increasing at an exponential rate worldwide. In the US it is estimated that 68% of the population are overweight, and 33.3% of men and 35.3% of women are obese.1, 2 Of pregnant women in the USA, 18-35% are estimated to be clinically obese.3 Further, children born to overweight/ obese women are at an increased risk of developing symptoms of the metabolic syndrome including obesity, insulin resistance, hyperglycemia, hyperlipidemia, hypertension, and cardiovascular disease.4, 5
In both rodents and sheep, there is a surge in leptin which occurs during the first 2-3 weeks of postnatal life6,7 and this leptin surge is an important trophic factor in the establishment of hypothalamic circuits that control energy homeostasis and appetite.8,9 Further, leptin has a powerful trophic action in the brain. In vitro exposure to leptin has been shown to increase the growth of axons from arcuate nucleus (ARC) neurons, which regulate feeding,10 and leptin administration to leptin-deficient ob/ob mice, only during the first few days of postnatal life, restored brain weight, and returned ARC projections to normal levels.10,11
Diet-induced obesity during pregnancy modifies the postnatal leptin surge in offspring of several mammalian species including both altricial (rodents) and precocial (sheep) species leading to increased appetite, adiposity, as well as insulin and leptin resistance in adulthood.7,12 Significant evidence indicates that insulin and leptin act on the brain as adiposity feedback signals.13 Additionally, neonatal leptin administration has been shown to normalize hyperglycemia and hyperinsulinemia and to increase insulin sensitivity.14
The “developmental origins of adult disease hypothesis”, proposes that an adverse environment in early life can alter normal growth and development predisposing to obesity and other related diseases in adulthood.15 In agreement with this concept, increasing evidence indicates that some diseases do not have their beginnings in adulthood.16,17 Evidence is now accumulating that demonstrates that the consequences of an environmental insult such as maternal overnutrition/obesity may not be limited to their immediate offspring (F1 generation), but may show multigenerational transfer to future generations.18,19 In rodent models, cardiometabolic traits induced by direct exposure to early environmental conditions such as maternal overnutrition and /or obesity prior to conception, during pregnancy, and /or while nursing were observed in the subsequent unexposed generations. 20,21 Similarly, studies in rodent models utilizing restricted diets (protein and/or energy) in F0 mothers during pregnancy and /or lactation have also reported programmed effects in both F1 and F2 generation offspring on birth weight, glucose tolerance, blood pressure and obesity.22-25 Human cohort studies especially the Dutch “winter hunger” studies have also provided additional evidence for multigenerational effects of maternal undernutrition on offspring adiposity and cardiometabolic disease. 26,27 Studies in this area are inherently difficult to perform in human subjects. Therefore, the majority of current research has focused on animal models. While interspecies differences, particularly during the establishment of pregnancy and fetal development, do occur, the ability to control maternal diet and examine comprehensive outcomes at different developmental stages in a precocious and monotocous species such as sheep provides important insights and understanding of the impact of maternal obesity on fetal and offspring development.
The sheep is similar to humans in that the young are generally delivered in fewer numbers, and born after a greater degree of intra-uterine development. Further, the weight of pregnant ewes is similar to that of pregnant women, as is the ratio of fetal to maternal weight. Due to its long gestational length, and relative ease of fetal and maternal instrumentation, the sheep has emerged as a major animal model for studying the impact of malnutrition on the developing fetus and resulting offspring. While sheep are ruminants, and humans are monogastrics and require different diets, the fetus and newborn are dependent on glucose from the mother as the major energy source, and similar metabolic disturbances have been observed in the offspring of overnourished/obese ewes and women including hyperphagia, hyperglycemia, hyperinsulinemia, increased adiposity, and insulin resistance.12,28,29
The objective of this study was to evaluate the multi-generational impact of maternal overfeeding/obesity in F0 on glucose:insulin dynamics of their adult female offspring (F1) when fed only to requirements during pregnancy, and to evaluate the subsequent phenotype of their granddaughters (F2).
MATERIALS AND METHODS
F1 generation
All procedures were approved by the University of Wyoming Animal Care and Use Committee. Seventeen singleton Rambouillet/Columbia cross female F1offspring - born to either control (CON) ewes (n=7) fed a highly palatable pelleted diet (88.5% DM, 17.4% CP, 93.9% in-vitro dry matter digestibility) at 100% of National Research Council (NRC)30 recommendations or to obese overnourished (OB) ewes fed the same diet at 150% of NRC recommendations (n=10) from 60 days prior to conception to term, were utilized in this study. During lactation, all ewes (F0) were given free choice access to high quality alfalfa hay and were supplemented with cracked corn to meet NRC recommendations for a lactating ewe. OBF1 and CONF1 ewe lambs were housed together and fed at 100% NRC requirements from weaning until sexual maturity (2 to 3 years of age). F1 ewes were checked for estrus twice daily with a single intact Rambouillet/Columbia cross ram at 0700 and 1700 hr. All ewes conceived at first estrus, and CONF1 and OBF1 ewes continued to be fed at 100% of NRC recommendations from mating through weaning of their lambs. All F1 ewes were weighed and body condition scored at weekly intervals throughout pregnancy to evaluate changes in body composition. Body Condition Score is highly related to carcass lipids and can be used to estimate energy reserves available to the ewe.31 Jugular blood samples were collected from F1ewes at d0 (first day of mating), d45, d75, and d135 of gestation. Blood was collected into vacutainer tubes (BD Vacutainer, Franklin Lakes, NJ; serum tube 16 × 100, 10mL) without anticoagulant, allowed to sit for 1 hr at room temperature, and then refrigerated at 4°C overnight. Serum was collected after centrifugation (2500 × g for 15 min) the following morning and frozen at −80°C until analyzed for selected hormones and metabolites. Conception was verified via real time ultrasonography, at d45 and confirmed again at d60 (Ausonics Microimager 1000 sector scanning instrument, Ausonics Pty Ltd., Sydney, Australia).
F1 Intravenous glucose tolerance test
Randomly selected pregnant F1 ewes (OBF1, n=6 and CONF1, n=6) were subjected to an intravenous glucose tolerance test (IVGTT) at d75 (midgestation) and again at d135 (late gestation). Ewes were fasted for 24 hrs prior to testing and then placed in individual pens with free access to water. Catheters (Abbocath, 14 ga, Abbott Laboratories, North Chicago, IL) were placed aseptically into the jugular vein 7h before blood sampling. A 124.5 cm extension set (Seneca Medical, Tiffin, OH) was attached to the catheter to allow for infusion and sampling without disturbing the animal. Baseline samples (~ 6 mL) were collected 15 min before and immediately prior to administration of a glucose bolus infusion (0 min) (50% Dextrose, Vedco Inc., St. Joseph, MO), which was administered at a dose of 250 mg/kg of body weight. Blood samples were collected into chilled tubes (BD Vacutainer, Franklin Lakes, NJ; 143 U.S.P. units sodium heparin per 10 mL) at −15, 0, +2, +5, +10, +15, +20, +30, +45, +60, +90, and +120 min. Tubes were centrifuged at 2500 × g for 15 min and plasma frozen at −20°C until time of assay for glucose and insulin.
F2 morphometrics and blood collection
F1 ewes were allowed to lamb unassisted. Twenty-five F2 lambs were born, CONF2, n=12 (5 males and 7 females) and OBF2, n=13 (7 males and 6 females). CONF1 ewes had 4 sets of twins and 4 singletons, while OBF1 ewes had 4 sets of twins and 5 singletons. Body weight and morphometric measurements (crown rump length, thoracic and abdominal circumferences, and biparietal distance) were recorded at birth. Blood samples were collected into chilled tubes (BD Vacutainer, Franklin Lakes, NJ; 143 U.S.P. units sodium heparin per 10 mL) from the lambs via jugular venipuncture at birth (pre-suckling), and then daily from postnatal d1 through d7 then again on d9 and d11. Lambs blocked by sex (male vs. female) and birth type (singletons vs. twins), were selected for postnatal hormone analysis.
Dual energy x-ray absorptiometry (DEXA)
To accurately determine bone mineral density (BMD), bone mineral content (BMC), lean tissue mass, and fat tissue mass, Dual Energy X-ray Absorptiometry (DEXA, GE Lunar Prodigy™ 8743, Madison, WI) was utilized as previously described32 and validated for sheep.33,34 Newborn F2 lambs were immobilized by wrapping them tightly in a large towel. This technique was less stressful on the lambs than tranquilizing them with ketamine, prevented excessive movement and had no effect on DEXA values (< 1% difference). The whole body scan mode was used for all sheep, and scan times were 1-2 min depending on the size of the lamb. A single, blinded, and experienced investigator performed all DEXA scans and quantified body fat percentage, bone mineral density, and bone mineral content. DEXA was calibrated, and quality assurance tests were performed daily prior to measurement and according to the manufacturer’s specifications and programmed acceptable limits.
Biochemical assays
Plasma glucose was measured colorimetrically in triplicate (Liquid Glucose Hexokinase Reagent Set, Point Scientific, Inc., Canton MI) using 96-well plates as previously described.35 Mean intra-and inter-assay CV were 1.1% and 3.7%, respectively, with a sensitivity of 25 mg/dL. Plasma insulin was measured in duplicate by commercial RIA kit (Siemens Healthcare Diagnostics, Deerfield, IL). Intra- and inter-assay CV for insulin were 6.3% and 11.3%, with a sensitivity of 2.6 μIU/mL. Concentrations of cortisol were determined as described previously by Dong et al.36 using Coat-A-Count Cortisol RIA with a sensitivity of 0.5 ng/mL (Siemens Medical Solutions Diagnostics, Los Angeles, CA) with an intra- and inter-assay CV of 3.6% and 4.6% respectively. Plasma leptin concentrations were measured in a single assay using a commercial RIA (LINCO Multispecies Leptin RIA, Linco Research, St. Charles, MO) with an intra-assay CV of 5.2% and a sensitivity of 0.5 ng/mL.
Statistical analyses
Significance was set at P < 0.05, with a tendency at P < 0.10. To calculate fasted glucose and insulin levels from the IVGTT, baseline samples were averaged across the −15 min and 0 min samples. Comparison of maternal blood samples collected during the IVGTT at d0 (mating), d45, d75, and d135 of gestation and F2 postnatal blood samples (days 1 to 7, and on days 9 and 11) were analyzed as repeated measures using the Proc Mixed procedure of SAS (SAS Inst. Inc., Cary, NC). Area under the curve was measured using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA). Gestation length, F2 lamb birth weights, percent fat, and bone mineral density at birth (determined by DEXA) was compared using the GLM procedure of SAS.
RESULTS
Gestation length of OBF1 (P < 0.05) ewes was shorter than that of CONF1 ewes (151.2 ± 0.4 vs. 153.6 ± 0.5 days, respectively). Gestation length was similar for singleton and twin pregnancies. No treatment (OBF1 vs. CONF1) differences were observed in either body weight or BCS from conception through lambing (data not shown). While BCS remained relatively constant throughout pregnancy, body weights remained relatively constant through 8-10 weeks of gestation then progressively increased thereafter in association with the increase in gravid uterine weight in both groups.
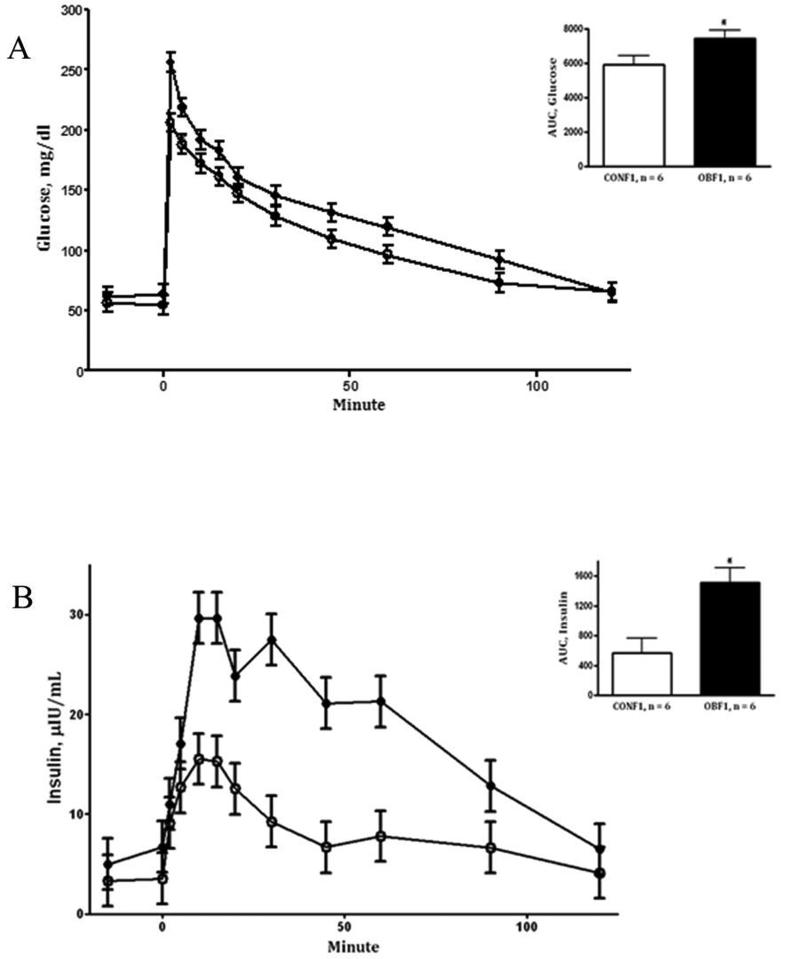
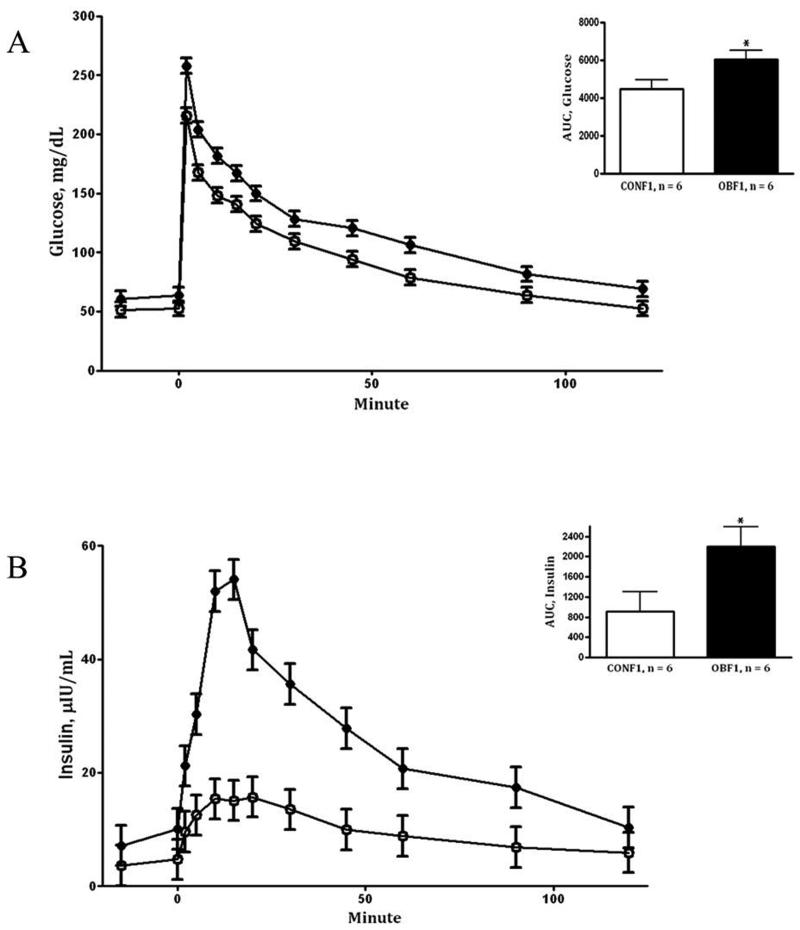
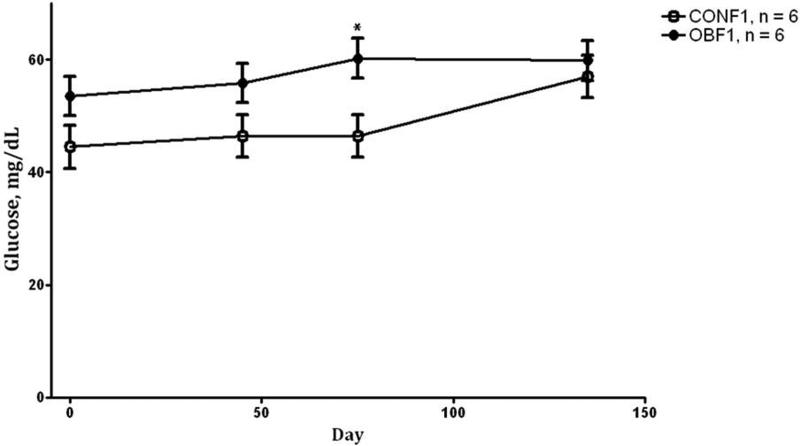
During the IVGTT at day 75 (midgestation), OBF1 ewes demonstrated greater (P < 0.05) fasting baseline glucose and insulin concentrations than CONF1 ewes (64.0 ± 4.0vs. 54.7 ± 1.3 mg/dL and 7.0 ± 2.2 vs. 3.4 ± 0.2 μIU/L, respectively). After glucose infusion, glucose concentrations remained greater in OBF1 ewes (area under the curve; P < 0.01, Fig.1A) than in CONF1 ewes during the post infusion period. Plasma insulin concentrations of OBF1 ewes during the IVGTT were also markedly increased (P < 0.05) when compared to CONF1 ewes (Fig.1B). During the IVGTT at day 135 (late gestation), fasted baseline glucose and insulin concentrations remained greater (P < 0.05) in OBF1 vs. CONF1 ewes (62.3 ± 2.0 vs. 52.0 ± 2.0 mg/dL and 8.6 ± 1.1 vs. 4.2 ± 1.1 μIU/L, respectively). Further, after glucose infusion, plasma glucose and insulin concentrations were markedly increased in OBF1 ewes compared to CONF1 ewes (area under the curve; P < 0.05, Fig. 2A and 2B). Blood glucose concentrations obtained at day 0 (conception), day 45, day 75, and day 135 were elevated (P < 0.01) in the OBF1 ewes in comparison to CONF1 ewes averaging 56.9 ± 3.6 and 48.8 ± 3.7 mg/dL, respectively over the whole period (Fig. 3).
Figure 1.
Glucose (A) and insulin (B) concentrations prior to and after glucose bolus infusion during an intravenous glucose tolerance test at day 75 of gestation in F1 ewes from obese (OBF1: ●) and control (CONF1: ○) dams fed at 100% NRC recommendations throughout gestation. Area under the curve (AUC) is located in the top right corner of each panel. *Means ± SEM differ (P < 0.05).
Figure 2.
Glucose (A) and insulin (B) concentrations prior to and after glucose bolus infusion during an intravenous glucose tolerance test at day 135 of gestation in F1 ewes from obese (OBF1: ●) and control (CONF1: ○) dams fed at 100% NRC recommendations throughout gestation. Area under the curve (AUC) is located in the top right corner of each panel. *Means ± SEM differ (P < 0.05).
Figure 3.
Glucose concentrations of F1 ewes from obese (OBF1: ●) and control (CONF1: ○) dams fed 100% NRC recommendations at day 0, 45, 75, and 135 of gestation. *Means ± SEM differ (P < 0.01).
Postnatal DEXA scans showed OBF2 lambs had a greater percentage of body fat (P < 0.01) than CONF2 lambs (9.7 ± 0.6% vs. 7.1 ± 0.6%, respectively) despite the similar birth weights in the two lamb groups (Table 1). Birth weight tended to be increased (P < 0.10) in male vs. female newborn F2 lambs and in singletons compared to twins. Total fat (g) was greater (P < 0.05) in OBF2 than CONF2 lambs, and also tended to be greater (P < 0.10) in males versus females and in singles versus twins (Table 1). Also, bone mineral density and bone mineral content were lower (P < 0.01) in female compared to male lambs (Table 1). Postnatal morphometric measurements, including crown rump length and right and left humerus length did not differ between treatment groups (Table 2). However, thoracic girth was decreased (P < 0.05) in OBF2 vs.CONF2 lambs and also in twins vs. singletons. Abdominal girth also tended to be decreased (P < 0.10) in OBF2 vs. CONF2 lambs.
Table 1.
Birth weight and % fat determined by DEXA scans in F2 newborn lambs.
| Treatment | Sex | Birth Type | ||||
|---|---|---|---|---|---|---|
| CONF2, n=12 | OBF2, n=13 | Female, n=13 | Male, n=12 | Single, n=9 | Twin, n=8 | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| Birthweight (kg) | 5.2 ± 0.2 | 5.0 ± 0.2 | 4.8 ± 0.2c | 5.4 ± 0.2d | 5.7 ± 0.3c | 5.1 ± 0.2d |
| Body Fat (%) | 7.1 ± 0.6a | 9.7 ± 0.6b | 8.1 ± 0.6 | 8.7 ± 0.6 | 8.7 ± 0.9 | 7.7 ± 0.5 |
| BMD (g) | 0.35 ± 0.01 | 0.33 ± 0.01 | 0.32 ± 0.01a | 0.38 ± 0.01b | 0.36 ± 0.02 | 0.35 ± 0.01 |
| BMC (g/cm2) | 121.6 ± 7.7 | 120.5 ± 7.2 | 106.5 ± 7.0a | 135.6 ± 8.0b | 130.9 ± 11.2 | 122.7 ± 6.6 |
| Fat (g) | 379.5 ± 26.5a | 493.0 ± 24.6b | 391.2 ± 24.1c | 481.2 ± 27.4d | 506.6 ± 38.3c | 407.7 ± 22.8d |
Means ± SEM differ (P < 0.05)
Means ± SEM differ (P < 0.05)
Means ± SEM differ (P < 0.10). Newborn F2 lambs born to offspring from obese ewes fed 150% NRC recommendations (OBF2) and control ewes fed 100% NRC recommendations (CONF2).
Means ± SEM differ (P < 0.10). Newborn F2 lambs born to offspring from obese ewes fed 150% NRC recommendations (OBF2) and control ewes fed 100% NRC recommendations (CONF2).
BMD= Bone Mineral Density; BMC=Bone Mineral Content.
Table 2.
Morphometrics at birth in F2 newborn lambs.
| Treatment | Sex | Birth Type | ||||
|---|---|---|---|---|---|---|
| CONF2, n=12 | OBF2, n=13 | Female, n=13 | Male, n=12 | Single, n=9 | Twin, n=8 | |
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |
| CRL (cm) | 52.2 ± 1.1 | 50.1 ± 1.0 | 50.1 ± 1.0 | 52.2 ± 1.2 | 52.0 ± 1.6 | 51.4 ± 1.0 |
| BPD (cm) | 15.6 ± 0.2 | 16.1 ± 0.2 | 15.8 ± 0.2 | 15.9 ± 0.2 | 16.4 ± 0.3 | 15.5 ± 0.2 |
| RH (cm) | 14.9 ± 0.4 | 14.9 ± 0.4 | 14.5 ± 0.3 | 15.4 ± 0.4 | 15.0 ± 0.6 | 14.7 ± 0.3 |
| LH (cm) | 15.0 ± 0.4 | 15.0 ± 0.4 | 14.6 ± 0.4 | 15.4 ± 0.4 | 15.2 ± 0.6 | 14.8 ± 0.4 |
| TG (cm) | 41.4 ± 0.8a | 38.5 ± 0.7b | 38.9 ± 0.7 | 41.1 ± 0.8 | 42.9 ± 1.1a | 39.9 ± 0.6b |
| AG (cm) | 42.2 ± 0.9c | 39.9 ± 0.9d | 40.2 ± 0.9 | 42.0 ± 1.0 | 43.7 ± 1.4 | 40.8 ± 0.8 |
Means ± SEM differ (P < 0.05)
Means ± SEM differ (P < 0.05)
Means ± SEM differ (P < 0.10). Newborn F2 lambs born to offspring from obese ewes fed 150% NRC recommendations (OBF2) and control ewes fed 100% NRC recommendations (CONF2). CRL= Crown Rump Length; BPD= Biparietal Distance; RH=Right Humerus Length; LH= Left Humerus Length; TG=Thoracic Girth; AG=Abdominal Girth.
Means ± SEM differ (P < 0.10). Newborn F2 lambs born to offspring from obese ewes fed 150% NRC recommendations (OBF2) and control ewes fed 100% NRC recommendations (CONF2). CRL= Crown Rump Length; BPD= Biparietal Distance; RH=Right Humerus Length; LH= Left Humerus Length; TG=Thoracic Girth; AG=Abdominal Girth.
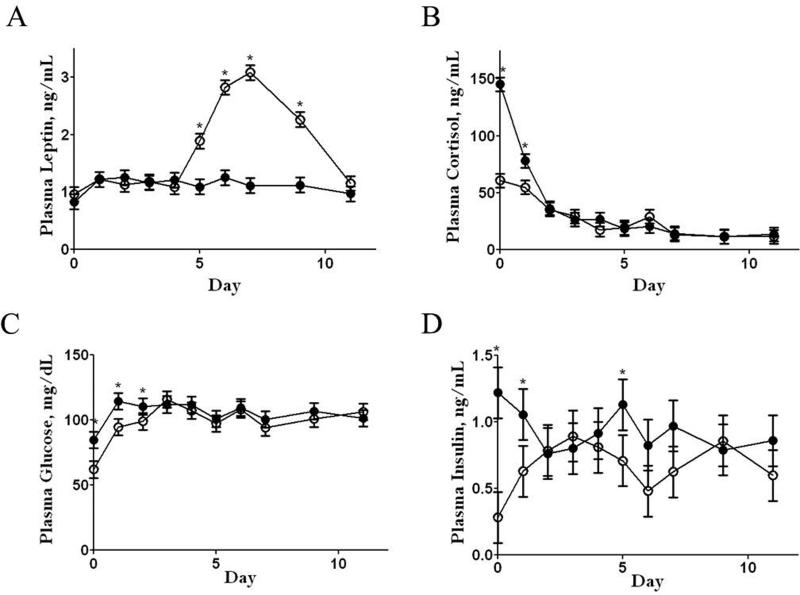
Leptin concentrations were similar for CONF2 and OBF2 lambs from postnatal day 1 to 4. Thereafter, plasma leptin concentrations of CONF2 lambs increased (P < 0.05) from postnatal day 4 to 7, remaining higher than values of OBF2 lambs from day 5 to day 9 before returning to pre-peak levels by day 11 (Figure 4, Panel A). In contrast, leptin concentrations of OBF2 lambs remained constant from day 1 through day 11. At birth and on day 1 of postnatal life, plasma cortisol was elevated (P < 0.01) in OBF2 lambs compared with CONF2 lambs (Figure 4, Panel B). Plasma glucose concentrations were also higher (P < 0.01) from birth through day 2 of postnatal life in OBF2 lambs (Figure 4, Panel C) compared to CONF2 lambs. At birth through day 1, and again on day 5, plasma insulin levels of OBF2 lambs were greater (P < 0.01) than those of CONF2 lambs. (Figure 4, Panel D).
Figure 4.
Plasma leptin, cortisol, glucose, and insulin in the early postnatal period (days 1 through 7 and again at days 9 and 11) in F2 lambs from obese (●) and control (○) grandmothers. *Means ± SEM differ (P < 0.01).
DISCUSSION
This is the first evidence using a large animal model demonstrating that maternal overnutrition/obesity can program granddaughters for increased adiposity at birth, and later in life. In the absence of excess nutrition or obesity, OBF1 ewes exhibited a greater insulin resistance, as evidenced by markedly increased insulin to glucose ratio in systemic blood, when compared to CONF1 ewes. We have previously reported, a positive correlation between insulin and glucose levels in the blood of OBF1 ewes demonstrating that more insulin is required per unit of glucose for transport into body tissues.28 This is consistent with research by Ahren and Pacini37 who reported that to compensate for insulin resistance, insulin output is enhanced, resulting in hyperinsulinemia in response to decreased insulin sensitivity in peripheral tissues. During normal human pregnancy, maternal pancreatic β-cells demonstrate remarkable plasticity. Significant adaptations are made in islet size and secretion capacity to meet the metabolic demand of pregnancy thus progressively increasing insulin secretion to compensate for insulin resistance, maintaining normal blood glucose concentrations.38,39 However, offspring of obese mothers may be incapable of increasing insulin secretion to maintain proper glucose metabolism due to pre-programmed β-cell dysfunction, leading to hyperglycemia. Inadequate β-cell function in OBF1 ewes is demonstrated by the higher glucose at day 0 (mating), day 45, day 75, and day 135 in pregnant OBF1 ewes compared to pregnant CONF1 ewes.
Since glucose uptake by maternal tissues appears to be impaired, excess maternal glucose may be redirected into the fetal compartment. Glucose transport across the placenta is driven by the maternal:fetal glucose ratio and thus maternal hyperglycemia can lead to fetal hyperglycemia, hyperinsulinemia, enhanced glycogen synthesis, and lipogenesis.40 Glucose is the principal substrate supplying the uterus, placenta, and fetus41 and is an essential component in fetal growth, with concentrations increasing as gestation progresses.40 These data demonstrate that throughout pregnancy, F1 females born to OB mothers exhibit marked increases in insulin resistance when compared to F1 females born to CON mothers independent of a difference in maternal diet, potentially subjecting their fetuses to the same blood glucose elevations as their mothers during fetal life. Although birth weight in the F2 generation did not differ between treatment groups, newborn OBF2 lambs born to OBF1 ewes demonstrated markedly greater adiposity, as well as hyperglycemia and hyperinsulinemia when compared to CONF2 lambs. Similar to OBF1 and OBF2 lambs during the postnatal period, rodent offspring from obese mothers fed a high-fat diet showed similar birth weights to offspring born to control-fed mothers accompanied by increased adiposity.42 According to Caluwaerts et al.42 exposure of the rat fetus to maternal glucose intolerance or a diabetic intrauterine environment imposed by a maternal obesogenic diet may alter fetal metabolic pathways, facilitating fat accumulation and glucose intolerance in postnatal life. One reason for glucose intolerance in offspring developing in an obese intrauterine environment may be an increase in adipocyte size.42 Accumulation of additional lipid can cause mature, lipid-containing adipocytes to enlarge during the differentiation process.43 Once the capacity of lipid storage within the adipocyte is reached, excess energy substrates are redistributed to other organs, such as the liver, muscle, and pancreas, accompanied by insulin resistance44 resulting in ectopic fat deposition. Abnormal deposition of lipid is characteristic of type II diabetes, including glucose overproduction by the liver, insulin resistance at the muscle, and decreased insulin secretion by the pancreas.44
Across treatment groups, singleton F2 lambs tended to be heavier than twins and male F2 lambs tended to be heavier than F2 female lambs at birth. Additionally, DEXA scans revealed that male F2 lambs exhibited markedly greater BMD and BMC than F2 females regardless of birth type. Some studies claim individuals with a higher body weight have higher BMD than those with lower body weight at the same age,45 while others have failed to find gender-related differences in bone mass using DEXA scans.46 In this study, higher BMD and BMC in male lambs may be attributed to greater longitudinal and periosteal bone growth than female lambs.47 Fat percentages were similar across sexes and birth types. This may indicate that both males and females and singletons and twins were responding in a similar manner to the maternal intrauterine environment. According to Harrington et al.48 alterations in adipose tissue deposition between growth-restricted and appropriate-for-gestational age (AGA) infants arises because of differences in subcutaneous fat rather than intra-abdominal fat, since no differences in intra-abdominal fat were prevalent using magnetic resonance imaging scans. These authors suggest that subcutaneous and intra-abdominal fat depots may be under different regulatory control during intrauterine development. The increase in body weight but similar body fat percentages in male and female F2 newborn lambs may be attributable to visceral fat deposition as opposed to subcutaneous. It is important to note that sheep and humans deposit fat differently. Human infants are more likely to first deposit fat subcutaneously rather than intra-abdominally, have more subcutaneous fat at birth, and have a greater percentage of their body weight accounted for by fat mass than lambs (15% vs. 3% fat, respectively).49,50 Further, it was reported by Stini51 that malnutrition in human infants reduces body fat from subcutaneous fat depots. Cnop et al.3 investigated the possible relationship between body fat distribution and insulin sensitivity and its relation to leptin levels in three different groups of individuals classified as lean insulin-sensitive (LIS), lean-insulin resistant (LIR), and obese insulin-resistant (OIR). These authors proposed that intra-abdominal fat was the most important predictor of insulin sensitivity, but fasting leptin levels (which were the highest in the OIR group) were more strongly associated with subcutaneous fat than intra-abdominal fat. Researchers have linked the increased deposition of intra-abdominal fat to insulin resistance52,53 and other disorders comprising the metabolic syndrome,54,55 suggesting that individuals with greater amounts of fat in this region experience disorders of the metabolic syndrome more often than those who distribute fat to peripheral locations.56,57 Therefore, alternative fat deposition from intrauterine programming effects may predispose offspring to future glucose intolerance and hyperleptinemia in later life. Although associations have been made among fat mass, BMD, and BMC, further research is necessary to determine the role intrauterine programming plays in fat deposition and bone formation.
The neonatal leptin peak was eliminated in both OBF17 and OBF2 lambs (present study) compared to CONF1 and CONF2 lambs, indicating a multi-generational effect. Bouret et al.58 has recently reported that leptin is essential for normal development of axonal projections from the arcuate nucleus to surrounding hypothalamic nuclei, thus programming the hypothalamic circuitry responsible for regulating appetite during postnatal life. Impairment in leptin receptor signaling may adversely affect axonal projections from the arcuate nucleus to their targets, affecting energy balance.58 In postnatal lambs, Muhlhausler et al.29 reported that mRNA expression of the leptin receptor in the arcuate nucleus of the hypothalamus was inversely related to fat mass. Elimination of this leptin peak may predispose rodent offspring of obese overnourished mothers to increased adiposity and decreased sensitivity to leptin in adulthood.10 Likewise, adult F1 females born to obese F0 ewes and subjected to ad libitum feeding exhibited markedly increased appetites, as well as glucose and insulin dysregulation, increased adiposity, and were hyperleptinemic when compared to F1females born to control-fed F0 ewes.12
OBF1 lambs7 and subsequently their offspring, OBF2 lambs (present study), exhibited elevated plasma cortisol levels on the day of birth and the beginning of postnatal life compared with CONF1 and CONF2 lambs. Cortisol has an important role in prenatal regulation of cell proliferation and differentiation to mature fetal tissues in preparation for extra-uterine life.59 Cortisol may cause premature differentiation of adipocytes, possibly altering the timing of the neonatal leptin peak and/or the quantity of leptin secreted. This idea is supported by Long et al.60 who reported that F2 offspring of ewes administered exogenous glucocorticoids during late gestation exhibited a similar elimination of the neonatal leptin peak as seen in offspring of overnourished/obese ewes and that this was associated with increased plasma cortisol from birth until day 2 of life. Further research is necessary to elucidate the mechanisms involving glucocorticoids, adipocyte differentiation, and leptin secretion.
Finally, these data do not conclusively demonstrate transgenerational epigenetic mechanisms, as the exposure of our Founder Generation (F0) to an obesogenic diet also resulted in the direct in utero exposure of the F1 generation, as well as F2 generation through germ-line exposure.19 They do, however, demonstrate a multigenerational effect resulting from a generationally recurring mechanism (e.g. gestational diabetes in MOF1) even in the absence of excess nutrition and obesity. Pregnancy may have constituted a “second hit”, as these F1 mothers failed to exhibit an altered phenotype in the nonpregnant state. As discussed, both OBF1 and OBF2 lambs exhibit increased plasma levels of glucose, insulin, and cortisol at birth, increased visceral adiposity and the elimination of a postnatal leptin peak, known to program appetitic centers for later life in comparison to CONF1 and CONF2 lambs. A combination of maternal hyperglycemia, dyslipidemia, altered insulin-signaling, and obesity may result in altered intrauterine programming of the fetus leading to the potential to develop the metabolic syndrome in later life. As we have seen in our sheep model, exposure of adult offspring from OB ewes to a bout of ad libitum feeding induces hyperphagia, leading to increased adiposity, and intensifies preprogrammed insulin resistance and hyperglycemia when compared to CONF1offspring. These data suggest that to avoid the adverse health risks of the metabolic syndrome, eating only to requirements may not only benefit the mother and her own health, but the health of her daughters and granddaughters.
ACKNOWLEDGMENTS
The authors thank the students of the Center for the Study of Fetal Programming for their assistance in animal care and data collection on the farm. The authors would also like to thank Adam Uthlaut and Robert Cordery-Cotter for animal care and management. This work was supported by National Institutes of Health (NIH) INBRE #P20 RR016474.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no competing interests, financial or otherwise.
REFERENCES
- 1.Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–487. doi: 10.1016/j.ajog.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;82:1–7. [PubMed] [Google Scholar]
- 3.Cnop M, Landchild MJ, Vidal J, Havel PJ, Knowles NG, Carr DR, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations: distinct metabolic effects of two fat compartments. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- 4.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 5.Mingrone G, Manco M, Mora ME, Guidone C, Iaconelli A, Gniuli D, et al. Influence of maternal obesity on insulin sensitivity and secretion in offspring. Diabetes Care. 2008;31:1872–1876. doi: 10.2337/dc08-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delahave F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, et al. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149:470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- 7.Long NM, Ford SP, Nathanielsz PW. Maternal obesity eliminates the neonatal lamb plasma leptin peak. J Physiol. 2011;589:1455–1462. doi: 10.1113/jphysiol.2010.201681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouret SG. Leptin, nutrition, and the programming of hypothalamic feeding circuits. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:25–35. doi: 10.1159/000281143. [DOI] [PubMed] [Google Scholar]
- 9.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 11.Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256:600–602. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- 12.Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, et al. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, adiposity, and glucose tolerance in adult offspring. J Anim Sci. 2010;88:3546–3553. doi: 10.2527/jas.2010-3083. [DOI] [PubMed] [Google Scholar]
- 13.Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389–411. doi: 10.1152/physrev.00007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 15.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 16.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanson MA, Gluckman PD. Developmental origins of health and disease: moving from biological concepts to interventions and policy. Int J Gynaecol Obstet. 2011;115(Suppl 1):S3–S5. doi: 10.1016/S0020-7292(11)60003-9. [DOI] [PubMed] [Google Scholar]
- 18.Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Integr Comp Physiol. 2010;299:R711–722. doi: 10.1152/ajpregu.00310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn GA, Bale TL. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology. 2009;150:4999–5009. doi: 10.1210/en.2009-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan M, Mitrani P, Sadhanandan G, Dodds C, Shbeir-Eldika S, Thamotharan S, et al. A high-carbohydrate diet in the immediate postnatal life of rats induces adaptations predisposing to adult-onset obesity. J Endocrinol. 2008;197:565–574. doi: 10.1677/JOE-08-0021. [DOI] [PubMed] [Google Scholar]
- 22.Zambrano E, Martınez-Samayoa P, Bautista C, Deas M, Guillen L, Rodriguez-Gonzalez G, et al. Sex differences in transgenerational alterations of growth and metabolism in progeny (f2) of female offspring (f1) of rats fed a low protein diet during pregnancy and lactation. J Physiol. 2005;566:225–236. doi: 10.1113/jphysiol.2005.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benyshek D, Johnston C, Martin J. Glucose metabolism is altered in the adequately-nourished grand-offspring (f 3 generation) of rats malnourished during gestation and perinatal life. Diabetologia. 2006;49:1117–1119. doi: 10.1007/s00125-006-0196-5. [DOI] [PubMed] [Google Scholar]
- 24.Jimenez-Chillaron JC, Isganaitis E, Charalambous M, Gesta S, Pentinat-Pelegrin T, Faucette RR, et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes. 2009;58:460–468. doi: 10.2337/db08-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison M, Langley-Evans SC. Intergenerational programming of impaired nephrogenesis and hypertension in rats following maternal protein restriction during pregnancy. Br J Nutr. 2009;101:1020–1030. doi: 10.1017/S0007114508057607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Painter R, Osmond C, Gluckman P, Hanson M, Phillips D, Roseboom T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 27.Lumey L, Stein AD, Kahn HS, Romijn J. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch hunger winter families study. Am J Clin Nutr. 2009;89:1737–1743. doi: 10.3945/ajcn.2008.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George LA, Uthlaut AB, Long NM, Zhang L, Ma Y, Smith DT, et al. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol. 2010;8:75. doi: 10.1186/1477-7827-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlhausler BS, Adam CL, Findlay PA, Duffield JA, McMillen IC. Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J. 2006;20:1257–1259. doi: 10.1096/fj.05-5241fje. [DOI] [PubMed] [Google Scholar]
- 30.National Research Council, editor. Nutrient requirements of sheep. 6th edn. National Academy. Press; Washington, DC, USA: 1985. [Google Scholar]
- 31.Sanson DW, West TR, Tatman WR, Riley ML, Judkins MB, Moss GE. Relationship of body composition of mature ewes with condition score and body weight. J Anim Sci. 1993;71:1112–1116. doi: 10.2527/1993.7151112x. [DOI] [PubMed] [Google Scholar]
- 32.Ford SP, Zhang L, Zhu M, Miller MM, Smith DT, Hess BW, et al. Maternal obesity accelerates fetal pancreatic beta-cell but not alpha-cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297:R835–843. doi: 10.1152/ajpregu.00072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercier J, Pomar C, Marcoux M, Goulet F, Theriault M, Castonguay FW. The use of dual-energy X-ray absorptiometry to estimate the dissected composition of lamb carcasses. Meat Sci. 2006;73:249–257. doi: 10.1016/j.meatsci.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 34.Pearce KL, Ferguson M, Gardner G, Smith N, Greef J, Pethick DW. Dual X-ray absorptiometry predicts carcass composition from live sheep and chemical composition of live and dead sheep. Meat Sci. 2009;81:285–293. doi: 10.1016/j.meatsci.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, et al. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci. 2007;85:1285–1294. doi: 10.2527/jas.2005-624. [DOI] [PubMed] [Google Scholar]
- 36.Dong F, Ford SP, Nijland MJ, Nathanielsz PW, Ren J. Influence of maternal undernutrition and overfeeding on cardiac ciliary neurotrophic factor receptor and ventricular size in fetal sheep. J Nutr Biochem. 2008;19:409–414. doi: 10.1016/j.jnutbio.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahren B, Pacini G. Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies. Eur J Endocrinol. 2004;150:97–104. doi: 10.1530/eje.0.1500097. [DOI] [PubMed] [Google Scholar]
- 38.Aerts L, van Assche FA. Animal evidence for the transgenerational development of diabetes mellitus. Int J Biochem Cell Biol. 2006;38:894–903. doi: 10.1016/j.biocel.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Dhawan S, Georgia S, Bhushan A. Formation and regeneration of the endocrine pancreas. Curr Opin Cell Biol. 2007;19:634–645. doi: 10.1016/j.ceb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruager-Martin R, Hyde MJ, Modi N. Maternal obesity and infant outcomes. Early Hum Dev. 2010;86:715–722. doi: 10.1016/j.earlhumdev.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Hay WW., Jr Placental-fetal glucose exchange and fetal glucose metabolism. Trans Am Clin Climatol Assoc. 2006;117:321–339. discussion 339-340. [PMC free article] [PubMed] [Google Scholar]
- 42.Caluwaerts S, Lambin S, van Bree R, Peeters H, Vergote I, Verhaeghe J. Diet-induced obesity in gravid rats engenders early hyperadiposity in the offspring. Metabolism. 2007;56:1431–1438. doi: 10.1016/j.metabol.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Bunnell A, Estes BT, Guilak F, Gimble JM. Differentiation of adipose stem cells. Methods Mo. Biol. 2008;456:155–171. doi: 10.1007/978-1-59745-245-8_12. [DOI] [PubMed] [Google Scholar]
- 44.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 45.Hannan MT, Felson DT, Anderson JJ. Bone mineral density in elderly men and women: results from the Framingham osteoporosis study. J Bone Miner Res. 1992;7:547–553. doi: 10.1002/jbmr.5650070511. [DOI] [PubMed] [Google Scholar]
- 46.Koo WW, Walters J, Bush AJ, Chesney RW, Carlson SE. Dual-energy X-ray absorptiometry studies of bone mineral status in newborn infants. J Bone Miner Res. 1996;11:997–102. doi: 10.1002/jbmr.5650110717. [DOI] [PubMed] [Google Scholar]
- 47.Clark EM, Ness AR, Tobias JH. Gender differences in the ratio between humerus width and length are established prior to puberty. Osteoporos Int. 2007;18:463–470. doi: 10.1007/s00198-006-0275-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrington TA, Thomas EL, Frost G, Modi N, Bell JD. Distribution of adipose tissue in the newborn. Pediatr Res. 2004;55:437–441. doi: 10.1203/01.PDR.0000111202.29433.2D. [DOI] [PubMed] [Google Scholar]
- 49.McCance RA, Widdowson EM. Fat. Pediatr Res. 1977;11:1081–1083. [PubMed] [Google Scholar]
- 50.Spray CM, Widdowson EM. The effect of growth and development on the composition of mammals. Br J Nutr. 1950;4:332–353. doi: 10.1079/bjn19500058. [DOI] [PubMed] [Google Scholar]
- 51.Stini WA. Body composition and nutrient reserves in evolutionary perspective. World Rev Nutr Diet. 1981;37:55–83. doi: 10.1159/000397997. [DOI] [PubMed] [Google Scholar]
- 52.Park KS, Rhee BD, Lee KU, Kim SY, Lee HK, Koh CS, et al. Intra-abdominal fat is associated with decreased insulin sensitivity in healthy young men. Metabolism. 1991;40:600–603. doi: 10.1016/0026-0495(91)90050-7. [DOI] [PubMed] [Google Scholar]
- 53.Rendell M, Hulthen UL, Tornquist C, Groop L, Mattiasson I. Relationship between abdominal fat compartments and glucose and lipid metabolism in early postmenopausal women. J Clin Endocrinol Metab. 2001;86:744–749. doi: 10.1210/jcem.86.2.7260. [DOI] [PubMed] [Google Scholar]
- 54.Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–309. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 55.Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism. 1987;36:54–59. doi: 10.1016/0026-0495(87)90063-1. [DOI] [PubMed] [Google Scholar]
- 56.Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 57.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr. 1956;4:20–34. doi: 10.1093/ajcn/4.1.20. [DOI] [PubMed] [Google Scholar]
- 58.Bouret SG, Bates SH, Chen S, Myers MG, Jr., Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32:1244–125. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fowden Al, Forhead AJ. The role of hormones in intrauterine development. In: Barker DJP, editor. Fetal Origins of Cardiovascular and Like Disease. Marcel Decker; New York, NY, USA: 2000. pp. 199–228. [Google Scholar]
- 60.Long NM, Smith DT, Ford SP, Nathanielsz PW. Elevated glucocorticoids during ovine pregnancy increase appetite and produce glucose dysregulation and adiposity in their granddaughters in response to ad libitum feeding at 1 year of age. Am J Obstet Gynecol. 2013;209:353, e1–9. doi: 10.1016/j.ajog.2013.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]